Abstract
Reelin signalling in the early developing cortex regulates radial migration of cortical neurons. Later in development, Reelin promotes maturation of dendrites and dendritic spines. Finally, in the mature brain, it is involved in modulating synaptic function. In recent years, efforts to identify downstream signalling events induced by binding of Reelin to lipoprotein receptors led to the characterization of novel components of the Reelin signalling cascade. In the present review, we first address distinct functions of the Reelin receptors Apoer2 and Vldlr in cortical layer formation, followed by a discussion on the recently identified downstream effector molecule n-cofilin, involved in regulating actin cytoskeletal dynamics required for coordinated neuronal migration. Next, we discuss possible functions of the recently identified Reelin-Notch signalling crosstalk, and new aspects of Reelin´s role in the formation of the dentate radial glial scaffold. Finally, progress in characterizing the function of Reelin in modulating synaptic function in the adult brain is summarized. The present review has been inspired by a session entitled “Functions of Reelin in the developing and adult hippocampus”, held at the Spring Hippocampal Research Conference in Verona/Italy, June 2009.
Keywords: Actin, Amyloid β, amyloid precursor protein, Cofilin, cortical development, Dab1, lipoprotein receptor, neuronal migration, neuronal polarity, Notch, radial glia, NMDA receptor, LTP
Introduction
The reeler mutant, subject of studies on cortical development and function for more than fifty years, is likely the best characterized mouse mutant with a neurological phenotype (Rakic & Caviness, 1995; Curran & D´Arcangelo, 1998; Lambert de Rouvroit & Goffinet, 1998; Rice & Curran, 2001; Tissir & Goffinet, 2003). The extracellular matrix molecule Reelin, a large glycoprotein secreted by Cajal-Retzius (CR) cells during early cortical development, is required to control proper migration and positioning of cortical neurons (D’Arcangelo et al., 1995, 1997; Frotscher, 1998; Soriano & Del Rio, 2005; Förster et al., 2006; Cooper, 2008). Lack of Reelin expression in mice results in the reeler phenotype (D´Arcangelo et al., 1995; Curran & D´Arcangelo, 1998). Reelin deficiency in the human cerebral cortex is accompanied by neuronal migration defects leading to the phenotype of lissencephaly (Hong et al., 2000).
Two lipoprotein receptors, the apolipoprotein E receptor 2 (Apoer2) and the very low density lipoprotein receptor (Vldlr) were identified as Reelin receptors in the mouse (Trommsdorff et al., 1999, D´Arcangelo et al., 1999, Hiesberger et al., 1999; for review see Bock & Herz, 2008). Binding of Reelin to Apoer2 and Vldlr leads to phosphorylation of the intracellular adapter protein Disabled-1 (Dab1) by Src-family tyrosine kinases (Howell et al., 1997; Arnaud et al., 2003a; Bock & Herz, 2003; Kuo et al., 2005). Each individual receptor, Apoer2 and Vldlr, exerts a different function in neuronal positioning. Thus, distinct neuronal migration defects may be related to dysfunction of only one of these receptors (Hack et al., 2007). In contrast to well characterized upstream signalling components, such as the lipoprotein receptors and the adapter protein Dab1, downstream signalling events of the Reelin signalling cascade functionally involved in radial migration have remained poorly characterized. Only recently, the actin associated protein n-cofilin (non-muscle cofilin, cofilin 1) was found to functionally couple upstream Reelin signalling events to downstream modulation of actin cytoskeletal dynamics (Chai et al., 2009). Another emerging topic is the recently identified signalling crosstalk between Reelin and Notch (Hashimoto-Torii et al., 2008; Sibbe et al., 2009). Canonical Notch signalling is an important pathway previously known for its role in radial glia maintenance, neurogenesis and dendrite development (Yoon & Gaiano, 2005; Ever & Gaiano, 2005; Louvi & Artavanis-Tsakonas, 2006).
Later in cortical development, Reelin has been shown to promote extension of dendritic processes and maturation of dendritic spines (Niu et al., 2004; Jossin et al., 2007; Niu et al., 2008). By the time radial neuronal migration comes to its end, Reelin is not only secreted by Cajal-Retzius cells in the marginal zone below the pial surface, but is then increasingly expressed by GABAergic interneurons (Alcántara et al., 1998; Drakew et al., 1998; Ramos-Moreno et al., 2006).
In the mature brain, interneuron-derived Reelin has been shown to play a role in modulating synaptic function (Weeber et al., 2002; for review see Herz & Chen, 2006). Recent evidence suggests that binding of Reelin to its postsynaptic receptors also interferes with the development of Alzheimer disease (Herz & Beffert, 2000; Beffert et al., 2002; Brich et al., 2003; Durakoglugil et al., 2009).
In the present review, we summarize and discuss recent progress in understanding functions of Reelin at different developmental stages, ranging from the embryonic to the mature cerebral cortex.
Stop or go? On the divergent roles of Reelin receptors Apoer2 and Vldlr
Histological characterization of neuronal malpositioning in reeler does not allow only one interpretation of how Reelin signalling may interfere with migrating neurons. Thus, in reeler radially migrating neurons are found to invade the marginal zone, future superficial layer I. By contrast, in wild-type layer I is not invaded by radially migrating neurons. This observation suggests that Reelin, being expressed by CR-cells in the marginal zone, acts as a stop signal for radially migrating neurons and prevents them from entering this layer (Frotscher, 1998). However, Reelin function may be interpreted differently when studying hippocampal development. Thus, in reeler, dentate granule cells fail to form a compact cell layer, but are distributed all over the dentate hilar region. When co-culturing a reeler hippocampal slice with a Reelin-expressing wildtype hippocampal slice, the reeler dentate granule cells were found to migrate towards the Reelin-expressing wild-type dentate gyrus where they formed a densely packed cell layer close to the Reelin-rich zone. This observation suggested that Reelin may initially attract newly generated neurons towards their final destination (Zhao et al., 2004; for review see Förster et al., 2006a, b).
How to explain the paradox of both attractive and repellent Reelin effects on migrating neurons? Studies using mutant mice lacking only one of the Reelin receptors, Apoer2 or Vldlr respectively, could provide a possible explanation to this question: Whereas double-knockout mutants for both lipoprotein receptors are indistinguishable from reeler, milder phenotypes are found in mutants deficient for one receptor, Apoer2 or Vldlr (Trommsdorff et al., 1999; Benhayon et al., 2003). Furthermore, migration defects in mice deficient for only one of the receptors are not only different from reeler, but also differ from each other (Trommsdorff et al., 1999; Benhayon et al., 2003, Hack et al., 2007). Thus, in mice lacking only Apoer2, few neurons were found in cortical layer I, similar to the situation in the wildtype. By contrast, in mice lacking only Vldlr, numerous neurons were found to invade cortical layer I (Fig. 1 A–C). Along the same line, by using hippocampal co-cultures, Zhao and colleagues found that Vldlr and Apoer2 exert different functions in coordinating the migration of dentate granule cells (unpublished data). These observations suggest that Vldlr mediates a stop signal for migrating neurons reaching the marginal zone (corresponding to the molecular layer in dentate gyrus), whereas Apoer2 seems to be required to promote granule cell migration, similar to the different functions of these Reelin receptors in the migration of neocortical neurons (Hack et al., 2007). Taken together, histological characterization of these migration defects suggests that different steps in neuronal positioning may be regulated in a receptor-specific manner. The question remains how Reelin signaling is coordinated when both receptors are present on migrating neurons. Besides the different affinities of Apoer2 and Vldlr to Reelin (Benhayon et al., 2003), developmental regulation, or different sorting of receptors to the cell surface or different membrane compartments of the migrating neuron could be involved in differentially activating each receptor type. Duit and collegues (2009) recently reported on the different sorting of Apoer2 to raft domains and of Vldlr to non-raft domains of the plasma membrane, thereby providing a possible mechanism allowing for differential receptor sorting and activation.
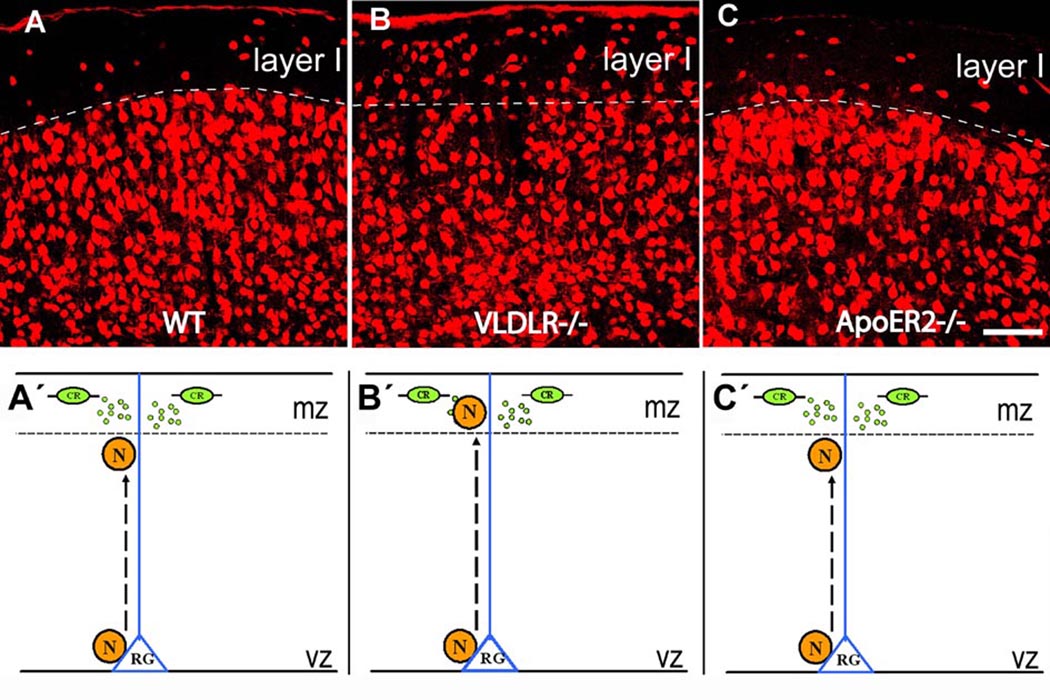
Figure 1. Reelin receptors Apoer2 and Vldlr differentially regulate positioning of radially migrating neurons.
Neurons were immunostained for the neuronal marker NeuN in coronal brain sections. In layer I of wildtype (A) and of Apoer2 deficient mice (C) only few NeuN positive cells are detected. In contrast, numerous NeuN-positive cells were found to invade layer I of Vldlr deficient mice (B).
Graphical representations A´-C´ illustrate radial migration underlying neuronal positioning in the developing embryonic cortex corresponding to the immunostained sections in A–C. In each pictogram a neuron (N, orange) is shown that migrates from its site of birth in the ventricular zone (vz) along the process of a radial glial cell (RG, blue) towards the marginal zone (mz), future layer I. Reelin is secreted by Cajal-Retzius cells (CR, green) in the marginal zone. In wildtype (A´), migrating neurons do not invade the mz. In Vldlr deficient mice (B´), neurons migrate into the marginal zone. In mice lacking Apoer2 (C´), neurons stop below the marginal zone, similar as in wildtype. Bar: 80 µm
To further characterize the individual roles of Reelin receptors in neuronal migration, the fate of cortical neurons was mapped with layer-specific markers or by BrdU-labelling in mice deficient for only one Reelin receptor (Benhayon et al., 2003; Beffert et al., 2006; Hack et al., 2007). In mutant mice deficient for Vldlr, the overall pattern of layer formation in the neocortex was similar to that of wild-type mice. That is, early generated neurons, identified by labeling for the transcription factors Er81 and Foxp2 of layer V and VI, or labeled by injecting BrdU at early stages of cortical development (E12-E13), were found in the deeper portion of the cortex, whereas late generated neurons, identified by labeling for the transcription factor Cux2, a marker for neurons in layer II-III, or labeled by BrdU-injection at later stages (E15-E16) of cortical development, were positioned in the outer regions of the cortical plate. A different pattern of layer formation was found in Apoer2-deficient mice when neuronal fate was mapped with layer-specific markers or by BrdU injection. Thus, early generated Er81- and Foxp2-positive neurons were located ectopically in more superficial positions of the cortex, and late generated Cux2-positive neurons formed two separate layers, one upper layer, right underneath the marginal zone, and one deeper layer closer to the ventricular zone. Consistent with Cux2-immunostaining, two separate BrdU-positive cell layers were observed in the neocortex of Apoer2-deficient mice when BrdU was injected at E15. By contrast, in wildtype cortex the majority of BrdU-positive cells was found in the superficial layers. These results further underline that the two Reelin receptors Vldlr and Apoer2 function differently in neuronal migration and cortical layer formation. An important question that remains to be addressed: How does Reelin signalling lead to coordinated changes in cytoskeletal dynamics that are required for the proper migration of cortical neurons during development?
How Reelin signalling is coupled to cytoskeletal dynamics
In recent years, upstream components of the intracellular Reelin signalling cascade, namely the lipoprotein receptors and the intracellular adapter protein Dab1, have been studied in great detail. Thus, the phenotype of mutant mice lacking Dab1 is indistinguishable from the reeler mutant, underscoring the importance of Dab1 in mediating the Reelin signal. Binding of Reelin to either Apoer2 or Vldlr induces phosphorylation of specific tyrosine residues of Dab1 by non-receptor tyrosine kinases of the Src-family. Tyrosine phosphorylation of Dab1 is required for Reelin signalling related to neuronal positioning (Howell et al., 2000), and may be induced by binding of Reelin to Apoer2 or Vldlr, respectively (Hiesberger et al., 1999; Howell et al., 1999; Bock & Herz, 2003; Arnaud et al., 2003a, b). In contrast to these well documented upstream signalling events, downstream effector molecules that functionally connect the Reelin signal at the plasma membrane to cytoskeletal dynamics in radially migrating neurons remained unclear.
Directed cell migration requires inducible changes in the dynamics of the actin cytoskeleton. Signalling related to cytoskeletal changes has been extensively studied in great detail in a variety of motile cell types, such as fibroblasts; breast carcinoma cells or T-cells (Moriyama et al., 1996; Dawe et al., 2003; Gosh et al., 2004; Burkhardt et al., 2008), Similar mechanisms are likely active in radially migrating neurons. The search for defects in downstream signalling events in the reeler mutant led to the recent identification of the actin associated protein n-cofilin as an effector molecule of the Reelin signalling cascade (Chai et al., 2009). The F-actin binding protein n-cofilin is known for its actin severing activity, i.e. its ability to promote the disassembly of actin filaments (Bamburg, 1999; Kiuchi et al., 2007). This activity of n-cofilin is controlled by the enzyme LIM-kinase 1 (LIMK1; Arber et al., 1998). LIMK1 phosphorylates n-cofilin at serine3 and thereby inhibits its actin severing activity. Phosphorylation of n-cofilin by LIMK1 required Reelin-induced activation of Dab1 and of phosphatidylinositiol 3-kinase (Bock et al., 2003; Chai et al., 2009). Reduced actin turnover stabilizes the actin cytoskeleton. In conditional knockout mice lacking n-cofilin expression in the cerebral cortex, cortical neurons are found to be malpositioned, reflecting the requirement of n-cofilin for proper neuronal migration (Bellenchi et al, 2007).
How to interpret the role of Reelin-induced phosphorylation of n-cofilin during neuronal migration? To better understand the function of n-cofilin phosphorylation in the developing cortex, it was important to localize accumulation of phosphorylated n-cofilin (p-cofilin) in the developing cortex. In immunostained cortical slices from E17.5 wildtype mice, p-cofilin was found to be enriched in the leading processes of radially migrating neurons when these processes reached the Reelin containing marginal zone. Reduced phosphorylation of n-cofilin in the absence of Apoer2, but not of Vldlr, appears to reflect a specific role of Apoer2 in mediating stabilization of the leading process. In Apoer2-deficient mice and in reeler, late born neurons are unable to migrate past earlier born neurons and therefore do not reach their final destinations in the superficial cortical layers. By contrast, only in the absence of Vldlr, but not of Apoer2, radially migrating neurons are found to invade the marginal zone of the developing cortex (Fig. 1 B–C).
How to relate differences in the stabilization of the leading process to the above described different phenotypes of Apoer2- or Vldlr-deficient mice? As one mode of radial migration used by late born neurons to bypass earlier born neurons, somal translocation has been described (Nadarajah et al., 2001; Nadarajah & Parnavelas, 2002) which has been shown to depend on F-actin dynamics in the leading process (Solecki et al., 2009). Stabilization of leading processes could be required to pull the neuronal cell soma of late born neurons past the barrier of earlier born neurons. In support of this interpretation, Miyata & Ogawa (2007) have shown that during somal translocation in the developing cortical plate, the leading process is under mechanical tension. Thus, binding of Reelin specifically to Apoer2-receptors might induce stabilization of the leading processes via LIMK1-induced phosphorylation of n-cofilin at serine3.
By E18, when late born neurons migrate radially, Reelin starts to be expressed by interneurons that by this time have reached their final destinations in layer V and VI by tangential migration (Alcántara et al., 1998; Soriano & Del Rio et al., 2005). Is Reelin derived from these interneurons involved in the control of neuronal migration? Interestingly, it has been shown that specification of the leading and the trailing process of radially migrating neurons to a dendrite or to an axon is determined during radial migration (Asada et al., 2007; Barnes et al., 2007; for review see Barnes & Polleux, 2009). The crucial time for process specification appears to overlap with radial migration of late born neurons past Reelin expressing interneurons. One possibility to be considered is that early Reelin-induced stabilization of the leading process might contribute to its dendritic specification. In turn, reduced stabilization may help to specify the trailing process to an axon, as has been shown for process specification in vitro (Bradke & Dotti, 1999). Presence of neurons with maloriented processes and with improperly specified processes, namely the occurrence of neurons with inverted axo-dendritic polarity in the reeler cortex support this interpretation (Pinto Lord & Caviness, 1979; Stanfield & Cowan, 1979; Terashima et al., 1983; Landrieu & Goffinet, 1981). SAD kinases implement axo-dendritic polarization of radially migrating neurons and exert this function, at least in part, by phosphorylation of microtubule associated proteins, including Tau (Kishi et al., 2005; Barnes et al., 2007). In turn, Reelin reduces phosphorylation of the microtubule-stabilizing protein Tau via PI3K–dependent inhibition of GSK3β (Beffert et al. 2002). Whether Reelin dependent modulation of Tau phosphorylation is involved in axo-dendritic specification remains to be determined. For instance, correction of process orientation of layer VI neurons has been shown in E13 reeler cortex explants within 4 hours after application of recombinant Reelin (Nichols & Olson, 2010).
Radial Glia and Neurons: Reelin´s Crosstalk with Notch signalling
Notch signalling represents a multifunctional intracellular pathway that has been known to play a role in various aspects of cortical development from neurogenesis to dendrite development (Yoon & Gaiano, 2005; Louvi & Artavanis-Tsakonas, 2006). Mammalian Notch receptors are transmembrane proteins that are activated by membrane-bound ligands such as Delta-like or Jagged. Activation of the Notch receptor by these ligands induces sequential proteolytic cleavages, ultimately leading to the liberation of the Notch intracellular domain (NICD) by γ-secretase. NICD is translocated to the cell nucleus and may, as part of a complex with the transcription factor CSL, induce the canonical Notch signalling pathway by modulating the expression of a variety of genes. The observation that in radial glial cells the target molecule brain lipid binding protein (Blbp) is regulated by both Reelin signalling and Notch signalling already suggested an interplay between both pathways (Gaiano et al. 2000; Hartfuss et al., 2003). The possibility of crosstalk between these two signalling pathways was bolstered by the finding that Reelin-deficient mice have reduced levels of the cleaved form of Notch intracellular domain (NICD) in the neocortex and hippocampus. Furthermore, transcription of the Notch1 downstream target genes Hes1 and Hes5 was found to be reduced in the neocortex and hippocampus of reeler mice. Loss of Notch signalling in migrating neurons resulted in malpositioning and morphological malformations, reminiscent of the migration defects seen in reeler (Hashimoto-Torii et al., 2008; Sibbe et al., 2009). In turn, Notch intracellular domain (NICD) overexpression in reeler cortical neurons largely rescued migration defects and morphological malformations (Hashimoto-Torii et al., 2008).
Where in the Reelin signalling cascade might the interference with Notch signalling take place and what are the underlying biochemical mechanisms of Reelin-dependent Notch1 activation? A direct interaction of Disabled (homolog to mammalian Dab1) with Notch has first been shown in Drosophila, which does not express Reelin (Giniger, 1998; Le Gall et al., 2008). NICD contains an NPXY-motif, a tetra-amino acid sequence that can bind to PTB domain-containing adapter proteins such as Dab1 (Trommsdorff et al., 1999; Howell et al., 1999). Indeed, Notch also interacts with Dab1 in mammals, as shown by co-immunoprecipitation experiments using lysates from embryonic mouse cortex (Hashimoto-Torii et al., 2008), or from a human neural progenitor cell line (Keilani & Sugaya, 2008). Moreover, Reelin-dependent Dab1 activation prevents the proteasomal degradation of NICD in postmitotic neurons (Hashimoto-Torii et al., 2008). Since Reelin signaling also promotes proteasomal Dab1 degradation (Arnaud et al., 2003b; Bock et al., 2004; Feng et al., 2007), it is possible that the protective effect of Reelin on NICD degradation might involve the function of activated Dab1 in regulating intracellular trafficking (Stolt & Bock, 2006; Hoe et al., 2006; Honda & Nakajima, 2006).
As indicated above, the Reelin-Notch crosstalk is clearly not restricted to neurons but also regulating the formation of the radial glial scaffold required for radial glia-guided neuronal locomotion, that differs from the earlier mentioned somal translocation (Nadarajah & Parnavelas, 2002). Evidence for a role of Notch signalling in the control of radial glia-guided migration has been provided for the dentate gyrus (Sibbe et al., 2009), but is likely to be valid also for the developing neocortex (Hartfuss et al., 2003; Gaiano, 2008; Hashimoto-Torii et al., 2008), although this has not been shown directly. Also the finding that Notch interacts with Dab1 in a human neural progenitor cell line supports the notion of Reelin-Notch crosstalk in radial glia, since radial glial cells represent neural progenitors (Malatesta et al., 2000; Noctor et al., 2001; Keilani & Sugaya, 2008). Unfortunately, mouse models that examine the consequences of conditionally inactivating Notch1 specifically in radial glial cells are not available. The effect of Notch signaling on dentate gyrus development in organotypic hippocampal slice cultures was blocked by using N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a pharmacological inhibitor of γ-secretase (Sibbe et al. 2009). The application of other pharmacological inhibitors like the Notch-sparing γ-secretase inhibitor begacestat (Martone et al., 2009), or the use of a recently described peptide inhibitor that specifically blocks Notch activity by interfering with the interaction of NICD with the CSL protein complex (Moellering et al., 2009) could help address if the inhibition of cleavage of other γ-secretase substrates including Apoer2 itself (May et al., 2003; Hoe & Rebeck, 2008) might have contributed to the effects of DAPT on dentate gyrus development.
Different from the development of other cortical regions, the incipient dentate gyrus is invaded by neuronal precursor cells which are guided from the ventricular zone by a primary radial glial scaffold to a secondary neurogenic zone in the dentate hilar region. A recent study revealed that these neurogenic precursor cells remain transiently located in close association with the pial meningeal surface of the developing dentate gyrus. Proper migration from this pia associated location towards the hilar region was found to depend on Reelin (Li et al., 2009). Around birth this pool of neurogenic cells contributes to the formation of a secondary radial glial scaffold required for the formation of the dentate granule cell layer. Moreover, it contributes to the subgranular zone required for the lifelong generation of granule cells. Whereas the primary radial glial scaffold seems to be largely unaffected in reeler, the secondary radial glial scaffold is severely disturbed, depending on the Reelin signalling cascade including Apoer2, Vldlr and Dab1 (Förster et al., 2002; Weiss et al., 2003; Zhao et al., 2004, 2006). Detailed studies on the expression of marker proteins such as Blbp, Vimentin or GFAP by dentate radial glial cells in reeler and wildtype mice should contribute to a better understanding of Reelin’s role in the formation and function of the postnatal dentate radial glial scaffold.
Emerging topics of Reelin function at the synapse
By the time radial migration of cortical neurons is terminated, many Reelin expressing CR cells disappear, whereas GABAergic interneurons continue to express Reelin into adulthood. What are the functions that Reelin expressed by interneurons exerts in the adult cortex?
Activity of glutamate receptors of the N-methyl-D-aspartate (NMDA)- and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-type was found to be modulated by binding of Reelin to postsynaptic Apoer2 and Vldlr (Chen et al., 2005; Herz & Chen, 2006; Qiu et al., 2006). Addition of recombinant Reelin to acute hippocampal slices resulted in enhanced long term potentiation (LTP; Weeber et al., 2002). Similar to Reelin signalling during development, this effect was found to depend on Reelin-induced activity of Src family kinases (Beffert et al., 2005; Chen et al., 2005; Qiu et al., 2006; Durakoglugil et al., 2009), which increase tyrosine phosphorylation of the NR2 subunit of NMDA-receptors (for review, see Sala & Sheng, 1999).
There is recent evidence that Reelin may antagonize suppressive effects on synaptic function induced by the oligomeric β-amyloid peptide (Durakoglugil et al., 2009). Abnormal processing of the amyloid precursor protein (APP) leads to β-amyloid (Aβ) plaque accumulation, which is characteristic for Alzheimer’s disease. Reelin was found to co-localize with Aβ in aged wild type mice (Doehner et al., 2010). Incubation of hippocampal neurons with Aβ-oligomers impaired endocytosis and trafficking of AMPA- and NMDA-receptors and thus decreased LTP (Hsieh et al., 2006; Kamenetz et al., 2003; Snyder et al., 2005). Interestingy, application of recombinant Reelin could reduce the Aβ-induced suppression of LTP. The reversal of Aβ-induced effects required the Reelin-dependent activation of Src family kinases (Durakoglugil et al., 2009). Conversely, β-amyloid seems to alter Reelin expression and proteolytic processing (Botella-Lopez et al., 2009). Moreover, it was shown that Reelin directly binds to APP (Hoe et al., 2009), which in turn interacts with Dab1 through its intracellular NPXY-motif (Trommsdorff et al., 1998; Howell et al., 1999), adding another level of complexity to the functional interactions of Aβ and Reelin-lipoprotein receptor signaling at the synapse (Hoe & Rebeck, 2008). It remains to be established whether the direct binding of Reelin to APP is also important for APP’s function in regulating neuronal migration in the neocortical plate, which was uncovered by in utero RNA interference (Young-Pearse et al., 2007). Together, these recent findings on Reelin function at the synapse allow one to establish a model in which Aβ, ApoE-receptors and Reelin modulate glutamatergic neurotransmission with Reelin binding to Apoer2 and Vldlr counteracting the suppressive effect of Aβ at the synapse (Fig. 2).
Fig. 2. A model of the complex interplay between Reelin, Apolipoprotein E receptors and amyloid precursor protein at the postsynaptic membrane.
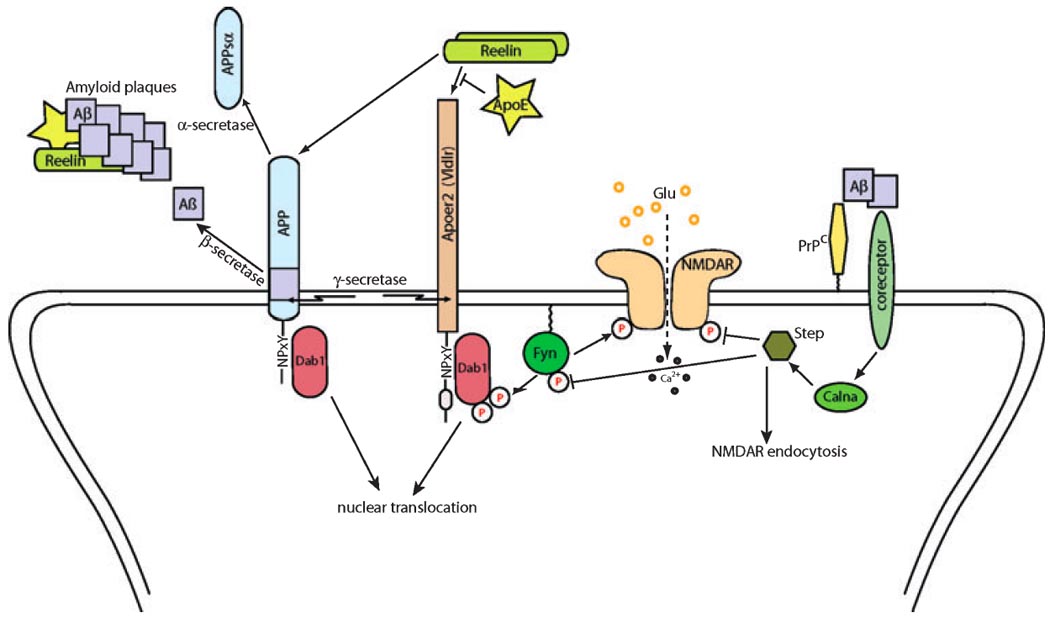
Binding of Reelin to its receptors Apoer2 and Vldlr induces Src family kinase Fyn-mediated tyrosine phosphorylation of the neuronal adapter protein Disabled-1, which in turn activates Fyn. The interaction of Reelin and its receptors can be modulated by Apolipoprotein E in an ApoE-isoform dependent manner. In addition to Apoer2 and Vldlr, Reelin also binds to the amyloid precursor protein, APP, which affects its trafficking and proteolytic processing by γ-secretase in a Dab1-dependent manner. In addition, Dab1 might modulate the nuclear trafficking and/or degradation of liberated intracellular domains of γ-secretase substrates, including Notch (not shown here). Intramembrane cleavage of the product of β-secretase-mediated APP processing by γ-secretase results in the extracellular release of neurotoxic amyloid-beta peptide (Aβ). It forms the main constituent of insoluble amyloid plaques, which can contain fragments of Reelin and ApoE as well. In contrast, ectodomain shedding by α-secretase releases the neuroprotective soluble extracellular domain of APP and prevents generation of Aβ.
Reelin-dependent activation of Fyn promotes tyrosine phosphorylation of the NMDA receptor subunit NR2 and thereby counteracts Aβ-induced downregulation of synaptic transmission. Oligomeric Aβhigh-affinity binding to the cellular form of prion protein (PrPC) inhibits LTP (Lauren et al. 2009) and might interfere with the interaction of Aβ with other co-receptors. Aβ binding to one of these coreceptors (possibly nicotinic acetylcholine receptor) activates calcineurin (Calna), which in turn activates striatal enriched phosphatase (Step) by dephosphorylation. Step-mediated NR2 dephosphorylation leads to increased endocytosis, reduced density of NMDA receptors and decreased glutamatergic transmission at the synapse.
Outlook
The latter studies on Reelin’s role in synaptic transmission underscore that Reelin effects are not restricted to developmental processes. Additional studies are required to document Reelin’s role for adult network function. At present it is difficult to tell to what extent Reelin functions in the adult are influenced by its functions during early development. As an example, studies in conventional reeler mutants are hampered by the fact that it is impossible to subject these mice to behavioural experiments. Reeler mutants suffer from cerebellar malformation resulting in ataxia. Moreover, even if the animals could be studied in behavioural paradigms, it would be impossible to determine whether an observed alteration in behaviour was due to maldevelopment or abnormal synaptic function (see Kowalski et al., 2010). Moreover, it is an open question to what extent Reelin’s role in modulating cytoskeletal dynamics during development may be neglected when studying Reelin effects on Tau phosphorylation in the adult. One way to disentangle adult from developmental Reelin functions would be to study conditional Reelin knock-out mice that developed normally before the Reelin gene is turned off. To this end J.H., H.H.B. and M.F. have developed a strategy to knock-out the Reelin gene in adult mice.
Reelin expression is decreased in a variety of neurological and psychiatric disorders, including schizophrenia, autism, major depression, temporal lobe epilepsy and Alzheimer’s disease (for review see Fatemi, 2008). Here again, it would be of utmost interest to figure out when precisely Reelin expression becomes altered. Either Reelin expression is decreased during ontogenetic development and leads to malformation, and disease develops when a “second hit” later on in the lifes of these patients affects critical network structures, or alternatively, some unknown environmental factors interfere with Reelin expression in adult life which in turn affects synaptic transmission, eventually resulting in disease. Undoubtedly, Reelin will continue to attract researchers from various fields in the neurosciences.
Acknowledgements
We apologise to authors whose research could not be cited directly owing to space constraints. Work in the laboratories of the authors is funded by the Deutsche Forschungsgemeinschaft (DFG; FO 223/6-1 to E.F.; SFB 592 TP A20 and SFB TR-3 TP D6 to M.F.; SFB 780 TP B5 to J.H., H.B. and M.F.; BO 1806/2-1 to H.B.) and by grants from the National Institutes of Health, the American Health Assistance Foundation, the Perot Family Foundation and the Consortium for Frontotemporal Dementia Research to J.H.. M.F. is supported by the Hertie Foundation.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- APP
amyloid precursor protein
- Aβ
β-amyloid
- Apoer2
apolipoprotein E receptor 2
- Blbp
brain lipid binding protein
- Calna
calcineurin
- Dab1
Disabled-1
- DAPT
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- GABA
γ-aminobutyric acid
- GSK3β
Glycogen synthase kinase 3β
- LTP
long term potentiation
- NICD
Notch intracellular domain
- NMDA
N-methyl-D-aspartate
- n-cofilin
non-muscle cofilin
- PI3K
Phosphoinositide 3-kinase
- Step
striatal enriched phosphatase
- SAD
Synapses of Amphids Defective
- Vldlr
very low density lipoprotein receptor
References
- Alcántara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J. Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanster H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr. Biol. 2003a;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell Biol. 2003b;23:9293–9302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J. Neurosci. 2007;24 doi: 10.1523/JNEUROSCI.1938-07.2007. 11769-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J. Neurosci. 2006;26:2041–2052. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J. Biol. Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhayon D, Magdaleno S, Curran T. Binding of purified Reelin to Apoer2 and Vldlr mediates tyrosine phosphorylation of Disabled-1. Brain Res. Mol. Brain Res. 2003;112:33–45. doi: 10.1016/s0169-328x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates Src family tyrosine kinases in neurons. Curr. Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Apolipoprotein E receptor 2 and very low density lipoprotein receptor: An overview. In: Fatemi SH, editor. Reelin Glycoprotein, Structure, Biology and Roles in Health and Disease. New York: Springer; 2008. pp. 15–35. [Google Scholar]
- Bock HH, Jossin Y, Liu P, Förster E, May P, Goffinet AM, Herz J. Phosphatidylinositol 3-kinase interacts with the adapter protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for Reelin-induced proteasomal degradation of the neuronal adaptor protein disabled-1. J. Biol. Chem. 2004;279:33471–33479. doi: 10.1074/jbc.M401770200. [DOI] [PubMed] [Google Scholar]
- Botella-López A, Cuchillo-Ibáñez I, Cotrufo T, Mok SS, Li QX, Barquero MS, Dierssen M, Soriano E, Sáez-Valero J. beta-amyloid controls altered Reelin expression and processing in Alzheimer's disease. Neurobiol Dis. 2009 doi: 10.1016/j.nbd.2009.12.006. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Brich J, Shie FS, Howell BW, Li R, Tus K, Wakeland EK, Jin LW, Mumby M, Churchill G, Herz J, Cooper JA. Genetic modulation of tau phosphorylation in the mouse. J. Neurosci. 2003;23:187–192. doi: 10.1523/JNEUROSCI.23-01-00187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Förster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J. Neurosci. 2009;29:288–299. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Curran T, D’Arcangelo G. Role of reelin in the control of brain development. Brain Res. Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- D´Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Doehner J, Madhusudan A, Konietzko U, Fritschy JM, Knuesel I. Co-localization of reelin and proteolytic AbetaPP fragments in hippocampal plaques in aged wild-type mice. J. Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-1333. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Dawe HR, Minamide LS, Bamburg JR, Cramer LP. ADF/cofilin controls cell polarity during fibroblast migration. Curr. Biol. 2003;13:252–257. doi: 10.1016/s0960-9822(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Duit S, Mayer H, Blake SM, Schneider WJ, Nimpf J. Differential functions of Apoer2 and VLDL receptor in Reelin signaling depend on differential sorting of the receptors. J. Biol Chem. 2009 doi: 10.1074/jbc.M109.025973. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc. Natl. Acad. Sci. U S A. 2009;106:15938–15943. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ever L, Gaiano N. Radial 'glial' progenitors: neurogenesis and signaling. Curr. Opin. Neurobiol. 2005;15:29–33. doi: 10.1016/j.conb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin Glycoprotein. Structure, Biology and Roles in Health and Disease. New York: Springer; 2008. [DOI] [PubMed] [Google Scholar]
- Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–2730. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Müller U, Frotscher M. Reelin, Disabled 1 and beta1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc. Natl. Acad. Sci. USA. 2002;99:13178–13183. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM. Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur. J. Neurosci. 2006a;23:901–909. doi: 10.1111/j.1460-9568.2006.04612.x. [DOI] [PubMed] [Google Scholar]
- Förster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat. Rev. Neurosci. 2006b;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr. Opin. Neurobiol. 1998;8:570–575. doi: 10.1016/s0959-4388(98)80082-2. [DOI] [PubMed] [Google Scholar]
- Gaiano N. Strange bedfellows: Reelin and Notch signaling interact to regulate cell migration in the developing neocortex. Neuron. 2008;60:189–191. doi: 10.1016/j.neuron.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Giniger E. A role for Abl in Notch signalling. Neuron. 1998;20:667–681. doi: 10.1016/s0896-6273(00)81007-7. [DOI] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of Apoer2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–3891. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Förster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Götz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, Radtke F, Gridley T, Sestan N, Rakic P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer's disease. Nat. Rev. Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet AM, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. DAB1 and Reelin Effects on Amyloid Precursor Protein and ApoE Receptor 2 Trafficking and Processing. J. Biol. Chem. 2006;281:35176–35185. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT, Pak DT, Bu G, Rebeck GW. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J. Neurosci. 2009;29:7459–7473. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Functional interactions of APP with the apoE receptor family. J. Neurochem. 2008;106:2263–2271. doi: 10.1111/j.1471-4159.2008.05517.x. [DOI] [PubMed] [Google Scholar]
- Honda T, Nakajima K. Mouse Disabled1 (DAB1) Is a Nucleocytoplasmic Shuttling Protein. J. Biol. Chem. 2006;281:38951–38965. doi: 10.1074/jbc.M609061200. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martín ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nature Genet. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Goffinet AM. Reelin Signals through Phosphatidylinositol 3-Kinase and Akt to Control Cortical Development and through mTor To Regulate Dendritic Growth. Mol. Cell. Biol. 2007;27:7113–7124. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Keilani S, Sugaya K. Reelin induces a radial glial phenotype in human neural progenitor cells by activation of Notch-1. BMC Dev. Biol. 2008;8:69. doi: 10.1186/1471-213X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Kiuchi T, Ohashi K, Kurita S, Mizuno K. Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J. Cell Biol. 2007;177:465–476. doi: 10.1083/jcb.200610005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J, Geuting M, Paul S, Dieni S, Laurens J, Zhao S, Drakew A, Haas CA, Frotscher M, Vida I. Proper layering is important for precisely timed activation of hippocampal mossy cells. Cereb. Cortex. 2010 doi: 10.1093/cercor/bhp267. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J. Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, Goffinet AM. The reeler mouse as a model of brain development. Adv. Anat. Embryol. Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- Landrieu P, Goffinet A. Inverted pyramidal neurons and their axons in the neocortex of reeler mutant mice. Cell. Tiss. Res. 1981;218:293–301. doi: 10.1007/BF00210345. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor Notch. Dev. Biol. 2008;313:556–567. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Kataoka H, Coughlin SR, Pleasure SJ. Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136:327–335. doi: 10.1242/dev.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, Gong X, Jin M, Kreft A, Harrison B, Mayer SC, Aschmies S, Gonzales C, Zaleska MM, Riddell DR, Wagner E, Lu P, Sun SC, Sonnenberg-Reines J, Oganesian A, Adkins K, Leach MW, Clarke DW, Huryn D, Abou-Gharbia M, Magolda R, Bard J, Frick G, Raje S, Forlow SB, Balliet C, Burczynski ME, Reinhart PH, Wan HI, Pangalos MN, Jacobsen JS. Begacestat (GSI-953): A novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease. J. Pharmacol. Exp. Ther. 2009;331:598–608. doi: 10.1124/jpet.109.152975. [DOI] [PubMed] [Google Scholar]
- May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- Miyata T, Ogawa M. Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter-cell migration. Curr. Biol. 2007;17:146–151. doi: 10.1016/j.cub.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Lida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas J. Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nichols AJ, Olson EC. Reelin promotes neuronal orientation and dendritogenesis during preplate splitting. Cereb. Cortex. 2010 doi: 10.1093/cercor/bhp303. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G. Reelin promotes hippocampal dendrite development through the Vldl/Apoer2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D'Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J. Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Caviness VS., Jr Cortical development: view from neurological mutants two decades later. Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- Ramos-Moreno T, Galazo MJ, Porrero C, Martinez-Cerdeno V, Clasca F. Extracellular matrix molecules and synaptic plasticity: immunomapping of intracellular and secreted Reelin in the adult rat brain. Eur. J. Neurosci. 2006;23:401–422. doi: 10.1111/j.1460-9568.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Sala C, Sheng M. The fyn art of N-methyl-d-aspartate receptor phosphorylation. Proc. Natl. Acad. Sci. U S A. 1999;96:335–337. doi: 10.1073/pnas.96.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbe M, Förster E, Basak O, Taylor V, Frotscher M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J. Neurosci. 2009;29:8578–8585. doi: 10.1523/JNEUROSCI.0958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA. The cells of Cajal-Retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. The morphology of the hippocampus and dentate gyrus in normal and reeler mice. J. Comp. Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Terashima T, Inoue K, Inoue Y, Mikoshiba K, Tsukada Y. Distribution and morphology of callosal commissural neurons within the motor cortex of normal and reeler mice. J. Comp. Neurol. 1985;232:83–98. doi: 10.1002/cne.902320108. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat. Rev. Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the Vldl receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Weiss KH, Johanssen C, Tielsch A, Herz J, Deller T, Frotscher M, Förster E. Malformation of the radial glial scaffold in the dentate gyrus of reeler mice, scrambler mice, and Apoer2/Vldlr-deficient mice. J. Comp. Neurol. 2003;460:56–65. doi: 10.1002/cne.10644. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Chai X, Bock HH, Brunne B, Förster E, Frotscher M. Rescue of the reeler phenotype in the dentate gyrus by wild-type coculture is mediated by lipoprotein receptors for reelin and disabled 1. J. Comp. Neurol. 2006;495:1–9. doi: 10.1002/cne.20846. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chai X, Förster E, Frotscher M. Reelin is a positional signal for the lamination of dentate granule cells. Development. 2004;131:5117–5125. doi: 10.1242/dev.01387. [DOI] [PubMed] [Google Scholar]