Abstract
Lipofibroblasts of the developing rat lung resemble adipocytes and produce soluble growth factors which stimulate neighboring alveolar type II epithelial cell differentiation. The mature adipocyte secretes leptin, a cytokine with an expanding array of functions. Leptin is expressed by the developing rat lung beginning on E17–E18, increasing by 7–10 fold by E20(term= E22). Leptin is expressed by fetal lung fibroblasts of both rat and human (WI38) origins; the leptin receptor is expressed by fetal rat lung type II cells and by human adult lung epithelial cells (H441). Expression of leptin by mesenchymal cells and its cognate receptor by epithelial cells of the developing alveolus is consistent with a paracrine signaling loop, as demonstrated by our experimental data: (1) leptin stimulates the de novo synthesis of surfactant phospholipid by both fetal rat type II cells (400%/100 ng/ml/24h) and by adult human airway epithelial H441 cells (85%/100ng/24h) in culture; (2) leptin is secreted by the mature (E21) lipofibroblast in cell culture in amounts which significantly stimulate type II cell surfactant phospholipid synthesis in vitro, and leptin secretion is stimulated by PTHrP; (3) epithelial cell secretions such as parathyroid hormone-related protein (PTHrP) and prostaglandin E2, and dexamethasone all significantly stimulate leptin mRNA expression by fetal lung fibroblasts; (4) treatment of E18 fetal rat lung explants with PTHrP or leptin stimulates the de novo synthesis of surfactant phospholipid (2–2.5-fold/24h) and up-regulates the expression of Surfactant Protein-B(>25-fold/24h), an effect which is blocked by leptin antibody; (5) PTHrP receptor antagonist blocks the expression of leptin mRNA by explants, but does not inhibit stimulation of surfactant phosphospholipid or SP-B expression, indicating that fibroblast-dependent PTHrP stimulation of type II cell maturation requires leptin expression by lipofibroblasts. This is the first demonstration of a paracrine loop mediated by endogenously produced growth factors of both epithelial and mesenchymal origins cooperatively interacting to induce alveolar acinar lung development.
Keywords: lung development, surfactant, type II cell, lipofibroblast, leptin
Introduction
Lipid-laden lipofibroblasts were first identified as a morphologically distinct cell-type in fetal and neonatal rat lung in 1970 (8). Both lipofibroblasts and adipocytes originate from mesoderm and are physically distinguished by the presence of large pools of triglyceride stores in their cytoplasm (11). The metabolism of lipid by these two cell types is also strikingly similar with respect to their lipogenic pathways, which are regulated by the same signal transduction pathway (11). Mature adipocytes express leptin, a 16-kDa cytokine product of the obesity(ob) gene (30). Fetal lung lipofibroblasts produce fibroblast pneumonocyte factor (FPF), a 10–20 kDa molecular weight peptide which stimulates surfactant phospholipid synthesis by fetal type II cells (17). Differentiation of both cell-types is stimulated by glucocorticoids (17,33), and down-regulated by androgens (10,12) Furthermore, previous studies from our laboratory had suggested that lung epithelial cell secretions such as prostaglandin E2 (25) and parathyroid hormone-related protein (PTHrP) (24) stimulate type II cell-fibroblast interactions via a soluble fibroblast growth factor of unknown identity. These observations, combined with the similarities between adipocytes and lipofibroblasts, and the recent reports that leptin is expressed in developing lung (9,30), prompted us to investigate the possible role of leptin as a lipofibroblast growth factor which might stimulate fetal lung development.
It has long been recognized that lung development is mediated by soluble paracrine growth factors which mediate epithelial-mesenchymal interactions (15). These signals are bi-directional (1,23) and the earliest known signals originate from the epithelium (22). We had previously identified PTHrP as a fetal rat alveolar type II cell product, which is expressed beginning in the glandular phase of fetal lung development (16), and stimulates type II cell differentiation indirectly by stimulating fetal lung fibroblast factors (14). PTHrP stimulates fibroblast maturation through a receptor-mediated signal transduction pathway involving the production of cyclic AMP and inositol phosphate (14), both of which induce fibroblast differentiation into adipocytes (34). Based on these observations, we hypothesized that leptin mediates the paracrine effect of PTHrP on alveolar type II cell surfactant synthesis.
Methods and materials
Reagents
leptin (rat, human), leptin antibody (rat, polyclonal) and were acquired from Linco, St. Charles, MO. The leptin radioimmunoassay kit was obtained from DSL, Webster, TX. PTHrP (1–34) and PTHrP(7–34) amide were obtained from Bachem, Torrance, CA. Dexamethasone and prostaglandin E2 were purchased from Sigma Biochemicals (St. Louis, MO).
Animals
Time-mated Sprague-Dawley rats (time E0=day of mating) were obtained from Charles River Breeders (Holister, CA). These experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Cell culture
WI38 and H441 cells were obtained from the American Type Culture Collection (Rockville, MD).
Isolation of Lipofibroblasts and type II cells from Fetal Lung
these methods have been used extensively in our laboratory (6). Three to five dams were used per preparation. The dams were killed with an overdose of pentobarbital (100 mg/ml, i.p.) and the pups were removed from the uterus by laparotomy and kept on ice. The lungs were removed en bloc in a laminar flow hood using sterile technique and put into ice-cold sterile Hanks’ balanced salt solution without calcium or magnesium. The solution was decanted and 5 volumes of 0.05% trypsin (Worthington) were added to the lung preparation.
The lungs were dissociated in a 37°C water bath using a Teflon stirring bar to physically disrupt the tissue. When the tissue had been completely dispersed into a unicellular suspension (approx. 20 min.) the cells were spun down at 500 × g for 10 min at room termperature in a 50 ml polystyrene centrifuge tube. The supernatant was decanted and the cell pellet was resuspended in minimal essential medium (GIBCO) containing 10% fetal bovine serum (MEM/fbs) to yield a mixed cell suspension of approximately 3 × 108 cells as determined by Coulter particle counter (Hayaleah, FL). The cell suspension was added to 75-cm2 culture flasks (Corning Glass Works, Corning, NY) for 30–60 min at 37°C in a CO2 incubator to allow for differential adherence of the fibroblasts (20). Fibroblasts were maintained in MEM until further processing.
Preparation of Type II Cell Cultures
the unattached cells from the above-described cell preparation were transferred to another 75-cm2 culture flask for an additional 60 min period. After this second culture period the medium and non-adherent cells were removed from the flask and diluted with 1 volume of culture medium. This diluted suspension was cultured overnight in a 75-cm2 flask at 37°C in a CO2 incubator to allow the TII cells to adhere (2). TII cells were identified by their appearance in culture under phase contrast microscopy, lamellar body content and microvillar processes, or by cytokeratin-positive staining.
Explant Culture
fetal rat lung tissue was cut into 1 mm cubes with a McIlwain tissue chopper (Brinkmann Instruments, Westbury, NY)and incubated in 0.5 ml Waymouth’s MB-252/l medium containing penicillin, streptomycin and fungizone (100 U/ml, 100 U/ml, 2.5μg/ml, respectively) while rocking on an oscillating platform (three cycles per min) in an atmosphere of 5% CO2-air at 37°C (26).
mRNA Extraction
Total cellular RNA was isolated using previously described methods (5).
Semi-Quantitative RT-PCR
The appropriate cDNA fragments were amplified using 400ng of total RNA from lung tissues or cells, avian myeloblastosis virus reverse transcriptase and random hexamers and deoxyribonucleotides. The reactions were run at 42°C for 75 min and terminated by heating at 95°C for 5 min. Co-amplification with GAPDH cDNA was used as an internal standard. PCR was initiated by Taq DNA polymerase and allowed to proceed for 30 cycles with an annealing temperature of 50°C. The following primers were used in the RT-PCR assay: rat SP-B (sense, 5′ TACACAGTACTTCTACTAGATG; antisense, 3′ ATAGGCTGTTCACTGGTGTTCC human leptin (sense, 5′-CCTATCTTTTCTATGTCCAAGC; antisense, 3′,-GTGAGGATCTGTTGGTAGACTG); human leptin receptor designed to detect all of the known isoforms (sense, 5′-TACTTTGGAAGCCCCTGATG; antisense, 3′-AAGCACTGAGTGACTGCACG); rat leptin(sense, 5′-TTATGTTCAAGCAGTGCCTATC; antisense, 3′-CATCCAACTGTTGAAGAATGTC); rat leptin receptor (sense, 5′-ACCTTCAGTTCCAGATTCGA; antisense, 3′-TGAGATTGGTCTGATTTCCC). The identities of all RT-PCR products were confirmed by Southern blotting. mRNA expression was quantitated by densitometry (Eagle Eye, Stratagene).
Phospholipid Assay
the rate of saturated phosphatidylcholine synthesis was determined as previously described (6) with modifications. Briefly, confluent monolayer cultures of fetal rat type II cells, H441 cells or fetal rat lung explants were treated with leptin, PTHrP, leptin antibody or PTHrP receptor antagonist and subsequently incubated with 3H-choline chloride (1 μCi/ml) for 4h at 37°C in 5%CO2/balance air. A lamellar body fractions were prepared from the cells and tissues as described by Snyder et al ( ). In order to correct for procedural losses, fetal rat lung tissue was incubated with [U-14C]glycerol (10 μCi/ml, 10 mCi/mmole, New England Nuclear Corp., Boston, MA) for 24h and lamellar bodies were prepared as previously described (21). Ten thousand dpm of this lamellar body preparation was added to each sample prior to processing for 3H-saturated phosphatidylcholine. Phospholipids were extracted from the lamellar body fractions by the method of Bligh and Dyer (3) and dried under a stream of nitrogen at 50°C. The phospholipid extracts were mixed with 0.5 ml of an osmium tetroxide solution (70 mg/100 ml carbon tetrachloride) and reacted for 15 min at room temperature (29). The reaction mixture was dried under a stream of nitrogen at 60°C and the dried extract was chromatographically separated by thin layer chromatography in chloroform:methanol:water::65:25:4 (v/v) (29). The phospholipids were subsequently scraped from the chromatography plates and analyzed for their radioactive content by liquid scintillation spectrometry.
Protein Determination
was made using the Bradford dye binding method (4).
Statistical Analyses
Analysis of Variance was used to compare data within experimental groups. The null hypothesis was rejected when p<0.05 was not obtained.
Results
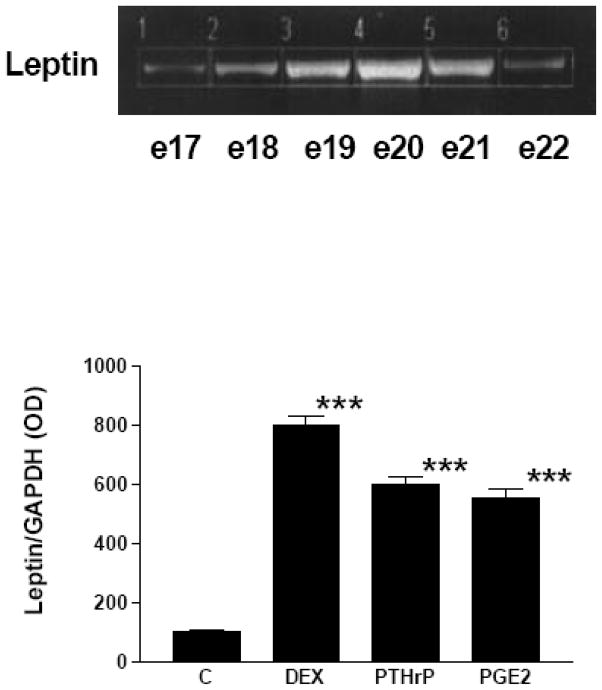
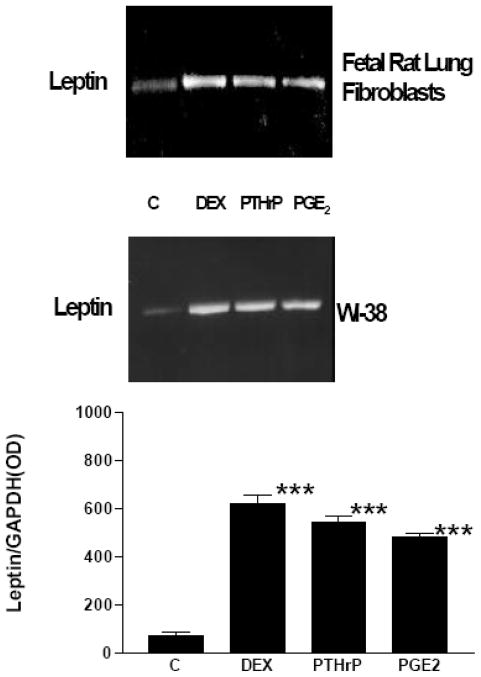
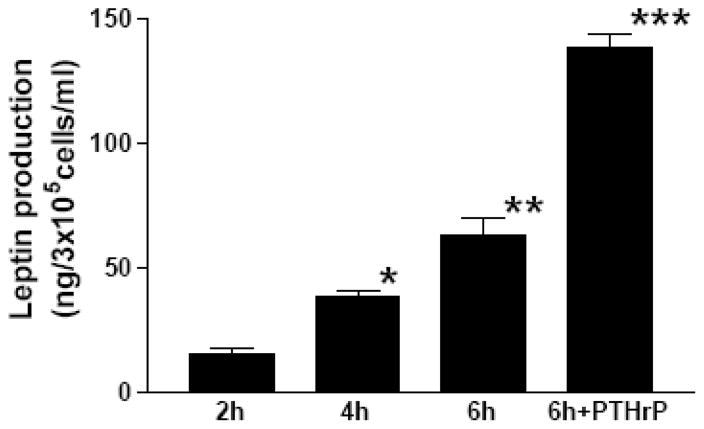
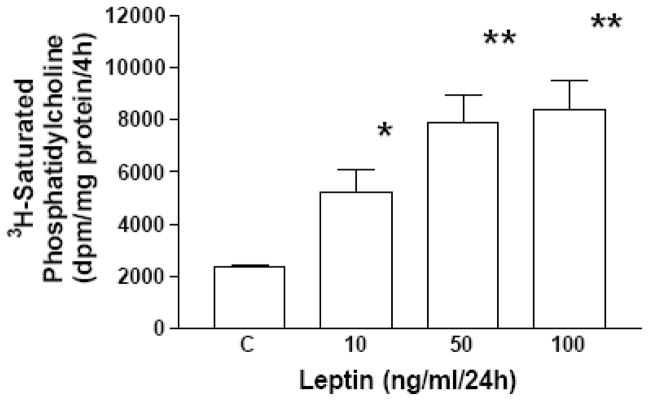
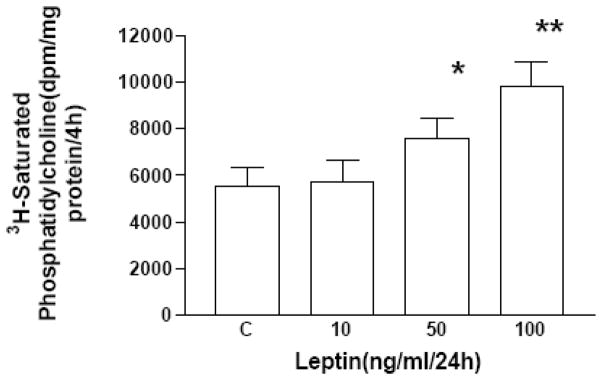
Initially, we examined the expression and ontogeny of leptin by fetal rat lung in the last third of gestation, during the phase of lung development when the fibroblast and type II cell mature. As can be seen in [Fig. 1], the expression of leptin increases progressively between E17 and E21, which corresponds to the transition from the glandular .E17), to the canalicular (E18, E19), to the saccular (.E20) stage of fetal rat lung development. The apparent decrease in leptin expression on E22 was observed consistently (n=5). Leptin expression by E19 fetal rat lung fibroblasts [Fig. 2, top graph] is significantly increased by dexamethasone (8-fold), PTHrP (6-fold) and PGE2 (6-fold), which are endocrine and paracrine factors known to stimulate lipofibroblast development. Similar effects on leptin expression were obtained [Fig. 2, bottom graph] by incubating human embryonic lung fibroblasts (WI38) with these same agonists. H441 human small cell carcinoma cells [Fig. 3] and E18 fetal rat type cells expressed the leptin receptor, whereas expression of the leptin receptor was faint in fetal rat lung fibroblasts and WI38 cells, consistent with the hypothesized paracrine nature of the leptin mechanism within the alveolar acinus. Fetal rat lung fibroblasts cumulatively secrete leptin into the culture medium over a 24 hour period [Fig. 4], increasing from 23 +/−8ng/ml/4h/106 cells, to 57+/−12ng/ml/8h, to 97+/−58ng/ml/24h/106 cells, equivalent to about 5ng/ml/h/106 cells; incubation of these cells with PTHrP increased secretion of leptin by greater than 2-fold more than the 6h rate. Incubation of fetal rat type II cells with leptin concentrations comparable to the amounts we had observed being produced by the fetal rat lung fibroblasts over a 24 hour period stimulated the incorporation of 3H-choline into lamellar body saturated phosphatidylcholine at 10,50 and 100 ng/ml by 150 to 400 percent, respectively [Fig. 5]. Leptin also stimulated choline incorporation into lamellar body saturated phosphatidylcholine by H441 cells, though the effect was lower than that of leptin on the fetal type II cells, i.e. there was no effect at 10 ng/ml, 40% at 50 ng/ml and 85% at 100 ng/ml [Fig. 6]. The stimulation of surfactant synthesis by these cells of human adult lung origin is evidence for the presence of functional leptin receptors on adult type II cells, which is consistent with the observed expression of leptin receptor mRNA by these cells [see Fig. 3].
Figure 1. Leptin ontogeny in fetal rat lung.
Semiquantitative RT-PCR was performed using RNA from fetal rat lung at the indicated gestational ages (e17–e22). Co-amplification with GAPDH cDNA was used as an internal standard for normalization of aliquot volumes for leptin mRNA expression.
Figure 2. Semi-quantitative RT-PCR of RNA extracts from fetal rat lung fibroblasts and WI38 human embryonic lung fibroblasts.
Primary fetal rat lung fibroblasts (E18) and WI38 human embryonic lung fibroblasts were treated with either dexamethasone (DEX, 1 × 10−8 M), parathyroid hormone-related protein (PTHrP, 5 × 10−7 M), or prostaglandin E2 (PGE2, 5 × 10−7 M) for 24 hours and subsequently analyzed for leptin mRNA expression using semi-quantitative RT-PCR. Co-amplification with GAPDH cDNA was used as an internal standard for normalization of the aliquot volumes for leptin mRNA expression. (Center panel) Representative blots for RT-PCR of leptin mRNA; the top and bottom graphs are densitometric quantitation of the corresponding blots. Each bar represents the mean ±standard deviation, n=5, ***p<.0001 by analysis of variance.
Figure 3. Semi-quantitative RT-PCR for leptin receptor mRNA.
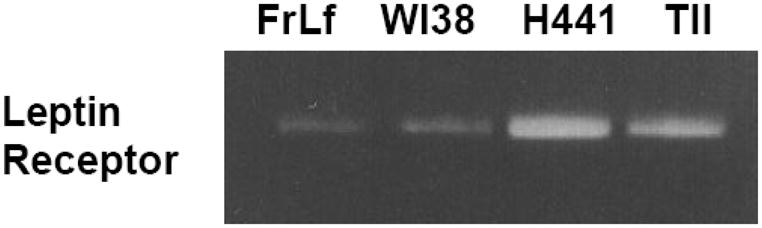
Primary fetal rat lung fibroblasts (FrLf) and type II cells, WI38 human embryonic lung fibroblasts and H441 adult lung epithelial cells were assayed for leptin receptor mRNA expression. Co-amplification with GAPDH cDNA was used as an internal standard for the normalization of the aliquot volumes for leptin receptor mRNA expression.
Figure 4. Fetal rat lung fibroblasts secrete leptin under the influence of PTHrP.
E21 fetal rat lung fibroblasts were cultured in 2 cm2 wells in serum-free DMEM containing 0.1% fatty acid-free bovine serum albumin for up to 6 hours plus or minus parathyroid hormone-related protein (PTHrP, 5 × 10−7 M). Leptin concentrations were determined by an enzyme linked immunosorbent assay for rat leptin. Each bar represents the mean±standard deviation, n=5, *p<.05, **p<.01, ***p<.001 by analysis of variance.
Figure 5. Leptin stimulates surfactant phospholipid synthesis by fetal lung type II cells.
E19 fetal rat lung type II cells were incubated with leptin for 24h and exposed to 3H-choline chloride for the last 4h. The type II cell lamellar body fractions were subsequently assayed for 3H-saturated phosphatidylcholine content. Each bar represents the mean±standard deviation, n=5,*p< .05, **p< .01, control(C) versus leptin treated by analysis of variance.
Figure 6. Leptin stimulates surfactant phospholipid synthesis by adult lung epithelial cells.
H441 adult lung epithelial cells were incubated with leptin for 24h and exposed to 3H-choline chloride for the last 4h. The H441 cell lamellar body fractions were subsequently assayed for 3H-saturated phosphatidylcholine content. Each bar represents the mean±standard deviation, n=5, *p< .05, **p< .01, control (C) versus leptin treated by analysis of variance.
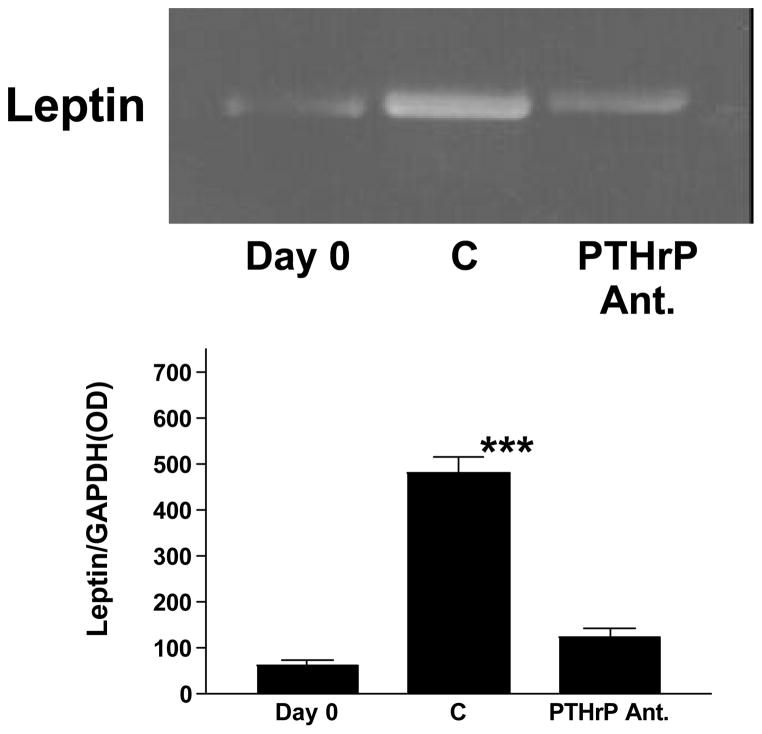
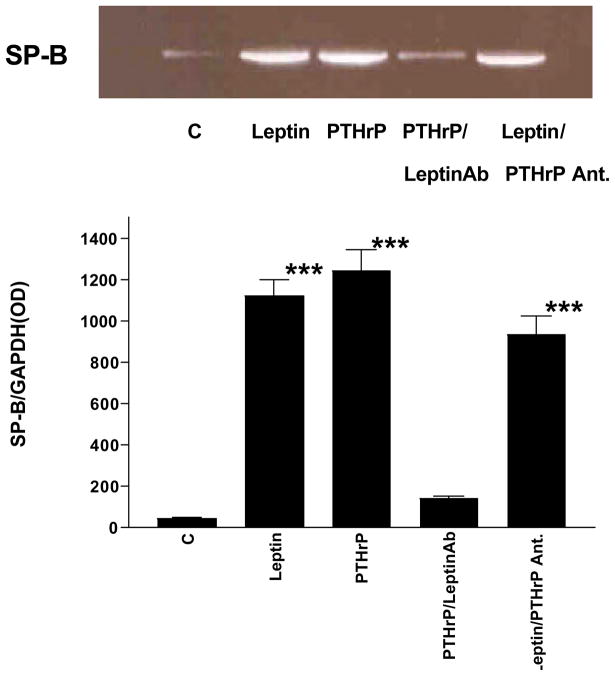
In subsequent experiments, we examined the interaction of PTHrP and leptin in fetal rat lung explant culture. Incubation of E18 fetal rat lung explants with leptin (200 ng/ml/24h) or PTHrP (5 × 10−7 M/24h) significantly increased the incorporation of 3H-choline into 3H-saturated phosphatidylcholine (more than 2-fold) [Fig. 7]. The concentration of leptin was chosen based on its maximally effective dose in monolayer culture (see effect of leptin on type II cells in monolayer culture, Fig. 4) and doubled to compensate for tissue penetration. The PTHrP dose was based on previous studies of its effect on surfactant synthesis in explant culture (14). The stimulatory effect of PTHrP was blocked by co-incubation with leptin antibody (1:100 dilution). However, the stimulatory effect of leptin was not blocked by co-incubation with the PTHrP receptor antagonist. Maintaining E18 fetal rat lung tissue in explant culture for 3 days resulted in a 10-fold increase in leptin mRNA expression [Fig. 8], consistent with its developmental up-regulation during this phase of lung maturation. This increase in leptin mRNA expression was blocked by co-incubating with the PTHrP receptor antagonist (PTHrP(7–34)amide, 5 × 10−6 M). Incubation of E18 fetal rat lung explants with leptin (200 ng/ml/24h) or PTHrP (5 × 10−7 M/24h) also stimulated the expression of Surfactant Protein-B (SP-B) [Fig. 9]; co-incubation of PTHrP-treated E18 explants with leptin antibody (1:100 dilution) blocked the PTHrP stimulation of SP-B expression. The same effect was observed when leptin-treated explants were co-incubated with leptin antibody (data not shown), as expected. In contrast to this, co-incubation of leptin-treated explants with the PTHrP receptor antagonist (5 × 10−7 M/24h) had no effect on leptin stimulation of SP-B expression.
Figure 7. Leptin antibody inhibits PTHrP stimulation of surfactant phospholipid.
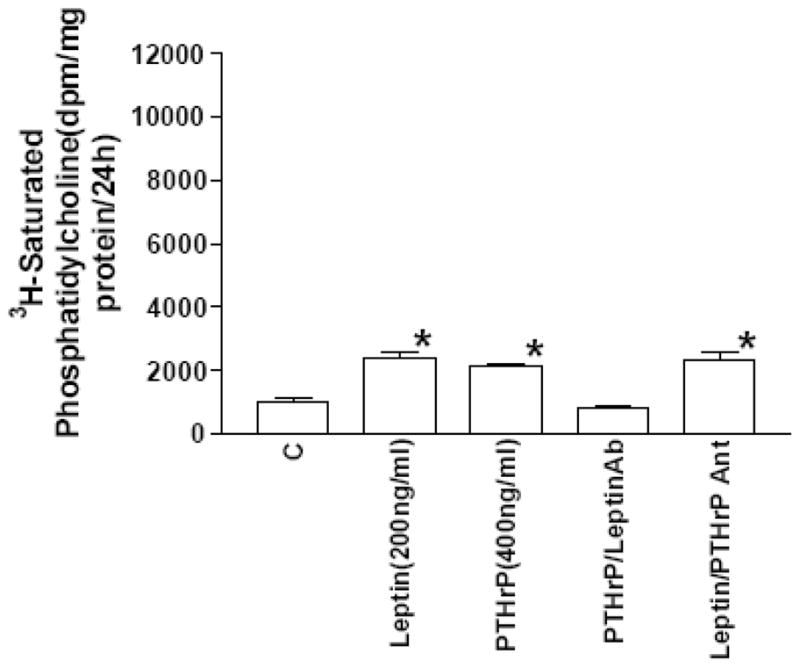
E18 fetal rat lung explants were maintained in culture for 24h. The explants were incubated with leptin during the 24h culture period, and were exposed to 3H-choline chloride for the last 4h. Leptin and parathyroid hormone-related protein (PTHrP) significantly increased 3H-saturated phosphatidylcholine synthesis (n=5,*p<.01, control(C) versus treated, by analysis of variance). Co-incubation of PTHrP-treated explants with leptin antibody (Leptin Ab, 1:100 dilution) blocked the stimulatory effect of PTHrP on saturated phosphatidylcholine synthesis; co-incubation of leptin-treated explants with PTHrP receptor antagonist (PTHrP Ant., 5 × 10−6 M) had no effect on saturated phosphatidylcholine synthesis.
Figure 8. Leptin mRNA expression is PTHrP-dependent.
E18 fetal rat lung explants were maintained in culture for 3 days plus or minus PTHrP receptor antagonist (PTHrP Ant., 1 × 10−6 M). The explants were subsequently analyzed for leptin mRNA expression by semi-quantitative RT-PCR. Co-amplification with GAPDH cDNA was used as an internal standard for normalization of the aliquot volumes for leptin mRNA expression. The graph represents densitometric quantitation of the leptin mRNA bands; each bar is the mean±standard deviation, n=5,***p<.0001 by analysis of variance.
Figure 9. PTHrP paracrine stimulation of Surfactant Protein-B (SP-B) is leptin-dependent.
E18 fetal rat lung explants were maintained in culture for 24h plus or minus leptin (200 ng/ml), parathyroid hormone-related protein (PTHrP, 5 × 10−7 M), PTHrP plus leptin antibody (PTHrP/LeptinAb) (1:100 dilution), or leptin plus PTHrP receptor antagonist (Leptin/PTHrP Ant., 5 × 10−6 M). Surfactant Protein-B (SP-B) mRNA expression was determined using semi-quantitative RT-PCR as shown in the representative blot. Co-amplification with GAPDH cDNA was used as an internal standard for normalization of the aliquot volumes for leptin mRNA expression. The bars in the graph below the blot represent the mean±standard deviation for control(C) versus treated groups; n=5,***p<.0001 by analysis of variance.
Discussion
The hypothesized role of leptin as a paracrine mediator of the lung type II cell-fibroblast interaction resulting in surfactant synthesis was supported by our experimental results. Leptin and its receptor are expressed by fetal rat lung fibroblasts and type II cells, respectively, prior to the onset of type II cell maturation beginning on E19–E20, and leptin expression is under both endocrine and paracrine control. Leptin stimulates both surfactant phospholipid and protein synthesis by type II cells by a PTHrP-dependent signaling pathway, based on the observation that the PTHrP receptor antagonist inhibits the developmental up-regulation of leptin expression in explant culture, and the leptin antibody blocks the PTHrP stimulation of surfactant phospholipid and protein expression, though the PTHrP receptor antagonist has no effect on (downstream) leptin-stimulated SP-B expression.
In the present series of experiments we have observed that leptin mRNA expression in the developing fetal rat lung increases during the period of alveolar lung differentiation when pulmonary surfactant phospholipid synthesis is induced (E18–20). Leptin expression appears to peak at E20 and then progressively declines on E21, E22. This phenomenon has also been observed for FPF (19), and may be due to the thinning of the alveolar wall which occurs during the process of alveolarization. The process of alveolar differentiation is a well-recognized hormonally-regulated paracrine mechanism (17) which depends upon interactions between the type II epithelial cell and mesenchymal fibroblast. Type II cells elaborate parathyroid hormone-related protein (PTHrP) (24), which promotes the differentiation of the mesenchyme into lipofibroblasts in a manner similar to adipocyte differentiation, including the expression of key adipocytic enzymes, triglyceride uptake, storage and secretion (11). These PTHrP-induced lipofibroblasts then apparently produce a soluble growth factor which stimulates type II cell surfactant phospholipid synthesis (14,24) and SP-B expression, as shown in this study; the other surfactant proteins (A, C, D) were not examined, though they also play an essential role in surfactant metabolism. With these principles in mind, we hypothesized that the mature lipofibroblast, like the mature adipocyte, would express leptin, a secreted product of the mature adipocyte. Since leptin belongs to the IL-6 family of cytokines (32), and IL-6 stimulates the synthesis of pulmonary surfactant by type II cells (13), PTHrP-stimulated expression of leptin by lipofibroblasts provides a closed paracrine loop from the type II cell to the lipofibroblast, and back to the type II cell.
It has long been recognized that alveolar type II cell differentiation is dependent on mesenchymal-epithelial interactions (15), which are mediated by low molecular weight soluble factors (7). Smith (19) was the first investigator to identify a specific hormonally-induced mesenchymal factor which stimulated surfactant phospholipid synthesis over twenty years ago (18), though the identity of this factor has remained undetermined. He termed this differentiation factor fibroblast pneumonocyte factor, or FPF. We subsequently discovered that FPF was down-regulated by androgens (6,12), providing further evidence for a biologic role of FPF in the timing of lung development, since there is a spontaneous sex difference in the rate of lung development and pulmonary surfactant synthesis (6,28) which is androgen-dependent (27). Leptin has many of the same characteristics as FPF: [1] its molecular weight (16-kDa) is within the range previously reported for FPF (18); [2] it is expressed by lung mesenchymal cells during fetal development in a pattern like that reported for FPF (i.e. beginning in the canalicular phase, peaking on E21 and then declining on E22) (18); [3] its expression is stimulated by glucocorticoids (18) and inhibited by both androgens (9) and TGF∃ (26,28). These similarities between leptin and FPF strongly support their common identity. Their functional similarities are further reinforced by their common cellular origins- leptin is produced by the ob gene of the mature adipocyte (31), which lung lipofibroblasts bear a strong resemblance to, both structurally and functionally (11). FPF is also produced by adepithelial lung fibroblasts (M. Post, personal communication). In summary, leptin may be the long sought after soluble factor which mediates the hormonal effects on fetal lung development which govern surfactant production.
Acknowledgments
This work was supported by NIH grant HL55268
References
- 1.Adamson IY, Young L, King GM. Reciprocal epithelial: fibroblast interactions in the control of fetal and adult rat lung cells in culture. Exp Lung Res. 1991;17:821–835. doi: 10.3109/01902149109062880. [DOI] [PubMed] [Google Scholar]
- 2.Battenburg JJ, MOtto-Verbeme CJ, Ten Have-Opbroek AA, Klazinza W. Isolation of alveolar type 11 cells from fetal rat lung by differnetial adherence in monolayer culture. Biochim Biophys Acta. 1990;960:441–453. doi: 10.1016/0005-2760(88)90053-7. [DOI] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJA. A rapid method of total lipid extraction and purification. Canad J Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Chomzynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Floros J, Nielsen HC, Torday JS. Dihydrotestosterone blocks fetal lung fibroblast pneumonocyte factor at a pretranslational level. J Biol Chem. 1987;262:13592–13598. [PubMed] [Google Scholar]
- 7.Grobstein C. Mechanisms of organic tissue interaction. Natl Cancer Inst Monogr. 1975;26:279–299. [PubMed] [Google Scholar]
- 8.Hithcock O’Hare K, Sheridan MN. Electron microscopic observations on the morphogenesis of the albino rat lung, with special reference to pulmonary epithelial cells. Am J Anat. 1970;127:181–206. doi: 10.1002/aja.1001270205. [DOI] [PubMed] [Google Scholar]
- 9.Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA. 1997;94:11073–8. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–74. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 11.McGowan S, Torday JS. Pulmonary Lipid Laden Fibroblasts. Annu Rev Physiol. 1999;59:45–64. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen HC, Torday JS. Sex Differences in Fetal Lung Development: Biology, Etiology and Evolutionary Significance. In: Mendelson CR, editor. Contemporary Endocrinology of the Lung: Development and Surfactant Synthesis. Humana Press, Inc; Totowa, NJ: 2000. pp. 141–159. [Google Scholar]
- 13.Rehan VR, Desai SH, Tantravahi JG, Pasquirello T, Steintroff MS, Paul SR, Torday JS, Rubin LP. Differential effects of IL-6 and IL-11 on lung epithelial cell surfactant production and cell kinetics. Am J Physiol, Lung Cellular and Molecular Physiol. in press. [Google Scholar]
- 14.Rubin LP, Kifor O, Hua J, Brown EM, Torday JS. Parathyroid hormone(PTH) and PTH-related protein stimulate surfactant phospholipid synthesis in rat fetal lung, apparently by a mesenchymal-epithelial mechanism. Biochim Biophys Acta. 1994;1223:91–100. doi: 10.1016/0167-4889(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 15.Rutter WJ. An analysis of pancreatic development: Role of mesenchymal factor and other extracellular factors. Symp Soc Dev Biol. 1978;35:205–207. doi: 10.1016/b978-0-12-612981-6.50019-3. [DOI] [PubMed] [Google Scholar]
- 16.Senior PV, Heath DA, Beck F. Expression of parathyroid hormone-related protein mRNA in the rat before birth: demonstration by hybridization histochemistry. J Mol Endocrinol. 1991;6:281–290. doi: 10.1677/jme.0.0060281. [DOI] [PubMed] [Google Scholar]
- 17.Smith B, Post M. Fibroblast-pneumonocyte factor. Am J Physiol. 1989;257:1,174–178. doi: 10.1152/ajplung.1989.257.4.L174. [DOI] [PubMed] [Google Scholar]
- 18.Smith BT. Lung maturation in the fetal rat: Acceleration by Injection of Fibroblast Pneumonocyte Factor. Science. 1979;204:1094–1095. doi: 10.1126/science.582216. [DOI] [PubMed] [Google Scholar]
- 19.Smith BT. Fibroblast-pneumonocyte factor: intercellular mediator of glucocorticoid effect on fetal lung. In: Stern L, editor. Neonatal Intensive Care. 2. New York: Masson; 1978. [Google Scholar]
- 20.Smith BT, Giroud CJP. Effects of cortisol on serially propagated fibroblast cell cultures derived from the rabbit fetal lung and skin. Canad J Physiol Pharmacol. 1975;53:1037–1041. doi: 10.1139/y75-144. [DOI] [PubMed] [Google Scholar]
- 21.Snyder JM, Longmuir KJ, Johnston JM, Mendelson CR. Hormonal regulation of the synthesis of lamellar body phosphatidylglycerol and phosphatidylinositol in fetal lung tissue. Endocrinology. 1983;112:1012–1018. doi: 10.1210/endo-112-3-1012. [DOI] [PubMed] [Google Scholar]
- 22.Sorokin S. Recent work on the developing lungs. In: deHaan RL, Ursprung H, editors. Organogenesis. New York: Holt; 1965. pp. 467–491. [Google Scholar]
- 23.Taderera JV. Control of lung differentiation in vitro. Dev Biol. 1967;16:489–512. doi: 10.1016/0012-1606(67)90061-9. [DOI] [PubMed] [Google Scholar]
- 24.Torday JS, Tsai S-W, Sun H, Rubin LP. The physiologic role of fluid deformation in fetal lung development. Cellular deformation: Mechanics and Mechanisms of Physiologic Response. Am J Med Sci. 1998;316:208–208. [Google Scholar]
- 25.Torday JS, Qin J, Sun H. Prostaglandin E2 integrates the effects of fluid distension and glucocorticoid on lung maturation. Am J Physiol. 1998;274:1,106–1,111. doi: 10.1152/ajplung.1998.274.1.L106. [DOI] [PubMed] [Google Scholar]
- 26.Torday JS. Cellular Timing of Fetal Lung Development. Semin Perinatol. 1992;16:130–139. [PubMed] [Google Scholar]
- 27.Torday JS. Fetal adrenal androgens delay lung maturation in vitro. Endocrinology. 1990;126:3240–3244. doi: 10.1210/endo-126-6-3240. [DOI] [PubMed] [Google Scholar]
- 28.Torday JS, Kourembanas S. Fetal rat lung fibroblasts produce a TFGP homolog that blocks alveolar type II cell maturation. Dev Biol. 1990;139:35–41. doi: 10.1016/0012-1606(90)90276-o. [DOI] [PubMed] [Google Scholar]
- 29.Torday JS, Carson L, Lawson EE. Saturated phosphatidylcholine in amniotic fluid and prediction of the respiratory distress syndrome. N Engl J Med. 1979;301:1013–1018. doi: 10.1056/NEJM197911083011901. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999;36:273–9. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- 31.Vernon RG, Barber MC, Travers MT. Present and future studies on lipogenesis in animals and human subjects. Proc Nutr Soc. 1999;58:541–9. doi: 10.1017/s0029665199000713. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H. Leptin receptor action in hepatic cells. J Biol Chem. 1997;272:16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 33.Wolf G. The molecular mechanism of the stimulation of adipocyte differentiation by a glucocorticoid. Nutr Rev. 1999;57:324–326. doi: 10.1111/j.1753-4887.1999.tb06907.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Puigserver P, Spiegelman BM. Transcriptional activathon of adipogenesis. Curr Opin Cell Biol. 1999;11:689–694. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]