Abstract
Carbon nanotubes (CNTs) have emerged as some of the most promising materials for the technologies of the future. One of the most significant limitations to furthering the understanding and application of these fascinating systems is the lack of atomic-level structural control in their syntheses. Current synthetic methods produce mixtures of structures with varying physical properties. In this article, we describe the potential advantages, recent advances, and challenges that lie ahead for the bottom-up organic synthesis of homogeneous carbon nanotubes with well-defined structures.
1. Introduction
Carbon nanotubes are allotropes of carbon that are made entirely of sp2-hybridized carbon wrapped into cylindrical structures [1–6]. In 2009, over 5000 journal articles were published on the subject of carbon nanotubes alone [7]. The enormous attention received is a direct result of the unique physical properties of CNTs. For example, aspect ratios (length-to-width) of over 10,000,000 have been observed for CNTs, which are significantly larger than any other material [8]. This large aspect ratio translates into a very high surface area, which has been exploited for numerous applications. In addition to high surface area, CNTs are some of the strongest materials known, with a tensile strength and specific strength far exceeding that of even steel [9].
Although the mechanical properties of CNTs are extraordinary, the electronic and optical properties are the most intriguing for future nanotechnologies. Certain types of CNTs are metallic, which can, in theory, support current densities of 4 × 109 A/cm2, more than 1000 times that of copper [10]. Other types of CNTs are semiconducting, with bandgaps that can vary depending on the diameter and arrangement of carbon atoms along the tube [11]. The wide variety of electronic properties of CNTs coupled with their nanometer dimensions have rendered CNTs extremely attractive for applications in the fields of electronics and energy, as well as for sensor applications. In addition to their electronic properties, upon excitation, carbon nanotubes can emit different wavelengths of light depending on their structure—a useful property for nanophotonic devices [12]. CNTs have the potential to impact a wide range of fields and, therefore, have emerged as one of the most promising materials in nanoscience research.
Unfortunately, one of the qualities that make CNTs such a powerful platform—the tunability of the electronic and optical properties—is the same quality that impedes their utilization. In order to take advantage of the wide spectrum of CNT properties, the ability to synthesize homogeneous batches of carbon nanotubes with a desired property is required. Currently, carbon nanotubes are produced as heterogeneous mixtures, and arduous purification techniques are necessary in order to enrich for a specific type of CNT [13–15]. The homogeneous synthesis of CNTs stands as one of the grand challenges for carbon nanotube research. In this article, we will briefly describe the limitations of current synthetic methodology and provide a rationale for why a bottom-up organic chemistry approach may lead to the ultimate solution. We will go on to describe the progress that has been made in this area and the challenges that need to be addressed in the future for this to be a truly viable strategy.
2. CNT Structure
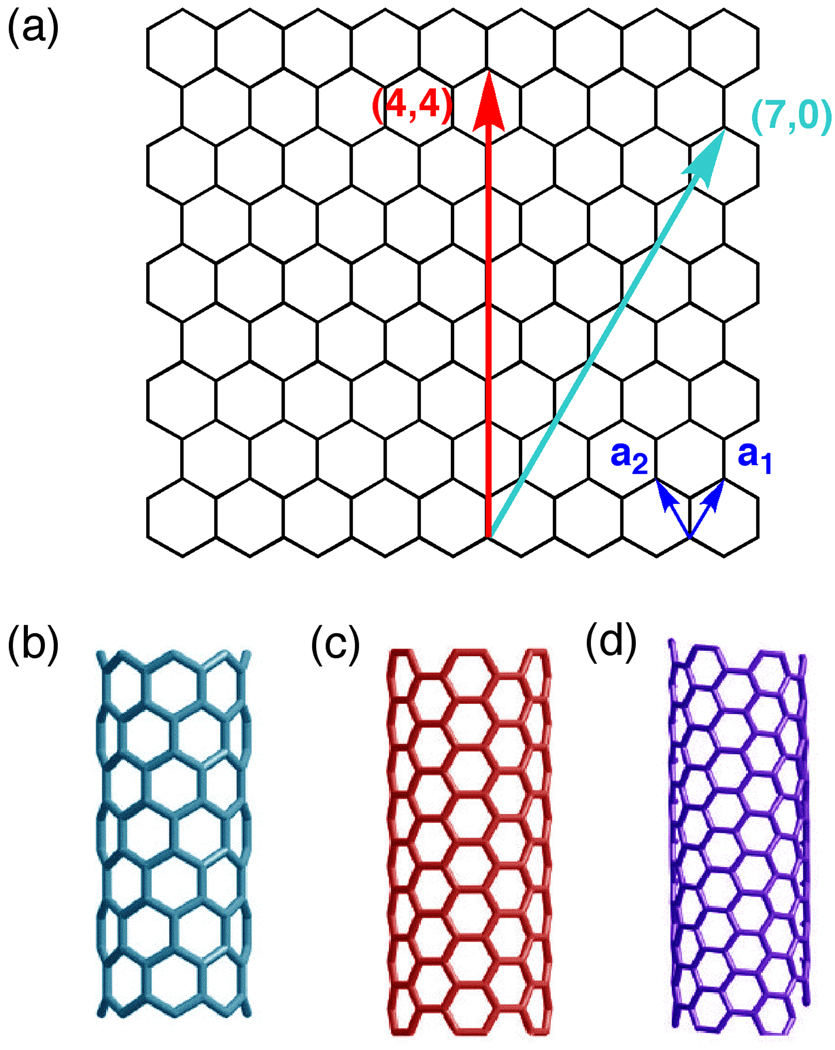
Carbon nanotubes have a wide range of optical and electronic properties. The diversity in properties is a direct consequence of the structural variation of the basic cyclindrical structure [16]. This difference in CNT structure is most easily visualized by imagining a carbon nanotube as a rolled-up sheet of graphene (Fig. 1a). Just as in the case of rolling up a sheet of paper, the graphene sheet can be rolled up at many different angles. The method for denoting the various CNT structures follows this concept. The method denotes the angle of wrapping relative to two unit vectors, a1 and a2 [16]. For instance, the cyan arrow illustrates a vector designated by the integers (7,0). These indices indicate moving 7 units along the a1 vector and 0 units along the a2 vector. If the end of the cyan arrow is wrapped such that it connects to the beginning of the arrow, a (7,0) CNT is generated. All CNT structures can be designated by this (m, n) index. CNTs with (m, 0) indices are referred to as zigzag tubes and have the general structure as shown in Fig. 1b, in which m phenyl rings are fused along the diameter of the tube. In contrast, if a cylinder is rolled up along the red vector, we generate a (4,4) CNT. CNTs with (m,n) indices where m = n are referred to as armchair CNTs and have the general structure shown in Fig. 1c. Note that in contrast to the zigzag CNTs, the phenyl rings in this case are linked by single bonds along the diameter and are fused along the long axis. All other CNTs are chiral and will have a helical pitch such as the one shown in Fig. 1d. Due to the different diameters, lengths, and chirality, a large number of CNT structures are possible—each possessing unique electronic and optical band structures. To add even greater diversity, CNTs can also change chirality along the tube axis, as well as incorporate structures other than 6-membered rings [17]. The seemingly limitless range of properties of carbon nanotubes is a fascinating consequence of quantum mechanics.
Figure 1.
CNTs have varying properties depending on the arrangement of carbon atoms.
3. Current Methods of CNT Production
Methods for carbon nanotube synthesis have been discussed extensively elsewhere, therefore, a comprehensive review of this topic will not be presented here. A brief discussion of CNT synthesis is appropriate, however, to familiarize the reader with the most common methods of production, as well as to the general limitations of these techniques.
Carbon nanotubes are produced today by methods that are in many ways very similar to those that resulted in their original, serendipitous discovery. Sumio Iijima’s arc discharge synthesis of CNTs in 1991 is often credited as the first preparation of carbon nanotubes [1]. In an arc discharge CNT synthesis, a current is passed between two graphitic electrodes, causing the graphite to vaporize and condense on the cathode, resulting in carbon nanotubes. This early method is still commonly used today and can produce carbon nanotubes in up to 30% yield. A few years later, a second important method for production of CNTs, termed laser ablation, was developed by Richard Smalley [18]. In this method, a pulsed laser is used to vaporize a graphite/catalyst target generating carbon nanotubes on the walls of the reactor. The laser ablation method can yield up to 70% CNTs, but is costly and not amenable to large-scale production. A third process for CNT synthesis, which is perhaps the most useful to date, is chemical vapor deposition (CVD) [19]. For CVD synthesis, a metal catalyst particle is deposited on a substrate and placed into a high temperature furnace. A carbon gas source, such as ethane or methane, is then passed through the furnace, resulting in growth of CNTs on the substrate. Advances in the CVD method have allowed for the bulk preparation of CNTs.
While attempts have been made to adapt these methods to a chirality-controlled synthesis, so far this has been relatively unsuccesful. Most recently, Harutyunyan reported a CVD technique in which the metallic-to-semiconducting ratio—typically 1:2 under standard growth conditions—can be shifted to 90:10 favoring metallic CNTs [20]. Harutyunyan clearly deomonstrated that by altering the catalyst annealing conditions, the catalyst morphology can be altered. The change in metallic-to-semiconducting ratio is attributed to this change in catalyst morphology, indicating that the nature of the catalyst can potentially control chirality. Although this conclusion is a logical one, how to extend this empirical result to a rational synthesis of a variety of CNTs with different chiralities is not clear. The same argument is true for reports of growth conditions that can dramatically alter the ratio of carbon nanotubes to favor semiconducting CNTs. While these types of growth conditions can produce up to 95% semiconducting CNTs, nanotubes of at least five different chiralities are produced [21]. Each of these individual CNTs will have different electronic and optical band structures. Here again, it is not obvious how one progresses from these albeit useful empirical observations to a rational chirality-controlled synthesis.
This inability to synthesize pure CNTs has thus fueled research in purification techniques. Perhaps most notably, centrifugation purification techniques have lead to the enrichment of CNTs of a specific chirality based on different densities of wrapped nanotubes. These techniques, however, are arduous and so far do not seem amenable to a scalable production [15]. Unquestionably, the more efficient process would be to synthesize a batch of CNTs in which 99% of the nanotubes are of a predetermined chirality as opposed to isolating a small fraction out of a mixture of CNTs. In order to fully exploit the electronic and optical properties of CNTs for advanced nanotechnologies, a more rational approach to carbon nanotubes of discrete chirality will ultimately be required.
4. A Bottom-up Template Approach to CNTs with Discrete Chirality
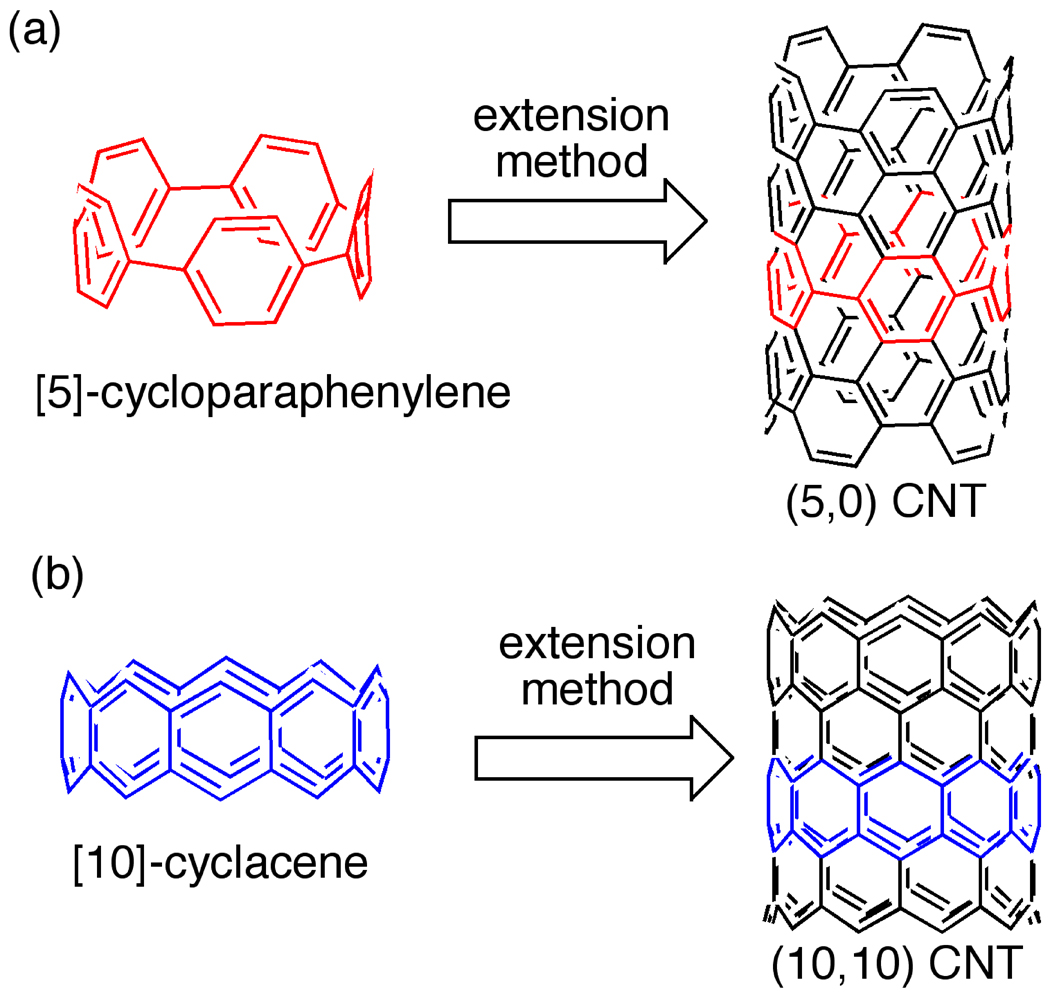
Synthetic organic chemistry can potentially provide a powerful solution to the CNT synthesis problem. Our approach to synthesizing carbon nanotubes with control of chirality relies on utilizing hoop-shaped carbon macrocycles—small fragments of CNTs that retain information regarding chirality and diameter— as templates for CNT synthesis (Fig. 2). For example, a (5,5) armchair carbon nanotube can be constructed by fusing additional phenyl rings to [5]cycloparaphenylene (Fig. 2a). In similar fashion, a (10,0) zigzag CNT can be constructed, in principle, from [10]cyclacene (Fig. 2b). Therefore, our strategy for the bottom-up synthesis of CNTs rests in two basic areas of research: 1) the synthesis of aromatic macrocyclic templates and 2) the development of polymerization reactions to extend these templates into longer CNTs. This templating approach is particularly attractive in that it would be amenable to both zigzag and armchair CNTs of different diameters, as well as to chiral CNTs with different helical pitches.
Figure 2.
Bottom-up, organic synthesis approach to CNTs with discrete chirality.
An organic synthesis approach to controlling CNT chirality has several advantages to offer over the current methods. First, the templating strategy is a rational approach that can be based on mechanistically well-understood reactions. Therefore, the process will be easier to troubleshoot and optimize. In contrast, predicting a priori which morphology of a catalyst particle will give a particular chirality of a CNT seems virtually impossible. Although much effort has been dedicated to this topic, the CNT growth process by current methods is not a well-understood process at the atomistic level. Secondly, organic synthesis is typically practiced at temperatures well below 200 °C whereas the current techniques for carbon nanotube growth are often at temperatures closer to 1000 °C. Reactions are more likely to be selective and lead to fewer side products at lower temperatures, where the number of energetically possible intermediates is fewer. Logically, producing carbon nanotubes of exact chirality in a selective manner should rely on kinetic processes, not thermodyanamic processes. Lastly, an organic synthesis approach will ultimately allow for the production of CNTs that are completely inaccessible by current methods. For instance, by judicious choice of reactions, structures that contain nitrogen or boron or sulfur could be incorporated into CNTs with exact positioning.
At first glance, the argument against a bottom-up, organic synthesis approach might seem to reside in the large size of carbon nanotubes. Organic synthesis is often associated with molecules of 1–2 nanometers in size, but perhaps not molecules of millimeters in length. We would like to remind the reader that polymers of extremely large size have been made with organic synthesis with superb control of structure. As is demonstrated by this templating concept, carbon nanotubes can also be viewed as polymeric structures—structures with a basic repeating unit.
5. Synthesis of Templates
5.1. The first synthesis of cycloparaphenylene: the smallest slice of an armchair CNT
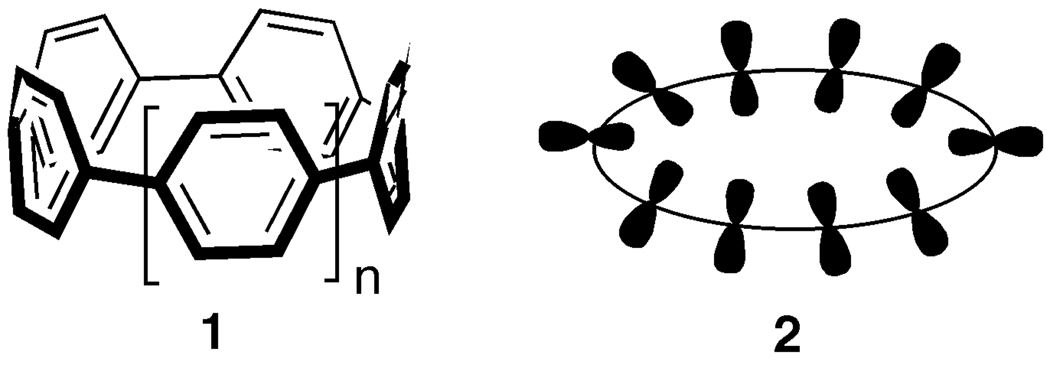
As an initial foray into this research area, we targeted the synthesis of cycloparaphenylenes (Fig. 3), which we refer to as “carbon nanohoops” due to their structural relationship with armchair CNTs. Although these compounds had been postulated in the literature dating back over 70 years [22], cycloparaphenylenes had never been realized in the laboratory, despite numerous synthetic attempts [23]. The heart of the synthetic challenge for these molecules lies in the strain energy of the bent aromatic system (Fig. 3, 2). In this macrocyclic system, the p orbitals are bent out of the normal preferred planarity, which results in a significant amount of strain energy (the smaller the macrocycle, the more severe the distortion). Our strategy was to build up strain sequentially during the synthesis, using a 3,6-syn-dimethoxy-cyclohexa-1,4-diene moiety as a masked aromatic ring in a macrocyclic intermediate (see structure 5, Figure 4) [24]. We reasoned that the cyclohexadiene unit would provide the curvature and rigidity necessary for macrocyclization to afford a marginally strained intermediate. Subsequent aromatization of the masked phenyl ring would then provide the driving force necessary to achieve the strain energy of the cycloparaphenylenes.
Figure 3.
“Bent” sp2-hybridized carbon structures are challenging synthetic targets due to strain energy.
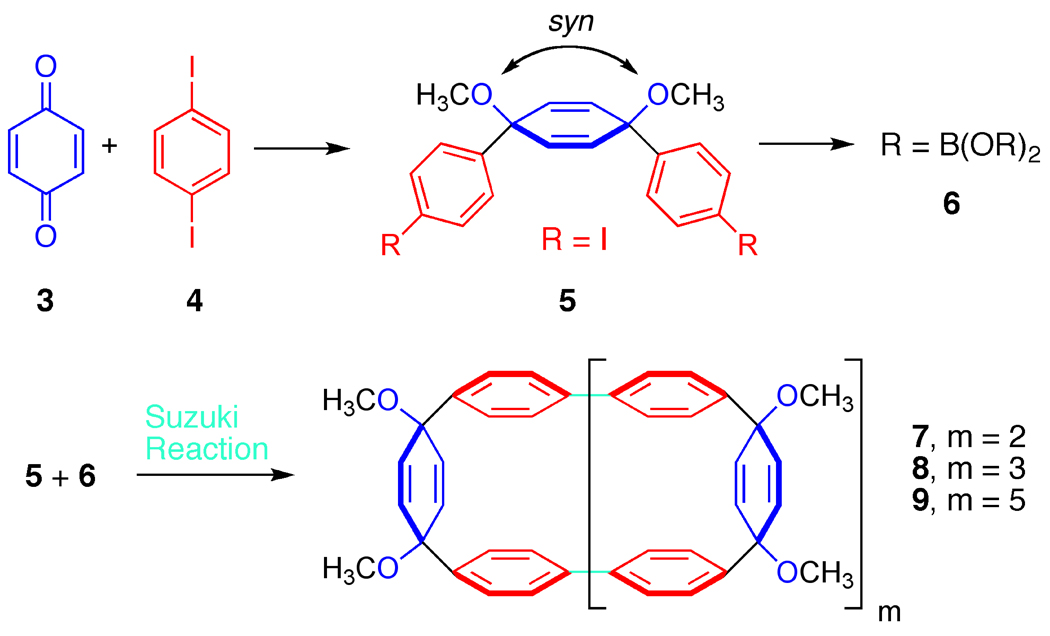
Figure 4.
Synthesis of macrocyclic precursors.
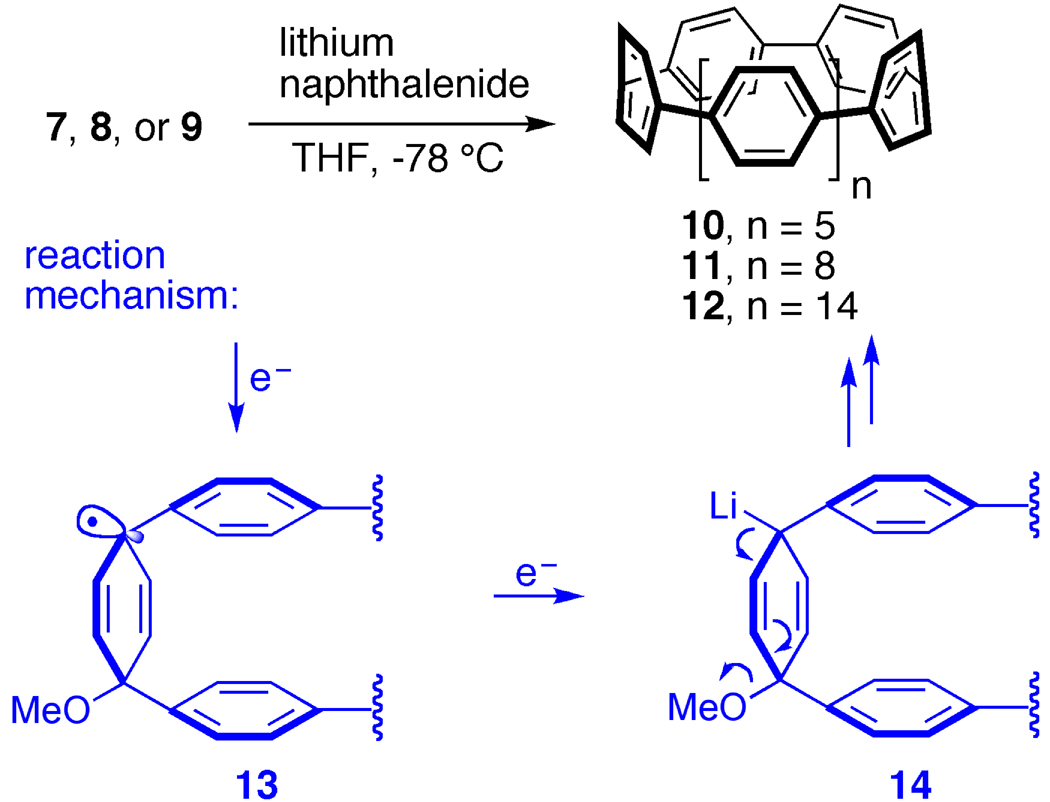
The synthesis of the macrocyclic precursors to cycloparaphenylenes is depicted in Figure 4 [25]. By treating diiodobenzene (4) with one equivalent of a lithiating agent, the aryl ring can be rendered nucleophilic by lithium-halogen exchange and subsequently reacted with benzoquionone to generate structure 5 [26]. One of the keys to this synthesis is that the product of this reaction is syn [27], meaning that the aryl groups are on the same side of the cyclohexadiene moiety. This generates the curvature necessary for the cyclization reaction. In order to connect these fragments together to form a macrocycle, we needed to construct aryl-aryl bonds. A common method for generating these types of bonds is termed a Suzuki-Miyaura coupling [28], a reaction which can combine an aryl halide with an aryl boronate to form a C-C bond. Therefore, we converted a portion of the aryl iodide 5 to boronate 6 utilizing a straightforward procedure [25]. At this stage, we were in position to investigate the C-C bond forming reactions. Our hypothesis was that the Suzuki-Miyaura coupling conditions would initially lead to the formation of intermolecular aryl-aryl bonds (shown in cyan). Due to the curvature and rigidity of the monomeric units, we anticipated the desired aryl-aryl bond formation, on occasion, would take place intramolecularly producing macrocyclic products. This outcome was indeed the case and we were able to generate and isolate macrocyles of three different ring sizes: one macrocycle comprised of 9 rings (7), another one containing12 rings (8), and a final macrocycle containing 18 rings (9). At this stage, we did not attempt to synthesize a macrocycle of a particular size selectively, but this has been show to be possible as well (vide infra).
With the macrocyclic precursors in hand, our attention turned to the aromatization reaction. Up until this time, no efficient reactions to convert the cyclohexadiene moiety to a para-substituted aryl ring had been reported in the literature [29]. Similar cyclohexadiene compounds had been aromatized under acidic conditions, but only with concomitant alkyl shifts generating 1,3-substituted aromatic compounds instead of 1,4 substituted compounds (as in cycloparaphenylenes) [30]. In order to avoid these cationic promoted alkyl shifts, we developed a novel aromatization reaction utilizing a one-electron reductant, lithium naphthalenide. As shown in Fig. 5, treatment of each macrocycle (7, 8, and 9) with lithium napthalenide at −78 °C afforded the corresponding cycloparaphenylenes (10, 11, and 12). Mechanistically, the reaction is envisioned to proceed via an initial one-electron transfer, generating stabilized radical intermediate 13 and an equivalent of lithium methoxide. A second electron transfer then produces the alkyl lithium 14 which can aromatize via loss of a second equivalent of lithium methoxide. Especially noteworthy is the formation of cycloparaphenylene 10, the most strained carbon nanohoop of the series, using these low-temperature conditions. Gratifyingly, three distinct cycloparaphenylenes, the shortest-possible fragments of armchair CNTs, had been synthesized for the first time and in only five synthetic steps.
Figure 5.
A novel reductive aromatization reaction.
5.2. Optical properties and theoretical characterization of the carbon nanohoop structures
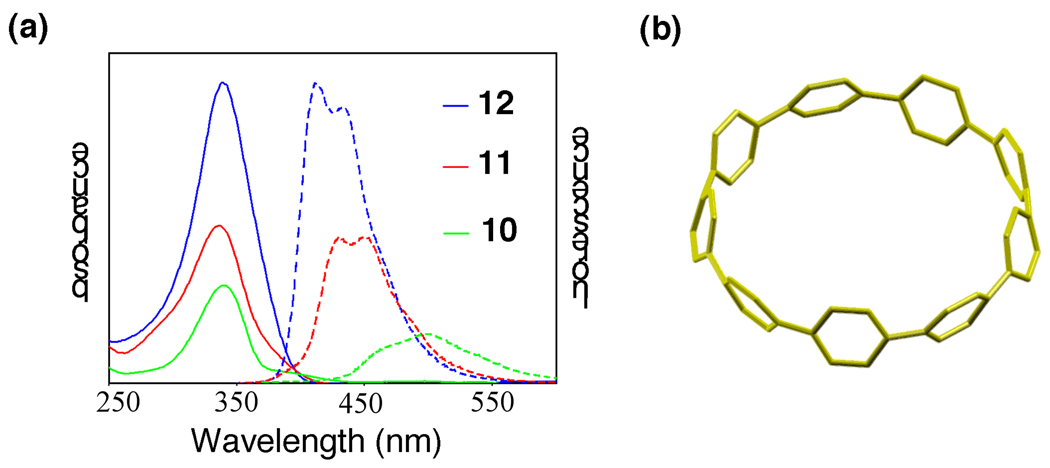
A bottom-up approach to carbon nanotubes provides unique opportunities to study the physical properties of discrete CNT structures. With cycloparaphenylenes—the shortest units of armchair CNTs—in hand for the first time, we analyzed their properties using a variety of methods [25]. Interestingly, the UV/visible absorption spectra showed symmetric peaks with a maximum at approximately 340 nm regardless of carbon nanohoop size (Fig. 6a). Surprisingly, the smallest carbon nanohoop (10), which has the poorest geometry for orbital overlap and fewest number of aryl rings, has a slightly smaller optical gap than the larger carbon nanohoops. This result stands in sharp contrast to the acyclic paraphenylenes in which a narrowing of the optical gap occurs with extended conjugation [31]. The fluorescence spectra also provided some unexpected results. As carbon nanohoop size decreased, the Stokes shift increased. Interestingly, the smallest carbon nanohoop 10 shows an especially broad spectrum with a large Stokes shift of approximately 160 nm.
Figure 6.
(a) Absorption (solid line) and fluorescence (dashed line) spectra of carbon nanohoops 10, 11, and 12; (b) Energy-minimized geometry of carbon nanohoop 10 by DFT calculations.
We further investigated the structural and optical properties of the carbon nanohoop molecules with computational methods based on density functional theory (DFT) [32]. Our calculations indicated the favored geometry for the even-membered nanohoops, [12]- and [18]cycloparaphenylene, is a staggered configuration in which the dihedral angle between two adjacent phenyl rings alternates between ±33 and ±34 degrees, respectively. This arrangement minimizes steric repulsion between neighboring aryl rings. For [9]cycloparaphenylene, the situation is slightly more complex. Reduced symmetry due to an odd number of phenyl rings results in a lowest energy conformation in which the dihedral angle varies between ±18, ±30, ±31, and ±33 degrees around the carbon nanohoop (Fig. 6b). We found that chiral Mobius-strip like arrangements, where the dihedral angle is successively increased by approximately 35 degrees around the ring, were higher in energy by at least 2 kcal/mol per phenyl ring. The calculated strain energies of carbon nanohoops 10, 11, and 12 were 47, 28, and 5 kcal/mol with diameters of 1.2, 1.7, and 2.4 nm, respectively.
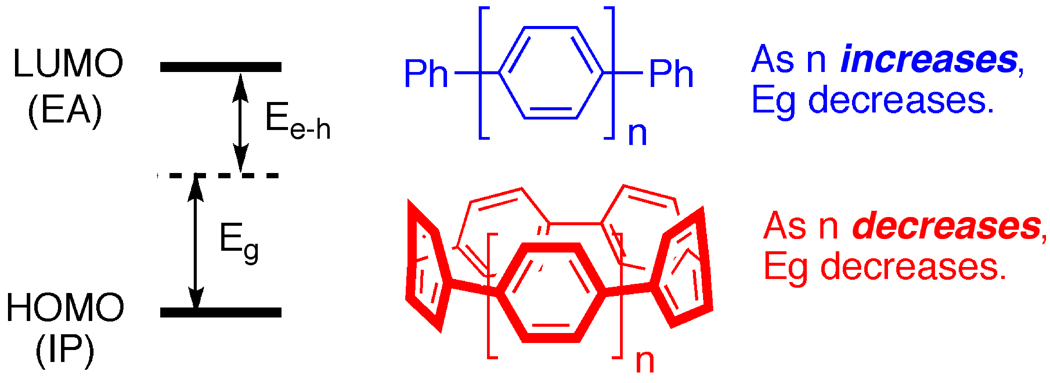
We also performed DFT calculations to understand the counter-intuitive trends in the observed optical data. We estimated the average optical absorption energy gap, Eg, with the following approximate formula: Eg = ionization potential (IP) − electron affinity (EA) − Ee-h, where Ee-h is the electron-hole interaction energy between the highest-occupied and lowest-unoccupied DFT one-electron wavefunctions [33–34] (Fig. 7). As expected, we observed that IP – EA decreases as the number of phenyl rings increase, in both the cyclic and acyclic cases. Remarkably, however, we found that the magnitude of Ee-h grows more dramatically with decreasing number of phenyl rings for carbon nanohoops than for their acyclic counterparts. In fact, this tendency can be directly related to the difference between linear finite (acyclic) and closed curved (cyclic) geometries. This difference in the computed trend in Ee-h more than compensates for the increase in IP – EA and results in an overall decrease in Eg with nanohoop diameter, in agreement with the experimentally-observed trends (Fig 7) [35]. We also rationalized the fluorescence data utilizing DFT calculations. The large Stokes shift observed in these spectra is governed by the degree of structural relaxation in the optically excited state. In the smaller nanohoops, enhanced curvature leads to greater sp3 hybridization and increasingly asymmetric p orbitals, with their smaller lobes oriented inside the nanohoops. This factor reduces steric interactions and facilitates a larger decrease in dihedral angle in the excited state for the smaller nanohoop. Thus smaller hoops have the potential for larger structural relaxation and Stokes shifts. Indeed constrained DFT calculations confirmed the observed trend that the smallest carbon nanohoop has the largest relaxation in its excited state. As we extend these carbon nanohoop structures into longer versions, the optical properties of these short CNTs and their relationship to diameter will continue to be an intriguing area of investigation.
Figure 7.
The surprising trends in optical data can be rationalized from quantum mechanics.
5.3. Other synthetic approaches to the cycloparaphenylenes
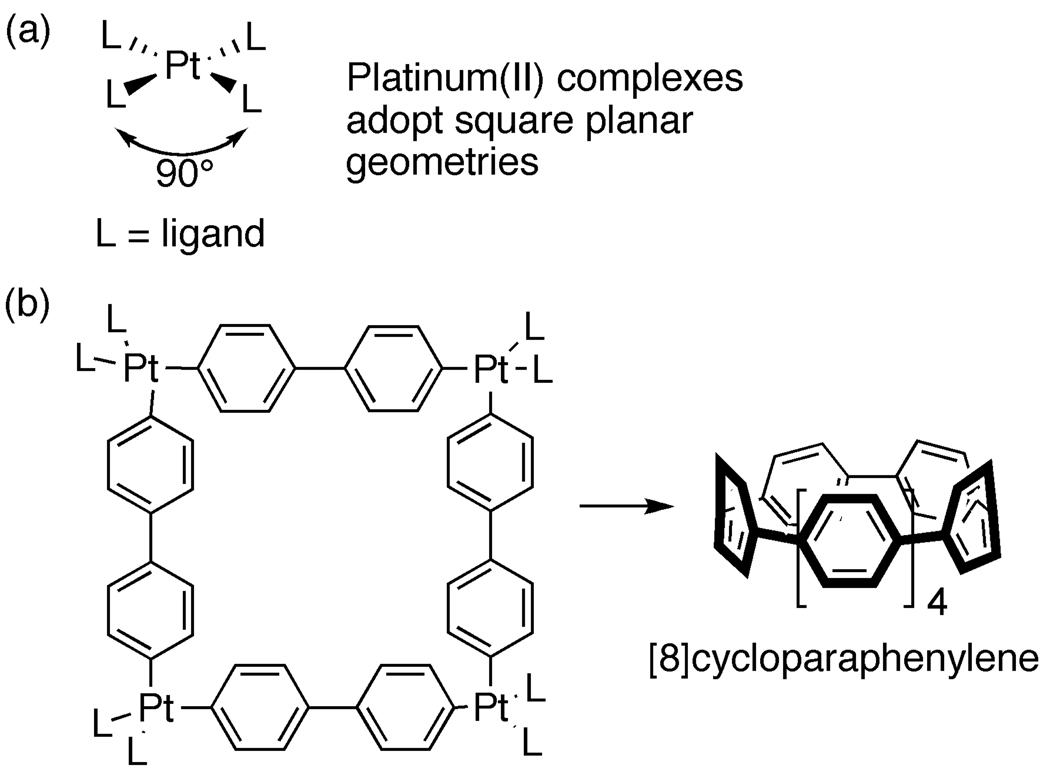
Our first synthesis of the cycloparaphenylenes demonstrated that these highly strained aromatic structures could be prepared at low temperatures utilizing organic synthesis. Since our initial report, several other groups have reported alternative synthetic strategies. For instance, by taking an iterative approach, Itami was able to prepare [12]cycloparaphenylene selectively [36]. Even more recently, Yamago utilized a strategy that relies on metal coordination geometry (Fig. 8a) to synthesize [8]cycloparaphenylene, the smallest cycloparaphenylene synthesized to date [37]. As shown in Figure 8, platinum in its +2 oxidation state prefers a square planar geometry. Yamago exploited this fact to construct the macrocycle shown in Figure 8b. This structure can then undergo a reductive elimination process—a common organometallic transformation—to deliver [8]cycloparaphenylene. This strategy is noteworthy for its efficiency (just 3 steps and 25% overall yield) and its ability to construct the smallest and therefore most strained cycloparapheneylene to date.
Figure 8.
Synthesis of [8]cycloparaphenylene.
5.4. Syntheses of other template molecules
The cycloparaphenylenes are the only CNT template molecules that have been currently synthesized, however, there are numerous other molecules of interest. The synthesis of cyclacenes (Fig. 2b), which would serve as templates for zigzag CNTs, has been a longstanding challenge. Numerous attempts at synthesizing these molecules have been reported, but all have ultimately failed in the final steps [38]. Professor Lawrence T. Scott, a pioneer of organic synthesis approaches to CNTs, has made significant progress in the syntheses of endcap structures (half fullerenes) that could template a variety of CNTs as well [39–40]. One can also imagine templates for a variety of chiral CNTs, as well as nanotube junctions. With the growing attention and importance of these molecules, it is likely that much progress will be made in the upcoming years in the synthesis of CNT templates.
6. Elongation Strategies
The second component required for a bottom-up synthesis of carbon nanotubes is a method to elongate the template structures into full-length CNTs. As illustrated in the previous section, synthesizing templates will take several synthetic steps and will likely require purification of numerous intermediates. The cost of this process can be offset, however, if efficient elongation strategies can be developed. For perspective, one milligram of a template grown to 1 mm long nanotubes would produce over 1 ton of CNTs. Thus, developing efficient elongation strategies that utilize cheap starting materials is essential. This area of research can be summarized as the development of new polymerization reactions that build aromatic rings iteratively from a template starting point. In the following sections, we highlight some of the potential elongation strategies that are currently being investigated.
6.1. Smalley Amplification Approach
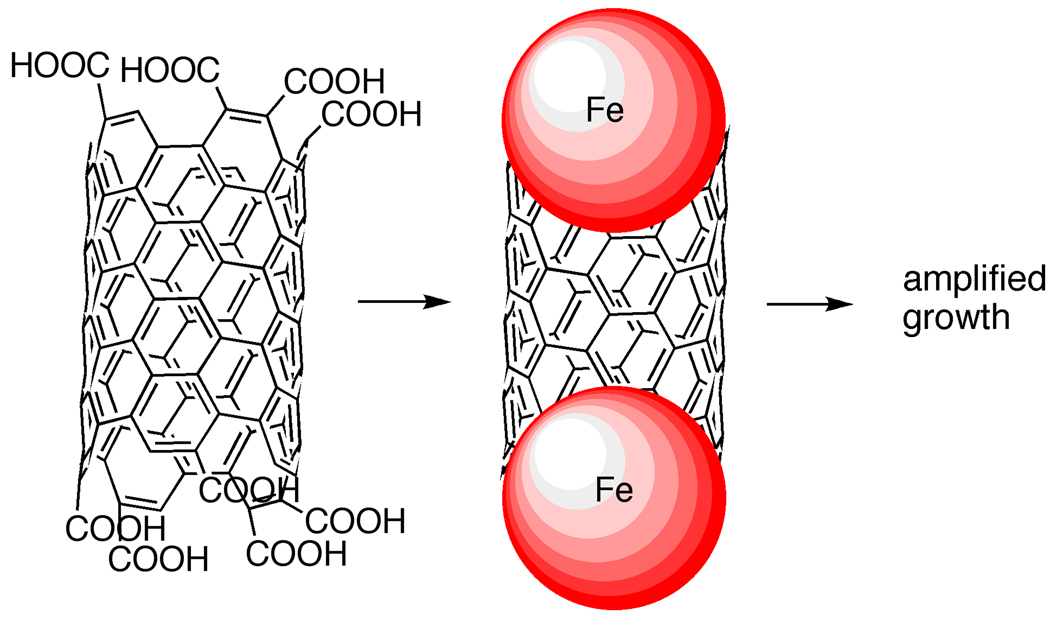
One potential solution to the elongation of CNT templates is an adaptation of Smalley’s amplification concept [41]. Smalley, Tour, et al. have proposed that by taking a single nanotube and cutting it into fragments, an amplification of a single chirality of CNT should be possible. This scheme is highlighted in Figure 9. A CNT is cut into short fragments under harsh oxidative conditions, with simultaneous installation of carboxylic acid groups at the ends of the CNT fragments. These carboxylates groups can then be used to bring an appropriate catalyst nanoparticle into proximity of the ends of these tubes. A reductive docking technique then generates a catalyst bound to the CNT fragment, an active intermediate in the growth process. By subjecting this structure to CVD growth conditions, Tour, et al. has been able to show that growth restarts and that the resulting CNT is the same diameter as the beginning CNT fragment. In this way, a CNT of a given chirality could potentially be cut into numerous small fragments and then subjected to re-growth conditions, resulting in an amplification process [42–43]. Although these results are promising, the chirality of the resulting CNT is yet to be proven conclusively to match that of the initial CNT. An obvious adaptation of the amplification concept would be to use small molecule templates (e.g. cycloparaphenylenes) in place of the CNT fragments and to restart the growth process. Although the CVD amplification concept is a potentially viable solution, the CVD process is still hampered by the fact that it is a high temperature process that inevitably leads to a variety of side-products.
Figure 9.
Amplification of CNT seeds utilizing CVD process.
6.2. Diels Alder Approach
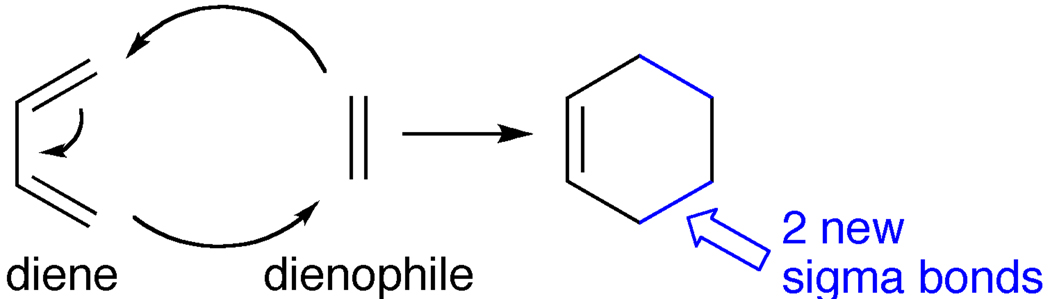
The Diels-Alder reaction [44] provides a tantalizing way of potentially extending template molecules into carbon nanotubes. The Diels-Alder pericyclic reaction, in its simplest form, is presented in Figure 10. A diene can react with an alkene (referred to as a dienophile) through a concerted, 6-membered transition state generating a cyclohexene product. The reaction is favorable thermodynamically in that 2 pi bonds are exchanged for 2 stronger sigma bonds. Although typically being thermodynamically favorable, the Diels-Alder reaction can be very slow from a kinetic standpoint. The reaction rate, however, can be accelerated by choice of the substituents on the diene and dienophile, as well as by the nature of the catalysts employed. Since its discovery in 1928, numerous studies have been dedicated to this reaction and it has been employed successfully in a large number of impressive syntheses [45].
Figure 10.
Diels-Alder cycloaddition reaction.
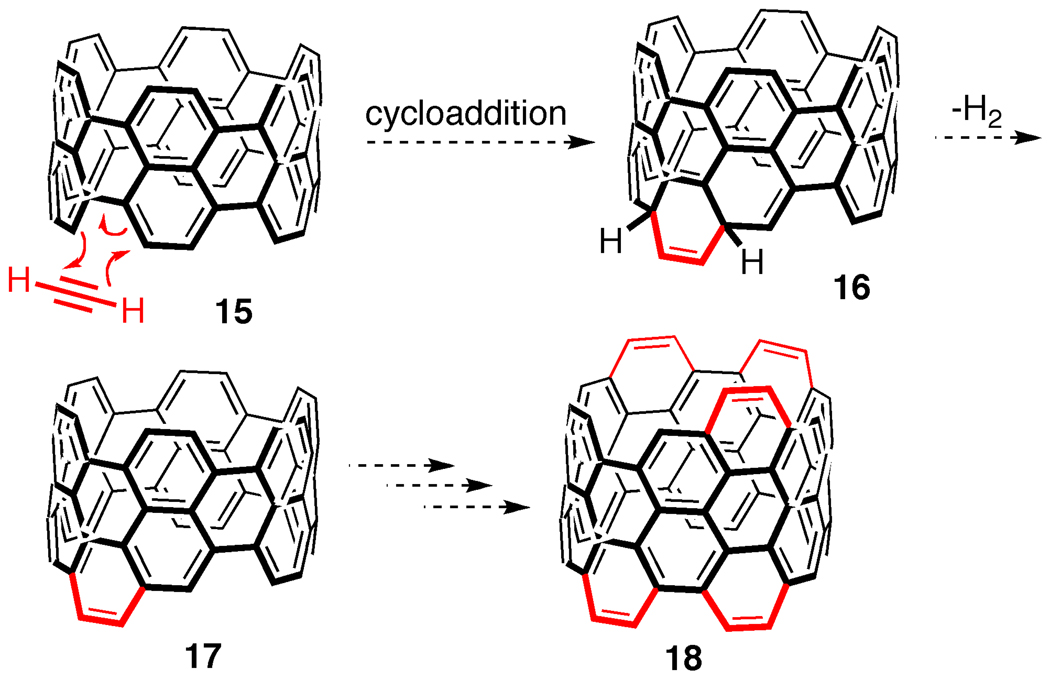
A Diels-Alder polymerization reaction would be an ideal candidate for extension methodology (Figure 11). We envision structure 15 undergoing a Diels-Alder reaction with acetylene or an acetylene equivalent [46] to deliver dearomatized product 16. Rearomatization of structure 16 by loss of hydrogen would then deliver extended CNT 17. In principle, this reaction could go through multiple iterations in the presence of excess acetylene to produce long armchair carbon nanotubes.
Figure 11.
Diels-Alder reaction to synthesize CNTs.
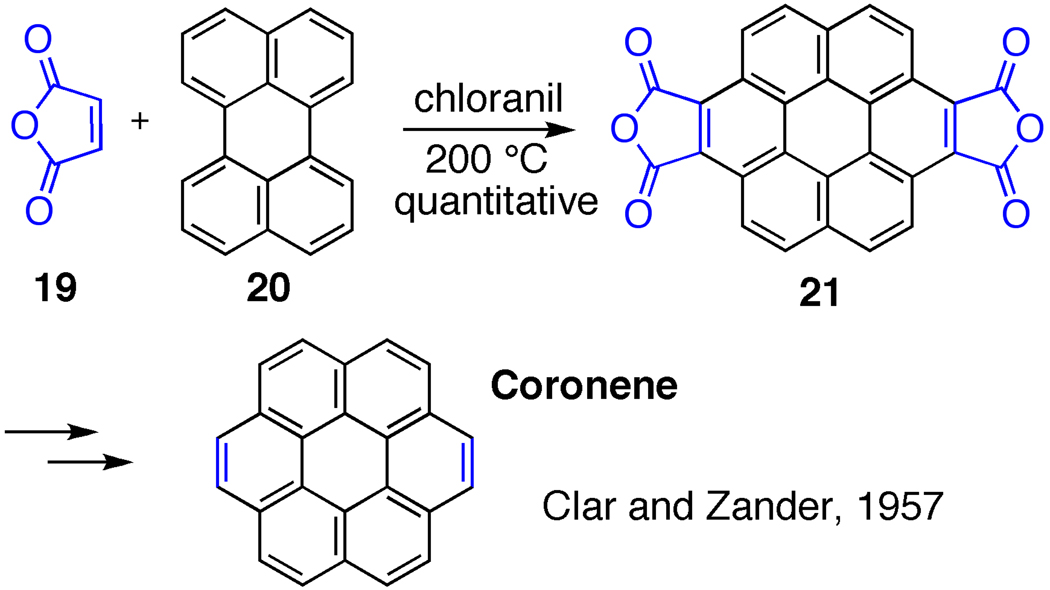
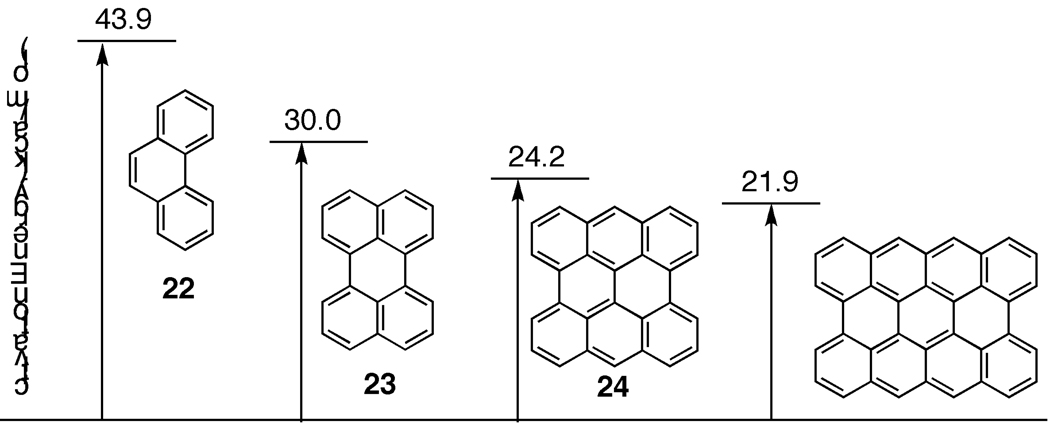
Numerous examples of Diels-Alder reactions have been carried out successfully for the extension of polyaromatic hydrocarbons. For example, Clar used a similar cycloaddition reaction in his classical synthesis of coronene (Fig. 12) [47]. By treating perylene (20) with excess maleic anhydride (19) in the presence of an oxidant, compound 26 was produced in quantitative yield. Hydrolysis and decarboxylation then delivered the desired corranulene product. More recently, Professor L. T Scott has reported both empircal and theoretical data suggesting the validity of the Diels-Alder process for extending CNTs [48]. Interestingly, as the CNT structure elongates, the Diels-Alder reaction should become more facile (Fig. 13). Structure 22, which is similar to the cyloparaphenylenes, requires an activation barrier of 43.9 kcal/mol to undergo Diels-Alder reaction. However, just by an extension of one more row of aromatic rings, the activation barrier drops dramatically to 30 kcal/mol. With this data in mind, a slightly wider version of the cycloparaphenylenes would be an ideal target for elongation by Diels-Alder reactions. It should be noted that this type of cycloaddition strategy would not be a viable extension strategy for zigzag templates (e.g. cyclacenes), but would be amenable to the extension of chiral CNTs.
Figure 12.
Extension of aromatic systems with Diels-Alder reaction.
Figure 13.
As a CNT grows in length, the activation energy for the Diels-Alder reaction will decrease.
6.3. Other approaches
Although the Diels-Alder reaction is an appealing strategy, numerous other approaches to the elongation of CNTs are potentially viable. Aromatic chemistry is a very rich field in organic chemistry and a variety of efficient reactions have been reported in the literature. For instance, a Friedel-Crafts type reaction has been known since 1877 [49] and is a very efficient way of adding a carbon unit to an aromatic ring. Another more modern set of reactions relying on C-H activation could potentially be very useful as well [50]. The field of C-H activation typically involves the use of catalytic organometallic reagents that are able to “activate” a C-H bond and make it susceptible to substitution. In that the open edges of CNTs are C-H functionalized, this reaction is an especially attractive method for regioselective reactions and extension methodology.
7. Challenges and Future Outlook
In the last few years, great progress has been made for the bottom-up organic synthesis of carbon nanotubes. It has been demonstrated for the first time that cycloparaphenylenes—the shortest possible slices of armchair CNTs—can be constructed utilizing organic synthesis (and at low temperatures). Undoubtedly, many more macrocyclic molecules will be synthesized that could serve as templates for a wide spectrum of different CNTs. The ultimate success of this strategy relies on development of efficient polymerization reactions to extend these templates. Although these reactions have not been demonstrated in the context of CNTs yet, this area of research in synthetic organic chemistry is an emerging field that has just begun to receive attention. With the continuing progress in organic chemistry and development of new reactions, CNTs of an exact chirality will eventually be synthesized utilizing a rational bottom-up approach. With these bottom-up approaches in hand, CNTs composed of heteroatoms or unusual ring sizes will also become accessible. The bottom-up synthesis of CNTs is a fascinating interdisciplinary area of organic synthesis that will not only lead to the accessibility of CNTs of pure chirality for new nanotechnologies, but also aid in the understanding of the physics of these unique molecules.
Acknowledgements
This work was initiated at the Molecular Foundry and currently is being conducted at Boston University. The authors thank Jeffrey B. Neaton and Joydeep Bhattacharjee for their extensive contributions to the theoretical understanding of the cycloparaphenylenes.
Biographies

Professor Jasti received his PhD in the field of organic chemistry at UC Irvine in 2006. As a graduate student, Dr. Jasti authored several highly cited articles regarding mechanistic and stereochemical aspects of the Prins cyclization reaction. Dr. Jasti then moved on to conduct his postdoctoral studies with Carolyn Bertozzi, working jointly at UC Berkeley and the Molecular Foundry—a nanoscience facility located at the Lawrence Berkeley National Lab. At Berkeley, Dr. Jasti addressed a longstanding and challenging problem at the interface of organic chemistry and materials science: the synthesis of the basic building blocks of carbon nanotubes. Dr. Jasti has since expanded on this work and continues to investigate the synthesis and properties of carbon-based nanomaterials in his laboratories at Boston University.

Carolyn Bertozzi is the T.Z. and Irmgard Chu Distinguished Professor of Chemistry and Professor of Molecular and Cell Biology at UC Berkeley, an Investigator of the Howard Hughes Medical Institute, and Director of the Molecular Foundry, a nanoscience institute at the Lawrence Berkeley National Laboratory. She completed her undergraduate degree in Chemistry from Harvard University in 1988 and her Ph.D. in Chemistry from UC Berkeley in 1993. After completing postdoctoral work at UCSF in the field of cellular immunology, she joined the UC Berkeley faculty in 1996. Prof. Bertozzi has been the recipient of numerous awards including the MacArthur Foundation Award, as well as being an elected member of the National Academy of Sciences.
Prof. Bertozzi’s research interests span the disciplines of chemistry and biology with an emphasis on studies of cell surface glycosylation pertinent to disease states. Her lab focuses on profiling changes in cell surface glycosylation associated with cancer, inflammation and bacterial infection, and exploiting this information for development of diagnostic and therapeutic approaches. In addition, her group develops nanoscience-based technologies for probing cell function and for medical diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iijima S. Nature. 1991;354:56. [Google Scholar]
- 2.Hamada N, Sawada S, Oshiyama A. Phys. Rev. Lett. 1992;68:1579. doi: 10.1103/PhysRevLett.68.1579. [DOI] [PubMed] [Google Scholar]
- 3.Odom TW, Huang J, Lieber CM. Ann. N.Y. Acad. Sci. 2002;960:203. [PubMed] [Google Scholar]
- 4.Dai H. Acc. Chem. Res. 2002;35:1035. doi: 10.1021/ar0101640. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Lee C, Han S, Li C, Zhou C. In: Molecular Nanoelectronics. Reed MA, Lee T, editors. 2003. p. 179. [Google Scholar]
- 6.Terrones M. Int. Mat. Rev. 2004;49:325. [Google Scholar]
- 7.This number was estimated based on a SciFinder Scholar search.
- 8.Wang X, Li Q, Xie J, Jin Z, Wang J, Li Y, Jiang K, Fan S. Nano Letters. 2009;9:3137. doi: 10.1021/nl901260b. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Lourie O, Dyer MJ, Moloni K, Kelly TF, Ruoff RS. Science. 2000;287:637. doi: 10.1126/science.287.5453.637. [DOI] [PubMed] [Google Scholar]
- 10.Tang ZK, Zhang L, Wang N, Zhang XX, Wen GH, Li GD, Wang JN, Chan CT, Sheng P. Science. 2001;292:2462. doi: 10.1126/science.1060470. [DOI] [PubMed] [Google Scholar]
- 11.Joselevich E. ChemPhysChem. 2004;5:619. doi: 10.1002/cphc.200301049. [DOI] [PubMed] [Google Scholar]
- 12.Misewich JA, Martel R, Avouris Ph, Tsang JC, Heinze S, Tersoff J. Science. 2003;300:783. doi: 10.1126/science.1081294. [DOI] [PubMed] [Google Scholar]
- 13.Kim WJ, Usrey ML, Strano MS. Chem. Mater. 2007;19:1571. [Google Scholar]
- 14.Arnold MS, Stupp SI, Hersam MC. Nano Letters. 2005;5:713. doi: 10.1021/nl050133o. [DOI] [PubMed] [Google Scholar]
- 15.Hersam MC. Nature Nanotech. 2008;3:387. doi: 10.1038/nnano.2008.135. [DOI] [PubMed] [Google Scholar]
- 16.Carbon nanotubes: Synthesis, Structure, Properties and Applications, Dresselhaus MS, Dresselhaus G, Avouris P, editors. Top. Appl. Phys. Vol. 80. Heidelberg, Germany: Springer-Verlag; 2001.
- 17.Yao Z, Postma HWC, Balents L, Dekker C. Nature. 1999;402:273. [Google Scholar]
- 18.Guo T, Nikolaev P, Thess A, Colbert DT, Smalley RE. Chem. Phys. Lett. 1995;243:49. [Google Scholar]
- 19.José-Yamacán M, Miki-Yoshida M, Rendón L, Santiesteban JG. Appl. Phys. Lett. 1993;62:657. [Google Scholar]
- 20.Harutyunyan AR, Chen G, Paronyan TM, Pigos EM, Kuznetsov OA, Hewaparakrama K, Kim SM, Zakharov D, Stach EA, Sumansekera GU. 2009;326:116. doi: 10.1126/science.1177599. [DOI] [PubMed] [Google Scholar]
- 21.Qu L, Du F, Dai L. Nano Letters. 2008;8:2682. doi: 10.1021/nl800967n. [DOI] [PubMed] [Google Scholar]
- 22.Parekh VC, Guha PC. J. Indian Chem. Soc. 1934;11:95. [Google Scholar]
- 23.Friederich R, Nieger M, Vögtle F. Chem. Ber. 1993;126:1723. [Google Scholar]
- 24.Srinivasan M, Sankararaman S, Hopf H, Varghese B. Eur. J. Org. Chem. 2003:660. [Google Scholar]
- 25.Jasti R, Bhattacharjee J, Neaton JB, Bertozzi CR. J. Am. Chem. Soc. 2008;130:17646. doi: 10.1021/ja807126u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The direct product of this reaction is the diol, which is subsequently converted to the methyl ether 5.
- 27.Alonso F, Yus M. Tetrahedron. 1991;47:7471. [Google Scholar]
- 28.Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]
- 29.The one relevant example in the literature produces a mixture of para- and meta-substituted aryl rings: Morrow GW, Schwind B. Synth. Commun. 1995;25:269.
- 30.Alonso F, Yus M. Tetrahedron. 1992;48:2709. [Google Scholar]
- 31.Nijegorodov NI, Downey WS, Danailov MB. Spectrochim. Acta, Part A. 2000;56:783. doi: 10.1016/s1386-1425(99)00167-5. [DOI] [PubMed] [Google Scholar]
- 32.Salahub DR, Castro M, Proynov EI. NATO ASI Series, Series B: Physics. 1994;318:411. [Google Scholar]
- 33.Jones RO, Gunnarsson O. Rev. Mod. Phys. 1989;61:689. [Google Scholar]
- 34.Hellman A, Razaznejad B, Lundqvist BI. J. Chem.Phys. 2004;120:4593. doi: 10.1063/1.1645787. [DOI] [PubMed] [Google Scholar]
- 35.These trends in optical data were further explored in the following article: Wong BM. J. Phys. Chem. C. 2009;113:21921. doi: 10.1021/jp9074674.
- 36.Takaba H, Omachi H, Yamamoto Y, Bouffard J, Itami K. Angew. Chem. Int. Ed. 2009;48:6112–6116. doi: 10.1002/anie.200902617. [DOI] [PubMed] [Google Scholar]
- 37.Yamago S, Watanabe Y, Iwamoto T. Angew. Chem. Int. Ed. 2010;49:757. doi: 10.1002/anie.200905659. [DOI] [PubMed] [Google Scholar]
- 38.Girreser U, Giuffrida D, Kohnke FH, Mathias JP, Philp D, Stoddart JF. Pur. Appl. Chem. 1993;65:119. [Google Scholar]
- 39.Hill TJ, Hughes RK, Scott LT. Tetrahedron. 2008;64:11360. [Google Scholar]
- 40.Steinberg BD, Scott LT. Angew. Chem. Int. Ed. 2009;48:5400. doi: 10.1002/anie.200901025. [DOI] [PubMed] [Google Scholar]
- 41.Wang YH, Kim MJ, Shan HW, Kittrell C, Fan H, Ericson L, Hwang WF, Arepalli S, Hauge RH, Smalley RE. Nano Letters. 2005;5:997. doi: 10.1021/nl047851f. [DOI] [PubMed] [Google Scholar]
- 42.Smalley RE, Li Y, Moore VC, Price K, Colorado R, Jr, Schmidt H, Hauge RH, Barron AR, Tour JM. J. Am. Chem. Soc. 2006;128:15824. doi: 10.1021/ja065767r. [DOI] [PubMed] [Google Scholar]
- 43.Ogrin D, Anderson RE, Colorado R, Jr, Maruyama B, Pender MJ, Moore VC, Pheasant ST, McJilton L, Schmidt HK, Hauge RH, Billups WE, Tour JM, Smalley RE, Barron AR. J. Phys. Chem. C. 2007;111:17804. [Google Scholar]
- 44.Berson JA. Tetrahedron. 1992;48:3. [Google Scholar]
- 45.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem. Int. Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Acetylene equivalents are masked versions of acetylene that are more reactive. For example, see: Williams RV, Chauhan K, Gadgil VR. J. Chem. Soc. 1994:1739.
- 47.Clar E, Zander M. J. Chem. Soc. 1957:4616. [Google Scholar]
- 48.Fort EH, Donovan PM, Scott LT. J. Am. Chem. Soc. 2009;131:16006. doi: 10.1021/ja907802g. [DOI] [PubMed] [Google Scholar]
- 49.Price CC. Org. React. 1946;3:1. [Google Scholar]
- 50.Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154. [Google Scholar]