Abstract
During Drosophila embryogenesis, establishment of ventral and lateral cell fates requires spatial regulation of an extracellular serine protease cascade composed of Nudel, Gastrulation Defective (GD), Snake, and Easter. Pipe, a sulfotransferase expressed ventrally during oogenesis, sulfates secreted targets that somehow confer positive spatial input to this cascade. Nudel and GD activation are pipe-independent, while Easter activation requires pipe. The effect of pipe on Snake activation has been unknown. Here we show that Snake activation is cascade-dependent but pipe-independent. These findings support a conclusion that Snake’s activation of Easter is the first spatially regulated step in the dorsoventral protease cascade.
Keywords: serine protease, fluorophosphonate (FP)-rhodamine, Snake, Drosophila, dorsoventral polarity, embryogenesis
1. Introduction
Spatial regulation of secreted signaling ligands is an essential and recurring theme during embryonic development. Specification of ventral and lateral cell fates in the early Drosophila embryo requires formation of a nuclear concentration gradient of the transcription factor Dorsal [1]. Translocation of Dorsal to the nucleus is the direct result of ventrally localized activation of the plasma membrane receptor Toll. Upstream of Toll, a morphogen gradient of its ligand, a proteolytically activated form of the NGF-related signaling molecule Spätzle, is produced within the extracellular perivitelline space by a proteolytic cascade comprising the serine proteases Nudel [2], GD [3], Snake [4], and Easter [5]. The final protease Easter activates Spätzle by cleavage [6,7].
Expression of a Golgi-localized sulfotransferase, Pipe, within a ventral subset of somatically-derived follicle cells surrounding the developing oocyte provides key spatial information essential for Spätzle activation [8]. Pipe sulfates several glycoprotein targets that are secreted and appear to become embedded in the vitelline membrane layer of the eggshell [9]. One or more of these Pipe targets, perhaps acting as positive cofactors, are required for activity of the protease cascade.
We have previously shown that processing of Nudel and GD proteases to apparently active forms occurs independently of pipe function [10,11], while Easter activation does not [12]. Processed forms of Snake in vivo have not been described, so the role of pipe in Snake activation has remained uncertain. It is essential that the first pipe-dependent step within the proteolytic cascade be identified in order to elucidate the mechanism of its spatial control. Here we report detection of a processed and active form of Snake that is cascade-dependent, yet pipe-independent. These findings significantly contribute to our understanding of the dorsoventral patterning pathway by demonstrating that Snake’s activation of Easter is the first spatially regulated step within the proteolytic cascade.
2. Materials and methods
2.1. Fly Stocks
The wild-type strain was Oregon R. Allelic combinations used to generate adult female flies mutant for individual dorsal-group genes included snk229/Df(3L)Exel8157, pip386/pip664, ndl111/Df(3)CH12, ea4/ea5022rx1, gd7/gd7, and gdVM90/gdVM90; information and references for all genes and alleles are available on FlyBase. Ovaries and embryos derived from these females are referred to as snk−, pipe−, ndl−, ea−, and gd−, respectively, because they lack critical maternal function in these genes. cDNA constructs expressing myc-tagged forms of wild-type (SM), catalytic serine mutated to alanine (SMSA), and zymogen cleavage site-mutated (SNKK) Snake zymogens have been previously described [11]. These constructs have been inserted into pGerm8 vector for constitutive expression in the female germ-line and mRNA transport into the oocyte [13]. Transgenic fly lines were generated in a w1118 background using conventional P element-mediated transformation [14]. The wild-type tagged SM has proteolytic activity in S2 cells [11] and can rescue embryos to hatching when injected as mRNA (E.K.L., unpublished).
2.2. Immunoblot analysis
Ovaries were dissected in cold Ringer’s solution. Equal numbers of Stage 14 egg chambers or nondechorionated laid eggs (0- to 4-hr collection, unless otherwise noted) were homogenized on ice in 2×Laemmli sample buffer containing 100 mM dithiothreitol (DTT) and 5×protease inhibitor cocktail. Samples were loaded on 13% SDS/PAGE gels. Typically, 12–15 stage 14 egg chambers or embryos were loaded per lane. Western blots of proteins separated on these gels were probed with affinity-purified rabbit polyclonal anti-SNKCAT (1:500; [15]), or with monoclonal anti-myc (9E10; 1:1000; Santa Cruz Biotechnology). Blots were incubated in appropriate HRP-conjugated secondary antibodies (1:80,000; Jackson ImmunoResearch) and detected with SuperSignal West Femto Substrate (Pierce).
2.3. Labeling active serine proteases
Constructs encoding functional HA-tagged GD and/or myc-tagged Snake (SM) were transiently transfected in Drosophila S2 cells (Invitrogen) using a calcium phosphate protocol [16]. Proteins from transfected cell media were precipitated with ammonium sulfate (80% saturation) for 1 hour at 4 °C. Precipitated proteins were dialyzed into 50 mM Tris, pH 7.5, then incubated with either monoclonal anti-HA (Covance, Princeton, NJ; [11]) or anti-myc (9E10; Santa Cruz Biotechnology) antibodies overnight at 4 °C at a dilution of 1:200. Protein-antibody complexes were captured on Protein G-Sepharose beads (Amersham) and labeled with fluorophosphonate (FP)-rhodamine (4 μM final concentration) in 50 mM Tris-HCl, pH 7.5 at room temperature for 1 hour. The reaction was quenched with 2×Laemmli sample buffer containing 100 mM DTT. Samples were run on 10% SDS/PAGE gels and analyzed with a Hitachi FMBio IIe flatbed fluorescence scanner (MiraiBio).
3. Results
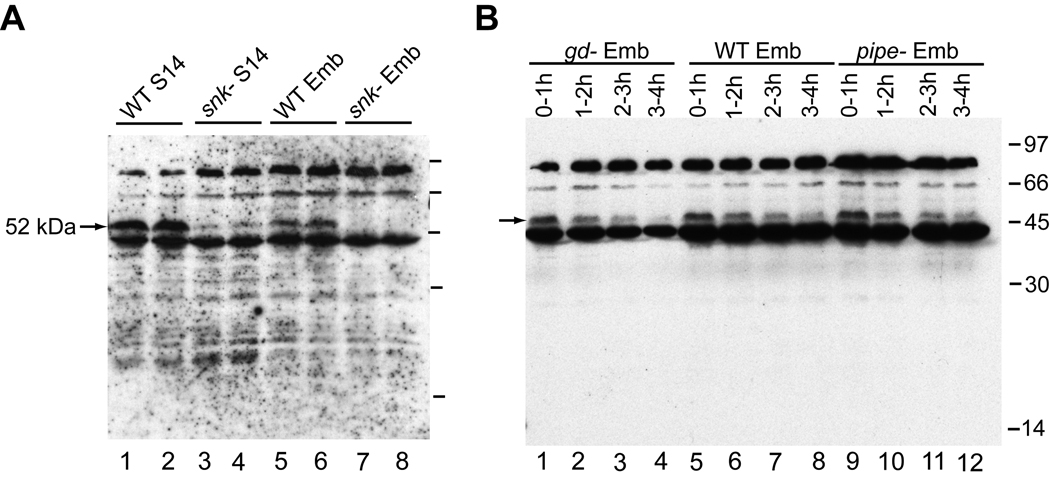
Anti-SNKCAT recognized a 52 kDa band corresponding to the size of the Snake zymogen in wild-type Stage 14 egg chambers that was absent in egg chambers of snk− mothers (Fig. 1A). This Snake zymogen band was consistently reduced in intensity in wild-type embryos compared to Stage 14 egg chambers and was lost over the first 3–4 hours of embryogenesis (Fig. 1B, lanes 5–8). This reduction of the Snake zymogen could also be observed in embryos derived from gd-mutant females (Fig. 1B, lanes 1–4), indicating that it is not cascade-dependent thus likely represents normal turnover of the protein. In no case were we able to detect processed forms of endogenous Snake with anti-SNKCAT.
Fig. 1.
Endogenous Snake zymogen was detected during oogenesis and embryogenesis. (A) A 52 kDa band was observed in wild-type (WT) Stage 14 egg chambers (lanes 1–2) and embryos (Emb; lanes 5–6), but was absent in snk− Stage 14 egg chambers (lanes 3–4) and embryos (lanes 7–8). (B) Snake zymogen was reduced over the first 3–4 hours of embryogenesis in WT (lanes 5–8), gd− (derived from gd7/gd7 females; lanes 1–4), and pipe− (lanes 9–12) embryos. In all Figures, positions of relevant Snake or GD bands are marked with arrows. Molecular weights are indicated with a straight line.
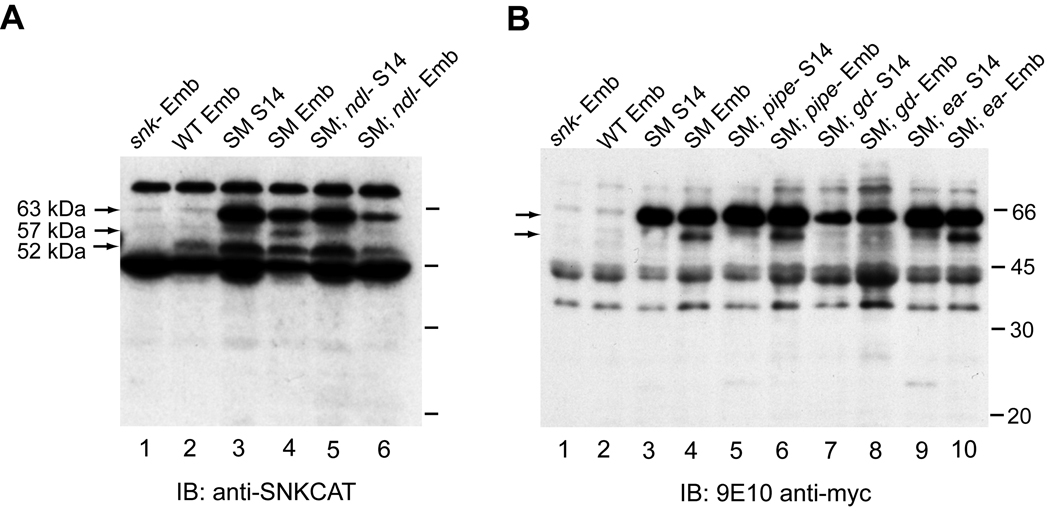
Using transgenic flies expressing myc-tagged wild-type Snake (SM), we were able to observe processing of the 63 kDa tagged zymogen to a C-terminal 57 kDa form recognized by both anti-SNKCAT and the anti-myc antibody. This processing occurred only in embryos and was not observed in Stage 14 egg chambers (Fig. 2A, lanes 3–4; Fig. 2B, lanes 3–4). The presence of this band relied on the activity of the upstream proteases Nudel (Fig. 2A, lane 6) and GD (Fig. 2B, lane 8) but not on the downstream protease Easter (Fig. 2B, lane 10), which is consistent with cascade-dependent activation of Snake. Interestingly, this cleavage product was formed perfectly well in embryos derived from pipe-mutant mothers (Fig. 2B, lane 6).
Fig. 2.
Processing of myc-tagged Snake (SM) is cascade-dependent but does not require Pipe. The SM zymogen (63 kDa) is detected in S14 egg chambers (lanes 3) and embryos (lanes 4) using either anti-SNKCAT (panel A) or anti-myc (panel B) antibodies. In embryos only (lanes 4), an additional 57 kDa processed product is seen, while the anti-SNKCAT antibody also recognizes the endogenous 52 kDa Snake zymogen (panel A). The 57 kDa embryo-specific processed SM form is not present in embryos lacking Nudel (lane 6, panel A) or GD (derived from gdVM90/gdVM90 females; lane 8, panel B), but is present in embryos lacking Easter (lane 10, panel B) or Pipe (lane 6, panel B).
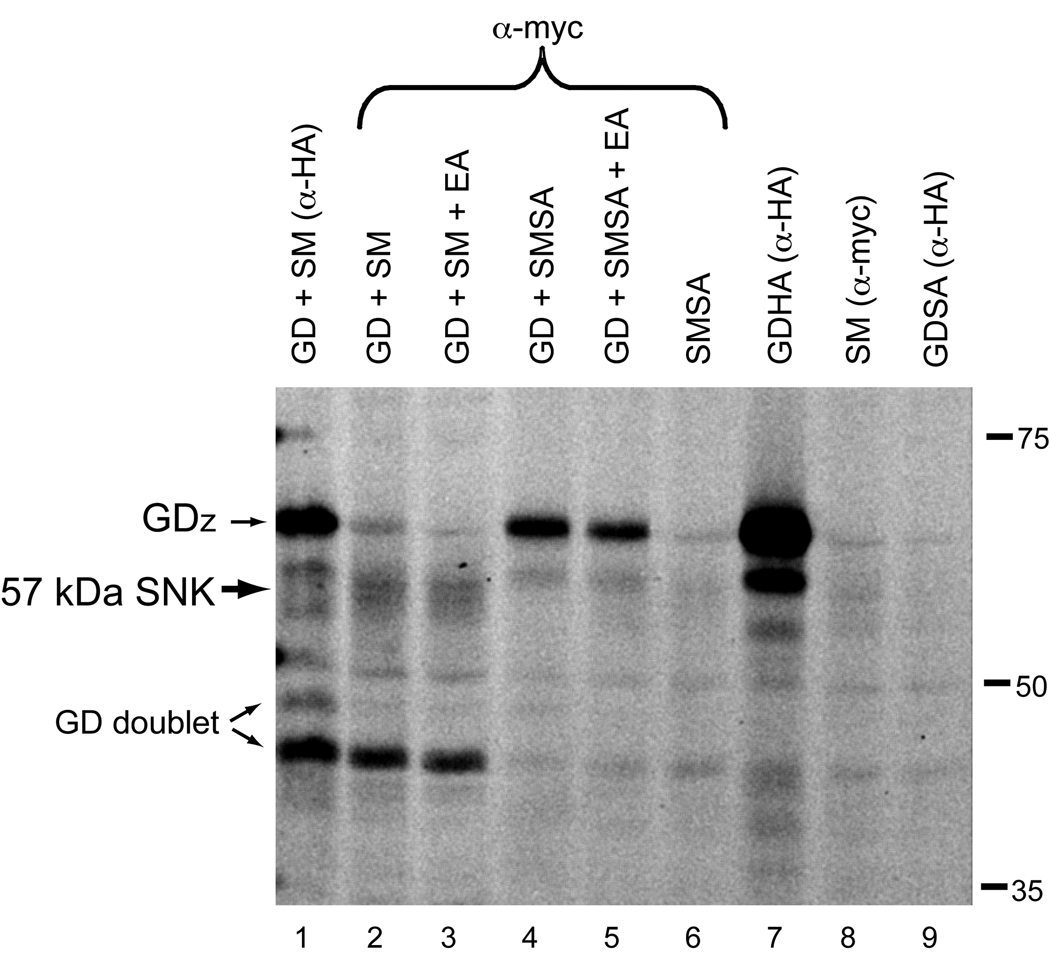
Processing of serine proteases typically occurs at the zymogen cleavage site and would be expected to produce an ~42 kDa form of SM (catalytic domain only plus tag; [11]). To confirm that the 57 kDa processed form of Snake represented an active form of the protein, we performed activity-labeling with FP-rhodamine. This small-molecule probe forms an irreversible covalent bond with the active site of serine proteases, but not with their inactive or inhibited forms [17–19]. In cell culture, an active Snake doublet at ~57 kDa was detected only in the presence of GD (Fig. 3, lanes 2 and 3), which biochemical and genetic studies suggest activates Snake [7,11,20,21]. The presence of Easter (lane 3) did not affect production of the 57 kDa form. No active forms of Snake were detected in transfections with SM alone (lane 8), demonstrating that the zymogen form is not active. Specificity of labeling was confirmed with a non-catalytic Snake construct in which the catalytic serine was mutated to alanine (SMSA). No active forms of Snake were labeled in samples with SMSA alone (lane 6). Furthermore, the 57 kDa Snake doublet was not present in transfections with non-catalytic Snake and GD (lanes 4 and 5). These findings confirm that the 57 kDa form of Snake, although atypically processed, is active. During these experiments, we also detected active forms of GD. A doublet corresponding to the previously described ~46–50 kDa GD doublet [11] was observed in the presence of Snake (lane 1). In addition, the GD zymogen (GDZ) also demonstrated strong activity-labeling (lanes 1,4,5, and 7).
Fig. 3.
Active forms of both Snake (57 kDa) and GD (50 and 46 kDa doublet) were detected. In addition, the GD zymogen (GDz) was activity-labeled. Proteins from co-transfections in S2 cells were immunoprecipitated with either anti-HA or anti-myc antibodies. Immunoprecipitates were incubated with FP-rhodamine, to label active serine proteases. Samples were separated on a gel and scanned directly for rhodamine fluorescence; the resulting gel is shown in grayscale.
4. Discussion
Processing of endogenous Snake at its conserved zymogen cleavage site was expected on reducing gels to produce either the Snake catalytic domain (~29 kDa) or a non-reducible covalent complex between the Snake catalytic domain and a serpin-type protease inhibitor (~80 kDa) as was seen for activated Easter [22]. However, no processed forms of endogenous Snake were observed, suggesting that the activated form of Snake is a minor species and difficult to detect.
Using transgenic flies that over-express SM, we detected a cascade-dependent 57 kDa cleavage product that appears to result from atypical processing within the pro-domain during embryogenesis. Detection of a similar Snake cleavage product has previously been reported in S2 cell and baculovirus expression systems for both SM and untagged Snake [15,23], and we have observed similar processing of Snake-HA following injection of its mRNA into embryos (E.K.L., unpublished). These findings suggest that this is a characteristic processed product of Snake arising independently of the presence or absence of a particular tag. However, it was uncertain if this product represented activated Snake at all, or perhaps a catalytic domain-serpin complex that had undergone an extra cleavage.
FP-rhodamine is a sensitive and specific reagent for detecting active forms of serine proteases, and is broadly reactive independent of substrate binding site specificity in contrast to previously described chloromethylketone reagents [20] that in our hands have not given reproducible results for the dorsoventral proteases. Using activity-labeling with FP-rhodamine we confirmed that the 57 kDa processed product represented an activated form of Snake, suggesting that Snake undergoes an atypical activating proteolysis within its pro-domain. In results described in Supplementary Data, we have also found that generation of the 57 kDa form in vivo does not rely on either Snake’s own catalytic activity (when catalytic serine-inactivated SMSA is the only Snake present, Fig. S1) or Snake’s conserved zymogen cleavage site (when mutant SNKK is the only Snake present, Fig. S2). These findings confirm that the 57 kDa form does not represent a catalytic domain-serpin complex since the catalytic serine is essential for forming a covalent bond with serpins [24]. Although SNKK undergoes processing to a 57 kDa form, this mutant has no rescuing activity by injection of its zymogen mRNA in snk-mutant embryos [23]. It is possible that mutations at the conserved zymogen cleavage site alter the conformation of the Snake catalytic domain independently of cleavage at that site, precluding the ability of a 57 kDa SNKK form to activate Easter.
In the FP-rhodamine labeling studies, we also detected a GD doublet at ~46–50 kDa that is active. This finding was consistent with processed forms of GD observed in cell culture and with a 46 kDa product observed in embryos but not ovaries [11]. Interestingly, the GD zymogen was also strongly labeled. Genetic studies have suggested that the GD zymogen possessed activity in vivo [20], consistent with our labeling results. In addition, we observed that the 46 kDa active form of GD co-precipitated with activated Snake (Fig. 3, lanes 2 and 3), suggesting the presence of an activated GD/Snake complex. Similarly, in preliminary immunoprecipitation results in embryos expressing both GDHA and SM proteins, we observed with anti-HA antibody the co-precipitation with GD of the 57 kDa Snake form but not the SM zymogen (E.K.L., unpublished). This result provides further indirect evidence that the 57 kDa processed form has functional relevance in vivo. In contrast, the active GD zymogen appeared to preferentially associate with the SMSA zymogen in anti-myc immunoprecipitations (Fig. 3, lanes 4 and 5). This result suggests that stabilization of a complex of the GD and Snake zymogens occurs when the Snake present is incapable of reciprocal processing of GD that appears to drive generation of the cleaved forms of these proteases in the S2 cell co-expression system [11]. Future investigations of such complexes in vivo may further elucidate the mechanisms regulating activities of the GD and Snake proteases.
Here we have identified an active form of Snake in vivo that is processed atypically within its pro-domain. We have shown that Snake activation occurs during early embryogenesis and depends on the upstream proteases, Nudel and GD, but not the downstream protease Easter. We also show that Snake activation is pipe-independent. Together with previous work on Nudel, GD, and Easter activation, these findings support a conclusion that Snake’s activation of Easter is the first spatially regulated step in the proteolytic cascade required for establishment of the Drosophila embryonic dorsoventral axis.
Supplementary Material
Acknowledgements
This work was supported by the NIH (E.K.L., R01GM067738) and the Damon Runyan Cancer Foundation (S.E.T., DRG 1978–08).
Abbreviations
- GD
Gastrulation Defective
- FP
fluorophosphonate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeMosy EK. Proteolytic regulatory mechanisms in the formation of extracellular morphogen gradients. Birth Defects Res C Embryo Today. 2006;78:243–255. doi: 10.1002/bdrc.20074. [DOI] [PubMed] [Google Scholar]
- 2.Hong CC, Hashimoto C. An unusual mosaic protein with a protease domain, encoded by the nudel gene, is involved in defining embryonic dorsoventral polarity in Drosophila. Cell. 1995;82:785–794. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- 3.Konrad KD, Goralski TJ, Mahowald AP, Marsh JL. The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc Natl Acad Sci U S A. 1998;95:6819–6824. doi: 10.1073/pnas.95.12.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLotto R, Spierer P. A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature. 1986;323:688–692. doi: 10.1038/323688a0. [DOI] [PubMed] [Google Scholar]
- 5.Chasan R, Anderson KV. The role of easter, an apparent serine protease, in organizing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1989;56:391–400. doi: 10.1016/0092-8674(89)90242-0. [DOI] [PubMed] [Google Scholar]
- 6.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spatzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72:141–148. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 7.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. Embo J. 2001;20:2387–2393. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Stevens LM, Stein D. Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 2009;19:1200–1205. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeMosy EK, Kemler D, Hashimoto C. Role of Nudel protease activation in triggering dorsoventral polarization of the Drosophila embryo. Development. 1998;125:4045–4053. doi: 10.1242/dev.125.20.4045. [DOI] [PubMed] [Google Scholar]
- 11.LeMosy EK, Tan YQ, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci U S A. 2001;98:5055–5060. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeMosy EK. Spatially dependent activation of the patterning protease, Easter. FEBS Lett. 2006;580:2269–2272. doi: 10.1016/j.febslet.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serano TL, Cheung HK, Frank LH, Cohen RS. P element transformation vectors for studying Drosophila melanogaster oogenesis and early embryogenesis. Gene. 1994;138:181–186. doi: 10.1016/0378-1119(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 14.Spradling AC. Drosophila: a Practical Approach. Oxford: IRL Press; 1986. [Google Scholar]
- 15.Tian S, LeMosy EK. Mutagenesis of the cysteine-rich clip domain in the Drosophila patterning protease, Snake. Arch Biochem Biophys. 2008;475:169–174. doi: 10.1016/j.abb.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 18.Jessani N, Liu Y, Humphrey M, Cravatt BF. Enzyme activity profiles of the secreted and membrane proteome that depict cancer cell invasiveness. Proc Natl Acad Sci U S A. 2002;99:10335–10340. doi: 10.1073/pnas.162187599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: the serine hydrolases. Proc Natl Acad Sci U S A. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JH, Lee SH, Tan YQ, LeMosy EK, Hashimoto C. Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo. Proc Natl Acad Sci U S A. 2000;97:9093–9097. doi: 10.1073/pnas.97.16.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CL, DeLotto R. Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature. 1994;368:548–551. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- 22.Misra S, Hecht P, Maeda R, Anderson KV. Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 23.Smith CL, Giordano H, Schwartz M, DeLotto R. Spatial regulation of Drosophila snake protease activity in the generation of dorsal-ventral polarity. Development. 1995;121:4127–4135. doi: 10.1242/dev.121.12.4127. [DOI] [PubMed] [Google Scholar]
- 24.Olson ST, Bock PE, Kvassman J, Shore JD, Lawrence DA, Ginsburg D, Bjork I. Role of the catalytic serine in the interactions of serine proteinases with protein inhibitors of the serpin family. Contribution of a covalent interaction to the binding energy of serpin-proteinase complexes. J Biol Chem. 1995;270:30007–30017. doi: 10.1074/jbc.270.50.30007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.