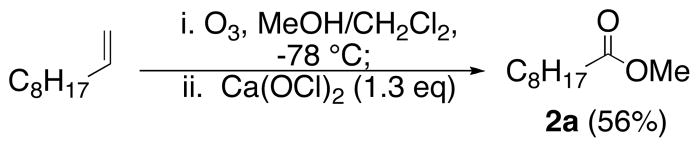Abstract
Hypochlorites efficently dehydrate hydroperoxyacetals to furnish the corresponding esters. The reaction, which can be accomplished with stoichometric Ca(OCl)2 or with catalytic amounts of t-BuOCl, appears to involve formation and heterolytic fragmentation of secondary chloroperoxides, species not previously described in solution chemistry.
Keywords: Hypochlorite, Hydroperoxyacetal, Chloroperoxide, Fragmentation, Ester
1. Introduction
Hydroperoxyacetals, readily available intermediates,1 are substrates for a number of useful fragmentations, including Fe(II)-mediated cleavage to alkoxy radicals,1c,2 heterolytic C-O bond migrations of tertiary peresters or persulfonates (Criegee rearrangement),3 and the base-promoted dehydration of hydroperoxyacetals or derived peresters or persulfonates.1c,4,5 In the course of investigations into the addition of oxygen nucleophiles to ozonolysis-derived carbonyl oxides,6 we observed the rapid dehydration of secondary hydroperoxyacetals in the presence of commercial bleach.7 We now report that Ca(OCl)2, t-BuOCl, and trichloroisocyanuric acid mediate the rapid heterolytic dehydration of hydroperoxyacetals through the apparent intermediacy of secondary chloroperoxides, species whose solution chemistry has not been previously described.
2. Results and Discussion
Most of the substrates employed in this study were prepared via ozonolysis of alkenes in the presence of an alcohol.1a Addition of Ca(OCl)2 (1.3 equiv) to CH3CN solutions of hydroperoxyacetals 1a–h furnished esters 2a–h (Table 1) after evaporation of solvent and filtration through a short silica column.7,8 The reaction could also be conducted in CH3OH/CH2Cl2; however reaction in CH2Cl2, THF, or toluene was limited by solubility of Ca(OCl)2. The dehydration proved compatible with a free primary alcohol (entry 4) or a chloroethyl acetal (entry 8). Little reaction was observed with aq. NaOCl.
Table 1.
Fragmentation of hydroperoxyacetals with Ca(OCl)2
 | |||||
|---|---|---|---|---|---|
| Entry | Subs. | R1 | R2 | Prod. | Yielda |
| 1 | 1a | octyl | Me | 2a | 75% (91) |
| 2 | 1b | octyl | Et | 2b | 86% (90) |
| 3 | 1c | octyl | i-Pr | 2c | 83% (93) |
| 4 | 1d | octyl | (CH2)2OH | 2d | 83% (86) |
| 5 | 1e | AcO(CH2)8 | Me | 2e | 85% (93) |
| 6 | 1f | BnO(CH2)3 | Me | 2f | 81% (94) |
| 7 | 1g | Ph(CH2)2 | Me | 2g | 80% (93) |
| 8 | 1h | octyl | (CH2)2Cl | 2h | 82% (92) |
Isolated yields on 0.5 mmol or (5 mmol) scale.
Comparable yields were available with t-BuOCl (Table 2).9 Reactions, although conducted for the same duration as for Ca(OCl)2 were now complete within 1 min (TLC).10 The rate and yield were not affected by protection from laboratory light (entry 2), use of CH2Cl2 as solvent (entry 3), or the presence of acid (entry 4). However, the presence of methanol slowed reactions considerably (not shown). Dehydration could be conducted with catalytic (0.25 eq) quantities of t-BuOCl (entries 5 and 8), although the reactions now required 15 min for completion.
Table 2.
Fragmentation of hydroperoxyacetals with t-BuOCl
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | t-BuOCl (eq) | t(min) | Product | Yielda |
| 1 | 1a | 1.2 | 10 | 2a | 77% |
| 2b | 1a | 1.2 | 10 | 2a | 77% |
| 3c | 1a | 1.2 | 10 | 2a | 79% |
| 4d | 1a | 1.2 | 10 | 2a | 84% |
| 5 | 1a | 0.25 | 15 | 2a | 78% |
| 6 | 1b | 1.2 | 10 | 2b | 85% |
| 7 | 1d | 1.2 | 10 | 2d | 84% |
| 8 | 1e | 0.25 | 15 | 2e | 85% |
| 9 | 1h | 1.2 | 10 | 2h | 84% |
Isolated yields on 0.5 mmol scale.
Protected from light (Al foil over flask).
CH2Cl2 as solvent.
Added HOAc (≥ 2 eq)
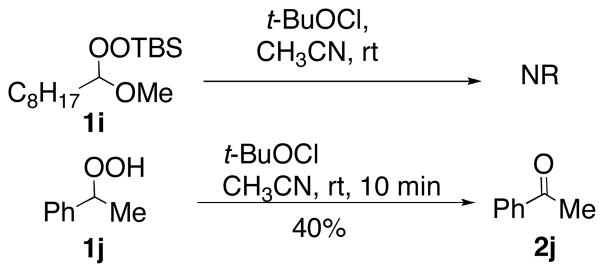
No reaction was observed betweeen t-BuOCl and a silylated hydroperoxyacetal (1i, Scheme 1). However, a secondary hydroperoxide (1j)11 underwent rapid dehydration; the low yield likely reflects product volatility. Dehydration of secondary allylic hydroperoxides (not shown) required excess t-BuOCl and furnished the expected α,β-unsaturated ketones as mixtures with significant amounts of byproducts lacking unsaturation.
Scheme 1.
Substrates other than hydroperoxyacetals
The fragmentation can be combined with alkene ozonolysis to afford a convenient one-pot synthesis of esters (Scheme 2).
Scheme 2.
Application in tandem with ozonolysis
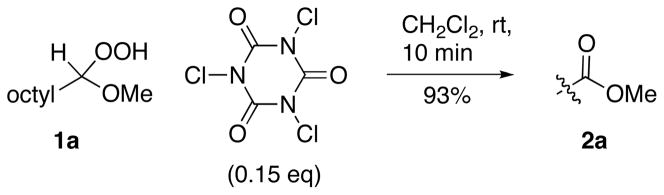
The fragmentation is likely to involve initial formation of chloroperoxides, species previously prepared only in tertiary systems.12 The intermediacy of ROOCl is consistent with the lack of reaction of silylated hydroperoxyacetal 1i. In an effort to access chloroperoxides with reagents other than hypochlorites, we discovered that commercially available trichloroisocyanuric acid promotes the dehydrative fragmentation as or more efficiently than t-BuOCl (Scheme 3).
Scheme 3.
Dehydration with trichloroisocyanuric acid.
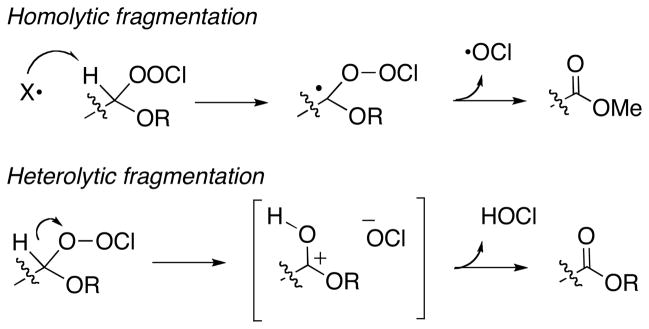
The conversion of the chloroperoxides to esters could in principle proceed through either homolytic or heterolytic pathways (Scheme 4), and several additional experiments were conducted to discriminate between these possibilities (Scheme 5).
Scheme 4.
Mechanistic possibilities
Scheme 5.
Additional substrates
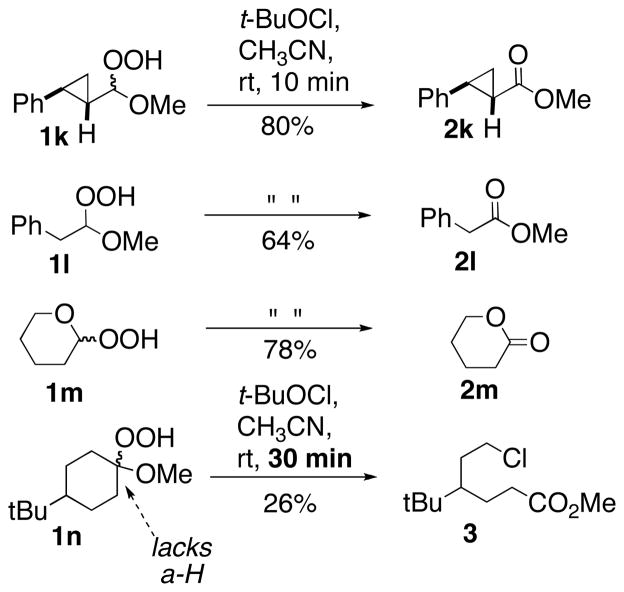
Chloroperoxides are reported to undergo homolytic scission to generate ROO• and Cl•.12,13 For this reason, we initially hypothesized the dehydrations involved a radical chain initiated by abstraction of the acetal C-H; the resulting carbon radical would be expected to fragment to the product ester and a propagating radical (•OCl). However, the dehydrations were insensitive to the presence or absence of visible light and did not occur in the presence of PhI(OTFA)2, a reagent known to promote peroxyl radical formation.14 Perhaps most convincingly, dehydration of 1k, a hydroperoxyacetal substrate incorporating a fast radical clock (Scheme 5), proceeded with no detectable formation of ring-opened products.15
The potential role of alkoxy radicals was probed with hydroperoxyacetals 1l and 1m16 (Scheme 5); the α-oxygenated alkoxy radicals derived from either substrate would be expected to readily undergo β-scission.17 However, both 1l and 1m undergo dehydration with no signs of radical cleavage. In contrast, a hydroperoxyketal unable to dehydrate (1n) undergoes a much slower (30 min) reaction to furnish chloroalkanoate 3, the product of alkoxy radical cleavage.
The results support fragmentation through the heterolytic pathway illustrated in Scheme 4, presumably through a mechanism analogous to Criegee or Hock fragmentation (activation of hydroperoxides by protonation or Lewis acid complexation).3,18 The specificity for migration of hydrogen relative to Ph or Bn (1j, 1l) under nonbasic conditions is interesting. However, as has recently been demonstrated for Baeyer-Villiger rearrangements of alkoxybromanes derived from hemiacetals,19 the nature of the activating reagents can have a strong influence on rearrangements to electron-deficient oxygen. The proposed mechanism predicts regeneration of HOCl, 20 and is consistent with the high conversion obtained in the presence of substoichiometric t-BuOCl or trichloroisocyanuric acid. The results may be of relevance to atmospheric decomposition of primary chloroperoxides.21
3. Conclusions
We have developed a new fragmentation of hydroperoxyacetals to esters based upon heterolytic fragmentation of intermediate chloroperoxides.
CAUTION: While we experienced no hazards in the course of this work, any preparative work with peroxides should be conducted with an awareness of the potential for spontaneous and exothermic decomposition reactions.22
Supplementary Material
Acknowledgments
This research was funded by NSF (CH-0749916) and the Nebraska Research Initiative and conducted in facilities remodeled with support from NIH (RR016544-01). NMR spectra were acquired, in part, on spectrometers purchased with NSF support (MRI 0079750 and CHE 0091975). We thank Dr. Chris Schwartz and Prof. Stephen DiMagno for helpful discussions.
Footnotes
1H and 13C NMR spectra for 1a–i, 1k–n, 2a–I, 2k–m, 3.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Reviews: Zmitek K, Zupan M, Iskra J. Org Biomol Chem. 2007;5:3895. doi: 10.1039/b711647k.(α-oxygenated hydroperoxides, transacetalization); Scarso A, Strukul G. In: Science of Synthesis. Berkessel A, editor. Vol. 38. Thieme; Stuggart: 2009. pp. 36–52.(trapping of carbonyl oxides); Dussault PH. In: Active Oxygen in Chemistry. Foote CS, Valentine JS, Greenberg A, Liebman JF, editors. Blackie A&P; London: 1995. pp. 141–203.(addition to enol ethers); Dussault PH, Lee IQ, Lee HJ, Lee RJ, Niu QJ, Schultz JA, Zope U. J Org Chem. 2000;65:8407. doi: 10.1021/jo991714z.(photooxygenation of enol ethers).
- 2.Ivanov SK, Kropf H. In: Methoden der Organischen Chemie. 4. Kropf H, editor. E13. Thieme; Stuttgart: 1988. pp. 1031–1037. [Google Scholar]
- 3.Criegee R. Lieb Ann. 1948;560:127. [Google Scholar]; Kropf H. In: Methoden der Organischen Chemie. 4. Kropf H, editor. E13. Thieme; Stuttgart: 1988. pp. 1095–1099. [Google Scholar]
- 4.Ellam RM, Padbury JM. J Chem Soc Chem Comm. 1972:1086.Base-promoted fragmentation of hydroperoxyacetals is likely involved in a direct ozonolytic conversion of terminal alkenes to methyl esters: Marshall JA, Garofalo AW. J Org Chem. 1993;58:3675. doi: 10.1021/jo961671b.Evano G. In: Science of Synthesis. Panek JS, editor. 20b. Thieme; Stuttgart: 2007. pp. 795–797.
- 5.Thompson QE. J Org Chem. 1962;27:4498. [Google Scholar]; Claus RE, Schreiber SL. Org Synth. 1986;64:150. [Google Scholar]
- 6.Schwartz C, Raible J, Mott K, Dussault PH. Tetrahedron. 2006;62:10747. [Google Scholar]
- 7.2.0 weight equiv of commercial solid bleach (~65% purity) delivers 1.3 molar equiv of Ca(OCl)2; other components include NaCl, CaCl2, Ca(ClO3)2, and water.
- 8.Typical procedure for reactions with Ca(OCl)2: To a flame-dried 8 mL vial equipped with stirbar and screw-top septa cap containing technical grade Ca(OCl)2 (1.3 eq, 1.0 mmol, 143 mg of 65% reagent) is added CH3CN (2 mL). A solution of the hydroperoxy acetal (1.0 eq, 0.50 mmol) in CH3CN (1 ml) is added as a single portion via syringe. The reaction is stirred vigorously for 10 min at which time the partially heterogeneous solution is filtered through a short plug of silica. The reaction vial is rinsed with a small amount of CH2Cl2 and this solution is also filtered through the silica plug. The silica plug is rinsed with another small portion of CH2Cl2 and the filtrate is concentrated.
- 9.Mintz MJ, Walling C. Org Synth. 1969;49:9. [Google Scholar]
- 10.Typical procedure for reactions with t-BuOCl: To a flame dried 8 mL vial equipped with stirbar and screw-top septa cap containing t-BuOCl (1.2 eq, 0.6 mmol, 65 mg) is added CH3CN (2 mL). The hydroperoxy acetal (1eq, 0.5 mmol) is dissolved in CH3CN (1 mL) and added in one portion via syringe. The reaction is stirred vigorously for 10 min and then worked up as before.
- 11.Prepared by Ag(I)-mediated displacement: Cookson PG, Davies AG, Roberts BP. J Chem Soc, Chem Commun. 1976:1022.
- 12.Osipov AN, Panasenko OM, Chekanov AV, Arnhold J. Free Rad Res. 2002;36:749. doi: 10.1080/10715760290032610. [DOI] [PubMed] [Google Scholar]
- 13.Marwah P, Marwah A, Lardy HA. Green Chem. 2004;6:570. [Google Scholar]
- 14.Ochiai M, Ito T, Takahashi H, Nakanishi A, Toyonari M, Suleda T, Goto S, Shiro M. J Am Chem Soc. 1996;118:7716. [Google Scholar]; Milas NA, Plesnicar B. J Am Chem Soc. 1968;90:4450. [Google Scholar]
- 15.kopening≥ 1011/sec for the unsubstituted 2-phenylcyclopropyl methyl radical: Tadic-Biadatti MHL, Newcomb M. J Chem Soc, Perkin Trans. 1996;2:1467.
- 16.1m was prepared via addition of H2O2 to dihydropyran: Milas NA, Peeler RL, Jr, Mageli OL. J Am Chem Soc. 1954;76:2322.
- 17.β-scission of oxygenated alkoxy radicals: Orlando JJ, Tyndall GS, Wallington TJ. Chem Rev. 2003;103:4657. doi: 10.1021/cr020527p.Erhardt S, Macgregor SA, McCullough KJ, Savill K, Taylor BJ. Org Lett. 2007;9:5569. doi: 10.1021/ol702534d.
- 18.Hock H, Kropf H. Angew Chem. 1957;69:313. [Google Scholar]; Dussault PH, Lee HJ, Liu X. Perkin. 2000;1:3006. [Google Scholar]
- 19.Ochiai M, Yoshimura A, Miyamoto K, Hayashi S, Nakanishi W. J Am Chem Soc. 2010;132:9236. doi: 10.1021/ja104330g. [DOI] [PubMed] [Google Scholar]
- 20.Chlorine test strips revealed the diluted aqueous layer from stoichometric reactions to have up to 50–60% of the theoretical amount of active chlorine.
- 21.Schnell M, Mühlhäuser M, Peyerimhoff SD. J Phys Chem A. 2004;108:1298. [Google Scholar]
- 22.For leading references to peroxide safety, see: Medard LA. Accidental Explosions: Types of Explosive Substances. Vol. 2 Ellis Horwood Limited; Chichester: 1989. Patnaik PA. Comprehensive Guide to the Hazardous Properties of Chemical Substances. 3. John Wiley & Sons; Hoboken, NJ: 2007. Zabicky J. In: The Chemistry of the Peroxide Group. pt 2. Rappoport Z, editor. Vol. 2. John Wiley & Sons; Chichester: 2006. pp. 597–773.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.