Abstract
Objectives
The study reported here assesses sexuality and sexual functioning among women treated for invasive cervical cancer over broad portions of the life cycle. Hysterectomy and oophorectomy, two widespread interventions in invasive cervical cancer, have potentially important effects on a woman's self-image and sexuality.
Methods
The investigation focused on women aged 29–69 with histories of invasive cervical cancer (n = 179) from the Connecticut Tumor Registry, 6–29 years postdiagnosis. Logistic regression analysis was used to assess the relative impact of time since cervical cancer diagnosis and treatment received, adjusting for age and social and economic background. Sexuality, sexual function, and potential correlates were assessed using the Sexual Adjustment Scale, the MOS-36, and the Center for Epidemiological Studies—Depression Scale (CES-D).
Results
Strong majorities of women in the study indicated that they were sexually active (81.1%) and both desired (81.4%) and enjoyed (90.9%) sexual activity. Neither time since cervical cancer diagnosis nor age significantly affected sexuality or sexual function. Women with hysterectomies (with or without oophorectomy) less often reported lack of interest in (odds ratio [OR] 0.36, p < 0.05) and lack of desire for (OR 0.26, p < 0.05) sexual activity than women who had not had hysterectomies. Among women with hysterectomies, those with oophorectomies had a greater risk (OR 21.1, p < 0.05) of not enjoying sex but did not differ otherwise from those without oophorectomies.
Conclusions
These findings suggest that cervical cancer survivors generally have a positive attitude toward sexuality and engage in satisfying sexual activity.
Introduction
Perhaps more than any malignancy, cervical cancer raises issues for long-term psychological and social adaptation and quality of life (QOL). In its invasive form, cervical cancer is not among the most frequently occurring female malignancies, yet high rates of long-term survival have resulted in large numbers of women in the United States with histories of this disease. Because of the involvement of gender-specific organs in cervical cancer, sexuality and sexual functioning constitute QOL dimensions of special relevance. Based on women residing in Connecticut who have survived cervical cancer for 6–29 years postdiagnosis, the research reported here examines several indicators of sexuality and sexual functioning to assess prevalence of problems in these areas and to identify risk factors associated with them.
The American Cancer Society estimated that 11,150 cases of invasive cervical cancer would be diagnosed in the United States during 2007.1 According to statistics from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program, the incidence rate of this disease declined by approximately 43% between 1975 and 2001.2 During the same period, the death rate from this disease declined by more than half, with 5-year survival occurring at a rate of 72.7% by 2001. For 2001, SEER estimated that over 220,000 women in the United States with histories of invasive cervical cancer were alive. Cervical cancer has a greater likelihood of occurring in early adulthood than most other malignancies. The population of women with a history of cervical cancer, then, includes many at the peak of their sexually active and family-building years.
Researchers have identified diverse areas of sexual concern associated with cancer as well as with its treatment.3–16 Survivors of cervical and other gynecological cancers have reported challenges in sexual functioning, childbearing, and marital relations.17 A number of studies have demonstrated significant challenges in the areas of sexuality and sexual function, including loss of interest in sex, pain associated with sex, a generally worsened sexual life, and reduced feelings of femininity18–23 Sexual dysfunction has also been reported among women who have had their ovaries removed for reasons other than cancer diagnosis.24,25 Of interest, however, is a study by Roussis et al., who report that women who received hysterectomies have little if any decrease in feelings of femininity, libido, or sexual activity.26
Much uncertainty remains about the degree to which women treated for cervical cancer experience lasting consequences from the disease and its treatment. Conditions detected soon after treatment, the subject of most studies, may disappear in the years or decades to follow; alternatively, significant sequelae may become manifest long after the malignancy has been successfully treated. Women encountering the posttreatment conditions described, moreover, may overcome them in ways that do not remove the sequelae themselves but mitigate their impact on day-to-day life.
The research reported here supplements current knowledge about sexuality and sexual functioning by focusing on long-term survivors. In addition, the study reported here had access to detailed information on the clinical features of individual women and their personal and social characteristics. This range of data made possible the assessment of factors associated with cervical cancer, such as stage, time since diagnosis, and treatment, independent of age, general health status, and social background.
Two distinct terms, “sexuality” and “sexual function,” are used here to avoid the implication that dimensions, such as lack of sexual interest or desire, necessarily signify dysfunction. A person strongly identified with sex, as an individual who considers sex important or who experiences strong sexual desire, may be thought of as “sexual,” but a person without such interests cannot be considered to have a dysfunction per se. This perspective reflects the inclusion of “marked distress or interpersonal difficulty” along with attitudinal and behavioral characteristics among the Diagnostic and Statistical Manual-V criteria for dysfunction.27 Similarly, recent conceptual28 and empirical29 reviews distinguish sexual dimensions, such as interest and desire, from dimensions to which the term dysfunction might be appropriately applied.
Materials and Methods
Subjects
A sample of women with histories of cervical cancer was obtained from the Connecticut Tumor Registry. The Connecticut Tumor Registry is part of a system of cancer registries under contract with and operating according to standards set by SEER.30,31 Women were selected from the Connecticut Tumor Registry who had been diagnosed with primary invasive cervical cancer between 1974 and 1996 and who had survived up to March 2000. Contact and interview procedures were approved by the Yale University Human Subjects Committee. Following clearance by physicians of record, letters were sent to potential subjects to explain the study. The letters contained an informed consent statement explaining the study, a return note card indicating interest in participation, and a postage-paid return envelope.
The research team identified 1724 records of women in the Connecticut Tumor Registry who met the study requirements. Among these, the records of 1446 listed a physician of record. Of the subjects for whom a physician was listed, the physician's permission was obtained to contact 793 of the women. Letters were mailed to these women; a total of 256 written responses were received from the potential subject or, if she was deceased, from her family. According to these responses, 11 women were deceased and 16 women refused, leaving a total of 229 women who consented to be interviewed. Among the women who initially agreed to participate, 21 later declined. A total of 208 women were eventually interviewed via telephone.
For the present report, only data from women >29 (the youngest woman interviewed) and <70 years of age were analyzed (n = 179). No assumption was made that sexuality and sexual function were unimportant to women ≥70; in the total sample, 26.9% of those ≥70 reported being sexually active. Rather, it was feared that factors associated with older age, such as loss of a partner and multiple comorbidity, might confound those associated with cervical cancer in explaining sexual function and sexuality. Of respondents ≤69, 90.9% had partners; of those ≥70, 46.4% had partners (p < 0.001). In addition, a strongly negative association between being ≥70 and most sexuality and sexual function variables was found in the present study. Inclusion of women >70 in the analysis, then, might mask important statistical relationships otherwise observable among women <70, comprising the vast majority (86.1%) of those interviewed.
Overall, the subjects interviewed closely resembled women in the Connecticut Tumor Registry who had been diagnosed with cervical cancer between 1975 and 1995 and who were alive in 2000. The research team found no statistically significant differences between interviewed and non-interviewed patients in age, stage, and county of residence.
Measures
The interview protocol requested basic demographic information and assessed health status, emotional state, health behavior, family life, lifestyle, and employment. Individual items from the MOS-3632 were used to indicate a number of dimensions of physical and mental health, such as the respondent's self-rating of her health status and the degree to which she considered herself a “happy person.” The MOS-36 has been extensively validated and widely applied.33
Depression, potentially a correlate of sexual dysfunction, was measured by the Center for Epidemiological Studies—Depression Scale (CES-D).34 The CES-D also generates a dichotomous measure of depression, a score of ≥16 indicating a subject who is depressed. Like the MOS-36, the CES-D has been widely used and has undergone extensive and repeated validation.35,36
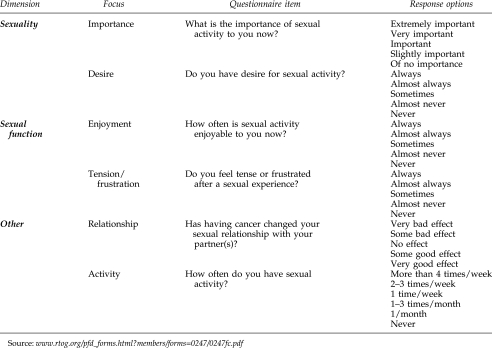
Items on sexually related thinking and actions were taken from the Sexual Adjustment Questionnaire (SAQ).37 Developed specifically for application in studies of cancer patients and survivors, the SAQ includes items focused on the separate dimensions of sexuality and sexual function, as described. The SAQ addresses sexuality through such items as the importance of and desire for sex and sexual function through such items as enjoyment of sex, tension or frustration following sexual activity, and engagement in sexual activity itself. A key SAQ item asks about the effects of illness on respondent's sexual relationships.
Construct validity of the SAQ was initially established by comparing responses from healthy subjects with those of individuals recently treated for cancer.37 Adoption of the SAQ for use in clinical trials by the Radiation Therapy Oncology Group (RTOG)38,39 has provided opportunity for subsequent revalidation in diverse patient populations through confirmatory factor analysis, comparison of younger and older age groups, and external validation via comparison of subject responses with physician assessments.40
To reduce respondent burden, selected items from the SAQ were used rather than the entire instrument. Actual items used, categorized by dimension and focus, are presented in Figure 1. The decision to use a reduced number of items is consistent with recent social research41 and the use of only single-item indicators of specific sex-related dimensions by a key national study.42
FIG. 1.
Sexuality and sexual function items from sexual adjustment questionnaire.
Data analysis
Summary statistics (percentages and means) were computed to provide an overview of characteristics of the sample and a profile of cervical cancer survivors. Logistic regression was used to assess the contributions of specific clinical and treatment factors, controlling for age and other demographics. Multivariate analysis of this kind was necessary because of the known effect of age on sexuality and sexual function42 and documented effects of race and other demographics on the outcomes of illness.43 Odds ratios (ORs) were computed from the logistic regression coefficients. In most instances, both independent and dependent variables were dichotomized so that the ORs would reflect differences in intuitively meaningful, single degrees on independent variables, such as diagnosis in stage I vs. late stage or depressed vs. nondepressed according to the CES-D.
To help identify possible effects of cervical cancer apparent within specific postdiagnosis time periods, the analysis included comparison of women in three survival time categories: 6–11 years, 12–15 years, and ≥16 years. Numbers in each of these categories represented about one third of the sample.
Results
Mean age of the subjects at the time of the study was 51.7 years (SD 8.7). Mean age at diagnosis was 37.8 years (SD 8.4). Age at the time of the study correlated strongly with age at diagnosis (r = 0.82, p < 0.01) and moderately with years since diagnosis (0.36, p < 0.01).
Table 1 presents characteristics of the sample, including social background, health and illness, cervical cancer treatment received, and sexuality and sexual function. Women included in this analysis were primarily Caucasian (92.7%), and married (67.0%), and had completed some college or a college or graduate degree (65.9%). A majority of the subjects (54.8%) had annual family incomes of ≥$60,000. A majority of subjects (63.8%) had been diagnosed with stage I (local) disease and had received surgical treatment (89.9%); it was determined on the basis of both tumor registry and interview data that 34.9% had had their ovaries removed; 20.9% had received radiation therapy alone or in combination with surgery.
Table 1.
Characteristics of Sample
| |
Percentage or mean |
|
|||
|---|---|---|---|---|---|
| Subject characteristic | Total sample | Survival 6–11 years | Survival 12–15 years | Survival ≥16 years | p value |
| Social background | |||||
| Education (some college, college, or graduate degree, % | 65.9 | 75.8 | 55.9 | 65.5 | |
| Annual family income $60,000, % | 54.8 | 58.9 | 50.0 | 54.9 | |
| Caucasian, % | 92.7 | 93.5 | 89.8 | 94.8 | |
| Married, % | 67.0 | 69.4 | 61.0 | 70.7 | |
| Health and illness | |||||
| General health good to excellent, % | 93.3 | 93.5 | 94.9 | 91.4 | |
| Happy all to a good bit of the time, % | 79.4 | 87.1 | 74.6 | 85.8 | |
| Depressed (CES-D ≥ 16), % | 47.1 | 54.1 | 49.1 | 37.5 | |
| Cancer diagnosed in stage I, % | 63.8 | 50.0 | 71.2 | 71.2 | <0.05 |
| Treatment received | |||||
| Surgery—all types, % | 89.9 | 86.2 | 96.0 | 89.9 | |
| Hysterectomy, % | 75.3 | 73.8 | 78.9 | 72.2 | |
| Ovary removal, % | 34.9 | 30.0 | 40.4 | 34.7 | |
| Radiation, % | 20.9 | 21.3 | 27.6 | 13.8 | |
| Hormonal, % | 42.5 | 35.5 | 49.2 | 43.1 | |
| Sexuality and sexual function | |||||
| Sexually active, % | 81.8 | 88.5 | 79.3 | 77.2 | |
| Sexual activity important to extremely important, % | 59.0 | 65.6 | 61.0 | 50.0 | |
| Desire sexual activity sometimes to always, % | 81.4 | 86.9 | 83.1 | 73.7 | |
| Sexual activity enjoyable sometimes to always, % | 90.9 | 98.1 | 90.9 | 82.2 | <0.05 |
| Tense/frustrated after sexual experience sometimes to always, % | 19.3 | 20.4 | 19.6 | 17.8 | |
| Cancer has had a negative effect, % on relationship(s), % | 33.8 | 28.6 | 37.3 | 35.3 | |
| n | 179 | 62 | 59 | 68 | |
Table 1 suggests that cervical cancer survivors generally enjoy a high QOL. Over 90% characterized their health as good to excellent, and nearly 80% indicated that they were happy all or a good bit of the time according to MOS-36 items. However, based on the CES-D composite score, 47.1% evidenced depression.
In general, the women in the sample appeared to be sexually interested and active. Over 80% of those interviewed reported being currently sexually active. A majority (59.0%) considered sexual activity extremely important, very important, or important, 81.4% indicated that they sometimes, almost always, or always desired sexual activity, and 90.9% indicated that they enjoyed sexual activity sometimes, almost always, or always. Only a minority (19.3%) indicated that they experienced tension or frustration sometimes, almost always, or always after a sexual experience. A minority (33.8%) reported that cervical cancer had had a negative effect on their relationships. For two of the variables presented in Table 1, stage of cancer diagnosis and enjoyment of sex, statistically significant differences were found among the three survival time categories.
Table 2 presents ORs computed on the basis of coefficients in logistic regression equations predicting the dimensions of sexuality and sexual function illustrated in Table 1. Independent variables include a broad array of personal, social background, and clinical factors. In Table 2, outcome variables are computed as dichotomous measures of reduced sexuality and sexual function. Thus, the dependent variables are sexual inactivity, lack of interest in sex (no vs. almost no interest), lack of desire (never or almost never have desire), lack of enjoyment (never or almost never enjoy), tension or frustration in sex (always or almost always), and perception that cervical cancer has damaged relationships (negative or somewhat negative effect). Two of the dichotomous variables indicating years since diagnosis that appear in Table 1 are included in the equations: 12–15 years and ≥16 years. Coefficients (and ORs) on these variables represent differences between individuals in the specified category on the dependent variables from individuals in the third category, 6–11 years, which is omitted from the equations. Caucasian race was omitted from the equations predicting tension or frustration and damage to relationships because excessively large confidence intervals were found when this independent variable was included. In both Table 2 and Table 3, equations were run on only patients for whom complete data existed; hence, different values of n are stated for each equation.
Table 2.
Subject Characteristics Predicting Sexuality and Sexual Dysfunction: ORs (95% CI)
| |
Sexuality and sexual function outcome variables |
|||||
|---|---|---|---|---|---|---|
| Sexual inactivity | Lack of interest | Lack of desire | Lack of enjoyment | Tension/frustration | Harm to relationship | |
| Subject characteristic | ||||||
| Age (years) | 1.04 (0.96–1.11) | 1.02 (0.97–1.07) | 1.01 (0.95–1.08) | 1.02 (0.97–1.07) | 0.95 (0.88–1.01) | 0.99 (0.94–1.05) |
| Stage I diagnosis | 0.44 (0.14–1.32) | 0.66 (0.80–1.46) | 1.09 (0.41–2.92) | 0.66 (0.30–1.46) | 0.99 (0.34–2.83) | 0.38* (0.16–0.89) |
| Education (some college or more) | 1.18 (0.38–3.61) | 0.73 (0.33–1.63) | 1.30 (0.48–3.50) | 0.73 (0.33–1.63) | 0.70 (0.24–2.08) | 1.15 (0.47–2.83) |
| Income ≥$60,000 | 0.26* (0.09–0.78) | 0.48 (0.22–1.04) | 0.42 (0.61–1.07) | 0.48 (0.22–1.04) | 0.52 (0.19–1.43) | 0.49 (0.21–1.15) |
| Caucasian | 0.16* (0.03–0.97) | 0.72 (0.14–3.81) | 0.74 (0.12–4.71) | 0.72 (0.14–3.81) | — | — |
| Health good to excellent | 0.54 (0.08–3.89) | 1.31 (0.26–6.52) | 0.66 (0.11–4.04) | 1.31 (0.26–6.52) | 0.37 (0.06–2.33) | 1.07 (0.20–5.61) |
| Depressed (CES-D) | 0.51 (0.178–1.49) | 1.07 (0.51–2.25) | 0.61 (0.24–1.54) | 1.07 (0.51–2.25) | 1.72 (0.64–4.63) | 1.72 (0.76–3.92) |
| Time since diagnosis (years) | ||||||
| 12–15 | 1.03 (0.31–5.51) | 0.97 (0.36–2.63) | 1.26 (0.36–4.34) | 0.97 (0.36–2.63) | 1.10 (0.31–3.90) | 2.16 (0.74–6.27) |
| ≥16 | 2.76 (0.724–10.49) | 1.84 (0.69–4.91) | 1.98 (0.61–6.42) | 1.84 (0.69–4.91) | 1.80 (0.49–6.61) | 2.24 (0.74–6.80) |
| n | 135 | 137 | 137 | 111 | 112 | 122 |
p < 0.05.
Table 3.
Selected Surgical Treatment, Sexuality, and Sexual Dysfunction: ORs (95% CI)
| |
Surgical treatment |
|||
|---|---|---|---|---|
| |
Hysterectomy (all) |
Hysterectomy with oophorectomy |
||
| Sexuality and sexual function outcome variables | OR (95% CI) | n | OR (95% CI) | n |
| Sexual inactivity | 0.29 (0.077–1.07) | 104 | 0.60 (0.06–5.57) | 83 |
| Lack of interest | 0.36* (0.35–0.962) | 116 | 3.05 (0.93–9.98) | 85 |
| Lack of desire | 0.26* (0.09–0.81) | 116 | 1.04 (0.20–5.25) | 85 |
| Lack of enjoyment | 0.36 (0.05–2.45) | 95 | 21.10* (1.83–243.09) | 74 |
| Tension/frustration | 0.81 (0.22–2.98) | 96 | 1.97 (0.49–7.87) | 75 |
| Harm to relationship | 0.80 (0.26–2.47) | 104 | 1.82 (0.49–6.72) | 77 |
p < 0.05.
According to Table 2, only income, Caucasian race, and diagnosis in stage I predict any of the outcome variables at the p < 0.05 level of significance. Sexual inactivity is less likely among women with family incomes of ≥$60,000 than among women with family incomes of ≤59,999. Sexual inactivity is less likely among Caucasian women than among women of other races. Women diagnosed in stage I are less likely to report harm to their relationships than women diagnosed in more advanced stages.
Table 3 presents ORs associated with hysterectomy and oophorectomy for the sexuality and sexual function variables. These ORs are computed from coefficients in equations containing the same independent variables as in Table 2. In Table 3, the column labeled Hysterectomy (all) reports coefficients on treatment with hysterectomy based on the entire sample. These coefficients indicate the effects of having a hysterectomy vs. not having this procedure. The column labeled Hysterectomy with oopherectomy reports coefficients on treatment including oophorectomy among only women who had hysterectomies. This subsample was used to assess the effects of oophorectomy because no women in the sample were believed to have had oophorectomy without hysterectomy.
Within the entire sample, women with hysterectomies were less likely to report lack of interest in sex and lack of desire than women who had not had this procedure. These relationships are significant at the 0.05 level. Only one statistically significant relationship was observed between oophorectomy and the sexuality and sexual function variables: women with oophorectomies were more likely to report lack of enjoyment of sex than women who had not undergone this procedure.
To address the possibility that women diagnosed at a relatively young age experienced more pronounced effects of cervical cancer and its treatment, the same equations on which Tables 2 and 3 are based were run for women ≤38 years of age (about one-half the total sample). No effects were found that were not visible in Tables 2 and 3, and the effects were of similar magnitudes to those based on the entire sample.
Discussion
The data presented here suggest that only a small percentage of long-term survivors of invasive cervical cancer experience significant diminution of their sexuality or sexual function, according to several key dimensions. According to most of the measures used here, sexuality and sexual function do not differ across survival time categories, suggesting an absence of late sequelae in this area. Neither hysterectomy nor oophorectomy, two of the most widespread treatments for invasive cervical cancer, appear to have comprehensive effects on sexuality or sexual function.
The study reported here did not compare cervical cancer survivors with women without histories of this disease. However, comparisons can be made with published surveys of women in the United States and elsewhere. The 2002 National Survey of Family Growth (NSFG)44 indicated that among women aged 35–39 and 40–44, 90.6%, and 89.2%, respectively, were sexually active (having sex with a male partner in the last 12 months). Among women in these age groups interviewed for the present study, 92.3% and 90.9%, respectively, were sexually active. Earlier, the National Health and Social Life Survey (NHSLS)42 found that among women aged 30–39, 40–49, and 50–59, 91.2%, 84.4%, and 70.0%, respectively, were sexually active. Among women in these age groups interviewed for the present study, 93.7%, 90.2%, and 81.1%, respectively, were sexually active. The NSFG, the NHSLS, and the survey of cervical cancer survivors reported here would not be expected to produce identical findings. The NSFG and NHSLS, of course, are national surveys, whereas the study reported here is confined to Connecticut. Results should also differ because of differences in methodology and sampling error. However, these comparisons suggest that sexual activity among the cervical cancer survivors studied here is no less prevalent than among women with no history of this disease.
The likelihood that long-term survivors of invasive cervical cancer retain levels of sexuality and sexual function akin to the general population is supported by the work of Jensen et al.22 These authors found that women who had undergone radical hysterectomy for cervical cancer were, up to 3 months after surgery, less likely to be sexually active than women free of cervical cancer. Between this time and the end of a 24-month follow-up period, however, the women treated for cervical cancer were no less likely to be sexually active than their cancer-free counterparts. According to Jensen et al.,22 women who had undergone radical hysterectomy for cervical cancer 24 months earlier exceeded women without cervical cancer histories by a statistically significant margin on only one of six sexual dysfunction dimensions, that of vaginal lubrication. The absence of statistically significant differences in sexual activity status, as well as other indicators of sexuality and sexual function, across survival time categories in the present study suggests that the resiliency reported by Jensen et al. remains stable for many years after treatment.
Despite the generally favorable findings reported here, it is important to acknowledge the potential complexity of individual responses to cervical cancer over the life course. As indicated, high percentages of the women studied here characterized their health as good to excellent and indicated that they were happy all or a good bit of the time, yet 47.1% of those studied evidenced depression according to the CES-D. Fully unraveling this complexity is beyond the scope of this paper, but evidence suggests that depression experienced by the women studied here tended to be limited and episodic. Among women classifiable as depressed according to the CES-D, for example, the vast majority (52.6%) reported feeling happy most of the time.
Treatment received by the women studied here had effects that were measurable but, in some respects, counterintuitive. According to several dimensions, women who had undergone hysterectomy were less likely to report reduced sexuality and sexual function than women who had not undergone such a procedure. This finding is consistent with studies of sexual function within 2 years of hysterectomy, which suggest that control of the condition for which hysterectomy was performed results in increased sexual desire and likelihood of sexual relations.45,46
Oophorectomy had no effect on dimensions such as sexual interest, desire, and activity, but did increase the risk of lack of enjoyment of sex by a statistically significant margin. One explanation of this finding may be that the physiological consequences of oophorectomy, for example, reduced vaginal lubrication,47 are likely to affect actual enjoyment of the sexual act more than interest or desire. Among the women interviewed for the present study, severe consequences of oophorectomy seemed infrequent: among those who had undergone oophorectomy, 85.1% indicated that they enjoyed sex sometimes, almost always, or always, whereas only 14.9% reported that they almost never or never enjoyed sex. The potential importance of factors other than strictly biological ones in determining sexuality and sexual functioning is illustrated by the ORs shown in Table 2. Features of the individual's social background, family income, and race were among the strongest predictors of sexual inactivity.
Several limitations in the study reported here must be acknowledged. Cervical cancer survivors in Connecticut are likely to have higher average income and education than in many other states. Although data were collected in this study on radiation and hormone treatment, they were not included in the analysis because they were thought to be insufficiently reliable and complete. Treatment data from the Connecticut Tumor Registry did not include intensity and duration of radiation and chemotherapy, and interview data on hormone treatment did not indicate the type of hormones used. More information on these modalities would have been desirable, as researchers have reported radiation treatment to be a predictor of sexual dysfunction in cervical cancer survivors48 and treatment with testosterone to be an effective intervention for sexual dysfunction.49
Limitations regarding the impact of hysterectomy deserve special emphasis. Significant differences in potential impact of simple abdominal vs. radical hysterectomy, for example, are possible Such differences could not be addressed in the present study because the tumor registry data were not always discriminatory among types of hysterectomy. In addition, the number of observations obtained was too small for definitive assessment of impact of each specific procedure.
Measurement poses challenges for studies such as the one reported here. An extensive, recent review of sex-related thinking and actions reports no consensus about the best approach to assessment in this area, whether clinical observation, imaging studies, laboratory values, or any of the numerous interview protocols or self-report questionnaires currently available.29 According to this review, however, consensus does seem to be emerging about the core dimensions that should be measured in assessing sexuality and sexual function. These include interest, desire, satisfaction, activity, and relationship, all of which are captured by SAQ items reported here. Consistent with this emerging consensus, the authors opted for selection of items focused on interest, desire, satisfaction, activity, and relationship rather than the physical assessments emphasized in some other studies, such as vaginal lubrication and perceived vaginal volume.
Aside from instrument validity, issues regarding potential response bias must be considered. Answers to researchers' questions about sex, it has been argued, are particularly subject to untruthful responses.50 However, an investigation comparing methods for assessing the responses of women to questions about sexual issues has reported that responses to retrospective questions were concordant with information from contemporaneous diaries of sexual activity.51 Notably, this investigation did not find major differences between responses to self-administered and interviewer-administered versions of the retrospective questions.
The possibility of participation bias, in which an unrepresentative set of potential subjects chooses to participate in a study or to answer questions in a particular area, must also be considered. Participation bias has been a major concern in research on sexual behavior.52 In the research reported here, however, participation bias did not appear to be present to any appreciable degree. The present study, identified with a highly regarded local institution, was not one specifically focused on sexuality; rather, it dealt with an extensive array of life experiences, areas of functioning, and means of adaptation among cervical cancer survivors. Of over 350 potential interview items, only 10 dealt with sexuality and sexual function. Very few participants chose not to respond to these items. Only two respondents, for example, chose not to respond to the question, “Do you have desire for sexual activity?” Only one did not select a response option to the item, “What is the importance of sexual activity in your life right now?” It is notable that interviews were not obtained from individuals in the sampling frame largely because the research team possessed insufficient address information for them. Of the 793 women to whom the researchers sent requests for participation, 537 were returned because the addressee did not live at the address used or because no forwarding address was available for her. Actual refusal to participate in the study occurred in only 37 cases.
Further research should address the questions posed here in other populations, but the high levels of sexual interest, activity, and enjoyment reported here should provide encouragement to women concerned that cancer and its treatment may adversely affect sexuality. Concern of this nature is widely reported, often associated with changes in body image.53,54 The findings reported here, however, reinforce those related to other female malignancies, that is, that a cancer history typically does not lead to decline in sexual health.55
The absence of strong and pervasive impact of disease and treatment factors on sexuality and sexual function in the study reported here has implications beyond those relevant to cancer. Approval of potentially more effective hormone-based treatment for female sexual issues may be forthcoming and, with it, high expectations for addressing chronic low libido.56,57 Researchers and clinicians already recognize the importance of a broad range of individual and social factors in sexuality and sexual function.58 It is notable in this study that oophorectomy, which has significant consequences for circulating sexual hormones, strongly affected only one dimension of sexuality and sexual function. Generally, it appears likely that at least some features of sexuality and sexual function depend only weakly on hormonal factors and may be addressed through psychotherapeutic, couple relationship, and similar interventions.
Footnotes
Funding for this study was provided by a grant from the National Cancer Institute (2001–345, 10.31.00–12.31.02).
Disclosure Statement
No competing financial interests exist.
References
- 1.American Cancer Society. Cancer facts and figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 2.Ries LAG, editor; Eisner MP, editor; Kosary CL, et al., editors. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 3.Davis RM. Cullin JW. Miller LS. Titus M. Physical rehabilitation. In: Haskell C, editor. Cancer treatment. Philadelphia: W.B. Saunders; 1986. pp. 940–945. [Google Scholar]
- 4.Dobkin PL. Morrow GR. Long-term side effects in patients who have been treated successfully for cancer. J Psychosoc Oncol. 1986;3:23–51. [Google Scholar]
- 5.Poplack DG. Brouwers P. Late CNS sequelae in long-term survivors of childhood leukemia. Proceedings of the fifth national conference on human values and cancer—1987; Atlanta. American Cancer Society; 1987. pp. 35–41. [Google Scholar]
- 6.Bonica JJ. Importance of the problem. In: Bonica JJ, editor; Ventafridda V, editor. Advances in pain research and therapy. New York: Raven; 1979. pp. 1–12. [Google Scholar]
- 7.Daut RL. Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. doi: 10.1002/1097-0142(19821101)50:9<1913::aid-cncr2820500944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Coyle N. Foley K. Pain in patients with cancer: Profile of patients and common pain syndromes. Semin Oncol Nurs. 1985;1:93–99. doi: 10.1016/s0749-2081(85)80042-5. [DOI] [PubMed] [Google Scholar]
- 9.Chapman CR. Syrjala K. Sargur M. Pain as a manifestation of cancer treatment. Semin Oncol Nurs. 1985;1:100–108. doi: 10.1016/s0749-2081(85)80043-7. [DOI] [PubMed] [Google Scholar]
- 10.Derogatis LR. Morrow GR. Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 11.Kornblith AB. Anderson J. Cella DF, et al. Hodgkin disease survivors at increased risk for problems in psychosocial adaptation. Cancer. 1992;70:2214–2224. doi: 10.1002/1097-0142(19921015)70:8<2214::aid-cncr2820700833>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Shands ME. Lewis F. Zahlis EH. Mother and child interactions about the mother's breast cancer: An interview study. Oncol Nurs Forum. 2000;27:77–85. [PubMed] [Google Scholar]
- 13.Lewis FM. Hammond MA. Psychosocial adjustment of the family to breast cancer: A longitudinal study. J Am Med Womens Assoc. 1996;47:194–200. [PubMed] [Google Scholar]
- 14.Yabroff KR. Lawrence FW. Clauser S, et al. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 15.Bradley CJ. Bednarek HL. Newmark D. Breast cancer survival, work, and earnings. J Health Econ. 2002;21:757–759. doi: 10.1016/s0167-6296(02)00059-0. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald HP. Dirks SJ. Borgatta EF, et al. Work disability among cancer patients. Soc Sci Med. 1989;29:1253–1259. doi: 10.1016/0277-9536(89)90065-8. [DOI] [PubMed] [Google Scholar]
- 17.Corney R. Everett H. Howells A, et al. The care of patients undergoing surgery for gynecological cancer: The need for information, emotional support and counseling. J Adv Nurs. 1992;17:667–671. doi: 10.1111/j.1365-2648.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 18.Bukovic D. Strinie T. Habek M, et al. Sexual life after cervical carcinoma. Coll Anthropol. 2003;27:173–180. [PubMed] [Google Scholar]
- 19.Bergmark K. Avall-Lundqvist E. Dickman PW, et al. Vaginal change and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1384–1389. doi: 10.1056/NEJM199905063401802. [DOI] [PubMed] [Google Scholar]
- 20.Schover LR. Fife M. Gershenson DM. Sexual dysfunction and treatment for early stage cervical cancer. Cancer. 1989;63:204–212. doi: 10.1002/1097-0142(19890101)63:1<204::aid-cncr2820630133>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Juraskova I. Butnow P. Robertson R. Post-treatment sexual adjustment following cervical and endometrial cancer: A qualitative insight. Psychooncology. 2003;12:267–279. doi: 10.1002/pon.639. [DOI] [PubMed] [Google Scholar]
- 22.Jensen PT. Groenvold M. Klee MC, et al. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. Cancer. 2004;100:97–106. doi: 10.1002/cncr.11877. [DOI] [PubMed] [Google Scholar]
- 23.Lindau ST. Gavrilova N. Anderson D. Sexual morbidity in very long term survivors of vaginal and cervical cancer: A comparison to national norms. Gynecol Oncol. 2007;106:413–418. doi: 10.1016/j.ygyno.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elit L. Esplem MJ. Butler K, et al. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Familial Cancer. 2001;1:149–156. doi: 10.1023/a:1021119405814. [DOI] [PubMed] [Google Scholar]
- 25.Robson M. Hensley M. Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281–287. doi: 10.1016/s0090-8258(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 26.Roussis NF. Waltrous L. Kerr A, et al. Sexual response in the patients after hysterectomy, total abdominal versus supracervical versus vaginal procedure. Am J Obstet Gynecol. 2004;190:1427–1428. doi: 10.1016/j.ajog.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 27.Balon R. Segraves RT. Clayton A. Issues for DSM-V: Sexual dysfunction, disorder, or variation along normal distribution: Toward rethinking DSM criteria of sexual dysfunctions. Am J Psychiatry. 2007;164:198–200. doi: 10.1176/ajp.2007.164.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Boran D. Wilwerding MB. Carpenter L. Loprinzi C. Libido as part of sexuality in female cancer survivors. Oncol Nurs Forum. 2004;31:599–607. doi: 10.1188/04.onf.599-610. [DOI] [PubMed] [Google Scholar]
- 29.Arrington R. Cofrancesco J. Wu AW. Questionnaires to measure sexual quality of life. Qual Life Res. 2004;13:1643–1658. doi: 10.1007/s11136-004-7625-z. [DOI] [PubMed] [Google Scholar]
- 30.Mettlin CJ. Menck HR. Winchester DP, et al. A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer. 1997;79:2052–2061. doi: 10.1002/(sici)1097-0142(19970515)79:10<2052::aid-cncr29>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Zippin C. Lum D. Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE. Sherbourne CD. The MOS-36 item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–482. [PubMed] [Google Scholar]
- 33.McHorney CA. Ware JE. Raczek AE. The MOS-36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Rodloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1:385–401. [Google Scholar]
- 35.Pandya R. Metz L. Patten SB. Predictive value of the CES-D in detecting depression among candidates for disease-modifying multiple sclerosis treatment. Psychosomatics. 2005;46:131–134. doi: 10.1176/appi.psy.46.2.131. [DOI] [PubMed] [Google Scholar]
- 36.Hann D. Winter K. Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the CES-D. J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 37.Waterhouse J. Metcalf MC. Development of the Sexual Adjustment Questionnaire. Oncol Nurs Forum. 1986;13:53–59. [PubMed] [Google Scholar]
- 38.Ratliff CR. Gershenson DM. Morris M, et al. Sexual adjustment of patients undergoing gracilis myocutaneous flap vaginal reconstruction in conjunction with pelvic exenteration. Cancer. 1996;78:2229–2235. [PubMed] [Google Scholar]
- 39.Feigenberg SJ. Lee WR. Desilvio ML, et al. Health-related quality of life in men receiving prostate brachytherapy on RTOG 98-05. Int J Radiat Oncol Biol Phys. 2005;62:956–964. doi: 10.1016/j.ijrobp.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 40.Bruner DW. Scott CB. McGowan D, et al. Validation of the sexual adjustment questionnaire (SAQ) in prostate cancer patients enrolled on Radiation Therapy Oncology Group (RTOG) studies 90-20 and 94-08. Int J Radiat Oncol Biol Phys. 1998;42(Suppl 1):202. [Google Scholar]
- 41.Moore KA. Halle TG. Vandivere S. Mariner CL. Scaling back survey scales: How short is too short? Sociol Methods Res. 2002;30:530–567. [Google Scholar]
- 42.Gagnon JH. Michael RT. Laumann EO, et al. The social organization of sexuality: Sexual practices in the United States. Chicago: University of Chicago Press; 1994. [Google Scholar]
- 43.Schultz PN. Stava C. Beck ML, et al. Ethnic/racial influences on the physiologic health of cancer survivors. Cancer. 2004;100:156–164. doi: 10.1002/cncr.11897. [DOI] [PubMed] [Google Scholar]
- 44.Moser WD. Chandra A. Jones J. Advance data from vital and health statistics, No. 363. Hyattsville, MD: National Center for Health Statistics; 2005. Sexual behavior and selected health measures: Men and women 15–44 years of age, United States. [PubMed] [Google Scholar]
- 45.Rhodes JC. Kjerulff KH. Langenberg PW. Guzinski GM. Hysterectomy and sexual functioning. JAMA. 1999;283:1934–1941. doi: 10.1001/jama.282.20.1934. [DOI] [PubMed] [Google Scholar]
- 46.Kuppermann V. Varner RE. Summitt RK, et al. Effect of hysterectomy vs. medical treatment on health-related quality of life and sexual functioning: The medicine or surgery (Ms) randomized trial. JAMA. 2004;291:1503–1504. doi: 10.1001/jama.291.12.1447. [DOI] [PubMed] [Google Scholar]
- 47.Basen-Engquist K. Paskett ED. Buzaglo J, et al. Cervical cancer: Behavioral factors related to screening, diagnosis, and survivors' quality of life. Cancer. 2003;98(Suppl):2009–2014. doi: 10.1002/cncr.11681. [DOI] [PubMed] [Google Scholar]
- 48.Frumowitz M. Sun CC. Schover RL, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 49.Shifren JL. Braunstein GD. Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 50.Lewontin RC. Sex, lies, and sociology. New York Review of Books, April 20, 1995.
- 51.Durant LE. Carey MP. Self-administered questionnaires versus face-to-face interviews in assessing sexual behavior in young women. Arch Sex Behav. 2000;29:309–322. doi: 10.1023/a:1001930202526. [DOI] [PubMed] [Google Scholar]
- 52.Bancroft J, editor. Researching sexual behavior: Methodological issues. Bloomington, IN: Indiana University Press; 1997. [Google Scholar]
- 53.Hawighorst-Knapstein S. Fusshoeller C. Franz C, et al. The impact of treatment for genital cancer on quality of life and body image—Results of a prospective longitudinal 10-year study. Gynecol Oncol. 2004;94:398–403. doi: 10.1016/j.ygyno.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 54.Elson J. Am I still a woman: Hysterectomy and gender identity. Philadelphia: Temple University Press; 2004. [Google Scholar]
- 55.Ganz PA. Desmond KA. Belin TR, et al. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17:2371–2380. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- 56.Ensernik M. Let's talk about sex—and drugs. Science. 2005;308:1578–1580. doi: 10.1126/science.308.5728.1578. [DOI] [PubMed] [Google Scholar]
- 57.Guzick DS. Hoeger K. Sex, hormones, and hysterectomies. N Engl J Med. 2000;343:730–731. doi: 10.1056/NEJM200009073431010. [DOI] [PubMed] [Google Scholar]
- 58.Avis NE. Stellato R. Crawford S, et al. Is there an association between menopause status and sexual functioning? Menopause. 2000;7:297–309. doi: 10.1097/00042192-200007050-00004. [DOI] [PubMed] [Google Scholar]