Abstract
Background
Current 2005 guidelines for advanced cardiac life support strongly recommend immediate defibrillation for out-of-hospital cardiac arrest. However, findings from experimental and clinical studies have indicated a potential advantage of pretreatment with chest compression-only cardiopulmonary resuscitation (CPR) prior to defibrillation in improving outcomes. The aim of this meta-analysis is to evaluate the beneficial effect of chest compression-first versus defibrillation-first on survival in patients with out-of-hospital cardiac arrest.
Methods
Main outcome measures were survival to hospital discharge (primary endpoint), return of spontaneous circulation (ROSC), neurologic outcome and long-term survival.
Randomized, controlled clinical trials that were published between January 1, 1950, and June 19, 2010, were identified by a computerized search using SCOPUS, MEDLINE, BIOS, EMBASE, the Cochrane Central Register of Controlled Trials, International Pharmaceutical Abstracts database, and Web of Science and supplemented by conference proceedings. Random effects models were used to calculate pooled odds ratios (ORs). A subgroup analysis was conducted to explore the effects of response interval greater than 5 min on outcomes.
Results
A total of four trials enrolling 1503 subjects were integrated into this analysis. No difference was found between chest compression-first versus defibrillation-first in the rate of return of spontaneous circulation (OR 1.01 [0.82-1.26]; P = 0.979), survival to hospital discharge (OR 1.10 [0.70-1.70]; P = 0.686) or favorable neurologic outcomes (OR 1.02 [0.31-3.38]; P = 0.979). For 1-year survival, however, the OR point estimates favored chest compression first (OR 1.38 [0.95-2.02]; P = 0.092) but the 95% CI crossed 1.0, suggesting insufficient estimate precision. Similarly, for cases with prolonged response times (> 5 min) point estimates pointed toward superiority of chest compression first (OR 1.45 [0.66-3.20]; P = 0.353), but the 95% CI again crossed 1.0.
Conclusions
Current evidence does not support the notion that chest compression first prior to defibrillation improves the outcome of patients in out-of-hospital cardiac arrest. It appears that both treatments are equivalent. However, subgroup analyses indicate that chest compression first may be beneficial for cardiac arrests with a prolonged response time.
Background
There are an estimated 294,851 emergency medical services (EMS)-assessed out-of-hospital cardiac arrests (OHCA) in the United States each year [1,2]. The most common underlying arrhythmias of witnessed arrests are ventricular tachycardia and ventricular fibrillation [3]. Despite major attempts to improve the chain of survival, survival rates for OHCA remain the same at 7.6% for over 30 years [4]. Average rates of survival to hospital discharge are as low as 0.3% in some communities [5,6] and depend strongly not only on the time to initiation of chest compressions but also on the time until defibrillation and the underlying rhythm [3]. While the first two factors can be influenced, they cannot be performed simultaneously. Controversy about priority has resulted from experimental and clinical data.
Current guidelines of the European Resuscitation Council (ERC) and the American Heart Association (AHA) were last updated in 2005 and emphasize the importance of early defibrillation. The International Liaison Committee on Resuscitation (ILCOR), ERC and AHA clearly prioritize early defibrillation [7,8]. However, the AHA guidelines state that in cases of nonwitnessed events, one cycle of cardiopulmonary resuscitation (CPR)/chest compressions may be considered before defibrillation (class IIb recommendation) [7]. The interval from compression to defibrillation is highly critical as impaired myocardial oxygenation distinctively decreases defibrillation success rates while myocardial preoxygenation may improve outcome [9,10].
There is, however, clinical equipoise whether professional chest compression only promptly followed by defibrillation could increase myocardial "readiness" for defibrillation. Data from the first randomized clinical trials (RCT) have shown conflicting results, but most studies were limited in size and underpowered to allow definite conclusions. A recent large-scale observational study indicated potential benefit for preshock chest compressions [11].
This is the first meta-analysis to systematically review the current research on chest compression first as compared to defibrillation first on outcomes in patients with OHCA.
Methods
The study was performed according to PRISMA guidelines (Additional file 1) [12]. Planning and study design were done by two authors (CS, PM), including creation of an electronic database with variables of interest (Microsoft Excel). Primary and secondary endpoints, variables of interest and search strategy (databases, sources for unpublished data) were defined in a strategy outline which can be obtained from study authors on request.
Data Sources and Searches
A search was conducted of SCOPUS, MEDLINE (via PubMed), BIOS, EMBASE, the Cochrane Central Register of Controlled Trials, International Pharmaceutical Abstracts database, and Web of Science from January 1, 1950, to June 19, 2010, supplemented by the conference proceedings of the American Heart Association (2006-2009), the American College of Cardiology (2006-2010), the European Society of Cardiology (2001-2009), the symposium on Transcatheter Cardiovascular Therapeutics (2006-2009), the World Congress of Cardiology (2006-2009) and the European Resuscitation Council Scientific Symposium (2006-2009). We also considered published review articles, editorials, and Internet-based sources of information (http://www.tctmd.com, http://www.theheart.org, http://www.europcronline.com, http://www.cardiosource.com, http://www.crtonline.com and Google scholar). For details on search strategy for MEDLINE, see Additional file 2. Similar but adapted search terms were used for the other literature databases.
Study selection
In a two-step selection process, two investigators (PM, BH) independently reviewed the titles and abstracts of all citations to identify potentially relevant studies and to exclude duplicates. The corresponding publications were reviewed in full text by three investigators (CS, PM, BH) to assess whether studies met the following inclusion criteria: 1) randomized treatment assignment to chest compression first versus defibrillation first, 2) human study and 3) included outcome data on one of the four following clinical outcomes: return of spontaneous circulation, survival to hospital discharge, neurological outcome at discharge or survival at 1 year (Figure 1). Reviewers were not blinded to study authors or outcomes. Final inclusion of studies was based on the agreement of three investigators (CS, PM, BH).
Figure 1.
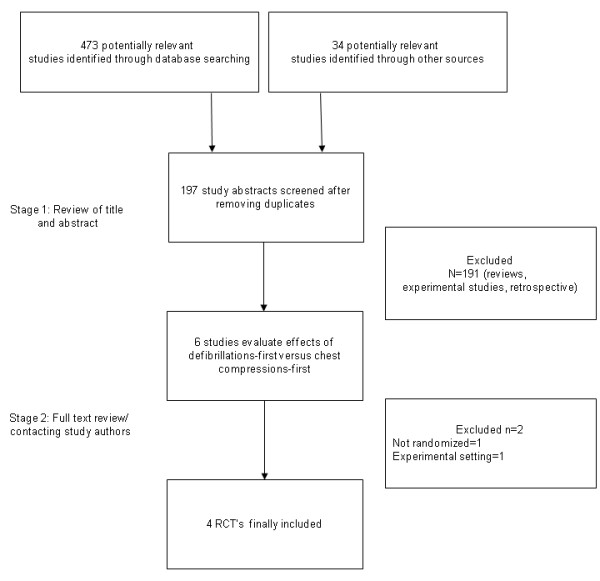
Flow chart depicting the outline of the search and selection strategy. RCT, randomized controlled trial.
Data extraction and quality assessment
Relevant information from the articles, including baseline clinical characteristics of the study population and outcome measures, were extracted by two reviewers (PM, BH) using the prepared standardized extraction database (MS Excel); data on outcome (see endpoint definition below), total patient numbers per group, and covariables of interest (average age, gender, witnessed arrest, bystander CPR, response time upon arrival of emergency medical service EMS as defined by each study) were extracted. The quality of each trial was assessed using the Jadad scale to ensure sufficient quality but was not implemented in the analysis due to relevant limitations of such approaches [13,14]. Absolute numbers were recalculated when percentages were reported. All corresponding authors of included trials were contacted to ensure accuracy of the data extraction and in an attempt to obtain more information and individual patient level data.
Endpoints
The primary endpoint of this analysis was survival to hospital discharge. However, the endpoints are presented in a chronologic order as follows:
1. Return of spontaneous circulation (ROSC)
2. Survival to hospital discharge
3. Favorable neurologic outcome at discharge (cerebral performance category (CPC) score 1 or 2)
4. Long-term outcome (survival at 1 year)
"Favorable neurological outcome" was defined as a CPC score of 1 or 2 (no or moderate cerebral disability).
Definition of a "clinically relevant" change for the primary endpoint
We regarded a relative change of at least 20-25% as clinically relevant. Power analyses of prospective randomized trials evaluating interventions for OHCA (predefibrillation chest compression, therapeutic hypothermia) used variable definitions for "clinically relevant" differences in survival, ranging from 32-550% [15-19]. Therapeutic hypothermia as one of few measures with proven benefits in OHCA showed a 35% increase in survival in a recent meta-analysis of randomized trials [20]. Since survival is such an essential endpoint, we regard a relative change of at least 20-25% as already clinically relevant, while on the other hand, a lower threshold would not be very meaningful in the context of the general low survival to discharge rate for OHCA (average 7.6%) [4]. This would increase the risk to detect incidental differences.
Data synthesis and analysis
All analyses were performed on an intent-to-treat basis. Data of included studies were combined to estimate the pooled treatment effect (odds ratio, OR) for the chest compression-first compared to the defibrillation-first groups. Calculations were based on a DerSirmonian and Laird random effects model [21]. Sensitivity analyses were conducted using alternative meta-analytical approaches such as the Hartung-Knapp method, which tends to be more conservative, and by meta-regression analyses (mixed-effects model) for the subgroups as defined below (R package "metafor") [22,23]. Continuity correction was used when no event occurred in one group to allow calculation of an odds ratio [24]. We used the rank correlation test to assess the risk for publication bias [25,26]. Heterogeneity among trials was quantified with Higgins's and Thompson's I2. I2 can be interpreted as the percentage of variability due to heterogeneity between studies rather than sampling error. On the basis of findings in a previous observational study, an a priori subgroup analysis of response time from event to EMS arrival (≤5 min versus >5 min) was also conducted [27]. Further, a meta-regression analysis was performed on the basis of the mean response intervals of each study using a mixed-effects model. Weighted average incidence of events for the chest compression-first and the defibrillation-first groups were calculated on the basis of a random effect analysis using a Freeman-Tukey double arcsine transformation and the inverse variance method [28]. Findings are presented as point estimates and 95% confidence intervals. Analyses have been performed by two investigators independently (GK, PM). All analyses were performed with R version 2.10.1 (packages "meta," "rmeta," and "metafor") [29].
Results
Description of included studies
A total of 245 abstracts were reviewed, and 79 of those were subsequently reviewed as full text articles; finally, four randomized trials enrolling 1503 subjects satisfied the predetermined inclusion criteria (Figure 1) [15-18]. Tables 1, 2, 3 summarize the characteristics and quality scores of the four trials.
Table 1.
Characteristics of included studies.
| Author | Year | Location | Group | Patients (n) | Age (yrs) | Male (%) | Witnessed (%) | Bystander CPR performed (%) | Response time (min) |
|---|---|---|---|---|---|---|---|---|---|
| Jost [15] | 2010 | France | Defi.-first | 424 | 62 | 79 | 86 | 21 | 10:54 |
| Compr.-first | 421 | 65 | 78 | 87 | 21 | 10:30 | |||
| Baker [16] | 2008 | Australia | Defi -first | 105 | 66* | 80 | 79 | 58 | 08:14 |
| Compr.-first | 97 | 65* | 84 | 84 | 59 | 07:41 | |||
| Jacobs [17] | 2005 | Australia | Defi -first | 137 | 62 | 80 | 74 | 54 | 09:00 |
| Compr.-first | 119 | 64 | 80 | 80 | 64 | 09:20 | |||
| Wik [18] | 2003 | Norway | Defi -first | 96 | 80* | 89 | 94 | 56 | 11:42 |
| Compr.-first | 104 | 71* | 85 | 91 | 62 | 12:00 |
*Median; Compr-first: chest compressions before defibrillation; Defi.-first: immediate defibrillation before chest compressions; response time: time-to arrival of ambulance.
Table 2.
Characteristics of included studies.
| Author | Year | Group | CPR pretreatment (sec) | Compression to ventilation ratio | No. of consecutive shocks |
|---|---|---|---|---|---|
| Jost | 2010 | Defi -first | Cardio-pump* | 3 | |
| Compr.-first | 60 | Cardio-pump* | 1 | ||
| Baker | 2008 | Defi -first | 3 | ||
| Compr.-first | 180 | 15:2 | 3 | ||
| Jacobs | 2005 | Defi -first | 3 | ||
| Compr.-first | 90 | 5:1 | 3 | ||
| Wik | 2003 | Defi -first | 3 | ||
| Compr.-first | 180 | 5:1 | 3 |
* Trademark (manufacturer: Ambu, Denmark). Compr-first: chest compressions before defibrillation;
Defi.-first: immediate defibrillation before chest compressions; sec: seconds
Table 3.
Quality of included studies (Jadad score).
| Author | Randomized | Appropriate randomization | Double blind | Appropriate blinding (single blind) | Drop outs appropriately declared | Score |
|---|---|---|---|---|---|---|
| Jost | Yes | Yes | No | Yes | Yes | 4/5 |
| Baker | Yes | Yes | No | Yes | Yes | 4/5 |
| Jacobs | Yes | Yes | No | Yes | Yes | 4/5 |
| Wik | Yes | Yes | No | Yes | Yes | 4/5 |
Outcomes
Return of spontaneous circulation (ROSC)
The pooled analysis did not reveal a relevant difference in the overall chance for ROSC between the chest compression-first and the defibrillation-first approach (OR 1.01 [0.82-1.26]; P = 0.979; heterogeneity: I2 = 0%, P = 0.79) (Figure 2a). The weighted average proportion of patients in whom ROSC was achieved was 39.2% [19.8-60.5%] for the chest compression-first group and 37.3% [17.0-60.2%] for the defibrillation-first group.
Figure 2.
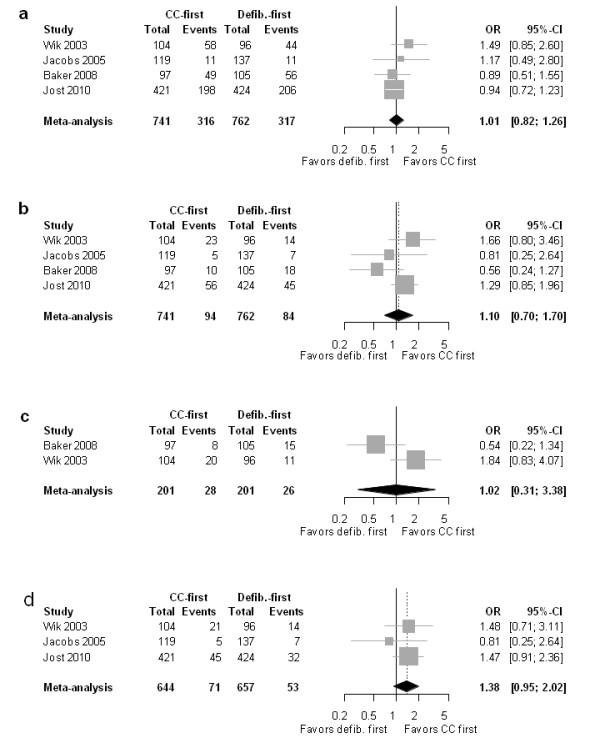
Forest plot of odds ratios (OR) of (a) ROSC, (b) survival to hospital discharge (primary endpoint), (c) favorable neurologic outcome, and (d) 1-year survival. Horizontal bars indicate 95% confidence intervals. Size of markers represents study weight in meta-analysis.
Survival to hospital discharge
As summarized for all response times in Figure 2b, the direct comparison between the chest compression-first and the defibrillation-first approach did not reveal a relevant difference (OR 1.10 [0.70-1.70]; P = 0.686; heterogeneity: I2 = 34.4%, P = 0.206). The average weighted proportion of patients able to leave the hospital after cardiac arrest was 12.0% [6.4-19.1%] for the chest compression-first group as compared to 11.4% [7.1-16.6%] for the defibrillation-first group.
Favorable neurologic outcome
The average weighted proportion of patients with favorable neurological status was 13.7% [4.9-25.9%] after chest compression first and 13.3% [9.0-18.3%] after defibrillation first. As seen in Figure 2c, patients who were treated with chest compression first did not show an increased likelihood of a "favorable neurologic outcome" (as defined by a CPC score of 1 or 2) compared to those with defibrillation first (OR 1.02 [0.31-3.38]; P = 0.979; heterogeneity: I2 = 74.9%, P = 0.05).
One-year survival
As shown in Figure 2d, the OR point estimates favored a chest compression-first approach (OR 1.38 [0.95-2.02]; P = 0.092; heterogeneity: I2 = 0%, P = 0.647). However, the 95% confidence intervals crossed 1.0, indicating insufficient precision of the effect size estimation and resulting in statistical nonsignificance. The average weighted proportion of patients able to leave the hospital after cardiac arrest with chest compression first it was 11.0% [4.8-19.5%] as compared to 8.6% [4.8-13.4%] for patients treated with defibrillation first.
Figure 3 summarizes the chance of survival of patients involved in the included trials after cardiac arrest up to 1 year after the event. As mentioned above, ROSC was achieved in approximately 40% of patients with OHCA included in these trials, chance for survival to hospital discharge was around 12.0% and similar between both treatment groups, while the survival chance at 1 year was 11.0% with chest compression first and 8.6% with defibrillation first.
Figure 3.
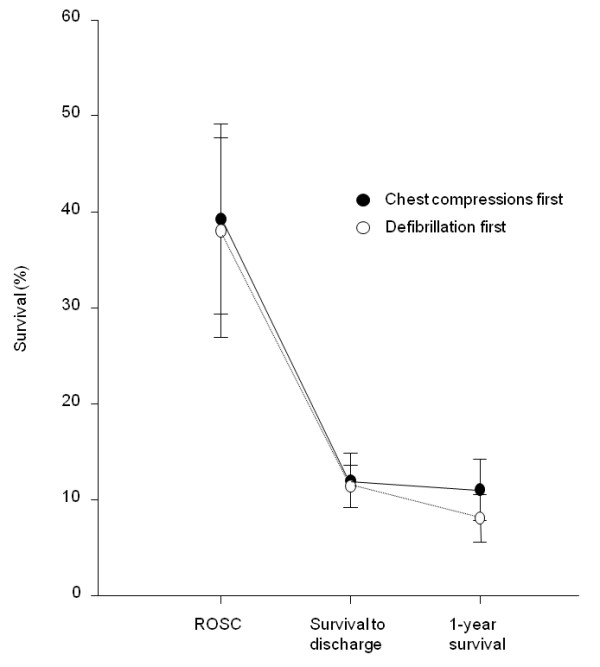
Survival of enrolled patients after cardiac arrest (average percentage and 95% confidence intervals).
Subgroup Analyses Based on Response Intervals (Call to EMS Arrival)
In Figure 4, the studies are ordered according to their average EMS response times. OR point estimates of studies with shorter EMS response times favored a defibrillation-first approach. The longer the EMS response times, the OR point estimates favored chest compression first followed by defibrillation. However, for all these OR estimates, the 95% confidence intervals crossed 1.0; thus, none of the differences were statistically significant.
Figure 4.
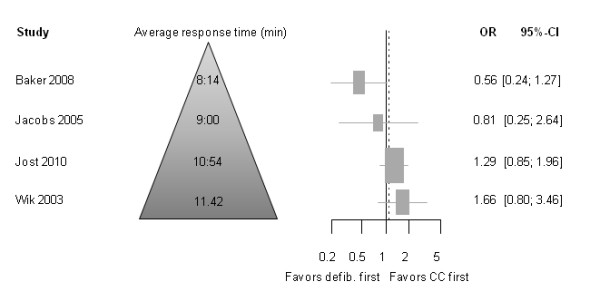
Odds ratio (OR) for primary endpoint "survival to hospital discharge" and response time. Horizontal bars indicate 95% confidence intervals. Size of markers represents study weight in meta-analysis.
Response Interval ≤5 minutes
ROSC
As shown in Figure 5a, for response time ≤5 minutes, the OR to achieve ROSC was not significantly different between chest compression first and defibrillation first (OR 1.05 [0.58-1.88]; P = 0.872; heterogeneity: I2 = 0%, P = 0.73).
Figure 5.
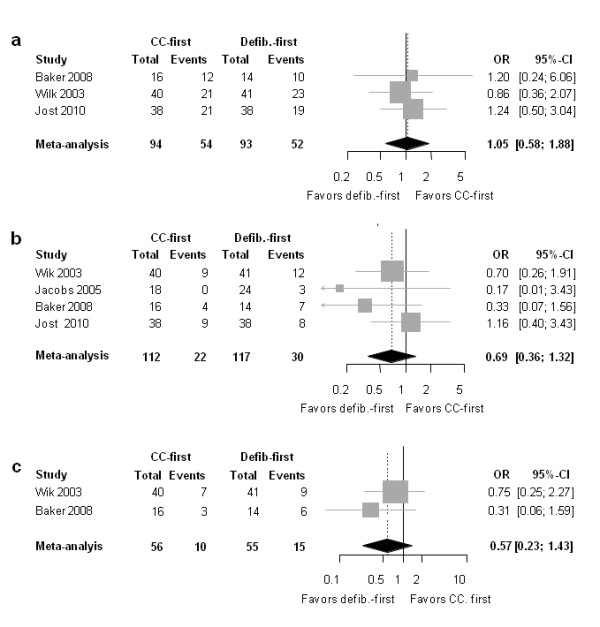
Forest plot of odds ratios (OR) of the subgroup of patients with response time ≤5 min for (a) ROSC, (b) survival to hospital discharge, and (c) favorable neurologic outcome. Horizontal bars indicate 95% confidence intervals. Size of markers represents study weight in meta-analysis.
Survival to discharge
The point estimates of the OR for this outcome were in disfavor of predefibrillation chest compressions (OR 0.69 [0.36-1.32]; P = 0.263; heterogeneity: I2 = 0%, P = 0.954) (Figure 5b). The 95% confidence interval crossed 1.0, indicating inadequate precision of the effect estimate, resulting in statistical nonsignificance.
Neurologic outcome
As Figure 5c shows, the OR point estimate was in disfavor of predefibrillation chest compression approach (OR 0.57 [0.23-1.43]; P = 0.300 (heterogeneity: 0%; P = 0.370). Again, the 95% confidence interval crossed 1.0, and the difference was therefore not statistically significant.
Response Interval >5 minutes
ROSC
No relevant differences were found for patients with a response time >5 minutes in ROSC (Figure 6a), the OR was 1.10 [0.67-1.78]; P = 0.705 (heterogeneity: 62.4%; P = 0.0712).
Figure 6.
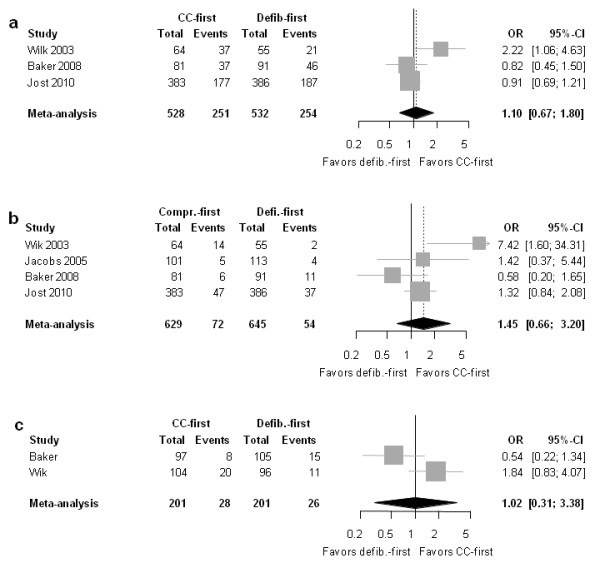
Forest plot of odds ratios (OR) of group with response time >5 min for (a) ROSC, (b) survival to hospital discharge and (c) good neurologic outcome. Horizontal bars indicate 95% confidence intervals. Size of markers represents study weight in meta-analysis.
Survival to discharge
The point estimate for the OR pointed toward superiority of chest compression first, but the confidence interval crossed 1.0; thus, the finding was not statistically significant (OR 1.45 [0.66-3.20]; P = 0.353; heterogeneity: 59.1%; P = 0.062) (Figure 6b).
Neurologic outcome
As Figure 6c illustrates, there was no relevant difference between the two groups (OR 1.02 [0.31-3.38]; P = 0.879; heterogeneity: I2 = 84.2%; P = 0.012).
Meta-regression analysis based on mean response intervals
This analysis showed a significant effect of the mean response interval of each study in the control arm on the effect of predefibrillation chest compression; the point estimates of the OR pointed toward inferiority of predefibrillation chest compression for studies with short mean response intervals but toward superiority for studies with longer mean response intervals (Additional file 3; Supplementary Figure 1). This response interval effect was statistically significant. The slope of the meta-regression was 0.0051 [0.0004-0.0097]; P = 0.033. That is, for every absolute increase of 1 time unit (1 second) in the response time, the log odds ratio increased by 0.0051 (in direction to superiority of a chest compression-first approach). At around 600 seconds (10 min) response time, the regression line crosses OR 1.0 (equipoise between the two interventions). Additional file 4, Supplementary Table 6 gives an overview of variable response intervals with corresponding predicted odds ratios.
Sensitivity analyses
The analysis performed with the Hartung-Knapp meta-analytical approach and by a mixed-effects meta-regression analysis revealed almost identical results (see Additional file 5, Supplementary Tables 3-5. Also, a sensitivity analysis was conducted without the study by Jost et al. [15], as this study did not exclusively test the effect of chest compression first, but also the effect of three consecutive shock applications versus a single shock at a time. Also, most patients did not receive bystander CPR; CPR was initiated in most cases by firefighters using a CPR device instead of manual compressions. When excluding this study, the results did not change despite the considerable weight (study size) of this study in this analysis (data not presented).
Publication bias assessment
Regarding the primary endpoint, the rank correlation test was not suggestive for publication bias, P = 0.588.
Discussion
This is the first meta-analysis evaluating the effect of chest compression first versus defibrillation first in patients having out-of-hospital cardiac arrest. We included four randomized, controlled clinical trials with 1503 subjects. Overall, our findings suggest that there was no significant difference between the two groups in general. However, our subgroup analyses of patients with a response interval >5 min found point estimates that pointed toward superiority of a chest compression-first approach and vice versa for the subgroup with response interval ≤5 min. The point estimate for the 1-year survival results pointed toward a lower 1-year mortality for chest compression-first patients, which was mainly driven by studies with longer EMS response times [15,18]. However, the 95% confidence intervals of these subgroup and long-term analyses crossed 1.0, indicating insufficient precision of the effect estimates and resulting in statistical nonsignificance. These analyses were based on smaller patient numbers.
Rational for Chest Compressions Prior to Defibrillation
Chest compressions serve to empty the right ventricle (RV) and to avoid RV distension during VF, which helps to reduce the risk of occurrence of "nonperfusing" postdefibrillation rhythms (e.g., pulseless electrical activity or asystole) [30,31]. Two experimental animal studies on ventricular defibrillation have demonstrated that chest compression first may improve defibrillation success in comparison to the standard defibrillation first approach. A randomized study in swine conducted by Berg et al. and a study by Niemann et al. in dogs both showed higher efficiency for chest compression prior to defibrillation [32,33]. Data from a study conducted on humans showed that even short preshock pauses were found to strongly correlate with lower defibrillation success [34]. Accordingly, a large observational study by Cobb et al. demonstrated improved survival for patients treated for out-of-hospital cardiac arrest after implementation of chest compression-first protocol compared to the preceding 42 months with the standard defibrillation-first approach [27]. Similarly, a study including 886 patients of Bobrow et al. performed in Arizona implementing a protocol of 200 uninterrupted chest compressions before defibrillation (single shock) showed a remarkable increase in survival-to-hospital discharge, from 1.8% to 5.4% after protocol implementation [35,36]. Yet, despite all of the above data from experimental and observational studies, our meta-analysis based on randomized clinical trials in humans shows that both treatments appear to be equivocal, with point estimates that favor chest compression first regarding long-term outcomes.
Several aspects could explain this controversy. First, findings from experimental animal studies may not apply to humans, especially since most models use electrical induction of ventricular fibrillation, which may not appropriately reflect the majority of cardiac arrests in humans [37]. In a more recent study in swine using an acute myocardial ischemia model, 24-hr survival with a favorable neurological outcome was less likely when chest compressions were performed prior to defibrillation [38]. Second, observational studies [27,35] are more prone to confounding than randomized trials. Because we decided a priori to include only randomized, controlled trials in our meta-analysis, our results may differ from these large observational studies. Finally, it may be that the treatment effect of chest compression first may be dependent on the response interval from the time of call to EMS response. Further research, with patient-level data, will need to be conducted to assess whether this finding is consistent.
Short- versus longer-duration cardiac arrest
The possible difference in treatment effect for longer-lasting (response interval >5 min) makes plausible sense from a pathophysiological standpoint. Cardiac arrest (due to ventricular tachycardia/fibrillation (VT/VF)) is definitively not a static event. Rather, it is a dynamic process with sometimes continuous transitions starting with VT, transforming into coarse and then into fine amplitude VF and finally into asystole; these different electrocardiogram morphologies are obviously associated with different degrees of defibrillation success [39]. During the course of VF high-energy phosphates are progressively depleted, which also decreases the chances for successful defibrillation [40].
Niemann et al. demonstrated the superiority chest compression first in a dog model [33], but found better outcomes for defibrillation first in a subsequent study [41]. In this second study, VF duration was relevantly shorter (5 min versus 7.5 min in the first study). Another study conducted in dogs specifically evaluated different VF durations, showing differential results based on the duration of VF. For short-lasting VF arrests (< 3 min), defibrillation first was superior to chest compression first [42]. It has to be considered, however, that most experimental animal studies used electrical induction of VF, which may not be identical to ischemia-induced VF [37]. The study by Cobb et al. included in our analysis showed the most prominent benefit for chest compression first if response time was >4 min [27].
In 2002, Weisfeldt et al. proposed a three-phase time-sensitive model for treatment of sudden cardiac arrest: the electrical phase (early phase during the first around 0-4 min where immediate defibrillation may be optimal, the circulatory phase (4-10 min) where predefibrillation chest compressions could be meaningful, and the metabolic phase (> 10 min), where survival rates are poor in general [39]. The authors stated in their editorial that "phase-specific research is needed to extend knowledge of the importance of time on resuscitation, such as testing early defibrillation and public access defibrillation programs during the electrical phase and testing chest compression and vasoconstrictors first during the circulatory phase." [39]. Our findings support the view of Weisfeld et al. as illustrated in Figure 4 and as shown in the subgroup analyses of patients with longer versus those with shorter response intervals.
Limitations of this study
It has to be considered that nonstratified overall results showed odds ratios very close to 1.0; that is, no treatment effect with fairly narrow confidence (precision) intervals and with very little heterogeneity. In contrast, OR point estimates pointed toward superiority of predefibrillation chest compressions for those cardiac arrests with prolonged EMS response, while in patients with shorter EMS intervals these OR estimates pointed toward superiority of a defibrillation-first approach (Figures 5 and 6). Owing to the smaller sample sizes in these subgroups, confidence intervals were wider due to reduced precision of these estimates. The confidence intervals for these subgroup analyses crossed 1.0; i.e., the result was statistically not significant. It is possible that there is in fact a difference that was not detected by our analysis due to limited statistical power. An interaction between optimal treatment and response time is further supported by the observation that the odds ratios were influenced by the average response intervals of the individual studies (Figure 3 and Additional file 1). However, the meta-regression analysis (Additional file 1), even though in line with the findings of the subgroup analyses, has to be interpreted with care because it is based on summary measure (mean response intervals of each study) and not on individual response intervals. Meta-analyses are useful for synthesizing the literature and to explore areas for further exploration rather than to provide a definitive conclusion. Future research based on this meta-analysis could be conducted with patient-level data to assess whether the overall pooled results are consistent with the individual-level data.
RCT data are considered the "golden standard" and superior to observational studies. Clearly, the latter are more prone to be biased by confounding, and, accordingly, we considered RCT exclusively in this meta-analysis. Nevertheless, there are caveats for RCT also [43]; this is especially true in the context of human emergency medicine research. The vast majority of patients assessed for inclusion in these trials were finally not eligible because of predefined exclusion criteria or owing to logistical reasons. Thus, the patient selection associated with RCT potentially complicates generalizability of findings into routine clinical practice. For example, bystander CPR rate ranged from 54-64% in three of the included trials, while the AHA estimates the average bystander CPR rate in the United States to be 31.4% [1]. Future research will need to be conducted on communities that may be more generalizable than the study populations in this analysis.
A further limitation of this study is the heterogeneity of the study protocols. Three of the four included trials use the 2000 guidelines with a "three-shock protocol" [16-18],
while one study utilized a single shock application (as advocated in the current 2005 guidelines) in the chest compression first group [15]. All four studies did not control for the quality of chest compressions. The quality of chest compressions has a key impact on outcome and is often insufficient, even for in-hospital cardiac arrests [34] and even in some experimental studies [44]. We cannot exclude that the quality of compressions in the included studies was insufficient, and as a consequence, the studies were unable to show a benefit. Because of the differences in study protocols, we chose to use a random effects model rather than a fixed-effect model for data analysis.
Finally, we did not have the complete set of individual patient data, and our analyses are thus based on study-level data. Therefore, we could not adjust the analysis for covariables. For example, the 1-year survival data for the study by Jost et al. [15] are based on Kaplan-Meier survival estimates, which showed a survival probability of 10.6% in the intervention group and 7.6% in the control group (P = 0.45).
Conclusions
The results of this meta-analysis demonstrate that survival is equivocal for the chest compression-first group as compared to the defibrillation-first group. Thus, current guidelines emphasizing early defibrillation still appear appropriate. However, the study revealed signals toward possible superiority of predefibrillation chest compressions for patients with a response interval of >5 min; the statistical power of this study was insufficient for such subgroup analyses, and none reached statistical significance. These signals suggest that the optimal treatment of cardiac arrest patients may depend on the duration of the event and the timeliness of the response. Future research will need to be conducted to assess whether this differential effect is seen in patients treated for out-of-hospital cardiac arrest. This may lead to different treatment guidelines based on the duration of the arrest and the interval of the response.
Abbreviations
AHA: American Heart Association; CPR: Cardiopulmonary resuscitation; ERC: European Resuscitation Council; EMS: Emergency medical services; ILCOR: International Liaison Committee on Resuscitation; OHCA: Out-of-hospital cardiac arrests; OR: Odds ratio; RCT: Randomized clinical trials; ROSC: Return of spontaneous circulation.
Competing interests
The authors declare that they have no competing interests to disclose. PM is supported by a postdoctoral fellowship grant from the Swiss National Research Foundation, Switzerland. The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Authors' contributions
PM, CS conceptualized and designed this meta-analysis. PM, BH, CS were substantially involved in data acquisition (literature search, study selection and data abstraction). PM, GK, CS performed the analyses and were substantially involved in data interpretation. PB, DJ, IJ provided data used for the analysis and relevantly contributed to the interpretation and intellectual content of the manuscript. PM and CS drafted the manuscript. All authors revised the manuscript critically for important intellectual content. All authors approved the final version.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Supplementary table 1. PRISMA statement checklist.
Supplementary table 2. Detailed literature search strategy with search terms used for Medline.
Supplementary figure 1. Meta-regression plot. Meta-regression plot of odds ratios (OR) versus response interval (seconds). Size of circles indicate study weights in a mixed-effects model.
Supplementary table 6. Predicted odds ratios for variable response intervals.
Supplementary tables 3 - 5. Sensitivity analyses with different meta-analytical approaches.
Contributor Information
Pascal Meier, Email: pascalmeier74@gmail.com.
Paul Baker, Email: Paul.Baker@health.sa.gov.au.
Daniel Jost, Email: chefscientifique.smu@pompiersparis.fr.
Ian Jacobs, Email: ian.jacobs@uwa.edu.au.
Bettina Henzi, Email: bettina.henzi@students.unibe.ch.
Guido Knapp, Email: guido.knapp@tu-dortmund.de.
Comilla Sasson, Email: comilla.sasson@ucdenver.edu.
Acknowledgements
We are especially grateful to Whitney Townsend, Librarian, Taubman Medical Library, University of Michigan, for her input during literature search and Dr. Jose Jalife, University of Michigan Center for Arrhythmia Research, for his help during this project. We also thank Dr. Petter A. Steen and Dr. Lars Wik, Ulleval University Hospital, Oslo, Norway for providing further information on their study and for data verification.
References
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Resuscitation Outcomes Consortium Investigators. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg M, Holmberg S, Herlitz J. The problem of out-of-hospital cardiac-arrest prevalence of sudden death in Europe today. Am J Cardiol. 1999;83(5B):88D–90D. doi: 10.1016/S0002-9149(98)01008-X. [DOI] [PubMed] [Google Scholar]
- Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3(1):63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- Dunne RB, Compton S, Zalenski RJ, Swor R, Welch R, Bock BF. Outcomes from out-of-hospital cardiac arrest in Detroit. Resuscitation. 2007;72(1):59–65. doi: 10.1016/j.resuscitation.2006.04.017. [DOI] [PubMed] [Google Scholar]
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(24 Suppl):IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- International Liaison Committee on Resuscitation. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 1: introduction. Resuscitation. 2005;67(2-3):181–186. doi: 10.1016/j.resuscitation.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Eftestol T, Sunde K, Steen PA. Effects of interrupting precordial compressions on the calculated probability of defibrillation success during out-of-hospital cardiac arrest. Circulation. 2002;105(19):2270–2273. doi: 10.1161/01.CIR.0000016362.42586.FE. [DOI] [PubMed] [Google Scholar]
- Valenzuela TD. Priming the pump: can delaying defibrillation improve survival after sudden cardiac death? JAMA. 2003;289(11):1434–1436. doi: 10.1001/jama.289.11.1434. [DOI] [PubMed] [Google Scholar]
- Garza AG, Gratton MC, Salomone JA, Lindholm D, McElroy J, Archer R. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119(19):2597–2605. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- Jost D, Degrange H, Verret C, Hersan O, Banville IL, Chapman FW, Lank P, Petit JL, Fuilla C, Migliani R, Carpentier JP, DEFI 2005 Work Group. DEFI 2005. a randomized controlled trial of the effect of automated external defibrillator cardiopulmonary resuscitation protocol on outcome from out-of-hospital cardiac arrest. Circulation. pp. 1614–1622. [DOI] [PubMed]
- Baker PW, Conway J, Cotton C, Ashby DT, Smyth J, Woodman RJ, Grantham H. Defibrillation or cardiopulmonary resuscitation first for patients with out-of-hospital cardiac arrests found by paramedics to be in ventricular fibrillation? A randomised control trial. Resuscitation. 2008;79(3):424–431. doi: 10.1016/j.resuscitation.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Jacobs IG, Finn JC, Oxer HF, Jelinek GA. CPR before defibrillation in out-of-hospital cardiac arrest: a randomized trial. Emerg Med Australas. 2005;17(1):39–45. doi: 10.1111/j.1742-6723.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, Steen PA. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289(11):1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Herkner H, Mullner M. Cochrane corner: hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Anesth Analg. 2010;110(4):1239. doi: 10.1213/ANE.0b013e3181ce8d34. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- Meier P, Knapp G, Tamhane U, Chaturvedi S, Gurm HS. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. BMJ. 2010;340:c467. doi: 10.1136/bmj.c467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankey SS, Weissfeld LA, Fine MJ, Kapoor W. An assessment of the use of the continuity correction for sparse data in meta-analysis. Commun Stat Simul Comput. 1996;25:1031–1056. doi: 10.1080/03610919608813357. [DOI] [Google Scholar]
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27(5):746–63. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, Antes G, Schumacher M. A test for publication bias in meta-analysis with sparse binary data. Stat Med. 2007;26(4):721–733. doi: 10.1002/sim.2588. [DOI] [PubMed] [Google Scholar]
- Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281(13):1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- Miller J. The inverse of the Freeman-Tukey double arcsine transformation. Am Stat. 1978;32:138. doi: 10.2307/2682942. [DOI] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria; 2010. ISBN 3-900051-07-0. [Google Scholar]
- Herlitz J, Bang A, Holmberg M, Axelsson A, Lindkvist J, Holmberg S. Rhythm changes during resuscitation from ventricular fibrillation in relation to delay until defibrillation, number of shocks delivered and survival. Resuscitation. 1997;34(1):17–22. doi: 10.1016/S0300-9572(96)01064-7. [DOI] [PubMed] [Google Scholar]
- Chamberlain D, Frenneaux M, Steen S, Smith A. Why do chest compressions aid delayed defibrillation? Resuscitation. 2008;77(1):10–15. doi: 10.1016/j.resuscitation.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Berg RA, Hilwig RW, Ewy GA, Kern KB. Precountershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Crit Care Med. 2004;32(6):1352–1357. doi: 10.1097/01.CCM.0000127780.01362.E5. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Cairns CB, Sharma J, Lewis RJ. Treatment of prolonged ventricular fibrillation. Immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation. 1992;85(1):281–287. doi: 10.1161/01.cir.85.1.281. [DOI] [PubMed] [Google Scholar]
- Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TL, Steen PA, Becker LB. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bobrow BJ, Clark LL, Ewy GA, Chikani V, Sanders AB, Berg RA, Richman PB, Kern KB. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008;299(10):1158–1165. doi: 10.1001/jama.299.10.1158. [DOI] [PubMed] [Google Scholar]
- Ramaraj R, Ewy GA. Rationale for continuous chest compression cardiopulmonary resuscitation. Heart. 2009;95(24):1978–1982. doi: 10.1136/hrt.2009.174268. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Rosborough JP, Youngquist S, Thomas J, Lewis RJ. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit Care Med. 2007;35(5):1356–1361. doi: 10.1097/01.CCM.0000261882.47616.7D. [DOI] [PubMed] [Google Scholar]
- Indik JH, Hilwig RW, Zuercher M, Kern KB, Berg MD, Berg RA. Preshock cardiopulmonary resuscitation worsens outcome from circulatory phase ventricular fibrillation with acute coronary artery obstruction in swine. Circ Arrhythm Electrophysiol. 2009;2(2):179–184. doi: 10.1161/CIRCEP.108.824862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288(23):3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- Kern KB, Garewal HS, Sanders AB, Janas W, Nelson J, Sloan D, Tacker WA, Ewy GA. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation. 1990;20(3):221–229. doi: 10.1016/0300-9572(90)90005-Y. [DOI] [PubMed] [Google Scholar]
- Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Ann Emerg Med. 2000;36(6):543–546. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8(3):157–163. doi: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118(12):1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- Sattur S, Kern KB. Increasing CPR duration prior to first defibrillation does not improve return of spontaneous circulation or survival in a swine model of prolonged ventricular fibrillation. Resuscitation. 2009;80(3):382. doi: 10.1016/j.resuscitation.2008.11.005. author reply 382-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. PRISMA statement checklist.
Supplementary table 2. Detailed literature search strategy with search terms used for Medline.
Supplementary figure 1. Meta-regression plot. Meta-regression plot of odds ratios (OR) versus response interval (seconds). Size of circles indicate study weights in a mixed-effects model.
Supplementary table 6. Predicted odds ratios for variable response intervals.
Supplementary tables 3 - 5. Sensitivity analyses with different meta-analytical approaches.


