Abstract
A meta-analysis was performed in order to inventory the immune epitope data related to viruses in the genus Flavivirus. Nearly 2000 epitopes were captured from over 130 individual Flavivirus-related references identified from PubMed and reported as of September 2009. This report includes all epitope structures and associated immune reactivity from the past and current literature, including: the epitope distribution among pathogens and related strains, the epitope distribution among different pathogen antigens, the number of epitopes defined in human and animal models of disease, the relationship between epitopes identified in different disease states following natural (or experimental) infection, and data from studies focused on candidate vaccines. We found that the majority of epitopes were defined for dengue virus (DENV) and West Nile virus (WNV). The prominence of DENV and WNV data in the epitope literature is likely a reflection of their overall worldwide impact on human disease, and the lack of vaccines. Conversely, the relatively smaller number of epitopes defined for the other viruses within the genus (yellow fever and Japanese encephalitis virus) most likely reflects the presence of established prophylaxis and/or their more modest impact on morbidity and mortality globally. Through this work we hope to provide useful data to those working in the area of Flavivirus research.
Introduction
The genus Flavivirus of the family Flaviviridae comprises more than 70 antigenically-related viruses, many of which represent important human pathogens (43,69). These include dengue virus (DENV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), West Nile virus (WNV), Murray Valley encephalitis virus (MVEV), tick-borne encephalitis virus (TBEV), St. Louis encephalitis virus (SLEV), Kunjin virus (KUN), and Louping ill virus (LIV). Flaviviruses, formerly known as group B arboviruses, were so-called due to their primary mode of transmission via an arthropod vector: mosquito (Aedes and Culex spp.) or tick (Ixodes spp.), with maintenance in a mammalian host (sylvatic transmission). The global burden of disease due to Flavivirus organisms is significant. Dengue fever (DF), along with its severe forms dengue hemorrhagic fever (DHF) and dengue shock syndrome, are estimated to cause 50–100 million infections per year worldwide. Mortality rates for DHF range between 2.5 and 20%, with the greatest impact on children (107). Japanese encephalitis virus has been identified as the leading cause of viral encephalitis in Asia (mostly in children), with up to 50,000 cases per year, and a case fatality ratio of 30% (12), and for yellow fever, the virus for which the genus was named, there are an estimated 200,000 cases per year with up to 15% mortality (108). In addition, West Nile virus has emerged in the Western hemisphere and is now the leading cause of human arboviral encephalitis in the United States, with more than 11,000 cases of neuroinvasive disease, 16,500 cases of non-neuroinvasive disease, and over 1000 deaths reported from 1999–2007 (13). Effective vaccines currently exist for yellow fever, Japanese encephalitis, and tick-borne encephalitis viruses (Europe only). However, while several veterinary vaccines are now in common use for West Nile virus (inactivated and live-attenuated), a candidate vaccine for use in humans is not yet available, and there is no vaccine currently available for dengue virus.
Global disease burden is further compounded by the fairly recent geographic expansion or resurgence of certain Flaviviruses. In fact, old nemeses like JEV, YFV, WNV, and DENV are classified as re-emerging pathogens by the National Institute of Allergy and Infectious Diseases (NIAID), a component of the United States National Institutes of Health (NIH). All of these deadly pathogens are now causing disease in areas heretofore not affected (and therefore not covered by immunization efforts), or are causing more severe disease in areas where more mild disease was once the norm (81,82,99). While highly-effective vaccines are available to combat the spread of YF and JEV, the lack of prophylaxis for DENV and WNV is troubling. As an example, the emergence of DENV as a major public health concern has been dramatic in the Americas. Since the 1970s, the transmission of DENV has increased dramatically worldwide, occurring in more than 100 tropic and sub-tropic countries. There also has been an increase in DENV virulence and disease severity, which has been attributed to the Southeast Asian genotypes (serotypes 2 and 3). These more virulent DHF-causing genotypes are now displacing (outcompeting), the less virulent “native” DF-causing genotypes in the Americas, leading to an increase in the incidence of severe disease on these continents (22,81).
A growing body of immune epitope-related data now exists for many of the viruses within this genus. Immune epitope data may be useful for the identification of targets for candidate vaccines, to help characterize important details related to the mechanisms of immunity and immunopathology, as a tool to more fully define immune reactivity to existing vaccines (such as YF and JEV), and as a tool to aid in immunosurveillance.
The Immune Epitope Database (IEDB) was created, with the support of NIAID, to provide the scientific community with a repository of freely accessible immune epitope data (www.immuneepitope.org). The IEDB contains epitope data curated from published literature, data submitted by the NIAID's high-throughput epitope discovery projects, and data imported from other databases. The database contains antibody and T-cell data for human, non-human primate, and rodent hosts, as well as a number of other animal species, and targets epitopes derived from a broad range of organisms and disease states, including bacteria, viruses, fungi, and parasites, as well as allergy, autoimmunity, and transplant rejection. Moreover, the IEDB hosts a broad range of analysis tools (e.g., epitope prediction, homology mapping, and conservancy analysis), and links directly to many established resources and related databases.
For each epitope, detailed experimental information is captured, along with the epitope structure, its source, and its chemical nature. The fields of the database are specifically designed to also capture information related to the immunization modality, the immunized host, and the assay in which the immune response was defined. Because the data are searchable at all levels, the IEDB can be used by the scientific community to assist in the identification and evaluation of potential targets for vaccine and diagnostic candidates, as well as to assist in better understanding disease immunobiology and pathogenesis. Moreover, these data can be integrated with data from other databases (genomic, transcriptomic, or proteomic), thereby increasing the utility and broad applicability of the analyses.
In this way, the IEDB provides a platform for detailed meta-analysis of immune epitope data for specific pathogens of interest. Based on a comprehensive inventory of all reported epitope structures and associated immune reactivity from the literature, these analyses include: the epitope distribution among pathogen and related strains, the epitope distribution among different pathogen products, the number of epitopes defined in humans and animal models of disease, the relationship between epitopes identified in different disease states following natural (or experimental) infection, and data from studies focused on candidate vaccines.
Meta-analyses have been conducted for several high-profile pathogens, such as influenza A virus, mycobacteria (M. tuberculosis and related species), anthrax, and botulinum toxins, and all Plasmodium species (8,11,101,112). These analyses have provided comprehensive catalogs of pathogen-specific epitope data, and at the same time have identified critical knowledge gaps and highlighted potential areas for further research. We report here a meta-analysis of all Flavivirus immune epitope data as of September 2009, including references published as early as the 1980s through the current year. Through this work we hope to provide useful data to those working in the area of Flavivirus research.
Review of Immunity and Immunopathology in Flaviviral Infections
In general, adaptive immune responses to flaviviruses comprise both humoral and cellular responses, including neutralizing and complement-fixing antibodies, CD8+ T cells/CTLs, and CD4+/T-helper cells (46,73). Humoral responses to Flavivirus infection are important for control of viral replication and dissemination, most of which is provided by neutralizing antibodies directed against surface glycoproteins. The main role of cellular immunity appears to be to provide help for B-cell activation (CD4+), and for direct viral clearance (CD8+). Eight of the 10 virus-encoded proteins have been shown to be targeted by B-cell responses (21,31,59), and all 10 proteins have been shown to elicit some type of T-cell response (2,5,30,57,87,92). However, the most commonly identified antigens include (in order of dominance) E (the major surface protein), preM/M, NS1, and NS3 (non-structural proteins). Of these, neutralizing antibodies are most often directed against E, preM/M, and NS1 (73), whereas the bulk of T-cell-specific responses target E and NS3 (10,52). In addition, while immunity to subsequent infections can be achieved following primary infection, in several cases significant immunopathology is observed for some viruses, and is described in more detail below. While the mechanisms involved in immunopathology remain the object of intense investigation, it has been hypothesized that cross-reactivity (for both B and T cells) following secondary infection leading to sub-neutralizing and/or low-affinity effectors might be involved.
Many of the mechanisms underlying Flavivirus immunity and immunopathology may be based in differences in determinant recognition, leading to protection or production of sub-neutralizing antibody and low-affinity T cells; therefore the study of these mechanisms at the molecular (epitope) level is important. Indeed, studies to date have examined the epitope conservation, variation, and cross-reactivity associated with disease (53,56,65,66,92). Additional genome-wide or individual antigen-based analysis of epitope reactivity from clinical isolates may help in elucidating such underlying differences. Epitope analysis of animal models may be useful for characterizing the roles of certain antibody isotypes and/or T-cell subtypes (CD4 and CD8). In the following sections we briefly review some of the knowledge related to the antigens recognized by adaptive responses to each virus, as a prelude to a detailed analysis of the information related to the specific epitopes recognized within each antigen.
Dengue virus
Associations have been made between protection from severe disease and the presence of neutralizing antibodies directed at the E protein, preM/M, and NS1 (48,52,83,86). However, neutralizing antibody and virus-specific T cells to these same antigens have also been associated with enhancement of disease (67). Indeed, while immunity to a homologous serotype can be achieved following primary infection, severe disease is most often observed following secondary, heterotypic infection. The mechanisms behind disease exacerbation are hypothesized to include antibody-mediated enhanced viral uptake and/or T-cell immunopathology.
Antibody-dependent enhancement (ADE) of infection describes the phenomenon of the dramatic increase in the infection of cells in the presence of non-neutralizing or sub-neutralizing levels of antibody. According to the ADE hypothesis, DENV-antibody complexes are formed and bind to the Fc receptors on cells such as macrophages, facilitating viral entry and replication. Increased viral loads resulting from ADE then drive the production of inflammatory mediators that increase vascular permeability. Increasing evidence supports a role for ADE in disease exacerbation following dengue infection (35); this phenomenon is proposed to occur following sequential infection with different dengue virus serotypes for which pre-existing immune sera is sub- or non-neutralizing for the heterologous virus. The phenomenon of more severe disease has also been observed in infants following passive transfer of maternally-derived antibodies, as the levels of DENV-specific antibodies wane with age, mimicking the sub-neutralizing situation. It has been proposed that ADE is one of the primary mechanisms leading to DHF and DSS in humans. However, the exact mechanisms involved in ADE have yet to be elucidated. To date, DHF/DSS has not yet been reported in an infant born to a DENV-naïve mother, providing the most compelling argument for the role of antibodies in DENV pathogenesis. Dengue-associated ADE has also been described after passive transfer of antibodies against YF virus and Langat encephalitis viruses (4,32,33,103). However, ADE was not observed after transfer of monoclonal or polyclonal antibodies against JEV or TBEV (45,47).
Similarly to the situation with the ADE, cross-reactive memory T cells have been studied intensively in recent years in order to understand their role in mediating protective versus immunopathological responses (5,40). In the phenomenon known as original antigenic sin, T cells generated during primary infection with one viral serotype, demonstrate low affinity to the secondary heterologous virus infection, leading to a suppression or delay of viral clearance. This, in turn, leads to higher viral loads and increased immunopathology caused by aberrant “hyperactive” T cells with associated cytokine storm (66). To date, variants of wild-type T-cell epitopes associated with low affinity and/or immunopathogenic effect have been identified in the NS3 and NS5 proteins (53,65,66,92).
It is important to note that the majority of published T-cell studies have focused predominantly on the role of T cells in DENV immunopathology. Few studies have examined the overall role of T cells in protection from disease. Therefore, the answer to the critical question of whether T cells contribute to protection against DENV infection, and/or to what extent, remains unknown. Such studies have largely been hampered by the lack of good in vitro and in vivo models of disease, and only recently have some potential models been developed. Animal models of DENV infection will be discussed further in a later section.
West Nile virus and Kunjin virus
Humoral immunity is considered an essential aspect of protective immunity (6,16,25,26,71). Passive transfer of immune sera can protect humans (hyperimmune globulin) (37), as well as B-cell-deficient mice from lethal infection (28). In mice, IgG has been shown to be protective through passive transfer studies, and IgM was shown to be critical for controlling initial infection and is predictive of disease outcome (26). While a majority of neutralizing antibodies are directed against regions of the WNV E protein, a subset recognizes the prM/M protein (29). Although neutralizing antibodies generated during WNV infection predominantly bind structural proteins, antibodies to the non-structural protein NS1 also protect mice against WNV infection (19). Antibody responses to NS3 and NS5 have also been observed during WNV infection (20,98,109); however, their functional significance is not fully understood. Antibody-mediated protection from WNV infection includes complement-dependent and independent, as well as Fc receptor-dependent and independent mechanisms (74).
Studies in humans and animal models have also demonstrated that T lymphocytes are an essential component of protection against WNV (16,44,54,90,91,105). Patients with hematologic malignancies or impaired T-cell function have been found to have an increased risk of neuroinvasive infection (70,76). Mice deficient in CD8+ T cells or MHC class I molecules have higher mortality rates due to sustained viral burdens in the spleen and CNS, despite a normal humoral response (90). Moreover, adoptive transfer of CD8+T cells decreased CNS viral burdens and enhanced survival. Studies in mice have shown that CD4+ T cells control WNV infection by priming B-cell and antibody responses, and by sustaining CD8+ T-cell activity. CD4+ T cells do not appear to be necessary for priming CD8+ T cells. Moreover, data suggest that CD4+ T cells restrict WNV pathogenesis in vivo, as CD4+ T-cell depletion or a genetic deficiency in MHC class II results in decreased WNV-specific antibody responses and increased lethality (93).
Yellow fever virus
A live-attenuated vaccine (YF strain 17D) was developed for yellow fever in the 1930s through serial passage of wild-type virus through chick embryos, inducing humoral immunity against the six currently known YF genotypes (64). The YF 17D vaccine has been one of the most successful vaccines developed to date, with an excellent safety record and conferring immunity for up to 35 years in as many as 99% of recipients (75,79). The effectiveness of this vaccine in humans and animal models has been attributed to the development of neutralizing antibodies against the E protein and complement-fixing antibodies against NS1 (9,75). In fact, active immunization with the E protein or NS1, or passive immunization using E- or NS1-specific monoclonal antibodies, was shown to protect mice and monkeys against lethal YF virus infection (9,78,89). Although it is known that the YF 17D vaccine induces long-lived humoral immunity, the exact immunological nature of this protection is not well understood. Similarly, while the nature and overall contribution of T-cell responses have yet to be fully elucidated, T cells have been shown to play a role in the protection generated after vaccination (21,80,100). Recent work using human volunteers showed a rapid primary CD8+ T-cell response following immunization with the YF 17D vaccine, which differentiated into a long-lived memory T-cell population (60).
Japanese encephalitis virus
A licensed vaccine has also been available for JEV since the 1950s. Developed in Japan, JE-VAX is an inactivated formulation prepared from whole virus grown on mouse brain (62). Again in the case of JEV, the E protein is the major target of neutralizing antibody (63), and protection is thought to be primarily antibody-mediated. Although neutralizing antibodies alone are sufficient to provide protection from infection (as seen by the killed vaccine), this observation does not rule out the potential importance of virus-specific T cells in immunity. Indeed, JEV-specific CTLs have been induced in mice following experimental infection (68), and the presence of T-helper, as well as regulatory T cells have been reported (49,58). The high serological cross-reactivity between JEV and DENV, as well as their overlap in global distribution, has significantly complicated diagnosis (15). At the amino acid level, 27–33% homology exists between the two viruses for the C protein, and 44–49% for the E protein. This level of homology suggests that group-specific determinants may exist that induce cross-reactive antibodies.
Tick-borne encephalitis virus, and Murray Valley, St. Louis encephalitis, and Louping ill viruses
Inactivated, whole-virus vaccines are currently produced in Russia, Austria, and Germany, covering the regions most greatly impacted by disease. Here again, the active immunological component of these formulations appears to be the E protein (3). And indeed, the E protein is believed to play a critical role in the induction of immunity to natural infection (38). Moreover, NS1 has also been shown to mediate protection against disease by inducing both humoral and cellular responses (41,96,97). Although there is no evidence that antibodies are associated with severe disease, non-neutralizing antibodies against E can enhance infectivity in mouse macrophages (72). Moreover, clinical studies of TBEV patients have revealed a correlation between low-level neutralizing antibodies and severe acute encephalitis (42).
Far less is known about MVEV and SLEV, despite the fact that before the introduction of WNV into North America, SLEV was the major cause of epidemic encephalitis in the U.S. (14). In addition, MVEV is endemic to Australia, causing sporadic epidemics of encephalitis in the heavily populated southeastern region. Instead, due to the relatedness among flaviviruses within the same antigenic group, much of what is understood about these viruses and their immunobiology appears to be extrapolated from more heavily studied viruses, like DENV, WNV, and JEV. However, two groups have identified MVEV peptides derived from the E protein that are recognized by CD4+ helper T cells, and elicit MVEV-specific antibody (55,84), suggesting a role for this antigen in MVEV immunity.
Inventory of Immune Epitope Database Epitope Data for all Flaviviruses
Flavivirus species and strain distribution
The IEDB contains data related to more than 1,900 structures derived from viruses within the Flavivirus genus, and were reported from more than 130 individual references. More than half of these structures (1,158 of 1,960) have been identified as either B- or T-cell epitopes defined in numerous host species (e.g., humans, mice, and non-human primates [NHP]). The remaining structures represent negative data relating to structures tested for reactivity and found to be non-immunogenic. Furthermore, it should be noted that in our analysis we have not included structures for which only MHC binding data in the absence of biological recognition is available, or structures in which synthetic peptides were used as both immunogen and antigen, and whole antigens/viruses were not utilized to either induce or detect responses.
To date, the IEDB houses immune epitopes from nine species within the Flavivirus genus: dengue viruses (DENV serotypes 1–4), JEV, WNV, KUN, YFV, MVEV, SLEV, TBEV, and LIV. Table 1 provides a summary breakdown of epitopes (total structures, positive structures, and negative structures) per group and/or serotype, and then classifies each of these as a B-cell or T-cell epitope.
Table 1.
Summary of Epitope Distribution Among All Flaviviruses
| Flavivirus species | Total structures in the IEDB | Total positive structures (epitopes) | Total negative structures | T-cell epitopes | Antibody epitopes |
|---|---|---|---|---|---|
| DENV 1 | 34 | 31 | 3 | 26 | 5 |
| DENV 2 | 854 | 501 | 353 | 122 | 379 |
| DENV 3 | 129 | 33 | 96 | 27 | 6 |
| DENV 4 | 37 | 28 | 9 | 22 | 6 |
| DENV total | 1,054 | 593 | 461 | 197 | 396 |
| WNV | 321 | 303 | 18 | 243 | 60 |
| JEV | 69 | 45 | 24 | 20 | 25 |
| MVEV | 26 | 19 | 7 | 6 | 13 |
| TBEV | 22 | 11 | 11 | 0 | 11 |
| YFV | 455 | 174 | 281 | 170 | 4 |
| SLEV | 9 | 9 | 0 | 0 | 9 |
| KUN | 3 | 3 | 0 | 3 | 0 |
| LIV | 1 | 1 | 0 | 0 | 1 |
| Totals | 1,960 | 1,158 | 802 | 639 | 519 |
Presented is the breakdown of epitopes according to virus and/or serotype and effector cell population. Total structures represent all peptides reported, both positive and negative. Negative data are used to identify non-reactive regions (non-epitopes) of tested antigens, and therefore are enormously valuable for use as control data. These data represent B- and T-cell responses only; MHC binding data were not included. Reactivity was defined only for epitopes identified using the whole organism or whole protein in combination with the peptide; data were excluded if both the immunizing agent and the assay antigen were epitope.
Perhaps not surprisingly, the largest number of epitopes has been defined for DENV. Nearly 600 epitopes (593 positive structures) have been reported for all four DENV serotypes, including multiple strains within each serotype. Among the four serotypes, DENV 2 dominates the epitope literature. DENV is followed distantly in number by WNV, for which 303 epitopes have now been reported. Many fewer epitopes have been reported for YF (174), JEV (45), MVEV (19), TBEV (11), SLEV (9), KUN (3), and LIV (1). The prominence of dengue virus and West Nile virus data in the epitope literature is likely a reflection of their overall worldwide impact on human disease, and the lack of vaccines. Conversely, the relatively smaller number of epitopes defined for the other viruses within the genus most likely reflects the presence of established prophylaxis and/or their more modest impact on morbidity and mortality globally.
Phenotype categories of Flavivirus epitopes
All Flavivirus epitopes reported to date in the IEDB are peptidic in nature. While the IEDB can capture non-peptidic epitopes (e.g., carbohydrate and lipid), our literature searches for this genus have not yielded references defining such epitopes, even though the structural and non-structural proteins of Flaviviruses are known to be glycosylated (e.g., E, NS1, and preM), all of the determinants defined to date have been strictly peptidic in nature. The identification of epitopes from the glycolic moiety of the most prominent antigens would be of interest as an area of further study.
B-cell epitopes have been identified in eight of the nine species of Flavivirus represented in the IEDB; however, the overall number of antibody epitopes varies widely among the different species (Table 1). Thus far, the greatest number of B-cell epitopes has been defined in dengue virus type 2 (379). This is in sharp contrast to the paucity of B-cell epitopes defined for the other three DENV serotypes (5 epitopes for DENV1 and 6 for DENV3 and DENV4). The number of B-cell epitopes described for DENV is followed distantly by the other Flaviviruses: WNV (60), JEV (25),TBEV (11), and MVEV (13), and with only a small number of B-cell epitopes being described in SLEV, YF, and LIV (9, 4, and 1 epitopes, respectively). Interestingly, a total of 95 discontinuous antibody epitopes have been reported to date. This number is greater than what has been reported in the past for pathogens in other meta-analysis. Most of these have been identified for either DENV2 or WNV. Of the reported antibody reactivities, only a very small fraction has included isotype information. Total IgG is reported in the majority of cases (DENV, WNV, JEV, and TBEV), followed by IgG1 and IgG2a (DENV, WNV, and JEV). Only 3 epitopes have been defined for IgG2b (WNV), and IgM (DENV, WNV, and JEV) (data not shown). These findings highlight a potential knowledge gap, as certain isotypes may be associated with protection from disease and others involved in, or associated with, immunopathology.
T-cell epitopes have been defined in six out of the nine Flaviviruses included in the IEDB (Table 1). The majority of T-cell epitopes have been identified for WNV (243), followed by DENV (197), again with DENV2 representing the largest proportion (122). Surprisingly, the dengue virus group did not represent the largest number of T-cell epitopes. Moreover, the overall distribution is somewhat skewed, with a disproportionate representation by the DENV 2 serotype. Yellow fever virus followed DENV with the greatest number of T-cell epitopes (170). Much smaller numbers have been reported for JEV (20), MVEV (6), and KUN (3). Thus far, no T-cell epitopes have been reported for SLEV, TBEV, or LIV.
For the Flavivirus T-cell epitopes currently recorded in the IEDB, we can also further specify the phenotype of the effector cell when provided by the authors or inferred from the assay used to measure the response (Table 2). The majority of DENV-specific T-cell epitopes are recognized by CD8+ T cells (97). This result is not surprising given the presumed role of CD8+ T cells in viral clearance and their potential role in immunopathology (although CD4+ T cells have also been implicated in the latter). Only 46 of the 197 T-cell epitopes described for DENV were recognized by CD4+, and another 54 were of an unspecified phenotype. Unspecified T-cell phenotype is a common finding in our meta-analyses, even among pathogens for which great attention has been paid to epitope mapping. This is due, in large part, to the routine use of peripheral blood mononuclear cells and/or splenocytes in assays in which the assignment of phenotype is ambiguous. This observation highlights a need for better resolution of effector cell phenotype and their role in viral clearance or immune pathogenesis.
Table 2.
Enumeration of B- and T-cell Epitopes by Flavivirus Species
| Flavivirus species | Linear B cell | Discontinuous B cell | T cell (unspecified) | CD4+/class II | CD8+/class I |
|---|---|---|---|---|---|
| DENV 1 | 3 | 2 | 0 | 9 | 17 |
| DENV 2 | 343 | 36 | 53 | 14 | 55 |
| DENV 3 | 2 | 4 | 0 | 13 | 14 |
| DENV 4 | 5 | 1 | 1 | 10 | 11 |
| WNV | 35 | 25 | 2 | 145 | 96 |
| JEV | 20 | 5 | 2 | 13 | 5 |
| MVEV | 12 | 1 | 1 | 1 | 4 |
| YFV | 0 | 4 | 0 | 120 | 50 |
| SLEV | 0 | 9 | 0 | 0 | 0 |
| TBEV | 4 | 7 | 0 | 0 | 0 |
| KUN | 0 | 0 | 0 | 0 | 3 |
| LIV | 0 | 1 | 0 | 0 | 0 |
| Totals | 424 | 95 | 59 | 325 | 255 |
The total number of antibody/B-cell and T-cell epitopes is enumerated according to virus and/or serotype (DENV1–4). The term “unspecified T cells” refers to effector cells identified generally by the authors as T cells, peripheral blood mononuclear cells, lymph node cells, lymphocytes, or splenocytes, and not according to subset. CD4+ and CD8+ T-cell assignments were made, when possible, by inference from the assay type (i.e., proliferation = CD4 or CTL = CD8). Antibody epitopes are broken down by type: linear or discontinuous (conformational).
Of the other Flaviviruses for which T-cell epitopes were described, a majority of CD4+ reactivity was reported for WNV (145); though numerous CD8+ epitopes were also described for this virus (96). A relatively unequal distribution of epitopes were reported for YFV (120 CD4+ and 50 CD8+), and 1 CD4+ and 4 CD8+ epitopes were described for MVEV. The small number of T-cell epitopes described for JEV was recognized by class II (13 CD4+ versus 5 CD8+). Only 3 CD8+ epitopes were reported for KUN.
MHC restriction (human and murine) of T-cell reactivity has also been captured by the IEDB for 6 of the 9 species within the Flavivirus genus (Table 3). The majority of MHC alleles that have been identified as binding Flavivirus peptides are associated with human infections from the dengue virus group (DENV 1, 2, 3, and 4), West Nile virus, and Yellow Fever virus, with a fairly broad distribution of human HLA-A and HLA-B alleles/serotypes represented. Human HLA-C alleles have not been identified, and class I alleles from several common laboratory mouse strains are also absent. While a few alleles/serotypes have been reported for JEV, KUN, and MVEV, these include only murine MHC types and no human data. No MHC restriction was reported for SLEV or TBEV T-cell epitopes. A broader distribution of class II alleles for either humans or rodents is also lacking. Thus far, only a small number of HLA-DR or -DP restricted epitopes have been defined. Defining the MHC restriction of T-cell epitopes in both humans and mice would facilitate a more detailed characterization of Flavivirus immunity and immunopathology.
Table 3.
MHC Allele Distribution Among Reported Flavivirus Species
| DENV 1 | DENV 2 | DENV 3 | DENV 4 | JEV | WNV | KUN | YF | MVEV |
|---|---|---|---|---|---|---|---|---|
| MHC class I | ||||||||
| A*1101 | A*1101 | A*1101 | A*1101 | A*0201 | A*0201 | |||
| A*11 | A*11 | A*11 | A*11 | A*2402 | A*2402 | |||
| A2 | A2 | A2 | A2 | A1 | A26 | |||
| A24 | A24 | A24 | A24 | A2 | B*0702 | |||
| B*5502 | B*3501 | B35 | B*3501 | B*0702 | B*3502 | |||
| B35 | B60 | B35 | B35 | B27 | ||||
| B62 | B62 | B7 | Cw3 | |||||
| B7 | B7 | |||||||
| B8 | ||||||||
| H-2-d class I | H-2-Kd | H-2-Kd | H-2-Kk | H-2-Kk | H-2-Kk | H-2-Kk | H-2-Kk | |
| H-2-Db | H-2-Kd | H-2-Kb | H-2-Kd | H-2-Kb | H-2-Kd | |||
| H-2-Kk | H-2-d | H-2-Kd | H-2-Db | H-2-d | ||||
| H-2-Kb | H-2-Db | |||||||
| H-2-Kd | ||||||||
| H-2-Ld | ||||||||
| MHC class II | ||||||||
| DP9 | DPw2 | DPw2 | DPw2 | DRB1*1501 | DRB1*0301 | |||
| DPw2 | DPw4 | DPw4 | DPw4 | DRB1*0301 | DRB1*0401 | |||
| DPw4 | DRB1*1501 | DR15 | DR1 | DRB1*0401 | DRB1*1501 | |||
| DQ1 | DRB1*1501 | DRB1*1501 | ||||||
| DQ5 | H-2-IAb | H-2-IAb | H-2-b | |||||
| DQ6 | H-2-k | |||||||
| DQ7 | ||||||||
| DQ8 | ||||||||
| DR1 | ||||||||
| DR15 | ||||||||
| DRB1*1501 | ||||||||
MHC restriction was reported for 6 of 9 species: DENV 1, DENV 2, DENV 3, DENV 4, JEV, WNV, KUN, YF, and MVEV, and include human and murine alleles only. No MHC restriction was reported for TBEV or SLEV. MHC-restricting alleles are classified by class I and class II. Human data are listed first, followed by mouse data.
Host distribution of the epitope reactivities
We investigated the overall distribution of Flavivirus epitopes among all relevant host species. For Flavivirus, these host species include: humans (from different geographic regions), mice, rabbits, horses, pigs, and NHP. The majority of epitopes reported for all viruses within this genus have been defined in either human or murine hosts (Tables 4a–d). For DENV, the majority of epitopes were derived from humans (445), followed by mice (294 from 9 strains), and then rabbits (15). Only two epitopes have been defined in a non-human primate species (both B-cell epitopes of the E protein). Given the genetic and immunological relevance of NHP species to humans, and therefore their potential utility as animal models of disease, a greater focus on Flavivirus epitope research using NHPs would be beneficial. Conversely, the majority of WNV epitopes have been defined in mice (321), followed closely by humans (227), with 34 epitopes defined in horses. The ratio of human to mouse epitope data is surprising, given the prevalence of this virus in human neuroinvasive disease in the Western hemisphere and the lack of a vaccine. However, a majority of the mouse data were generated in human HLA-transgenic mouse strains.
Table 4a.
Epitope Distribution Among Host Species (Dengue Virus)
| Host species | Total | B-cell linear | B-cell discontinuous | T-cell (total) | T-cell (unspecified) | CD4+/class II | CD8+/class I |
|---|---|---|---|---|---|---|---|
| Humans | 445 | 277 | 3 | 165 | 41 | 48 | 76 |
| Chimpanzees | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| Mouse, unspecified | 167 | 85 | 41 | 37 | 13 | 5 | 19 |
| BALB/c | 85 | 56 | 7 | 18 | 13 | 0 | 5 |
| C3H/HeJ | 2 | 0 | 0 | 2 | 2 | 0 | 0 |
| CBA/Ca | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| NHI Swiss | 19 | 19 | 0 | 0 | 0 | 0 | 0 |
| Outbred | 11 | 11 | 0 | 0 | 0 | 0 | 0 |
| Transgenic mice | |||||||
| HLA-DR2 Tg | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| HLA-DR3 Tg | 3 | 0 | 0 | 3 | 0 | 3 | 0 |
| HLA-DR4 Tg | 4 | 0 | 0 | 4 | 0 | 4 | 0 |
| Rabbit, unspecified | 11 | 11 | 0 | 0 | 0 | 0 | 0 |
| New Zealand Whites | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
Epitopes are enumerated by Flavivirus species, by host species, and then by effector cell phenotype. Murine hosts have been separated into inbred/outbred and transgenic species. Note that the total number of pathogen-specific epitopes per host will not necessarily equal those numbers generated in Table 1, as the same epitope (aa sequence) can be identified in multiple hosts, and is therefore counted more than once. In Table 1 unique molecular structures are counted only once.
Table 4d.
Epitope Distribution Among Host Species (MVEV, TBEV, YFV, SLEV, KUN, and LIV)
| Host species | Total | B-cell linear | B-cell discontinuous | T-cell (total) | T-cell (unspecified) | CD4+/class II | CD8+/class I |
|---|---|---|---|---|---|---|---|
| MVEV | |||||||
| Mouse, unspecified | 11 | 11 | 0 | 0 | 0 | 0 | 0 |
| BALB/c | 11 | 6 | 1 | 4 | 1 | 1 | 2 |
| C57BL/6 | 3 | 2 | 0 | 1 | 0 | 1 | 0 |
| CBA/Ca | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| TBEV | |||||||
| Mouse, unspecified | 8 | 1 | 7 | 0 | 0 | 0 | 0 |
| BALB/c | 3 | 3 | 0 | 0 | 0 | 0 | 0 |
| YFV | |||||||
| Humans | 131 | 0 | 1 | 130 | 0 | 101 | 29 |
| Mouse, unspecified | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| BALB/c | 39 | 0 | 0 | 39 | 0 | 21 | 18 |
| C57BL/6 | 3 | 0 | 0 | 3 | 0 | 1 | 2 |
| CBA/Ca | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| HLA-A2 Tg | 3 | 0 | 0 | 3 | 0 | 0 | 3 |
| HLA-A24 Tg | 15 | 0 | 0 | 15 | 0 | 0 | 15 |
| HLA-B7 Tg | 6 | 0 | 0 | 6 | 0 | 0 | 6 |
| HLA-DR2 Tg | 24 | 0 | 0 | 24 | 0 | 24 | 0 |
| HLA-DR3 Tg | 45 | 0 | 0 | 45 | 0 | 45 | 0 |
| HLA-DR4 Tg | 39 | 0 | 0 | 39 | 0 | 39 | 0 |
| SLEV | |||||||
| Mouse, unspecified | 9 | 0 | 9 | 0 | 0 | 0 | 0 |
| KUN | |||||||
| BALB/c | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| CBA/Ca | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| LIV | |||||||
| Mouse, unspecified | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
Epitopes are enumerated by Flavivirus species, by host species, and then by effector cell phenotype. Murine hosts have been separated into inbred/outbred and transgenic species. Note that the total number of pathogen-specific epitopes per host will not necessarily equal those numbers generated in Table 1, as the same epitope (aa sequence) can be identified in multiple hosts, and is therefore counted more than once. In Table 1 unique molecular structures are counted only once.
Table 4b.
Epitope Distribution Among Host Species (West Nile Virus)
| Host species | Total | B-cell linear | B-cell discontinuous | T-cell (total) | T-cell (unspecified) | CD4+/class II | CD8+/class I |
|---|---|---|---|---|---|---|---|
| Humans | 227 | 1 | 4 | 222 | 2 | 138 | 82 |
| Mouse, unspecified | 10 | 0 | 10 | 0 | 0 | 0 | 0 |
| BALB/c | 16 | 1 | 12 | 3 | 0 | 1 | 2 |
| C57BL/6 | 17 | 0 | 3 | 14 | 0 | 6 | 8 |
| CBA/Ca | 4 | 0 | 0 | 4 | 0 | 0 | 4 |
| Transgenic mice | |||||||
| HLA-DR2 Tg | 50 | 0 | 0 | 50 | 0 | 50 | 0 |
| HLA-DR3 Tg | 38 | 0 | 0 | 38 | 0 | 38 | 0 |
| HLA-DR4 Tg | 38 | 0 | 0 | 38 | 0 | 38 | 0 |
| HLA-A2 Tg | 80 | 0 | 0 | 80 | 0 | 40 | 40 |
| HLA-A24 Tg | 48 | 0 | 0 | 48 | 0 | 24 | 24 |
| HLA-B7 Tg | 20 | 0 | 0 | 0 | 0 | 10 | 10 |
| Horses | 34 | 33 | 1 | 0 | 0 | 0 | 0 |
Epitopes are enumerated by Flavivirus species, by host species, and then by effector cell phenotype. Murine hosts have been separated into inbred/outbred and transgenic species. Note that the total number of pathogen-specific epitopes per host will not necessarily equal those numbers generated in Table 1, as the same epitope (aa sequence) can be identified in multiple hosts, and is therefore counted more than once. In Table 1 unique molecular structures are counted only once.
Table 4c.
Epitope Distribution Among Host Species (Japanese Encephalitis Virus)
| Host species | Total | B-cell linear | B-cell discontinuous | T-cell (total) | T-cell (unspecified) | CD4+/class II | CD8+/class I |
|---|---|---|---|---|---|---|---|
| Humans | 8 | 8 | 0 | 0 | 0 | 0 | 0 |
| Chimpanzee | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Mouse, unspecified | 5 | 4 | 1 | 0 | 0 | 0 | 0 |
| BALB/c | 22 | 4 | 1 | 17 | 1 | 13 | 3 |
| C3H/He | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| C3H/HeJ | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| FVB/J | 3 | 3 | 0 | 0 | 0 | 0 | 0 |
| CBA/Ca | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| Swiss albino | 6 | 3 | 0 | 3 | 3 | 0 | 0 |
| Pig, unspecified | 3 | 2 | 0 | 1 | 1 | 0 | 0 |
| Pig, Yorkshire | 2 | 1 | 0 | 1 | 1 | 0 | 0 |
Epitopes are enumerated by Flavivirus species, by host species, and then by effector cell phenotype. Murine hosts have been separated into inbred/outbred and transgenic species. Note that the total number of pathogen-specific epitopes per host will not necessarily equal those numbers generated in Table 1, as the same epitope (aa sequence) can be identified in multiple hosts, and is therefore counted more than once. In Table 1 unique molecular structures are counted only once.
The relatively small number of human T- and B-cell epitopes described for JEV (8) was also unexpected. As mentioned above, this lack of data is likely due to the existence of the JEV vaccine, and therefore the relatively lower epitope-specific research effort for this particular pathogen. Moreover, while mouse data do exist for this species, the total numbers seem low given the overall significance of this virus. JEV shows a total of 40 murine epitopes (from 7 strains). The data for YF virus are a bit more impressive: a total of 131 epitopes have been defined in humans, and 179 epitopes have been identified using 10 murine strains (6 human HLA transgenic). For MVEV, TBEV, SLEV, and LIV, only B-cell epitopes defined in murine species have been reported to date (27, 11, 9, and 1, respectively). For KUN, all 3 T-cell epitopes were also defined in mice. Note that the total number of pathogen-specific epitopes per host will not necessarily equal those numbers generated in Table 1, as the same epitope (aa sequence) can be identified in multiple hosts, and is therefore counted more than once. In Table 1 unique molecular structures are counted only once.
Genomic distribution of defined epitopes
The Flavivirus genome is composed of a linear, single-stranded positive-sense RNA molecule. This genomic RNA is translated into a single polyprotein precursor comprising only one ORF, and containing approximately 3,400 amino acids. The polyprotein is co-translationally processed by host- and virus-specific proteases to yield 10 individual proteins: three structural proteins (capsid [C], membrane [prM/M], and envelope [E]); and seven non-structural proteins involved primarily in replication (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5).
We have mapped the reported epitope reactivity among the structural and non-structural proteins encoded by the Flavivirus genome (Table 5). Epitopes have been reported from all 10 of the Flavivirus virus proteins; however, comprehensive data for all 10 are not available for any one virus. The most comprehensive distribution of epitope data is for DENV and WNV. Not surprisingly, the greatest number of epitopes described for the genus as a whole has been derived from the E glycoprotein (576) (both B- and T-cell, mostly CD4+), followed by NS3 (almost exclusively CD4+ and CD8+ T cells) (209). These observations are in accordance with information regarding dengue immunobiology from the literature, suggesting that NS3 and E are major targets of immune responses to dengue virus (73).
Table 5.
Epitope Mapping Among Flavivirus Proteins
| C | prM/M | E | NS1 | NS2a | NS2b | NS3 | NS4a | NS4b | NS5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| DENV | 20 | 11 | 368 | 42 | 3 | 2 | 103 | 11 | 14 | 14 |
| WNV | 2 | 8 | 95 | 10 | 10 | 7 | 60 | 6 | 16 | 20 |
| JEV | 7 | 2 | 19 | 6 | 1 | 0 | 6 | 0 | 0 | 0 |
| MVEV | 0 | 0 | 13 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| TBEV | 0 | 0 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| YFV | 8 | 0 | 61 | 9 | 7 | 6 | 34 | 5 | 2 | 37 |
| SLEV | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KUN | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| LIV | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Totals | 37 | 21 | 576 | 68 | 21 | 15 | 209 | 22 | 32 | 71 |
The Flavivirus genomic RNA is translated into a single ORF polyprotein that is post-translationally processed into 10 viral proteins: 3 structural (C, prM/M, and E), and 7 non-structural (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). The total number of epitopes mapped per protein is indicated for each virus. The totals represent the relative distribution of epitopes among the antigens in the Flavivirus genome. However, the data are not comprehensive (all proteins not equally tested in the literature), therefore the data presented here cannot be considered indicative of immune dominance. Note that the total number of epitopes per protein for each species will not necessarily equal the totals generated in Table 1, as each epitope structure is counted only once if the same sequence has been called both CD4 and CD8, or both T-cell and B-cell. In Table 1 unique molecular structures are counted only once, but that table does take into account the phenotype, not just the structure.
Two Flaviviruses, DENV and WNV, dominate the epitope literature, and as noted above, only two of the 10 potentially antigenic proteins stand out prominently in the epitope data. While the emphasis on these two viruses was not unexpected given their impact on global disease burden and lack of vaccine, the narrow focus of epitope mapping was unexpected. Seemingly absent are T- and B-cell epitopes derived from the C and preM/M proteins. Indeed, these proteins represent conserved structural antigens involved in viral replication; moreover, the M protein, which is a glycoprotein known target of antibody responses, has been previously identified as a protective antigen in mice (102). Together, epitopes from C and prM/M were defined in less than half of the viruses. Of the non-structural proteins, more epitopes were expected to have been defined for NS1, as it has also been identified as a target of protective humoral responses (89). However, epitopes were mapped to this antigen in only 5 of the 9 species. While we did expect to find that viruses for which vaccines existed would have a lower number of reported epitopes, we did not anticipate the degree to which this would be true. Indeed, a fuller understanding of the mechanisms involved in vaccine-generated (active) immunity at the epitope level for these successfully managed viruses, may provide critical insights for the challenges facing DENV and WNV vaccine development.
Epitope data associated with different Flavivirus disease states
Clinical manifestations of Flavivirus infection in humans range from self-limiting febrile illness to hemorrhagic syndromes to severe neuroinvasive disease (34,85). The characterization of epitope reactivity in different disease states can be used to gain a better understanding of the mechanisms and players involved in protective versus pathological immunity. Such information may help distinguish reactivity consistent with severe disease from uncomplicated disease resolution, and help discern certain aspects of host- and pathogen-mediated outcome. When available, detailed information pertaining to clinical disease state (e.g., DF, DHF, and YF), and stage (acute, post, or chronic) is captured within the IEDB, along with other data detailing the target population (age, sex, geographic location, and MHC type). We have compiled data representing all Flavivirus epitopes associated with defined human disease states (Table 6). These disease states included: dengue fever (DF), dengue hemorrhagic fever (DHF), yellow fever (YF), Japanese encephalitis (JE), and West Nile fever (WNF).
Table 6.
Human Disease States
| Disease | B cell | T cell | Total |
|---|---|---|---|
| Dengue fever | 265 | 40 | 305 |
| Dengue hemorrhagic fever | 22 | 69 | 91 |
| West Nile fever | 4 | 22 | 26 |
| Japanese encephalitis | 20a | 1 | 21 |
| Yellow fever | 1 | 0 | 1 |
These 20 antibody epitopes were defined for dengue virus from individuals with Japanese encephalitis (see Table 9d).
Flavivirus disease states include dengue fever, dengue hemorrhagic fever, West Nile fever, Japanese encephalitis, and yellow fever. No data were reported for St. Louis encephalitis, Murray Valley/Australian encephalitis, or tick-borne encephalitis. Note that these data do not include individuals that may be considered exposed without evidence of disease (seropositive, from an endemic region), but these data do exist in the IEDB and can be reported separately.
Thus far, the vast majority of epitopes are defined for DF (305) versus DHF (91), in a ratio of approximately 3:1. The majority of epitopes defined for DF are B-cell epitopes (265), whereas the bulk of epitopes defined for DHF are T-cell epitopes (69, mostly CD8+ or unspecified T-cell). This finding is consistent with observations in the literature that suggest that neutralizing antibodies play a critical role in the normal course of DF disease, and further reflects the notion that T-cell dysfunction is thought to be responsible for the immunopathology leading to DHF. However, this does not mean that antibodies are not important in DHF, or that CD8+ T cells do not play a role in DF; it is just a reflection of the focus of the epitope literature to date. In addition, the overall role of T cells in DENV infection has not been fully examined. A total of 21 epitopes have been defined in individuals with Japanese encephalitis, the majority of which are B-cell epitopes. However, these epitopes were mapped to DENV using B cells taken from JEV-infected individuals (DENV exposure was not addressed), thus defining potential cross-reactivity. This study likely reflects the fact that several viruses within this genus are co-endemic for vast regions around the world, and that co-infection may be quite common. Surprisingly, only a relatively small number of epitopes have been reported for humans with West Nile fever (26), and none of these describe severe neuroinvasive disease. Another unexpected finding was that only one epitope has been defined for YF. To date, no epitopes have been described for SLEV, MVEV, or TBEV during clinical disease in humans. We conclude from these data that the mapping of epitope reactivity during clinical disease in humans should be a priority for future studies, with an emphasis on those viruses for which immunopathology may be manifested at the epitope level.
Epitope data from different geographic regions
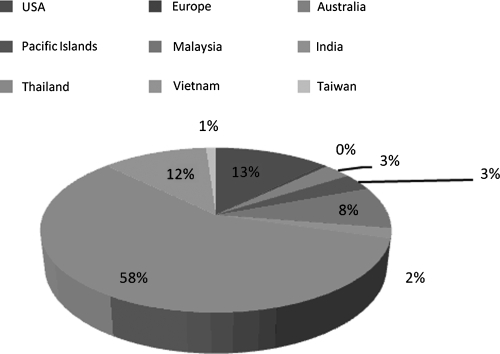
The broad distribution of flaviviruses around the globe, genomic and protein sequence homology among viral species, and overlapping endemicities, are some of the greatest challenges to studying this group of viruses. Indeed, the insect vectors responsible for harboring Flavivirus disease are found on nearly every continent in the world. When available, the IEDB records the geographic residence of the sampled populations. This information can then be used to help gain insight into relative population coverage of epitope identification. Such information was collected for dengue virus epitopes, since detailed human data are available for this virus. Figure 1 shows the relative distribution of epitopes (B and T) defined from populations living in different geographical regions, including North America (U.S.), Europe, India, and countries in Southeast Asia. As shown in the figure, the vast majority of epitopes have been defined for populations (adults and children) living in Thailand, followed by Vietnam; and the number of T-cell epitopes far exceeds that of B-cell epitopes (data not shown). The largest number of B-cell epitopes has been defined in people living in Malaysia. Epitopes defined in Western populations (the U.S. and Europe) are all representative of experimental vaccine trials. The predominance of DENV epitopes defined in populations living in Southeast Asia is not surprising, as this was the first region in which severe disease due to dengue was reported.
FIG. 1.
Epitope distribution among different geographic regions for dengue virus. Epitopes were reported from multiple endemic and non-endemic populations around the world, including the United States (13%), Europe (0%), Australia (3%), the Pacific Islands (3%), Malaysia (8%), India (2%), Thailand (58%), Vietnam (12%), and Taiwan (1%). This pie chart shows the relative percentage of regions explicitly stated in the patient histories (these data are not available in all references).
Epitopes associated with protection in animal models
In general, B- and T-cell epitopes associated with protection and/or protective immunity can be identified using animal models in which the epitopes are used to immunize against a pathogen challenge. These types of experiments are rarely feasible in humans. Therefore the identification of immune epitopes with protective activity in humans has to rely on animal models, including HLA transgenic mice; studies utilizing in vitro assays correlated with in vivo protection, such as neutralizing antibody titers, or correlated the pattern of epitope recognition with different clinical outcomes, which is discussed in the following section.
Animal models are essential for performing studies aimed at examining the mechanisms underlying immune protection and disease pathogenesis. A review of the literature shows that numerous animal models have been used to study different aspects of Flavivirus infection, including hamsters (YF, WNV, SLEV, and JEV), mice (all), rabbits (JEV), guinea pigs (JEV), piglets (JEV), dogs (TBEV), and NHP (DENV, YF, WNV, JEV, and SLEV) (17,95,110). However, to date, no one model adequately represents all manifestations of disease, making it difficult to fully characterize many aspects of immunobiology and pathogenesis. Much of this difficulty lies with the lack of host susceptibility to viral infection (replication), and the subsequent development of clinical disease.
While murine models manifest certain elements of Flavivirus disease (neuropathology, hematological changes, and even death), mice are in many cases resistant to infection and of limited value in the study of protective capacity, and conversely immunopathogenesis, for some flaviviruses. For example, wild-type mice (common lab strains) are susceptible to infection with WNV and develop neuropathology; however, most common lab strains are resistant to infection with DENV. Therefore researchers have made use of mouse-adapted or engineered virus strains in immunocompetent mice, chimeric mice transplanted with susceptible human cells (humanized), or severely immunocompromised mouse strains (7,17,18,88,100,111). Development of a genetically tractable murine model in which relevant immune parameters can be controlled would represent a key advance in the study of Flavivirus immunity and immune-mediated pathogenesis.
At the other end of the spectrum, NHPs (monkeys) are considered natural hosts for DENV (104) and YF (61) as part of the sylvatic cycle. Moreover, these animals, when experimentally infected, develop viremia and some degree of virus-specific immunity; however, results vary with the viral species used. Evidence of DENV-induced disease and demonstrable hematological changes are mostly lacking (36), and in the case of YF, viral pathogenesis is more severe than that seen in humans, with much higher mortality (62). So while NHP models represent genetically and immunologically relevant species, their use for studying Flavivirus immunobiology is limited.
Table 7 shows epitopes associated with protection as measured in vivo after live challenge in mice. For DENV, three B-cell epitopes have been associated with survival after lethal challenge with DENV 2 (A15 strain) in BALB/c mice (102). All of these epitopes were derived from the preM/M protein of DENV 2: preM (117–145), M (71–106), and M (183–207). These same epitopes/regions have not been identified in humans in the course of natural infection (see Table 9 below). For JEV, four B-cell and one T-cell epitope were reported. Antibody-mediated protection was observed using epitopes derived from the E protein [E (667–693), E (39–48), E (151–163), and E (334–343)] in BALB/c and FVB/J mice (24). T-cell-mediated protection was seen following immunization with M (17–26). Only one B-cell epitope has been defined as protective against TBEV challenge in mice: NS1 (813–831). As expected, no Flavivirus epitopes have been used to immunize humans. This analysis highlights a relative lack of protective epitope data in the current literature for all host species (natural and experimental), and suggests a need for further work in this area.
Table 7.
Epitopes Associated with Protection As Measured In Vivo After Live Challenge
| Epitope sequence | Antigen/position | Host organism | Response measured |
|---|---|---|---|
| Dengue virus–antibody epitopes | |||
| LTTRNGEPHMIVMRQEKGKSLLFKTGDGV | D2 prM (117–145) | Mouse (BALB/c) | Survival after challenge (D2) |
| RQNEPEDIDCWCNSTSTWVTYGTCTTTGEHRREKRS | D2 M (71–106) | Mouse (BALB/c) | Survival after challenge (D2) |
| NSTSTWVTYGTCTTTGEHRREKRSV | D2 M (183–207) | Mouse (BALB/c) | Survival after challenge (D2) |
| Japanese encephalitis virus–antibody epitopes | |||
| EMEPPFGDSYIVVGRGDKQINHHWHKA | Env (667–693) | Mouse (FVB/J) | Survival after challenge |
| PTLDVRMINI | Env (39–48) | Mouse (BALB/c) | Survival after challenge |
| NHGNYSAQVGASQ | Env (151–163) | Mouse (BALB/c) | Survival after challenge |
| TLDVRMINIE | Env (334–343) | Mouse (BALB/c) | Survival after challenge |
| Japanese encephalitis virus–T-cell epitope | |||
| EAWLDSTKAT | M (17–26) | Mouse (BALB/c) | Survival |
| Tick-borne encephalitis virus–antibody epitope | |||
| ETLGALASAIKETFEEGTC | NS1 (813–831) | Mouse (BALB/c) | Survival after challenge |
Epitopes are shown according to virus, and include the epitope phenotype, the epitope sequence, antigen/position, host organism (challenged organism), and response measured. Mouse strain and DENV serotype are shown in parentheses (D2). Flavivirus source protein data come directly from the NCBI and are therefore reported as “genome polyprotein” in some cases, and as the specified protein (e.g., NS3 or Env) in others. These assignments are not made by the IEDB. Variation in nomenclature and epitope position is therefore expected.
Table 9a.
T-cell Reactivity Following Natural Infection in Humans (DENV)
| Infecting virus | Epitope sequence | Antigen/position | Response | Phenotype |
|---|---|---|---|---|
| DENV 1 | QYSDRRWCF | NS3 (557–565) | T cell | CD8 |
| KPWDVIPMV | NS5 (2822–2830) | T cell | CD8 | |
| EMAEALKGMPIRYQT | NS3 (231–245) | T cell | CD8 | |
| KLVMAFIAFLRFL | C (45–57) | T cell | CD4 | |
| NREGKIVGLYGNGVV | NS3 (141–155) | T cell | CD4 | |
| SSIGKMFEATARG | E (396–408) | T cell | CD4 | |
| YRILQRGLLGRSQ | NS3 (23–35) | T cell | CD4 | |
| LDNINTPEGIIPALFEPERE | NS3 (496–515) | T cell | ND | |
| NYADRRWCF | NS3 (556–564) | T cell | ND | |
| LRGEARKTFVELMRR | NS3 (526–540) | T cell | ND | |
| TIENTTANISLTAIA | NS4b (2300–2314) | T cell | ND | |
| WHYDEDNPYKTWAYHGSYEV | NS5 (2784–2803) | T cell | ND | |
| DENV 2 | INYADRRWCF | NS3 (556–564) | T cell | CD8 |
| ILGDTAWDFG | E (96–105) | T cell | CD8 | |
| PFNMLKRERNRVSTVQQLTK | C (12–31) | T cell | ND | |
| RVSTVQQLTKRFSLGMLQGR | C (22–41) | T cell | ND | |
| TAGILKRWGTIKKSKANVL | C (62–81) | T cell | CD4 | |
| IKKSKAINVLRGFRKEIGRM | C (72–91) | T cell | CD4 | |
| LGELCEDTITYKCPLLRQNE | PreM/M (41–60) | T cell | ND | |
| MSSEGAWKHVQRIETWILRH | PreM/M (111–130) | T cell | ND | |
| QRIETWILRHPGFTMMAAI | PreM/M (121–139) | T cell | ND | |
| SGGSWVDIVLEHGSC | E (16–30) | T cell | ND | |
| FVEGVSGGSWVDIVL | E (11–25) | T cell | ND | |
| LRKYCIEAKLTNTTT | E (56–70) | T cell | ND | |
| TLVTFKNPHAKKQDV | E (236–250) | T cell | ND | |
| VTMECSPRTGLDFNE | E (181–195) | T cell | ND | |
| MENKAWLVHRQWFLD | E (201–215) | T cell | ND | |
| TFHTMWHVTRGAVLM | NS3 (45–59) | T cell | CD4 | |
| IEPSWADVKKDLISY | NS3 (65–79) | T cell | CD8 | |
| ADVKKDLISYGGGWK | NS3 (70–84) | T cell | CD8 | |
| AVSLDFSPGTSGSPI | NS3 (125–139) | T cell | CD8 | |
| FSPGTSGSPIIDKKG | NS3 (130–144) | T cell | CD8 | |
| KVVGLYGNGVVTRSG | NS3 (145–159) | T cell | CD4 | |
| TKRYLPAIVREAIKR | NS3 (200–214) | T cell | CD4 | |
| GLRTLILAPTRVVAA | NS3 (215–229) | T cell | ND | |
| ILAPTRVVAAEMEEA | NS3 (220–234) | T cell | CD8 | |
| EMEEALRGLPIRYQT | NS3 (230–244) | T cell | CD8 | |
| LRGLPIRYQTPAIRA | NS3 (235–249) | T cell | ND | |
| IRYQTPAIRAEHTGR | NS3 (240–254) | T cell | ND | |
| EHTGREIVDLMCHAT | NS3 (250–264) | T cell | CD4 | |
| EIVDLMCHATFTMRL | NS3 (255–269) | T cell | CD4 | |
| LSPVRVPNYNLIIMD | NS3 (270–284) | T cell | ND | |
| VPNYNLIIMDEAHFT | NS3 (275–289) | T cell | ND | |
| LIIMDEAHFTDPASI | NS3 (280–294) | T cell | ND | |
| EAHFTDPASIAARGY | NS3 (285–299) | T cell | CD8 | |
| EMGEAAGIFMTATPP | NS3 (305–319) | T cell | ND | |
| AGIFMATATPPGSRDP | NS3 (1785–1799) | T cell | ND | |
| KKVIQLSRKTFDSEY | NS3 (380–394) | T cell | ND | |
| NDWDFVVTTDISEMG | NS3 (400–414) | T cell | ND | |
| LDNINTPEGIIPSMF | NS3 (495–509) | T cell | CD8 | |
| TPEGIIPSMFEPERE | NS3 (500–514) | T cell | CD8 | |
| GDLPVWLAYRVAAEG | NS3 (540–554) | T cell | ND | |
| INYADRRWCFDGVKN | NS3 (555–569) | T cell | ND | |
| EGERKKLKPRWLDAIY | NS3 (2060–2076) | T cell | ND | |
| KLKPRWLDARIYSDP | NS3 (590–604) | T cell | ND | |
| WLDARIYSDPLALKE | NS3 (595–609) | T cell | ND | |
| LATVTGGIFLFLMSGRGIGK | NS4a (61–80) | T cell | ND | |
| RGFRKEIGRMLNILNRRRS | C (82–100) | T cell | CD4 | |
| KKDQVVVLGSQEGAM | E (526–540) | T cell | ND | |
| TVWFVPSIK | NS3 (358–366) | T cell | CD8 | |
| KLAEAIFKL | NS5 (3054–3062) | T cell | ND | |
| YILRDVSKK | NS5 (517–525) | T cell | CD8 | |
| DVFFTPPEK | NS5 (2622–2630) | T cell | CD8 | |
| EALRGLPIR | NS3 (233–241) | T cell | CD8 | |
| NYADRKWCF | NS3 (2031–2039) | T cell | CD8 | |
| RVSTVQQLTK | C (22–31) | T cell | ND | |
| SLVLVGVVTL | E (478–487) | T cell | ND | |
| ELERAADVK | NS2b (15–23) | T cell | ND | |
| ILIRTGLLVI | NS2b (1442–1451) | T cell | ND | |
| RIKQKGIL | NS3 (1499–1506) | T cell | CD8 | |
| RIEPSWADVK | NS3 (1539–1548) | T cell | ND | |
| AIKRGLRTL | NS3 (1686–1694) | T cell | ND | |
| DKKGKVVGL | NS3 (1616–1624) | T cell | CD8 | |
| RVIDPRRCMK | NS3 (1896–1905) | T cell | ND | |
| ALSELPETL | NS4a (2137–2145) | T cell | ND | |
| LLLLTLLATV | NS4a (2148–2157) | T cell | ND | |
| LLLTLLATV | NS4a (56–64) | T cell | ND | |
| LEKTKKDL | NS4b (2249–2256) | T cell | CD8 | |
| AIIGPGLQAK | NS4b (2362–2371) | T cell | ND | |
| VLNPYMPSV | NS5 (2673–2681) | T cell | ND | |
| KITAEWLWK | NS5 (2866–2874) | T cell | ND | |
| DENV 3 | GESRKTFVE | NS3 (211–220) | T cell | ND |
| DENV 4 | LAPTRVVAAEME | NS3 (221–232) | T cell | ND |
| EIVDLMCHAT | NS3 (255–264) | T cell | CD4 | |
| DENV, unspecified | GTSGSPIADKK | NS3 (1608–1618) | T cell | ND |
| GTSGSPIIDK | NS3 (133–143) | T cell | ND | |
| GTSGSPIIDKK | NS3 (133–143) | T cell | ND | |
| GTSGSPIINR | NS3 (133–142) | T cell | CD8 | |
| GTSGSPIINRK | NS3 (133–143) | T cell | ND | |
| GTSGSPIVDR | NS3 (1608–1617) | T cell | CD8 | |
| NYADRRWCF | NS3 (556–564) | T cell | CD8 |
See footnote to Table 9d for details.
In addition to the in vivo protection data, epitopes have been associated with potentially protective responses in vitro. Here we define as protective those functional assays that define correlates of in vivo protection, namely viral clearance (i.e., TCID50), reduction of viral titer, inhibition of infectivity (e.g., virus neutralization or inhibition assays), and cytolysis (CTL assays). Table 8 describes protective epitopes for dengue virus, West Nile virus, and Japanese encephalitis virus. Interesting observations include: (1) nearly all of the data were derived from mice, (2) nearly all of the epitopes defined are B-cell epitopes, and most of these are specific to the major envelope protein, (3) all of the epitopes defined for DENV come from serotype 2, and (4) all of the protective epitopes defined for WNV are non-linear, conformational (discontinuous) epitopes.
Table 8.
Epitopes Associated with Protective Responses As Measured In Vitro When Used to Immunize
| Epitope sequence | Antigen/position | Host organism | Response measured |
|---|---|---|---|
| Dengue virus | |||
| QLKLDWFKKGSS | D2 Env (386–397) | Rabbit (New Zealand White) | Neutralization/inhibition (D2) |
| AKNKPTLDFELIKTEAKQPAT | D2 Env (35–55) | Mouse (BALB/c) | Neutralization/inhibition (D2) |
| ITVNPIVTEKDSPVNIE | D2 Env (368–397) | Mouse (BALB/c) | Neutralization/inhibition (D2) |
| QLKLNWFKKGSS | D2 Env (386–397) | Mouse (BALB/c) | Neutralization/inhibition (D2) |
| West Nile virus | |||
| W391, G394, G396* | Env18 | Mouse (BALB/c) | Neutralization/inhibition |
| P365, T366, R389, G396, L397, S465, E481* | Env53 | Mouse (BALB/c) | Neutralization/inhibition |
| H371, D373, A376, S465, R526* | Env7H7 | Mouse (BALB/c) | Neutralization/inhibition |
| E339, K570* | Env113 | Mouse (BALB/c) | Neutralization/inhibition |
| S465, E481, R483, S484* | Env121 | Mouse (BALB/c) | Neutralization/inhibition |
| W507, N512* | Env48 | Mouse (BALB/c) | Neutralization/inhibition |
| H553* | Env100 | Mouse (BALB/c) | Neutralization/inhibition |
| A454, A463, S465* | Env101 | Mouse (BALB/c) | Neutralization/inhibition |
| Kunjin virus | |||
| GYISTRVEL | NS3 (299–307) | Mouse (BALB/c) | CTL |
| Japanese encephalitis virus | |||
| SENHGNYSAQVGASQ | Env (149–163) | Mouse | CTL |
| EAHNEKRADSSYVCKQGFTDRGWGNGC | Env (373–399) | Mouse (C3H/He) | CTL |
| EMEPPFGDSYIVVGRGDKQINHHWHKA | Env (667–693) | Mouse (FVB/J), Yorkshire pig | Neutralization/inhibition |
| GRGDKQINHHWHKA | Env (680–693) | Mouse (FVB/J) | Neutralization/inhibition |
| NHGNYSAQVGASQ | Env (445–457) | Mouse (FVB/J) | Neutralization/inhibition |
| EAWLDSTKAT | M (17–26) | Mouse (Swiss) | Enhancement of Ab, neutralization/inhibition |
| SVRTTTDSGKLITD | NS1 (297–310) | Mouse (Swiss) | Enhancement of Ab, Neutralization/inhibition |
| SIGGVFNSIGKAVHQ | Egp (439–455) | Mouse (Swiss) | Enhancement of Ab, neutralization/inhibition |
| AADKPTLDIRMMNIEA | Env (210–225) | Mouse (BALB/c) | Neutralization/inhibition |
| YYVMTIGTKHFLVHREWFNDLLLP | Env (201–224) | Mouse (BALB/c) | Neutralization/inhibition |
Epitopes are shown according to virus, and include the epitope sequence, antigen/position, host organism, and response measured. Mouse strain and DENV serotype are shown in parentheses (D2) or in bold. Discontinuous epitope immunogens were also considered here (*residues are separated by commas). Position assignments may vary, as there are differences in the numbers reported (individual proteins versus the polyprotein). The epitope phenotype is determined here by the response measured.
Epitope reactivity following natural infection in humans
Tables 9a–d provide lists of epitope reactivity following natural infection in humans by virus and/or serotype, and the type of immune response and phenotype if determined, for DENV (9a and b), WNV (9c), and JEV (9d). These data were not available for the other flaviviruses. For DENV, a large number of epitopes have been identified over the course of natural infection. For this, the data have been divided into T-cell reactivity (9a) and B-cell reactivity (9b). Thus far T-cell reactivity has been defined against 8 of the 10 antigens (excluding NS2a and NS1). While the vast majority of these T-cell epitopes were unclassified with regard to phenotype, for those that were reported, CD8+ T-cell epitopes (25) outnumber CD4+ T-cell (13) epitopes. We do see antibody reactivity against NS1 (9b); however, the vast majority of antibody reactivity defined to date is against the E protein. A total of 243 B-cell epitopes were identified alone for DENV2. Table 9b shows only a fraction of these in the interest of brevity.
Table 9d.
B-Cell Reactivity Following Natural Infection in Humans (JEV)
| Infecting virus | Epitope sequence | Antigen/position | Response |
|---|---|---|---|
| JEV | AELTGYGTVT | D2 E (146–155) | B cell |
| ALHQVFGAIYGAAFSGVSWT | D2 E (435–454) | B cell | |
| ATLRKYCI | D2 E (54–61) | B cell | |
| AWLVHRQWFLDLPLPW | D2 E (178–193) | B cell | |
| GTIVIRVQYEG | D2 E (291–301) | B cell | |
| IGISNRDFV | D2 E (4–12) | B cell | |
| IGQMFETTMR | D2 E (398–407) | B cell | |
| KILIGVIITWIGM | D2 E (456–468) | B cell | |
| MVDRGWGNGCGL | D2 E (96–107) | B cell | |
| MVLLQM | D2 E (196–201) | B cell | |
| NLEYTIVITP | D2 E (107–116) | B cell | |
| PFGDSYIIIGVE | D2 E (345–356) | B cell | |
| QLKLNWF | D2 E (386–392) | B cell | |
| RHVLGRLITVNP | D2 E (318–329) | B cell | |
| RTGLDFN | D2 E (161–167) | B cell | |
| SGNLLFTGHL | D2 E (247–256) | B cell | |
| SRSTSLSVSLVLVGVVTLYLGAMV | D2 E (443–466) | B cell | |
| TAWDFGSLGGVFTSIG | D2 E (418–433) | B cell | |
| TQGSNWIQ | D2 E (199–206) | B cell | |
| VSGGSWVDIVLE | D2 E (15–26) | B cell |
Epitope data are enumerated by dengue serotype, and include epitope sequence, epitope name, type of response (T or B), and effector phenotype. Data were derived from multiple human reactivities; therefore peptide position may reflect only slight differences: (a) DENV T cell, (b) DENV B cell, (c) WNV T cell (only, no B-cell data), and (d) JEV B-cell (only, no T-cell data). **The 20 peptides shown in 9b are DENV 2 epitopes recognized by individuals infected with JEV (cross-reaction). This number takes into account only those epitopes recognized by JEV-infected humans, which is in contrast to Table 11, wherein the same peptide may be present in more than one species/serotype. Flavivirus source protein data come directly from the NCBI and are therefore reported as “genome polyprotein” in some cases, and as the specified protein (e.g., NS3 or Env) in others. These assignments are not made by the IEDB. Variation in nomenclature and epitope position is therefore expected. Assignments in these tables were made to establish the protein of origin and relative position based on available information in NCBI/the originating paper.
Abbreviations: ND = not determined; NA = not applicable.
Table 9b.
B-Cell Reactivity Following Natural Infection in Humans (DENV)
| Infecting virus | Epitope sequence | Antigen/position | Response |
|---|---|---|---|
| DENV 1 | DSGCVVSWKNKELKC | NS1 (29–43) | B cell |
| ETLVTFKN | E (208–215) | B cell | |
| IVTCAMF | E (113–119) | B cell | |
| KILIGVIITWIGM | E (456–468) | B cell | |
| LDFELI | E (41–66) | B cell | |
| QLKLNWF | E (386–392) | B cell | |
| RHVLGRLITVNP | E (318–329) | B cell | |
| SGNLLFTGHL | E (247–256) | B cell | |
| AELTGYGTVT | E (146–155) | B cell | |
| ALHQVFGAIYGAAFSGVSWT | E (435–454) | B cell | |
| ATLRKYCI | E (54–61) | B cell | |
| AWLVHRQWFLDLPLPW | E (178–193) | B cell | |
| GKNRAINMLKRGLPR | C (9–23) | B cell | |
| GTIVIRVQYEG | E (291–301) | B cell | |
| IDAVNKRGRKQNKRG | C (92–106) | B cell | |
| IGISNRDFV | E (4–12) | B cell | |
| IGQMFETTMR | E (398–407) | B cell | |
| MVDRGWGNGCGL | E (97–107) | B cell | |
| MVLLQM | E (196–201) | B cell | |
| NLEYTIVITP | E (107–116) | B cell | |
| PFGDSYIIIGVE | E (345–356) | B cell | |
| QDVVVLGS | E (221–228) | B cell | |
| RTGLDFN | E (161–167) | B cell | |
| SRSTSLSVSLVLVGVVTLYLGAMV | E (443–466) | B cell | |
| TAWDFGSLGGVFTSIG | E (418–433) | B cell | |
| TQGSNWIQ | E (199–206) | B cell | |
| VSGGSWVDIVLE | E (15–26) | B cell | |
| DENV 2** | ADTQGS | E (187–202) | B cell |
| AELTGY | E (146–151) | B cell | |
| AKMLSTELH | NS1 (149–157) | B cell | |
| CAMFTC | E (89–94) | B cell | |
| CGLFGK | E (78–83) | B cell | |
| CKKNME | E (94–99) | B cell | |
| DFELIK | E (42–47) | B cell | |
| DFNEMV | E (165–170) | B cell | |
| DSGCVVSWK | NS1 (29–37) | B cell | |
| EAELTGYG | E (172–179) | B cell | |
| ECSPRT | E (157–162) | B cell | |
| ELRYSWKTWGKAKMLSTELH | NS1 (138–157) | B cell | |
| FETTMR | E (375–380) | B cell | |
| FGAIYG | E (440–445) | B cell | |
| FGDSYI | E (346–351) | B cell | |
| GAIYGA | E (441–446) | B cell | |
| GATEIQ | E (239–244) | B cell | |
| GPVSQHNNR | NS1 (53–61) | B cell | |
| HGTIVI | E (290–295) | B bell | |
| HQVFGA | E (437–442) | B bell | |
| HRQWFL | E (182–187) | B bell | |
| IGISNR | E(4–9) | B bell | |
| IGVIIT | E (459–464) | B bell | |
| KAWLVH | E (177–182) | B bell | |
| KGGIVT | E (83–88) | B bell | |
| KKNMEG | E (95–100) | B bell | |
| LEYTIV | E (108–113) | B bell | |
| LFGKGG | E (80–85) | B bell | |
| LFTGHL | E (251–256) | B bell | |
| MAILGD | E (412–417) | B bell | |
| MAKNKP | E (34–39) | B bell | |
| MECSPR | E (156–161) | B bell | |
| NEMVLL | E (167–172) | B bell | |
| NLEYTI | E (107–112) | B bell | |
| NLLFTG | E (249–254) | B bell | |
| PLPWLP | E (190–195) | B bell | |
| PWHLGKLEM | NS1 (267–275) | B bell | |
| PWLPGA | E (192–197) | B bell | |
| QGSNWI | E (200–205) | B bell | |
| QLKLNW | E (386–391) | B bell | |
| QPTELRYSW | NS1 (135–143) | B bell | |
| RDFVEG | E (9–14) | B bell | |
| RGAKRM | E (407–412) | B bell | |
| RHVLGR | E (318–323) | B bell | |
| SGNLLF | E (247–252) | B bell | |
| SGVSWT | E (449–454) | B cell | |
| SLNEEQ | E (81–86) | B cell | |
| TQGSNW | E (199–204) | B cell | |
| TRLENLMWK | NS1 (61–69) | B cell | |
| TTASGKLIT | NS1 (329–337) | B cell | |
| VFTSIG | E (428–433) | B cell | |
| VGVVTL | E (482–487) | B cell | |
| VHTWTEQYK | NS1 (25–33) | B cell | |
| WGNGCG | E (74–79) | B cell | |
| WKTWGKAKM | NS1 (145–151) | B cell | |
| WLPGAD | E (193–198) | B cell | |
| YGMEIRPLK | NS1 (331–339) | B cell | |
| YLGAMV | E (461–466) | B cell | |
| YSMCTG | E (299–304) | B cell | |
| DENV 3 | ALHQVFGAIYGAAFSGVSWT | E (435–454) | B cell |
| ATLRKYCI | E (54–61) | B cell | |
| AELTGYGTVT | E (146–155) | B cell | |
| AWLVHRQWFLDLPLPW | E (178–193) | B cell | |
| DSGCVVSWKNKELKC | NS1 (29–43) | B cell | |
| ETLVTFKN | E (208–215) | B cell | |
| GTIVIRVQYEG | E (291–301) | B cell | |
| IGISNRDFV | E (4–12) | B cell | |
| IVTCAMF | E (113–119) | B cell | |
| KILIGVIITWIGM | E (456–468) | B cell | |
| MVDRGWGNGCGL | E (96–107) | B cell | |
| MVLLQM | E (196–201) | B cell | |
| NLEYTIVITP | E (107–116) | B cell | |
| PFGDSYIIIGVE | E (345–356) | B cell | |
| QDVVVLGS | E (221–228) | B cell | |
| QLKLNWF | E (386–392) | B cell | |
| RHVLGRLITVNP | E (318–329) | B cell | |
| RTGLDFN | E (161–167) | B cell | |
| SGNLLFTGHL | E (247–256) | B cell | |
| SRSTSLSVSLVLVGVVTLYLGAMV | E (443–466) | B cell | |
| TAWDFGSLGGVFTSIG | E (418–433) | B cell | |
| TQGSNWIQ | E (199–206) | B cell | |
| VSGGSWVDIVLE | E (15–26) | B cell | |
| YSMCTG | E (299–304) | B cell | |
| DENV 4 | AELTGYGTVT | E (146–155) | B cell |
| ALHQVFGAIYGAAFSGVSWT | E (435–454) | B cell | |
| ATLRKYCI | E (54–61) | B cell | |
| AWLVHRQWFLDLPLPW | E (178–193) | B cell | |
| DSGCVVSWKNKELKC | NS1 (29–43) | B cell | |
| ETLVTFKN | E (208–215) | B cell | |
| GTIVIRVQYEG | E (291–301) | B cell | |
| IGISNRDFV | E (4–12) | B cell | |
| IVTCAMF | E (113–119) | B cell | |
| KILIGVIITWIGM | E (456–468) | B cell | |
| LDFELI | E (41–46) | B cell | |
| MVDRGWGNGCGL | E (96–107) | B cell | |
| MVLLQM | E (196–201) | B cell | |
| NLEYTIVITP | E (107–116) | B cell | |
| PFGDSYIIIGVE | E (345–356) | B cell | |
| RHVLGRLITVNP | E (318–329) | B cell | |
| SGNLLFTGHL | E (247–256) | B cell | |
| YSMCTG | E (299–304) | B cell | |
| DENV, unspecified | FLIDGPDTSECPNERRA | NS1 (133–149) | B cell |
| ILEENMEVEIWTREGEKKKL | NS3 (572–591) | B cell | |
| KFQPESPARLASAILNA | NS1 (33–49) | B cell | |
| MKFREGSSEVC | NS1 (169–179) | B cell | |
| WCCRSCTLPPLRYRGEDGCW | NS1 (311–330) | B cell | |
| WYGMEIRPLSEKEENMV | NS1 (330–346) | B cell |
See footnote to Table 9d for details.
Table 9c.
T-Cell Reactivity Following Natural Infection In Humans (WNV)
| Infecting virus | Epitope sequence | Antigen/position | Response | Phenotype |
|---|---|---|---|---|
| WNV | SVGGVFTSV | E (430–438) | T cell | ND |
| RLDDDGNFQL | NS2b (78–87) | T cell | ND | |
| YTMDGEYRL | NS3 (518–526) | T cell | ND | |
| SLFGQRIEV | NS4b (68–76) | T cell | ND | |
| SLTSINVQA | NS4b (15–23) | T cell | ND | |
| ATWAENIQV | NS5 (862–870) | T cell | ND | |
| ARIMLDNINMPNGLIAQF | NS3 (491–511) | T cell | ND | |
| CWMAEVPGTKIAGMLLL | NS4a (93–109) | T cell | CD8 | |
| GVIMPNGSYISAIVQGER | NS3 (152–169) | T cell | ND | |
| KGAWMDSTKATRYLVK | M (15–30) | T cell | CD8 | |
| KKELGTLTSAINRRSSK | C (85–101) | T cell | ND | |
| KTKSDISSLFGQRIEVK | NS4b (7–23) | T cell | CD8 | |
| LITAAAVTLWENGASSVW | NS4b (201–219) | T cell | CD8 | |
| MPNGLIAQF | NS3 (501–509) | T cell | CD8 | |
| NMPNGLIAQFYQPEREKV | NS3 (499–516) | T cell | ND | |
| RDFLEGVSGATWVDLVL | E (7–24) | T cell | CD8 | |
| SGATWVDLV | E (17–26) | T cell | CD8 | |
| SGATWVDLVLEGDSCVTI | E (15–32) | T cell | CD8 | |
| SLFGQRIEVKENFSMGEF | NS4b (13–31) | T cell | CD8 | |
| TFLVHREWFMDLNLPW | E (205–220) | T cell | ND | |
| WDFGSVGGVFTSVGKAVH | E (421–438) | T cell | CD8 | |
| WMDSTKATRY | M (111–120) | T cell | CD8 |
See footnote to Table 9d for details.
Far fewer epitopes have been identified for WNV (all T-cell) and JEV (all B-cell); however, these include both major and minor antigens. The epitopes reported as recognized by JEV patients were all derived from DENV2, thus showing a significant degree of potential cross-reactivity. These data could be used in the clinical setting to compare reactivities among patients with different disease outcomes, or could be used analytically (or computationally) to evaluate sequence variations among DENV strains associated with more severe disease.
Epitopes identified during the course of natural infection can also help elucidate the issue of epitopes associated with immunopathology. For example, the epitopes from Tables 9a–d could be further evaluated for disease state in order to generate a subset of reactive peptides recognized only during severe disease (i.e., DHF). This subset could then be used to compare reactivity (by serotype) to individuals with less severe disease (i.e., DF). The current data are, however, limited in their capacity to adequately answer this basic question, as the vast majority of epitope mapping in humans with defined disease is not comprehensive (with each peptide tested in both DF and DHF). It is important to note that while studies to date have shown associations between certain epitopes (B- and T-cell) and different disease states, much more work remains to be done in order truly correlate particular peptides to immunopathology.
Zivna et al. have demonstrated that T-cell responses to an HLA-B07-restricted epitope (LAPTRVVAAEME) on DENV NS3 correlated with severe disease in Thai children; the frequency of responses to this peptide was higher for those with DHF than for those who had DF (113). Mongkolsapaya et al. analyzed CTL responses during acute dengue infection and found an association between DHF and the A11-restricted epitope (GTSGSPIIDKK) on NS3, for which there are at least six naturally-occurring variants among the four serotypes (66). Here as well, responses to the epitope were more frequent in individuals with more severe disease. Loke et al. found three epitopes from NS3 (TVWFVPSIK, NYADRRWCF, and EALRGLPIR), and two from NS5 (YILRDVSKK and DVFFTPPEK), that were recognized by the CD8+ T cells of DHF patients (53). Simmons et al. studied T-cell responses from DHF patients from Vietnam with secondary dengue infection, and found acute T-cell responses to natural variants of E (ILGDTAWDFGSL) and NS3 (GINYADRRWCF). Interestingly, they found that the overall breadth and magnitude of T-cell responses were not significantly related to disease severity (92), strengthening the argument for cross-reactivity leading to altered T-cell functionality. By contrast, very few antibody epitopes have been defined that are associated with severe disease. Falconar has studied antibody reactions in human patients with DF and DSS, and found that antibody responses to NS1 epitopes (RPQPTELRY, QPTELRYSW, and TELRYSWKT) sharing the ELR motif, were stronger in DSS patients than in those with DF (29). Finally, enhanced infectivity in mice was associated with antibody responses to the preM/M epitope (CPFLKQNEPEDIDCW) (39). Table 10 depicts those epitopes captured in the IEDB that have been associated with either ADE or T-cell immunopathology in the literature, and have been associated with different disease outcome; all but one of these epitopes were defined in humans.
Table 10.
Dengue Virus Epitopes Associated with Immunopathology
| Epitope sequence | Epitope name | Effector cell | Host | Read-out/association |
|---|---|---|---|---|
| Antibody-dependent enhancement (ADE) | ||||
| CPFLKQNEPEDIDCW | Pre M (167–181) | B cell/antibody | Mouse (BALB/c) | Epitope-specific Abs enhanced cell infectivity in vitro |
| G106, F107, H317 | Env, discontinuous | B cell/Antibody | Variant of mAb1A5 epitope (L107F) | |
| Cross-reactive T cells or “original antigenic sin” | ||||
| GTSGSPIIDKK | NS3 (130–144) | CD8+ T cells [HLA-A11] | Human | Correlate disease severity with pattern of epitope-specific response (apoptosis) in vitro from infected patients |
| NYADRRWCF | A24-NYA D2 NS3 (2031–2039) | CD8+ T cells [HLA-A24] | Human | Epitope-specific T cells showed suboptimal degranulation and high inflammatory cytokine production in vitro; epitope-specific response in patients with DHF |
| TVWFVPSIK | D2 NS3 (1833–1841) | CD8+ T cells [HLA-A1, A2] | Human | Epitope-specific response in patients with DHF |
| EALRGLPIR | D2 NS3 (1708–1716) | CD8+ T cells [HLA-A1, A33] | Human | Epitope-specific response in patients with DHF |
| YILRDVSKK | D2 NS5 (3008–3016) | CD8+ T cells [HLA-A24] | Human | Epitope-specific response in patientsith DHF |
| DVFFTPPEK | D2 NS5 (2622–2630) | CD8+ T cells [HLA-A2, A11] | Human | Epitope-specific response in patients with DHF |
The epitopes included here have been identified from the peer-reviewed literature as being associated with DENV immunopathology and are also included in the IEDB. Structures are categorized as either antibody-dependent enhancement (ADE) or cross-reactive T cells. Details include epitope sequence, epitope name, effector cell type, host species (and strain), and read-out or association with immunopathology. Position assignments may vary, as there are differences in the numbers reported (individual proteins versus the polyprotein). See footnote to Table 11 for significance of bold type.
Flavivirus cross-reactivity
The identification of cross-reactivity within Flavivirus species is now a major focus of research, as it is hypothesized to be one of the primary causes of B- and T cell-mediated immunopathology, and therefore the basis of severe disease. In general, antibody-mediated enhancement of disease is thought to result when neutralizing antibodies generated against the serotype of the primary infection fail to, or poorly, neutralize the serotype of the secondary infection, and lead to increased uptake of virus through Fcγ II-bearing cells (36,51). The surface-exposed structural proteins E and preM/M are thought to play a significant role in ADE; however, NS1 is also implicated (29). In the “original antigenic sin” model, T cells generated upon primary dengue infection are able to respond to variant epitopes during secondary heterologous infection. The selective expansion of these lower-avidity cross-reactive memory T cells from the initial infection may outcompete naïve T cells with higher avidity for the second serotype (56). It appears that more conserved, non-structural proteins play a dominant role in this phenomenon.
The characterization of the cross-reactivity of epitopes from different serotypes is highly relevant for vaccines or diagnostic applications. It is therefore critical that a broader understanding of the antigens and epitopes involved in these phenomena be more fully elucidated. Indeed, the development of an efficacious vaccine will require a better understanding of epitope cross-reactivity, as any candidate formulation will have to ensure a lack of potential disease enhancement. The identification of non-cross-reactive epitopes is therefore an area of keen interest going forward.
Table 11 shows serologically heterologous epitopes defined in human subjects following natural infection with DENV or JEV; similar cross-reactivity data are shown for experimental DENV immunization in human subjects (see Table 12 below). We see a fairly broad range of cross-reactivity with DENV 2 epitopes. Reactivity to these epitopes was observed following infection with all other DENV serotypes (1, 3, and 4), as well as JEV, KUN, and MVEV. JEV is known to be cross-reactive with DENV (see also Table 9d), making serodiagnosis difficult, and leaving the question open as to its possible role in dengue disease. The prominence of DENV 2 in these data may reflect some degree of experimental bias. Some studies have suggested that DENV 2 (especially Asian genotypes) is more often associated with more severe disease, and is therefore more heavily studied (50,106). However, the data collected here are mostly associated with DF. Interestingly, those cross-reactive epitopes defined for subjects with DHF were all identified from NS3, which is a known target of T-cell responses, and had been implicated in immunopathology (66) [see example peptide in bold Tables 10 and 11].
Table 11.
Examples of Cross-Reactive Epitopes Defined in Humans
| Epitope source | Epitope sequence | Antigen/position | Infecting virus | Response | DS |
|---|---|---|---|---|---|
| JEV | GKNRAINMLKRGLPR | C (8–22) | DENV 1 | B | DF |
| JEV | DTGCAIDITRKEMRC | NS1 (795–809) | DENV | B | DF |
| JEV | IDAVNKRGRKQNKRG | (91–105) | DENV 1 | B | DF |
| KUN | GYISTRVEL | NS3 (1804–1812) | DENV 2 | T | EXP |
| MVEV | GYIATRVEA | NS3 (299–307) | DENV 2 | T | EXP |
| DENV 1 | QYSDRRWCF | NS3 (556–564) | DENV 2 | T | DHF |
| DENV 2 | DSGCVVSWKNKELKC | NS1 (1–15) | DENV 3 | B | DF |
| DENV 2 | NYADRRWCF | NS3 (556–564) | DENV 1 | T | DHF |
| DENV 2 | ALRGLPIRY | NS3 (234–242) | DENV 3 | T | EXP |
| DENV 2 | DVKKDLISY | NS3 (71–79) | DENV 3 | T | EXP |
| DENV 2 | TPEGIIPSL | NS3 (500–508) | DENV 4 | T | EXP |
| DENV 2 | TPEGIIPSM | NS3 (500–508) | DENV 4 | T | EXP |
| DENV 2 | LDFELI | E (41–46) | DENV 1 | B | DF |
| DENV 2 | MVLLQM | E (196–201) | JEV | B | JE |
| DENV 2 | YSMCTG | E (299–304) | DENV 4 | B | DF |
| DENV 2 | IGISNRDFV | E (4–12) | DENV 1 | B | DF |
| DENV 2 | VSGGSWVDIVLE | E (15–26) | DENV 4 | B | DF |
| DENV 2 | ATLRKYCI | E (54–61) | DENV 1 | B | DF |
| DENV 2 | MVDRGWGNGCGL | E (96–107) | JEV | B | JE |
| DENV 2 | IVTCAMF | E (113–119) | DENV 1 | B | DF |
| DENV 2 | NLEYTIVITP | E (134–143) | DENV 3 | B | DF |
| DENV 2 | AELTGYGTVT | E (173–182) | DENV 3 | B | DF |
| DENV 2 | RTGLDFN | E (188–194) | DENV 1 | B | DF |
| DENV 2 | AWLVHRQWFLDLPLPW | E (205–220) | DENV 3 | B | DF |
| DENV 2 | TQGSNWIQ | E (226–233) | DENV 1 | B | DF |
| DENV 2 | ETLVTFKN | E (235–242) | DENV 1 | B | DF |
| DENV 2 | QDVVVLGS | E (248–255) | DENV 3 | B | DF |
| DENV 2 | SGNLLFTGHL | E (274–283) | DENV 3 | B | DF |
| DENV 2 | GTIVIRVQYEG | E (318–328) | DENV 1 | B | DF |
| DENV 2 | RHVLGRLITVNP | E (345–356) | DENV 1 | B | DF |
| DENV 2 | PFGDSYIIIGVE | E (372–383) | DENV 1 | B | DF |
| DENV 2 | QLKLNWF | E (386–392) | JEV | B | JE |
| DENV 2 | IGQMFETTMR | E (398–407) | JEV | B | JE |
| DENV 2 | TAWDFGSLGGVFTSIG | E (418–433) | JEV | B | JE |
| DENV 2 | ALHQVFGAIYGAAFSGVSWT | E (435–454) | DENV 1 | B | DF |
| DENV 2 | KILIGVIITWIGM | E (456–468) | DENV 4 | B | DF |
| DENV 2 | SRSTSLSVSLVLVGVVTLYLGAMV | E (470–493) | DENV 3 | B | DF |
| DENV 2 | KPWDIIPMV | NS5 (2821–2829) | DENV 1 | T | DF |
| DENV 2 | KPWDVVPMV | NS5 (2821–2829) | DENV 1 | T | DF |
| DENV 2 | KPWDVLPMV | NS5 (2821–2829) | DENV 1 | T | DF |
| DENV 2 | KPWDVLPTV | NS5 (2821–2829) | DENV 1 | T | DF |
| DENV 3 | KYTDRKWCF | NS3 (2030–2038) | DENV 2 | T | DHF |
| DENV 3 | GYISTRVGM | NS3 (1772–1780) | DENV 4 | T | EXP |
| DENV 3 | TPEGIIPAL | NS3 (500–508) | DENV 4 | T | EXP |
| DENV 3 | KPWDVVPTV | NS5 (2819–2827) | DENV 1 | T | DF |
| DENV 4 | SYKDREWCF | NS3 (2030–2038) | DENV 2 | T | DHF |
| DENV 4 | APTRVVAAE | NS3 (222–230) | DENV 2 | T | DHF |
| DENV 4 | LAPTRVVAAEMEE | NS3 (221–232) | DENV 2 | T | DHF |
Cross-reactive epitopes were defined by those assays in which the immunizing virus was of a different serotype than the source of the reacting peptide. These are enumerated by epitope source (virus), and include epitope sequence, antigen/position, infecting virus, response type (T or B), and disease state (DS). Disease states are thus defined: dengue fever (DF), dengue hemorrhagic fever (DHF), and exposure without evidence of disease (EXP). This last designation refers to individuals living in endemic regions who are likely exposed to the pathogens on a regular basis. Bold type indicates epitope overlap with Table 10. Flavivirus source protein data come directly from the NCBI and are therefore reported as “genome polyprotein” in some cases, and as the specified protein (e.g., NS3 or Env) in others. These assignments are not made by the IEDB. Variation in nomenclature and epitope position is therefore expected. Assignments in this table were made to establish the protein of origin and its relative position based on available information in the NCBI/the originating paper. In the interest of simplicity, in this table we did not try to report all possible Flavivirus cross-reactivity. The same peptide may be present in more than one species/serotype, making the number of potential cross-reactive peptide/immunogen combinations greater than what is shown here.
Table 12.
Human Reactivity Following Experimental Immunization with Attenuated/Inactivated Virus
| Virus used for immunization | Epitope as antigen | Epitope sequence |
|---|---|---|
| DENV 1 | D1 NS4b (181–189) | ILLMRTTWA |
| D2 NS4b (111–119) | FLLVAHYAI | |
| D2 E (211–219) | FLDLPLPWL | |
| D2 NS4a (56–64) | LLLTLLATV | |
| D3 NS4b (181–189) | LLLMRTSWA | |
| D4 E (211–219) | FFDLPLPWL | |
| D3 E (211–219) | FFDLPLPWT | |
| D1 E (211–219) | FLDLPLPWT | |
| D3 NS4a (56–64) | LLLGLMILL | |
| D4 NS4b (181–189) | LLLMRTTWA | |
| D4 NS4b (111–119) | LVMLLVHYA | |
| D1 NS4a (56–64) | MLLALIAVL | |
| D4 NS4a (56–64) | MLVALLGAM | |
| D3 NS4b (111–119) | VLLLVTHYA | |
| D2 NS4b (181–189) | VLLMRTTWA | |
| D1 NS4b (111–119) | VLMLVAHYA | |
| DENV 2 | D1 NS4b (111–119) | VLMLVAHYA |
| D1 NS4a (56–64) | MLLALIAVL | |
| D2 NS4b (181–189) | VLLMRTTWA | |
| D3 E (211–219) | FFDLPLPWT | |
| D4 E (211–219) | FFDLPLPWL | |
| D2 E (211–219) | FLDLPLPWL | |
| D1 E (211–219) | FLDLPLPWT | |
| D2 NS4b (111–119) | FLLVAHYAI | |
| D1 NS4b (181–189) | ILLMRTTWA | |
| D3 NS4a (56–64) | LLLGLMILL | |
| D3 NS4b (181–189) | LLLMRTSWA | |
| D4 NS4b (181–189) | LLLMRTTWA | |
| D2 NS4a (56–64) | LLLTLLATV | |
| D4 NS4b (111–119) | LVMLLVHYA | |
| D4 NS4a (56–64) | MLVALLGAM | |
| D3 NS4b (111–119) | VLLLVTHYA | |
| DENV 3 | D1 NS3 (141–155) | NREGKIVGLYGNGVV |
| D1 NS3 (223–237) | PTRVVASEMAEALKG | |
| D1 NS3 (251–265) | HTGKEIVDLMCHATE | |
| D1 E (211–219) | FLDLPLPWT | |
| D2 NS3 (141–155) | DKKGKVVGLYGNGVV | |
| D3 E (211–219) | FFDLPLPWT | |
| D2 E (211–219) | FLDLPLPWL | |
| D2 NS4b (111–119) | FLLVAHYAI | |
| D2 NS4a (56–64) | LLLTLLATV | |
| D1 NS4b (11–119) | VLMLVAHYA | |
| D2 NS3 (223–237) | PTRVVAAEMEEALRG | |
| D2 NS3 (251–265) | HTGREIVDLMCHATE | |
| D3 NS3 (141–155) | NREGKVVGLYGNGVV | |
| D3 NS3 (348–362) | GNEWITDFVGKTVWF | |
| D3 NS3 (223–237) | PTRVVAAEMEEAMKG | |
| D3 NS3 (251–265) | HTGREIVDLMCHATE | |
| D3 NS4b (111–119) | VLLLVTHYA | |
| D3 NS4a (56–64) | LLLGLMILL | |
| D3 NS3 (527–536) | GESRKTFVEL | |
| D3 NS3 (132–142) | GTSGSPIINRE | |
| D4 NS3 (141–155) | NRKGKIVGLYGNGVV | |
| D4 NS3 (223–237) | PTRVVAAEMEEALRG | |
| D4 NS3 (251–265) | HTGREIVDLMCHATE | |
| D4 NS4b (111–119) | LVMLLVHYA | |
| D4 NS4a (56–64) | MLVALLGAM | |
| D4 E (211–219) | FFDLPLPWL | |
| DENV 4 | D1 C (81–92) | LRGFKKEISNML |
| D1 NS3 (186–200) | RKLTIMDLHPGSGKT | |
| D1 NS3 (584–598) | KEGERKKLRPRWLDA | |
| D2 NS3 (584–598) | KEGERKKLKPRWLDA | |
| D2 C (81–92) | LRGFRKEIGRML | |
| D2 NS3 (186–200) | RKLTIMDLHPGAGKT | |
| D3 C (81–92) | LKGFKKEISNML | |
| D3 NS3 (186–200) | RNLTIMDLHPGSGKT | |
| D3 NS3 (584–598) | KEGEKKKLRPRWLDA | |
| D4 NS3 (500–508) | TPEGIIPTL | |
| D4 C (81–92) | LIGFRKEIGRML | |
| D4 NS3 (186–200) | KRLTIMDLHPGAGKT | |
| D4 NS3 (584–598) | REGEKKKLRPRWLDR | |
| YF 17D vaccine | 17D NS2b (110–118) | HPFALLLVL |
| 17D NS1 (356–365) | HAVPFGLVSM | |
| 17D E (332–340) | IPVIVADDL | |
| 17D NS3 (349–358) | WNTGHDWILA | |
| 17D NS3 (1728–1736) | DVKFHTQAF | |
| 17D NS3 (1746–1754) | IDAMCHATL | |
| 17D NS3 (1702–1710) | RRRLRTLVL | |
| 17D NS3 (184–192) | TPEGIIPAL | |
| JEV vaccine | C (1–15) | TKKPGGPGKNRAINM |
| C (8–22) | GKNRAINMLKRGLPR | |
| C (34–48) | MSLLDGRGPVRFVLA | |
| C (65–79) | LGRWKAVEKSVAMKH | |
| C (78–92) | KHLTSFKRELGTLID | |
| C (91–105) | IDAVNKRGRKQNKRG | |
| C (96–110) | KRGRKQNKRGGNEGS |
Epitope reactivities were defined following experimental immunization of human subjects with inactivated or attenuated dengue virus (all four serotypes), or with the commercially available YF 17D and JE-VAX vaccines. Data are shown according to immunizing virus (immunogen), and include the antigen/position and the epitope sequence. Bold type (e.g., D1 and D2) denotes the dengue virus serotype, if known. Flavivirus source protein data come directly from the NCBI and are therefore reported as “genome polyprotein” in some cases, and as the specified protein (e.g., NS3 or Env) in others. These assignments are not made by the IEDB. Variation in nomenclature and epitope position is therefore expected. Assignments in this table were made to establish the protein of origin and relative position based on available information in the NCBI/the originating paper.
In addition to its potential role in disease exacerbation, cross-reactivity and sequence variation among Flavivirus species also contributes to difficulties in epidemiological surveillance. This is true in the human population, as well as in animal reservoirs (native or agricultural). The characterization of epitope reactivity for certain antigens routinely used for serodiagnosis between and among Flavivirus species may be useful to provide higher fidelity. Such data would allow researchers to make important distinctions between virus endemicities, which would then help the world community gain a greater understanding of the prevalence of specific flaviviruses in specific regions and which ones overlap. It may also help make distinctions among strains associated with higher virulence, and may help identify infected versus vaccinated individuals. To date, detailed analyses have been performed by other groups to investigate cross-reactivity between species within the Flavivirus genus (1,23,94), and these types of studies will be useful going forward for serodiagnosis, as well as vaccine development.
The potential utility of epitopes in Flavivirus vaccine development
Of the vaccines available for species in the Flavivirus genus, all are whole-organism formulations (YF, JEV, and TBEV), and indeed, these have demonstrated high levels of protective efficacy. Moreover, the vast majority of experimental vaccines tested in humans for dengue virus include whole-organism preparations. Here the epitope data can be queried to identify those epitopes found to be reactive following experimental immunization. Table 12 shows all epitope-specific reactivity in humans following active immunization (experimental or routine) with whole inactivated/attenuated dengue virus by serotype, as well as for the YF 17D vaccine and the JEV vaccine. These data show a broad array of antigen reactivity representing 7 of the 10 viral proteins, and also indicate a high degree of cross-reactivity among DENV serotypes. The ultimate goal would be to use this type of data, perhaps in concert with epitope data from natural infection, to help identify candidate antigens for those viruses lacking a vaccine, and to better define the components leading to the protection provided by the established vaccines YF 17D and JE-VAX.
The potential lack of YF vaccine coverage in certain areas may lead to disease re-emergence, and the lack of epidemiological understanding hinders surveillance and therefore predictability. For example, the epidemiology of YF has been well defined in West Africa, but not in East Africa, where the disease shows an unpredictable focal periodicity, long inter-epidemic periods, and the potential for large-scale epidemics (27). Indeed, the re-emergence of decades-old virus strains has been identified following outbreaks in Kenya in 1992–1993, as well as in the Sudan in 2003 and 2005. In addition, the current YF 17D vaccines essentially constitute quasi-species of closely related viral variants. Because neutralizing epitopes of YFV have not been fully mapped for humans, it cannot be ruled out that naturally-occurring neutralization escape variants might be present in 17D vaccine preparations (77). A greater understanding of the YF 17D vaccine at the molecular level is surely warranted, not only to uncover the underlying components of its success, but also to gain a better understanding of the interaction of this vaccine with its recipient's immune responses in the global setting.
Summary of Findings
What we have learned, knowledge gaps, and future directions
The Flavivirus genus consists of a complex group of antigenically-related pathogens with worldwide distribution. Disease sequelae of Flavivirus infection range from mild febrile illness to severe neuroinvasive disease or hemorrhagic syndromes. The viral genome consists of just 10 total proteins (structural and non-structural); however, the exact role of each protein in pathogenesis is not yet fully understood. Successful immunization strategies have existed for decades for some members, while vaccine development for other viral species remains problematic due to complexities of the immunobiology. The overall disease burden is therefore weighted more heavily upon those viral species lacking prophylaxis. While a broad array of animal models has been employed to study Flavivirus infection and immunity, none adequately represents all manifestations of disease; moreover, common laboratory animals are resistant to infection with some flaviviruses. Good human data exist for some flaviviruses; however, not all disease states are represented, and these data lack a broad global distribution among populations affected by viruses in this genus. And indeed, to a large extent we find that all of this complexity is reflected in the epitope literature.
Of the nearly 1,200 epitopes identified from viruses within the Flavivirus genus, the majority belong to the dengue virus group, followed by WNV and YFV. The prominence of DENV and WNV data in the epitope literature is likely a reflection of their overall worldwide impact on human disease, and the lack of vaccines. Conversely, the relatively smaller number of epitopes defined for the other viruses within the genus most likely reflects the presence of established prophylaxis, and/or their more modest impact on morbidity and mortality globally. All of the epitopes reported to date are peptidic in nature, raising the question of their overall role, and the relative interest in pursuing the identification of immunogenic carbohydrate moieties from prominent antigenic glycoproteins, such as the E protein.
Looking at the genus as a whole, there is a fairly even distribution of reported B-cell and T-cell epitopes. B-cell epitopes have been identified in 8 of 9 species of Flavivirus present in the IEDB. However, the overall number of B-cell epitopes varied greatly among the different virus species. For example, the greatest number of B-cell epitopes has been defined for dengue virus type 2, and these greatly outnumber the B-cell epitopes defined for all other species. The relative lack of B-cell epitopes defined for other viruses within this genus is somewhat surprising, given the overwhelming evidence demonstrating the importance of neutralizing antibodies in Flavivirus immunity. Whether the relative paucity of antibody data is due to experimental or biological factors (or a combination thereof ) cannot be determined from this analysis. Nevertheless, the identification of B-cell epitopes should be a priority for the future.
T-cell epitopes have been reported for all but 3 (TBEV, SLEV, and LIV) of the 9 flaviviruses captured in the IEDB. To date, DENV, WNV, and YFV represent the largest numbers of T-cell epitopes. The overall distribution for DENV is, however, somewhat skewed due to the disproportionate representation by the DENV 2 serotype, which highlights a need for better resolution of T-cell epitopes for the other three serotypes. Both CD4 and CD8 T-cell epitopes have been defined; however, dengue epitopes are predominantly CD8+ T-cell epitopes, whereas CD4 T-cell epitopes dominate the other viruses (WNV and JEV). Further identification of both CD4+ and CD8+ T-cell epitopes is certainly warranted given the putative role of these effector cells in both disease resolution and immunopathology. The MHC data reflect a need for the identification of more HLA-C alleles for humans and class I alleles for mice, as well a broader distribution of class II alleles for both humans and rodents.
Our analysis of host distribution of epitope reactivities showed, not surprisingly, that the majority of Flavivirus epitopes have been defined in either humans or mice. That the majority of DENV epitopes were defined in humans was not unexpected; however, the lack of human epitopes for JEV, and the lower numbers for WNV and YFV, was surprising. WNV has been responsible for a significant increase in neuroinvasive disease in the Western hemisphere since its introduction 1999, and millions of people worldwide have been vaccinated against JEV and YFV. Despite their high cost and limited availability, the identification of epitopes in NHP models remains of interest, because they represent natural hosts and have biological/immunological relevance to humans.
The genomic distribution of defined epitopes shows that structures have now been reported from all 10 of the Flavivirus viral proteins. However, comprehensive data for all 10 proteins have not been reported for any one virus. The best overall distribution of epitope data is for DENV and WNV (10 out of 10 for both), and the greatest number of epitopes described for the genus as a whole has been derived from E and NS3. The narrow focus of epitope mapping for this genus was somewhat surprising, as only two of the 10 potentially antigenic proteins are prominent in the epitope literature. Further epitope mapping is warranted for all other Flavivirus proteins.
The analysis of epitope data associated with different Flavivirus disease states identified both B- and T-cell epitopes recognized in patients with dengue fever, dengue hemorrhagic fever, yellow fever, Japanese encephalitis, and West Nile fever. A further category, “exposure without evidence of disease,” could be included to describe those individuals who have either been confirmed to have existing immunity (seropositive) or were assumed to have been exposed as a result of living in an endemic region. Most of the human epitope data were collected from populations living in Southeast Asia, a region highly endemic for several Flavivirus species. An overwhelming number of epitopes was defined for DF, and these were almost entirely antibody epitopes. A much smaller number of epitopes was defined for DHF, most of which were T-cell. These data highlight the need for additional epitope identification between the two primary DENV disease states, and for both effector types. Completely lacking are T-cell epitopes defined in WNF, JE, and YF infections in humans. While several groups have published well-controlled clinical studies in human Flavivirus patients, many of these have focused on DENV. Thus we conclude that the mapping of epitope reactivity during clinical disease in humans should be a priority in the future, at least for DENV, WNV, and YFV. Indeed, studies conducted in patients following mild and severe dengue virus infection suggest that the immune system is the critical component in determining disease outcome, and therefore the importance of expanded epitope mapping during clinical disease is clear.
To date, a small number of protective epitopes have been reported for some flaviviruses: DENV (3), JEV (5), and TBEV (1). This paucity of data is likely due to the lack of suitable animal models of infection and disease. While many models exist for studying different aspects of Flavivirus infection, the murine model is the standard for many. However, the use of mice to study certain flaviviruses is problematic due to this animal's relative resistance to infection and disease development, thereby making it difficult to use these animals to reliably define protective immunity. Progress is being made in the development of humanized and/or susceptible mouse models in which viral replication and disease symptoms more closely mimic those observed in humans. Until better models are found, the ability to define protective epitopes will be limited, and will mostly rely on extrapolation for clinical studies. Correlates of immunity defined by in vitro assays have identified potentially protective epitopes, and may therefore be useful to identify key antigens. However, these studies have almost exclusively focused on the E glycoprotein, and have been conducted in mice/rodents only.
A broad array of epitopes has been identified during natural infection in humans for DENV, WNV, and JEV, including both major and minor antigens (8 out of 10 Flavivirus proteins). However, the data are heavily weighted toward DENV. Nevertheless, the data may be useful for characterizing general antigenic reactivity during natural infection, and may further help elucidate the issue of variant (cross-reactive) epitopes responsible for immunopathology. The current data are somewhat limited in their capacity to adequately answer all questions related to natural infection and immunopathology, as the vast majority of epitope mapping in humans with defined disease is not comprehensive.
The identification of cross-reactivity within Flavivirus species is becoming a major focus of research, due to the association with cross-reactivity as the primary cause of B- and T-cell-mediated immunopathology. The characterization of the cross-reactivity of epitopes from different serotypes is therefore highly relevant for vaccines or diagnostic applications. Studies to date have shown correlations between certain epitopes (B- and T-cell) and more severe disease; however, the number of epitopes (or the amount of data on any one epitope) is thus far inadequate to begin a substantive discussion. Moreover, while certain epitopes have been associated with severe disease in humans, these same structures have not been evaluated in terms of cross-reactivity with other viral strains. The reason for this lack of information is that in the vast majority of clinical cases, the primary and secondary serotype is not determined or is unknown. Further work to define cross-reactivity is therefore of great interest, and much work is focused on the development of appropriate animal models for investigating this phenomenon.
Herein we have provided an overview of Flavivirus immunobiology and related immune epitope data. The aim of this meta-analysis was to offer a comprehensive inventory of all published epitope structures and associated immune reactivity in the context of the broader issues and challenges facing Flavivirus research. Through this work, we have tried to demonstrate the utility of epitope data for studying immunity and pathogenesis, uncovering critical knowledge gaps, and highlighting potential areas for further research. Ultimately, we hope that this information will be useful and meaningful to those working in the area of Flavivirus research, and seek to solicit valuable feedback from this scientific community.
Acknowledgments
We gratefully acknowledge the helpful contribution of Alison Deckhut, Sujan Shresta, and Lauren Yauch in reviewing this manuscript. The La Jolla Institute of Allergy and Immunology is supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, contract number HHSN2662004000 0 6C, under the Immune Epitope Database and Analysis Program.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Allwinn R. Doerr HW. Emmerich P. Schmitz H. Preiser W. Cross-reactivity in flavivirus serology: new implications of an old finding? Med Microbiol Immunol. 2002;190:199–202. doi: 10.1007/s00430-001-0107-9. [DOI] [PubMed] [Google Scholar]
- 2.Appanna R. Huat TL. See L. Tan PL. Vadivelu J. Devi S. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia. Clin Vaccine Immunol. 2007;14:969–977. doi: 10.1128/CVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett PN. Dorner F. Tick-borne encephalitis vaccine. In: Plotkin SA, editor; Mortimer EA, editor. Vaccines. 2nd. WB Saunders Company; Philadelphia: 1994. pp. 715–727. [Google Scholar]
- 4.Barrett AD. Gould EA. Antibody-mediated early death in vivo after infection with yellow fever virus. J Gen Virol. 1986;67:2539–2542. doi: 10.1099/0022-1317-67-11-2539. [DOI] [PubMed] [Google Scholar]
- 5.Bashyam HS. Green S. Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous virus serotypes. J Immunol. 2006;176:2817–2824. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Nathan D. Lustig S. Tam G. Robinzon S. Segal S. Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 7.Blaney JE. Johnson DH. Manipon GG. Firestone CY. Hanson CT. Murphy BR. Whitehead SS. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002;300:125–139. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]
- 8.Blythe M. Zhang Q. Vaughan K, et al. An analysis of the epitope knowledge related to Mycobacteria. Immunome Res. 2007;3:10. doi: 10.1186/1745-7580-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandriss MW. Schlesinger JJ. Walsh EE. Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986;67(Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- 10.Brinton MA. Kurane I. Methew A. Zeng L. Shi PY. Rothamn A. Ennis FA. Immune mediated and inherited defences against flaviviruses. Clin Diagn Virol. 1998;10:129–139. doi: 10.1016/s0928-0197(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 11.Bui HH. Peters B. Assarsson E. Mbawuike I. Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci USA. 2007;104:246–251. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention 1: Questions and Answers About Japanese Encephalitis. www.cdc.gov/ncidod/dvbid/jencephalitis/qa.htm www.cdc.gov/ncidod/dvbid/jencephalitis/qa.htm
- 13.Centers for Disease Control and Prevention 2. West Nile activity–United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:556–559. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention 3. SLEV. www.cdc.gov/ncidod/dvbid/sle/Sle_FactSheet.html www.cdc.gov/ncidod/dvbid/sle/Sle_FactSheet.html
- 15.Calisher CH. Karabatsos N. Dalrymple JM. Shope RE. Porterfield JS. Westaway EG. Brandt WE. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70(Pt 1):37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 16.Camenga DL. Nathanson N. Cole GA. Cyclophosphamide-potentiated West Nile viral encephalitis: Relative influence of cellular and humoral factors. J Infect Dis. 1974;130:634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- 17.Charlier N. Leyssen P. De Clerq E. Neyts J. Rodent models for the study therapy against flavivirus infections. Antiviral Res. 2004;63:67–77. doi: 10.1016/j.antiviral.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen HC. Lai SY. Sung JM, et al. Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J Med Virol. 2004;73:419–431. doi: 10.1002/jmv.20108. [DOI] [PubMed] [Google Scholar]
- 19.Chung KM. Nybakken GE. Thompson BS. Engle MJ. Marri A. Fremont DH. Diamond MS. Antibodies against West Nile virus nonstructural (NS)-1 protein prevent lethal infection through Fc gamma receptor-dependent and independent mechanisms. J Virol. 2006;80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churdboonchart V. Bhamarapravati N. Peampramprecha S. Sirinavin S. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am J Trop Med Hyg. 1991;44:481–493. doi: 10.4269/ajtmh.1991.44.481. [DOI] [PubMed] [Google Scholar]
- 21.Co MDT. Terajima M. Cruz J. Ennis FA. Rothman AL. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology. 2002;293:151–163. doi: 10.1006/viro.2001.1255. [DOI] [PubMed] [Google Scholar]
- 22.Cologna R. Armstrong PM. Rico-Hesse R. Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol. 2005;79:853–859. doi: 10.1128/JVI.79.2.853-859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crill WD. Trainor NB. Chang GJJ. A detailed mutagenesis study of flavivirus cross-reactive epitopes using West Nile virus-like particles. J Gen Virol. 2007;88:1169–1174. doi: 10.1099/vir.0.82640-0. [DOI] [PubMed] [Google Scholar]
- 24.Dewasthaly SS. Bhonde GS. Shankarraman V. Biswas SM. Ayachit VM. Gore MM. Chimeric T helper-B cell peptides induce protective response against Japanese encephalitis virus in mice. Protein Pept Lett. 2007;14:543–551. doi: 10.2174/092986607780990028. [DOI] [PubMed] [Google Scholar]
- 25.Diamond MS. Shrestha B. Marri A. Mahan D. Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond MS. Sitati EM. Friend LD. Higgs S. Shrestha B. Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis BR. Barrett AD. The enigma of yellow fever in East Africa. Rev Med Virol. 2008;18:331–346. doi: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- 28.Engle MJ. Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77:12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falconar AKI. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin Vaccine Immunol. 2007;14:493–504. doi: 10.1128/CVI.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagnon SJ. Zeng W. Kurane I. Ennis FA. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. J Virol. 1996;70:141–147. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia G. Vaughn DW. Del Angel RM. Recognition of synthetic oligopeptides from nonstructural proteins NS1 and NS3 of dengue-4 virus by sera from dengue virus-infected children. Am J Trop Med Hyg. 1997;56:466–470. doi: 10.4269/ajtmh.1997.56.466. [DOI] [PubMed] [Google Scholar]
- 32.Gould EA. Buckley A. Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J Gen Virol. 1989;70:1605–1608. doi: 10.1099/0022-1317-70-6-1605. [DOI] [PubMed] [Google Scholar]
- 33.Gould EA. Buckley A. Groeger BK. Cane PA. Doenhoff M. Immune enhancement of yellow fever virus neurovirulence for mice: studies of mechanisms involved. J Gen Virol. 1987;68:3105–3112. doi: 10.1099/0022-1317-68-12-3105. [DOI] [PubMed] [Google Scholar]
- 34.Gould EA. Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 35.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 36.Halstead SB. Shotwell H. Casals J. Studies on the pathogenesis of dengue infection in monkeys: Clinical laboratory response to heterologous infection. J Infect Dis. 1973;128:15–22. doi: 10.1093/infdis/128.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Hamdan A. Green P. Mendelson E. Kramer MR. Pitlik S. Weinberger M. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002;4:160–162. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 38.Holzmann H. Utter G. Norrby E. Mandl CW. Kunz C. Heinz FX. Assessment of the antigenic structure of tick-borne encephalitis virus by the use of synthetic peptides. J Gen Virol. 1993;74:203–2035. doi: 10.1099/0022-1317-74-9-2031. [DOI] [PubMed] [Google Scholar]
- 39.Huang KJ. Yang YC. Lin YS, et al. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- 40.Imrie A. Meeks J. Gurary A. Sukhbataar M. Kitsutani P. Effler P. Zhao Z. Differential functional avidity of dengue virus-specific T cell clones for variant peptides representing heterologous and previously encountered serotypes. J Virol. 2007;81:10081–10091. doi: 10.1128/JVI.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs SC. Stephenson JR. Wilkinson GWG. Protection elicited by a replication-defective adenovirus vector expressing the tick-borne encephalitis virus non-structural glycoprotein NS1. J Genl Virol. 1994;75:2399–2402. doi: 10.1099/0022-1317-75-9-2399. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser R. Holzmann H. Laboratory findings in tick-borne encephalitis. Correlation with clinical outcome. Infection. 2000;28:78–84. doi: 10.1007/s150100050051. [DOI] [PubMed] [Google Scholar]
- 43.Karabatsos N. International Catalogue of Arboviruses. 3rd. American Society of Tropical Medicine and Hygiene; San Antonio, Texas: 1985. [Google Scholar]
- 44.Kesson AM. Blanden RV. Mullbacher A. The primary in vivo murine cytotoxic T cell response to the flavivirus, West Nile. J Gen Virol. 1987;68:2001–2006. doi: 10.1099/0022-1317-68-7-2001. [DOI] [PubMed] [Google Scholar]
- 45.Kimura-Kuroda J. Yasui K. Topographical analysis of antigenic determinants on envelope glycoprotein V3 (E) of Japanese encephalitis virus, using monoclonal antibodies. J Virol. 1983;45:124–132. doi: 10.1128/jvi.45.1.124-132.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King NJ. Getts DR. Getts MT. Rana S. Shrestha B. Kesson AM. Immunopathology of flavivirus infections. Immunol Cell Biol. 2007;85:33–34. doi: 10.1038/sj.icb.7100012. [DOI] [PubMed] [Google Scholar]
- 47.Kreil TR. Eibl MM. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J Virol. 1997;71:2921–2927. doi: 10.1128/jvi.71.4.2921-2927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurane I. Brinton MA. Samson AL. Ennis FA. Dengue virus-specific human CD4+, CD8-cytotoxic T cell clones: multiple patterns of virus cross-reactivity recognized by the NS3-specific T cell clones. J Virol. 1991;65:1823–1828. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kutubuddin M. Kolaskar AS. Galande S. Gore MM. Ghosh SN. Banerjee K. Recognition of helper T cell epitopes in envelope (E) glycoprotein of Japanese encephalitis, West Nile and Dengue viruses. Mol Immunol. 1991;28:149–154. doi: 10.1016/0161-5890(91)90098-5. [DOI] [PubMed] [Google Scholar]
- 50.Leitmeyer KC. Vaughn DW. Watts DM, et al. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Littaua R. Kurane I. Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 52.Lobigs M. Arthur CE. Mullbacher A. Blanden RV. The Flavivirus non-structural protein NS3 is a dominant source of cytotoxic T cell peptide determinants. Virology. 1994;202:195–201. doi: 10.1006/viro.1994.1335. [DOI] [PubMed] [Google Scholar]
- 53.Loke H. Bethell DB. Phuong CX, et al. Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis. 2001;184:1369–1373. doi: 10.1086/324320. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y. Blanden RV. Mullbacher A. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J Gen Virol. 1989;70:565–573. doi: 10.1099/0022-1317-70-3-565. [DOI] [PubMed] [Google Scholar]
- 55.Mathews JH. Allan JE. Roehrig JT. Brubaker JR. Hunt AR. T-helper cell and associated antibody response to synthetic peptides of the E glycoprotein of Murray Valley encephalitis virus. J Virol. 1991;65:5141–5148. doi: 10.1128/jvi.65.10.5141-5148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathew A. Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–313. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 57.Mathew A. Kurane I. Rothman AL. Zeng LL. Brinton MA. Ennis FA. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J Clin Invest. 1996;98:1684–1691. doi: 10.1172/JCI118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathur A. Kulshreshtha R. Rawat S. Chaturvedi UC. Memory suppressor T cells in latent Japanese encephalitis virus infection. Immunology. 1987;60:71–74. [PMC free article] [PubMed] [Google Scholar]
- 59.Matveeva VA. Popova RV. Kvetkova EA. Chernicina LO. Zlobin VI. Puchovskaya NM. Morozova OV. Antibodies against tick-borne encephalitis virus (TBEV) non-structural and structural proteins in human sera and spinal fluid. Immunol Lett. 1995;46:1–4. doi: 10.1016/0165-2478(95)00021-v. [DOI] [PubMed] [Google Scholar]
- 60.Miller JD. van der Most RG. Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 61.Monath TP. Yellow fever. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Vol. 5. CRC Press; Boca Raton, FL: 1989. pp. 139–123. [Google Scholar]
- 62.Monath TP. Arroyo J. Levenbook I. Zhang Z-X. Catalan J. Draper K. Guirakhoo F. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J Virol. 2002;76:1932–1943. doi: 10.1128/JVI.76.4.1932-1943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monath TP. Heinz FX. Flaviviruses, Chapter 31. In: Fields BN, editor; Knipe DM, editor; Howley PM, editor; Chanock RM, editor; Melnick JL, editor; Monath TP, editor; Roizmann B, editor. Fields Virology. Lippincott-Raven; Philadelphia: 1996. pp. 961–1034. [Google Scholar]
- 64.Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 65.Mongkolsapaya J. Duangchinda T. Dejnirattisai W, et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 66.Mongkolsapaya J. Dejnirattisai W. Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 67.Morens DM. Halstead SB. Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol. 1990;71:2909–2914. doi: 10.1099/0022-1317-71-12-2909. [DOI] [PubMed] [Google Scholar]
- 68.Murali-Krishna K. Ravi V. Manjunath R. Cytotoxic T lymphocytes raised against Japanese encephalitis virus: effector cell phenotype, target specificity and in vitro virus clearance. J Gen Virol. 1994;75(Pt 4):799–807. doi: 10.1099/0022-1317-75-4-799. [DOI] [PubMed] [Google Scholar]
- 69.Murphy FA, editor; Fauquet CM, editor; Bishop DHL, et al., editors. Virus Taxonomy: Classification and Nomenclature of Virus. Springer-Verlag; New York: 1995. pp. 415–421. [Google Scholar]
- 70.Murray K. Baraniuk S. Resnick M, et al. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134:1325–1332. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliphant T. Engle M. Nybakken GE, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillpotts RJ. Stephenson JR. Porterfield JS. Antibody-dependent enhancement of tick-borne encephalitis virus infectivity. J Gen Virol. 1985;66(Pt 8):1831–1837. doi: 10.1099/0022-1317-66-8-1831. [DOI] [PubMed] [Google Scholar]
- 73.Pierson TC. Diamond MS. Molecular mechanisms of antibody-mediated neutralization of Flavivirus infection. Expert Rev Mol Med. 2008;10:e12. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pierson TC. Fremont DH. Kuhn RJ. Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of Flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poland JD. Calisher CH. Monath TP. Downs WG. Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 76.Pruitt AA. Central nervous system infections in cancer patients. Semin Neurol. 2004;24:435–452. doi: 10.1055/s-2004-861538. [DOI] [PubMed] [Google Scholar]
- 77.Pugachev KV. Ocran SW. Guirakhoo F. Furby D. Monath TP. Heterogeneous nature of the genome of the ARILVAX yellow fever 17D vaccine revealed by consensus sequencing. Vaccine. 2002;20:996–999. doi: 10.1016/s0264-410x(01)00439-x. [DOI] [PubMed] [Google Scholar]
- 78.Putnak JR. Schlesinger JJ. Protection of mice against yellow fever virus encephalitis by immunization with a vaccinia virus recombinant encoding the yellow fever virus non-structural proteins, NS1, NS2a and NS2b. J Gen Virol. 1990;71:1697–1702. doi: 10.1099/0022-1317-71-8-1697. [DOI] [PubMed] [Google Scholar]
- 79.Querec T. Bennouna S. Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinhardt B. Jaspert R. Niedrig M. Kostner C. L'age-Stelvr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159–167. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 81.Rico-Hesse R. Dengue virus evolution and virulence models. Clin Infect Dis. 2007;44:1462–1466. doi: 10.1086/517587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson SE. Hull BP. Tomori O. Bele O. LeDuc JW. Esteves K. Yellow fever: A decade of reemergence. JAMA. 1996;276:1157–1162. [PubMed] [Google Scholar]
- 83.Roehrig JT. Bolin RA. Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 84.Roehrig JT. Hunt AR. Johnson AJ. Hawkes RA. Synthetic peptides derived from the deduced amino acid sequence of the E-glycoprotein of Murray Valley encephalitis virus elicit antiviral antibody. Virology. 1989;171:49–60. doi: 10.1016/0042-6822(89)90509-6. [DOI] [PubMed] [Google Scholar]
- 85.Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv Virus Res. 2003;60:397–419. doi: 10.1016/s0065-3527(03)60010-2. [DOI] [PubMed] [Google Scholar]
- 86.Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rothman AL. Kurane I. Ennis FA. Multiple specificities in the murine CD4+ and CD8+ T-cell response to dengue virus. J Virol. 1996;70:6540–6546. doi: 10.1128/jvi.70.10.6540-6546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sabin AB. Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 89.Schlesinger JJ. Brandriss MW. Walsh EE. Protection against 17D yellow fever encephalitis in mice by passive transfer monoclonal antibodies to the non-structural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985;135:2805–2809. [PubMed] [Google Scholar]
- 90.Shresta S. Kyle JL. Snider HM. Basavapatna M. Beatty PR. Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78:2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shresta S. Sharar KL. Prigozhin DM. Beatty PR. Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simmons CP. Dong T. Chau NV, et al. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J Virol. 2005;79:5665–5675. doi: 10.1128/JVI.79.9.5665-5675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sitati EM. Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stiasny KS. Kiermayr S. Holzmann H. Heinz FX. Cryptic properties of a cluster of dominant Flavivirus cross-reactive antigenic sites. J Virol. 2006;80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tesh RB. Guzman H. da Rosa AP, et al. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 96.Timofeev AV. Ozherelkov SV. Pronin AV. Deeva AV. Karaganova GG. Elbert LB. Stephenson JR. Immunological basis for protection in a murine model of tick-borne encephalitis by a recombinant adenovirus carrying the gene encoding the NS1 non-structural protein. J Gen Virol. 1998;79:689–695. doi: 10.1099/0022-1317-79-4-689. [DOI] [PubMed] [Google Scholar]
- 97.Timofeev AV. Butenko VM. Stephenson JR. Genetic vaccination of mice with plasmids encoding the NS1 non-structural protein from tick-borne encephalitis virus and dengue 2 virus. Virus Genes. 2004;28:85–97. doi: 10.1023/B:VIRU.0000012266.04871.ce. [DOI] [PubMed] [Google Scholar]
- 98.Valdes K. Alvarez M. Pupo M. Vazquez S. Rodriguez R. Guzman MG. Human dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol. 2000;7:856–857. doi: 10.1128/cdli.7.5.856-857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Hurk AF. Ritchie SA. Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 100.van der Most RG. Harrington LE. Giuggio V. Mahar PL. Ahmed R. Yellow fever virus 17D envelope and NS3 proteins are major targets of the antiviral T cell response in mice. Virology. 2002;296:117–124. doi: 10.1006/viro.2002.1432. [DOI] [PubMed] [Google Scholar]
- 101.Vaughan K. Blythe M. Greenbaum J. Zhang Q. Peters B. Doolan D. Sette A. Meta-analysis of immune epitope data for all Plasmodia: overview and applications for malarial immunobiology and vaccine-related issues. Parasite Immunol. 2009;32:78–97. doi: 10.1111/j.1365-3024.2008.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vazquez S. Guzman M. Guillen G, et al. Immune response to synthetic peptides of dengue prM protein. Vaccine. 2002;20:1823–1830. doi: 10.1016/s0264-410x(01)00515-1. [DOI] [PubMed] [Google Scholar]
- 103.Wallace MJ. Smith DW. Broom AK. Mackenzie JS. Hall RA. Shellam GR. McMinn PC. Antibody-dependent enhancement of Murray Valley encephalitis virus virulence in mice. J Gen Virol. 2003;84:1723–1728. doi: 10.1099/vir.0.18980-0. [DOI] [PubMed] [Google Scholar]
- 104.Wang E. Ni H. Xu R. Barrett AD. Watowich SJ. Gubler DJ. Weaver SC. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74:3227–3234. doi: 10.1128/jvi.74.7.3227-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang T. Scully E. Yin Z, et al. IFNγ-producing γδ-T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 106.Watts DM. Porter KR. Putvatana P. Vasquez B. Calampa C. Hayes CG. Halstead SB. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization 1. Dengue fact sheet. www.who.int/topics/dengue/en/ www.who.int/topics/dengue/en/
- 108.World Health Organization 2. Yellow fever fact sheet. www.who.int/topics/yellow_fever/en/ www.who.int/topics/yellow_fever/en/
- 109.Wong SJ. Boyle RH. Demarest VL, et al. Immunoassay targeting nonstructural protein 5 to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and from flavivirus vaccination. J Clin Microbiol. 2003;41:4217–4223. doi: 10.1128/JCM.41.9.4217-4223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao SY. Zhang H. Guzman H. Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- 111.Yauch LE. Shresta S. Mouse models of dengue virus infection and disease. Antiviral Res. 2008;80:87–93. doi: 10.1016/j.antiviral.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zarebski L. Vaughan K. Sidney J, et al. Analysis of epitope information related to Bacillus anthracis and Clostridium botulinum. Expert Rev Vaccines. 2008;7:55–74. doi: 10.1586/14760584.7.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zivna I. Green S. Vaughn DW, et al. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]