Abstract
The purpose of this project was to evaluate the preclinical efficacy of pyridoxine, or vitamin B6. Rats received a 3.0 mm unilateral controlled cortical impact (CCI) injury of the sensorimotor cortex or sham surgery. Treatment with vitamin B6 (600 or 300 mg/kg IP) or vehicle was administered at 30 min and 24 h post-CCI. Somatosensory dysfunction was evaluated with the vibrissae–forelimb placing and bilateral tactile adhesive removal tests. Sensorimotor dysfunction was evaluated with the locomotor placing and the forelimb asymmetry tests. On the forelimb asymmetry test both treatment groups displayed no asymmetry bias on any of the testing days post-CCI and were statistically no different than the shams. Both vitamin B6 groups displayed a significant improvement in behavioral performance on the locomotor placing test compared to the vehicle-treated group. Administration of 600 mg/kg also significantly reduced tactile adhesive removal latencies on days 2, 4, 6, and 12 post-CCI. Both treatment groups were improved in their rate of recovery post-CCI on the vibrissae–forelimb placing test, but only the recovery seen in the 600-mg/kg group was significantly improved compared to vehicle. Finally, the 600-mg/kg dose resulted in significant cortical sparing compared to the vehicle-treated group. In general, the effects of vitamin B6 on recovery of function were dose-dependent, with the 600-mg/kg dose consistently showing greater recovery than the 300-mg/kg dose. More experimental analyses are warranted to evaluate the potential preclinical efficacy and mechanistic action of vitamin B6.
Key words: B-vitamin, controlled cortical impact, recovery of function, treatment, traumatic brain injury, vita-nutrient, vitamin B6
Introduction
Traumatic brain injury (TBI) can lead to debilitating sensory, motor, and cognitive deficits in humans and animal models (Narayan et al., 2002; Saatman et al., 2008; Statler et al., 2001). Annually, from 1989 to 1998, the Centers for Disease Control and Prevention (CDC) estimated that more than 50,000 Americans suffered fatal outcomes following a head injury (Adekoya et al., 2002). Medical advances have helped to lower hospitalization rates by more than half, reducing mortality rates to 3.3% of all people who suffer a TBI (Thurman and Guerrero, 1999). This decrease in TBI-related deaths has increased the number of TBI survivors, many of whom will carry out the remainder of their lives with a reduced quality of life. Thus, TBI remains a major public health issue in the United States which is expected to worsen, considering the current level of military activity (Gondusky and Reiter, 2005). Extensive preclinical animal research has been conducted in hopes of developing effective treatments for TBI. Despite these efforts, no pharmacological or non-pharmacological treatment has been successfully translated into common clinical practice (Statler et al., 2001). Of the several therapies that have advanced as far as Phase II and III clinical trials, nearly all have failed to consistently improve functional outcome post-TBI (Narayan et al., 2002).
Many preclinical pharmacological treatments that attenuate post-injury sequelae have been shown to provide neuroprotection in various injury models (Saatman et al., 2008; Vink and Nimmo, 2009). Included in these studies are vita-nutrients such as magnesium, nicotinimide (vitamin B3), vitamin E, and riboflavin (vitamin B2), all of which have shown significant therapeutic effects on recovery of function (Barbre and Hoane, 2006; Hoane and Barth, 2001; Hoane et al., 2005, 2008b; Van Den Heuvel and Vink, 2004). Vita-nutrients attract investigative attention because they are natural compounds that in general exhibit less severe side effects than other pharmacological treatments, and they tend to offer multiple mechanisms of action for neuroprotection (Hoane, 2004; Hoane et al., 2003, 2005).
Vitamin B3 has been shown to reduce astrocytic reactivity, improve behavioral performance on cognitive and sensorimotor tasks, reduce edema formation, reduce lesion volume, and maintain blood–brain barrier integrity post-TBI (Cernak et al., 2002; Hoane et al., 2003, 2006a, 2006b, 2006c, 2008a, 2008b; Holland et al., 2008; Quigley et al., 2009). Recent reports describing the multiple mechanisms of action of vitamin B3 have been published and point to antioxidant activity at the level of the mitochondrion, PARP inhibition, and general neuroprotection through the sirtuin receptor system (Chong and Maiese, 2008; Maiese et al., 2009). Other vita-nutrients such as riboflavin (vitamin B2), a strong antioxidant, and vitamin E have also been reported to improve behavioral outcome, reduce edema formation, provide antioxidative protection, and reduce astrocytic reactivity, as well as protect against lipid peroxidation following TBI and ischemia (Barbre and Hoane, 2006; Betz et al., 1994; Hoane et al., 2005; Inci et al., 1998). Although of these all appear to hold great potential for the treatment of TBI, it is crucial to continue to develop and test new vita-nutrient-based treatments (Hoane et al., 2003). Given the beneficial effects that have been found with some of the B-vitamins, it is likely that others may also hold therapeutic promise. A strong candidate vita-nutrient is pyridoxine, or vitamin B6, which has not been previously studied in conjunction with TBI.
The background on vitamin B6 suggests that it may play an interesting role in TBI. Deficiencies of pyridoxine can result in ischemia, edema formation, free radical production, neurotoxicity, and long-term cognitive impairments (Bender, 1999; Cabrini et al., 1998; Cuskelly et al., 2001; Friso et al., 2004; Kelly et al., 2003). Thus pyridoxine is essential for proper functioning of the mechanisms that control neurotoxicity, edema formation, free radical production, and mediate cognitive function. Considering that these deficiency-induced problems are also commonly observed post-injury, it is reasonable to assume that increasing overall pyridoxine levels after TBI may have a neuroprotective effect. Furthermore, more active forms of vitamin B6 have been shown to be neuroprotective following ischemia-induced brain damage (Hwang et al., 2007).
The purpose of the present study was to evaluate the potential neuroprotective effect of vitamin B6 administration following a unilateral cortical contusion of the sensorimotor cortex in rats. Although it has never been used in a rodent model of TBI, there is some dosing literature for vitamin B6 in rodents. Typically dosing has ranged from a high-dose range (1200 mg/kg IP), an intermediate-dose range (600 mg/kg IP), and a low-dose range (200 mg/kg IP) in a model of transient ischemia in the gerbil, where beneficial effects were attributed to GABAergic modulation in the hippocampus (Hwang et al., 2007). The current study examined vitamin B6 within the intermediate- and low-dose ranges, and assessed recovery of function following TBI to determine the initial preclinical efficacy of vitamin B6.
Methods
Subjects
Thirty-one adult male Sprague-Dawley rats (weight 325–350 g) were individually housed in standard acrylic cages on a 12-h light/dark schedule with food and water freely available. All experimental procedures were reviewed and approved by the Southern Illinois University Institutional Animal Care and Use Committee, and the study was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care. All researchers were blind to individual group assignments throughout testing. One animal was removed from the low-dose group because of severe morbidity post-injury and was not replaced.
Surgery
The surgical procedure was performed under aseptic conditions. The controlled cortical impact (CCI) model utilized was based on previous studies (Hoane et al., 2008b; Quigley et al., 2009). Animals were anesthetized with a combination of isofluorane (2–4%) and oxygen (0.8 L/min). Normal body temperature (37°C) was maintained with a warm water recycling pump and bed system during the surgical procedure and recovery. Following a midline incision, a 4.0-mm craniotomy was made over the sensorimotor cortex portion of the left cortex 0.5 mm anterior to the bregma and −4.0 mm lateral to the midline (Kozlowski et al., 2004). An electromagnetic CCI device fitted with a 3.0-mm impactor tip was positioned at an angle of 18° to ensure that the impactor surface and cortex were parallel. The injury was induced with 2.0 mm compression of the cortex at a velocity of 2.7 m/sec. Following surgery, the animals were sutured closed and placed in a heated recovery chamber until they regained locomotor ability, after which they were placed back in their home cages. Rats receiving sham surgery were anesthetized, surgically prepared, and received a midline incision, a craniotomy, and suturing, before being placed in the recovery box.
Drug administration
The animals were randomly assigned to one of four groups: vitamin B6 600 mg/kg IP (n = 8); vitamin B6 300 mg/kg IP (n = 7); vehicle (1 mL/kg saline [n = 8]), and sham (1 mL/kg saline [n = 8]). Thirty minutes post-CCI, the animals were treated with vitamin B6 (P4722; Sigma-Aldrich Co., St. Louis, MO) or vehicle, followed by an identical booster administration 24 h later. The vitamin B6 was dissolved in 0.9% sterile saline.
Vibrissae–forelimb placing task
Sensorimotor dysfunction was evaluated with the vibrissae-mediated forelimb placing reaction (Hoane et al., 2004, 2008b; Schallert and Woodlee, 2005). Baseline performance was recorded prior to injury. Each rat was held by the trunk, while ensuring that the forelimbs were free to move. One side of the rat was oriented parallel to an acrylic glass surface and then slowly moved until the vibrissae on that side touched the surface. In an uninjured rat, a reliable lateralized placing response should be elicited each time the vibrissae made contact with the surface. A successful forelimb-placing response was recorded if the animal raised its forelimb and placed it on the surface in response to stimulation of the vibrissae ipsilateral to that forelimb. Each rat was given 10 trials for each forelimb per testing day. If a placing response was not elicited within 5 sec of vibrissae stimulation, the trial was recorded as unsuccessful. The animals were tested on postoperative days 2, 4, 6, 8, 10, 12, 14, 18, and 21.
Bilateral tactile adhesive removal task
Somatosensory dysfunction was also evaluated following injury with the bilateral tactile adhesive removal test, as has been previously described (Barth et al., 1990; Hoane et al., 2004, 2008b; Schallert and Woodlee, 2005). The rats were tested twice daily on postoperative days 2, 4, 6, 8, 10, 12, 14, 18, and 21. A small, bright orange adhesive disc 0.5 inches in diameter was applied to the radial aspect of each forelimb. Latency to remove the adhesive from each forelimb was recorded for each animal. Trials were ended when the rat either removed both stimuli or after 2 min had elapsed.
Locomotor placing task
This test assessed recovery of coordinated limb placing during locomotion, and has been previously discussed in detail elsewhere (Barth et al., 1990; Hoane et al., 2004, 2008b; Schallert and Woodlee, 2005). The rats were tested once per test day on post-CCI days 3, 9, 15, and 21. During testing each rat was placed on an elevated grid floor (56.5 × 54.0 cm) with openings of 3.0 × 3.0 cm. The rats were allowed to explore for 3 min while being videotaped. While traversing the grid an intact animal occasionally made a “foot-fault,” occurring when a rat inaccurately placed a forelimb and it fell through an opening in the grid floor. Intact rats initially made foot-faults with both forelimbs, but these faults declined over time. During video analysis the number of foot-faults with both forelimbs were recorded. The data are represented for the locomotor placing test as total foot-faults (contralateral foot-faults – ipsilateral foot-faults).
Forelimb asymmetry task
This task measures somatosensory dysfunction following injury. The animals were placed in a glass chamber (25 × 25 × 25 cm) and were allowed to explore for 5 min while being videotaped (Becerra et al., 2007; Hoane et al., 2008b). While exploring the chamber, animals typically rear, pressing their forelimbs against the vertical surface (Schallert and Woodlee, 2005). Intact animals use their forelimbs (right and left) equally, and do not show an asymmetry bias. During video analysis, the number of times each (left or right) forelimb and both forelimbs were used for support on the vertical glass wall was recorded. One score was assigned per rear. The data are represented as the percentage of preference for the contralateral limb, calculated using the following formula: [(contralateral forelimb/(contralateral + ipsilateral)) × 100]. A derived score of 50% reflects equal use of both forelimbs, and a score greater than 50% represents a reliance on the intact forelimb and increasing neglect of the affected forelimb. The animals were tested on post-CCI days 3, 9, 15, and 21.
Histology
At 23 days post-injury, the rats were anesthetized with urethane and transcardially perfused with 0.9% phosphate-buffered saline (PBS), followed by 10% phosphate-buffered formalin (PBF). The brains were post-fixed in PBF following removal from the cranium. A 30% sucrose solution was used to cryopreserve the brains for 3 days prior to frozen sectioning. Serial sections (40 μm thick) were sliced using a sliding microtome with an electronic freezing stage and collected in phosphate buffer. A series of coronal sections, mounted on gelatin-subbed microscope slides, were stained with a cresyl violet nissl stain, dehydrated, and cover-slipped.
Lesion analysis
The extent of the lesion was analyzed with an Olympus microscope (BX-51; Olympus Corp., Center Valley, PA) and DP-70 camera. Images of the sections throughout the extent of the injury were captured using the digital capturing system, and area measurements of the lesioned tissue were determined using ImageTool software. The Calvalieri method was used to calculate the volumes of the remaining, intact, and healthy cortex at 1.70, 1.00, and 0.00 mm relative to the bregma (Paxinos and Watson, 2005). The number of sections and the section thickness (40 μm) were multiplied by the mean area of the lesion cavity (Coggeshall, 1992). The extent of cortical injury was measured by calculating the volume of remaining cortex at the level of the injury by calculating the percent reduction in the ipsilateral cortex compared to the contralateral cortex using the formula (1 − (ipsilateral/contralateral) × 100) (Hoane et al., 2007, 2008b; Quigley et al., 2009).
Statistical analysis
For the behavioral data factorial analysis of variance (ANOVA) tests were performed using the procedures for general linear models (SPSS 15.0 for Windows; SPSS Inc., Chicago, IL) with the option for repeated measures where appropriate. Huynh-Feldt (HFP) probabilities were used for the assessment of the repeated measures factor and corrected degrees of freedom are provided. The HFP is a more conservative estimate of probability, which reduces the probability of committing a type I error. Post-hoc analyses were conducted using Fischer's least significant difference (LSD) for comparison of means. Anatomical data were analyzed with one-way ANOVAs and appropriate post-hocs. For all statistical tests, a p value of 0.05 was considered significant.
Results
Vibrissae–forelimb placing
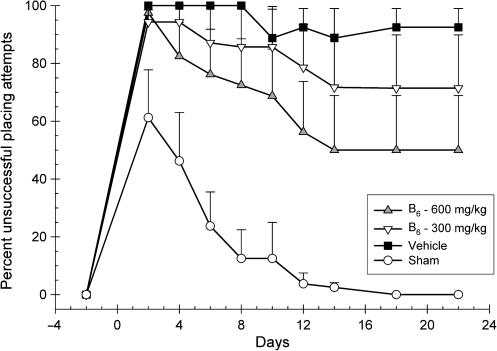
Placing responses were analyzed with a 4 × 9 repeated-measures ANOVA for both forelimbs. Group (600 mg/kg vitamin B6, 300 mg/kg vitamin B6, vehicle, and sham), and day (2, 4, 6, 8, 10, 12, 14, 18, and 22 post-CCI), served as the between-group and within-group factors, and day was treated as the repeated measure. The rats displayed no deficits in their ability to successfully place with the ipsilateral forelimb (p > 0.05). An injury effect was observed with the contralateral forelimb as represented by the significant main effect for group [F(3,27) = 12.21; p < 0.001; Fig. 1]. There was also a significant main effect for day [F(2.78,75.17) = 10.31; p < 0.001], indicating significant recovery over time. However, the interaction effect was not significant [F(8.35,75.17) = 1.24; p > 0.29]. Though dose-dependent recovery was observed in both treatment groups, post-hoc analysis of the significant main effect of group found that only the intermediate-dose group performed statistically better than vehicle [LSD(14) = 27.92; p < 0.05]. On day 14, the intermediate-dose group displayed approximately a 50% deficit, the low-dose group displayed approximately a 75% deficit, and the vehicle-treated group continued to display approximately a 95% deficit in successful placing attempts. These deficits remained consistent through the conclusion of testing.
FIG. 1.
Effects of administration of vitamin B6 (600 or 300 mg/kg) or vehicle on the vibrissae–forelimb placing test. Plotted are the mean (±standard error of the mean) percentages of unsuccessful placing attempts. Significantly fewer unsuccessful placing attempts were observed over time for both treatment groups in a dose-dependent fashion, but only improvement in the 600-mg group was significant compared to vehicle. All groups appeared to reach a steady state of performance on this task by approximately day 14 post-injury.
Tactile adhesive removal
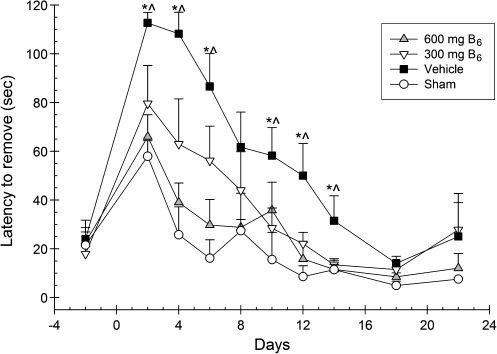
Latencies to remove the tactile adhesive from the contralateral forelimb were analyzed using a 4 × 9 ANOVA. Group (600 mg/kg vitamin B6, 300 mg/kg vitamin B6, vehicle, and sham), and day (2, 4, 6, 8, 10, 12, 14, 18, and 22 post-CCI) served as the between-group and within-group factors, and day was treated as the repeated measure. A significant main effect for day [F(7.29,196.85) = 37.23; p < 0.001] was observed, indicating a decrease in removal latencies over time. The effect of group was significant [F(3,27) = 6.82; p < 0.001].There was also a significant interaction for group × day, indicating significant recovery [F(21.87,196.85) = 2.52; p < 0001]. Post-hoc analyses revealed that when compared to vehicle-treated animals, the 600-mg/kg group had significantly lower latencies to remove the tactile adhesive from the contralateral limb on days 2, 4, 6, 12, and 14 post-CCI [LSD(14) = 46.69; p < 0.005]; [LSD(14) = 69.13; p < 0.001]; [LSD(14) = 56.81; p < 0.001]; [LSD(14) = 34.69; p < 0.004]; and [LSD(14) = 19.94, p < 0.02], respectively (Fig. 2). In addition the 300-mg/kg group had significantly lower latencies compared to vehicle on days 2, 4, 10, 12, and 14 post-CCI [LSD(13) = 33.05; p < 0.05]; [LSD(13) = 45.26; p < 0.01]; [LSD(13) = 29.76; p < 0.05]; [LSD(13) = 11.55; p < 0.02]; and [LSD(13) = 18.07; p < 0.04], respectively.
FIG. 2.
Effects of administration of vitamin B6 (600 and 300 mg/kg) or vehicle on the bilateral tactile adhesive removal test. Plotted are the mean (±standard error of the mean) latencies to remove the adhesive from the contralateral forelimb. Significant improvements in latencies to remove the adhesive were observed in the 600-mg group (∧p < 0.05), but not the 300-mg group (*p < 0.05).
Forelimb asymmetry
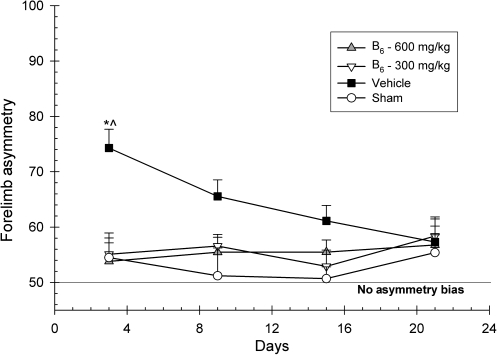
A 4 × 4 ANOVA was implemented to analyze the extent of forelimb asymmetry. Group (600 mg/kg vitamin B6, 300 mg/kg vitamin B6, vehicle, and sham), and day (3, 9, 15, and 21 post-CCI) served as the between-group and within-group factors, and day was treated as the repeated measure. Forelimb asymmetries did not significantly change over time, as there was no significant main effect for day [F(3,81) = 1.43; p > 0.05]. However, there was a significant main effect for group [F(3,27) = 6.20; p < 0.002]. The group × day interaction was also significant [F(9.00,81.00) = 2.00; p < 0.05]. Post-hoc analysis revealed that the 600-mg/kg group was significantly different from vehicle on days 3 and 9, and that the 300 mg/kg group was significantly different from vehicle on days 3 and 15 (p < 0.05). Furthermore, on all four testing days, both treatment groups were statistically indistinguishable from the sham group (Fig. 3).
FIG. 3.
Effects of administration of vitamin B6 (600 and 300 mg/kg) or vehicle on the forelimb asymmetry test. Plotted is the percentage (±standard error of the mean) of contralateral forelimb use. Vitamin B6 eliminated the initial behavioral deficit in both treatment groups (*p < 0.05). On all days, both treatment groups were not significantly different from shams.
Locomotor placing
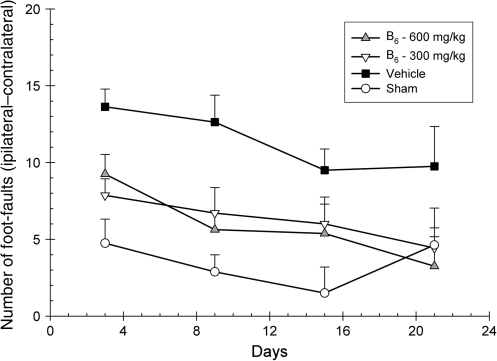
The number of foot-faults occurring with the contralateral forelimb were analyzed using a 4 × 4 ANOVA. Group (600 mg/kg vitamin B6, 300 mg/kg vitamin B6, vehicle, and sham) and day (3, 9, 15, and 21 post-CCI) served as the between-group and within-group factors, with day being treated as the repeated measure. While traversing the grid, the rats made fewer foot-faults over time, as the main effect for day was significant [F(2.55,68.92) = 6.15; p < 0.002]. The main effect for group [F(3,27) = 5.36; p < 0.005] was also significant, indicating the presence of an injury effect. There was not a significant group × day interaction [F(7.66,68.92) = 1.33; p > 0.05], suggesting that the rate of recovery between groups was not significant. However, post-hoc analysis of the significant main effect of group revealed that both the 600- and 300-mg/kg groups displayed significantly fewer foot-faults than did vehicle-treated animals [LSD(14) = 5.50; p < 0.01] and [LSD(13) = 5.13; p < 0.01], respectively (Fig. 4).
FIG. 4.
Effects of administration of vitamin B6 (600 or 300 mg/kg) or vehicle on the locomotor placing test. Plotted are the mean (±standard error of the mean) numbers of foot-faults with the contralateral forelimb. Significant improvements in behavioral performance were observed in both the 600- and 300-mg/kg vitamin B6–treated animals compared to vehicle-treated animals.
Lesion analysis
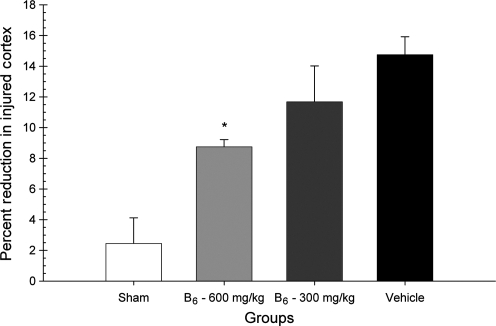
To assess the extent of cortical tissue loss, the percent volume reduction was analyzed in a one-way ANOVA, with group (600 mg/kg vitamin B6, 300 mg/kg vitamin B6, vehicle, and sham) as the factor in the analysis. There was a significant difference in the extent of cortical volume lost; the main effect for group was significant [F(3,27) = 12.40; p < 0.001]. Post-hoc analysis revealed that the 600-mg/kg group showed a significant reduction in lesion cavity magnitude compared to the vehicle-treated group [LSD(14) = 5.99; p < 0.001; Fig. 5). Representative histology is presented in Figure 6.
FIG. 5.
Effects of administration of vitamin B6 (600 or 300 mg/kg) or vehicle on the reduction of lesion volume. Plotted are the mean (±standard error of the mean) percent reductions in lesion volumes. Treatment with vitamin B6 significantly reduced the cortical volume loss in a dose-dependent manner, with only the 600-mg group being significantly different from vehicle-treated animals (*p < 0.05).
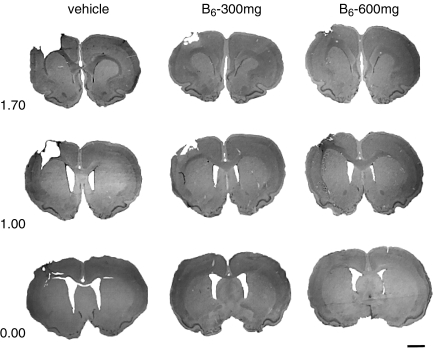
FIG. 6.
Shown are coronal brain sections (40 μM, cresyl violet) at three levels through the injury cavity (1.70, 1.00, and 0.00 mm relative to the bregma). A large cortical cavity can be seen in the representative vehicle-treated brain compared to the vitamin B6–treated brains (scale bar = 2.0 mm).
Discussion
The present data indicate that administration of vitamin B6 following unilateral CCI of the sensorimotor cortex leads to a dose-dependent effect on recovery of function. Although both doses provided some degree of improvement, functional recovery and cortical loss were most improved following administration of the intermediate dose (600 mg/kg) compared to the low dose (300 mg/kg). More specifically, the intermediate dose of vitamin B6 significantly reduced the initial behavioral deficit seen on the tactile adhesive removal test, forelimb asymmetry test, and locomotor placing test, while the low dose significantly reduced the initial behavioral deficit seen on the forelimb asymmetry and foot-fault tests. No noticeable forelimb asymmetry bias was observed for either treatment group, as both the intermediate-dose and low-dose groups were behaviorally indistinguishable from shams. Vitamin B6 increased the rate of recovery for the intermediate-dose group but not the low-dose group, in the bilateral tactile adhesive removal test. It should be noted that although the animals in the low-dose group were not significantly different than those in the vehicle-treated groups on the bilateral tactile adhesive removal test, they were statistically indistinguishable from intermediate-dose animals, which performed significantly better than vehicle-treated rats.
On the tactile adhesive removal test, a consistent dose-dependent behavioral response was also observed. The difference between the low-dose group and the vehicle-treated group was half as strong as the difference between the intermediate-dose group and the vehicle-treated group. In addition, recovery was seen in a dose-dependent fashion in the vibrissae–forelimb placing test. Once the rat's behavior had reached a steady state of recovery by approximately day 14, the difference between the low dose group and the vehicle-treated group was half as strong as the difference between the intermediate-dose group and the vehicle-treated group. Furthermore, the lesion analysis revealed a dose-dependent trend similar to what was observed with the vibrissae–forelimb placing test data. The difference between the low-dose group and the vehicle-treated group was again half as strong as the difference between the intermediate-dose group and the vehicle-treated group. Taken together, the vibrissae–forelimb placing test data and the lesion analysis data indicate that for doses administered at acute time points (and not exceeding 600 mg/kg vitamin B6 per day), an increase in dose may result in a proportional increase in the degree of neuroprotection.
Vitamin B6 possesses a variety of physiological actions that may prove to be neuroprotective. Although elucidating the physiological mechanism of action underlying vitamin B6's neuroprotective effect was not the intent of this project, these potentially neuroprotective mechanisms combined with the case literature were the basis of this investigation. Vitamin B6 is a precursor for pyridoxal 5′-phosphate, which upregulates erythrocyte affinity for O2 (Shen et al., 2010), independent of cerebral blood flow (CBF). Administration of vitamin B6 post-TBI may accelerate hemoglobin (Hgb) production, thus providing increased availability of oxygen to tissue subject to infarction. Upregulating erythrocyte affinity for oxygen may help to compensate for the hypoxic condition brought on by the TBI-induced reduction in CBF, thus preventing the remaining tissue from entering an anoxic state. Vitamin B6 also aids in glycogenolysis, and vitamin B6 deficiencies reduce glycogen phosphorylase (GPh) activity in the rodent (Okada et al., 1991; Oka, 2001). GPh converts stored glycogen into glucose-1-phosphate, which is phosphorylated by the enzyme phosphoglucomutase, resulting in glucose-6-phosphate (G6P). Administering vitamin B6 may upregulate GPh activity, thereby increasing G6P and glycolytic metabolism, which may facilitate ATP production in damaged or hypoxic tissues.
Another possible mechanism underlying vitamin B6's neuroprotective effect involves its anti-excitotoxic potential. It has long been understood that vitamin B6 is crucial to the proper functioning of the glutamate-degrading enzyme glutamate decarboxylase (GAD), and that heightened levels of vitamin B6 may also significantly increase GAD activity (Roberts et al., 1964). Vitamin B6 is also crucial in the breakdown of homocysteine, a compound with excitotoxic potential similar to glutamate. Homocysteine can cause direct neurotoxicity via NMDA receptor activation, and stroke and TBI can increase homocysteine levels in the CNS (Lipton et al., 1997). Furthermore, it has been reported that homocysteine can induce neuronal apoptosis in rat hippocampal neurons, in addition to increasing neuronal vulnerability to excitotoxicity (Kruman et al., 1997). Thus vitamin B6 has many possible mechanisms by which it may have improved recovery of function and prevented tissue loss in the present studies. Future studies are needed to elucidate their mechanisms of action.
Unlike some of the other B-vitamins, there is a degree of care that should be taken with vitamin B6 therapy. Long-term administration of high doses has been correlated with neuropathy; however, the mechanism of action has not been fully uncovered and has yielded disparate results (Perry et al., 2004). What has been shown is that vitamin B6 intoxication manifests primarily as structural and functional changes in large sensory neurons, with the secondary effects being on axonal processes within the dorsal root ganglia and the trigeminal nerve (Parry and Bredesen, 1985; Xu et al., 1989). The primary changes were characterized by the formation of swollen membranous profiles in the axon hillock and initial segment of the axon. This change occurred within 24 h of exposure, followed by axonal degeneration that preceded a secondary degeneration of the axonal processes (Krinke et al., 1985). It has also been reported that chronic, daily doses of 200 mg of vitamin B6 can also induce this syndrome (Krinke et al., 1980). No studies have reported irreversible sensory neuropathy after only 2 days of administration at the 600-mg/kg dose. Our current dosing regimen of 600 or 300 mg/kg given twice within 24 h is well below the levels at which reversible neuropathies have occurred in the literature. No observable sensory neuropathy occurred following either dose of vitamin B6 in the current study. It is likely that the sensitive battery of sensorimotor tests would have detected any sensory disturbances caused by the peripheral neuropathology.
The data presented here indicate that the effects of vitamin B6 administration following unilateral CCI of the sensorimotor cortex in rats is neuroprotective and improves both behavioral and anatomical outcomes. The beneficial effects were in general dose-dependent, with the 600-mg/kg dose consistently showing better recovery of behavioral function and neuroprotection than the 300-mg/kg dose. More studies are warranted to evaluate specific dosing parameters, most importantly the window of opportunity for treatment. The initiation of treatment at 30 min post-CCI in the present study has limited clinical utility; however, it has served as proof of principle for vitamin B6's neuroprotective ability. Vitamin B3 was initially demonstrated to be beneficial at 30 min post-CCI, and has since been shown to have a fairly wide window of opportunity (6–8 h) following CCI (Hoane et al., 2003, 2008b). Thus, more research is needed to determine if vitamin B6 represents a potential therapeutic avenue for the treatment of TBI.
Acknowledgments
Funding was provided by National Institutes of Health grant NS045647.
Author Disclosure Statement
No competing financial interests exist.
References
- Adekoya N. Thurman D. White D. Webb K. Surveillance for traumatic brain injury deaths—United States. Morb. Mortal. Weekly Rep. 2002;51:1–14. [PubMed] [Google Scholar]
- Barbre A.B. Hoane M.R. Magnesium and riboflavin combination therapy following cortical contusion injury in the rat. Brain Res. Bull. 2006;69:639–646. doi: 10.1016/j.brainresbull.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Barth T.M. Grant M.L. Schallert T. Effects of MK-801 on recovery from sensorimotor cortex lesions. Stroke. 1990;21:III-153–III-157. [PubMed] [Google Scholar]
- Becerra G.D. Tatko L.M. Pak E.S. Murashov A.K. Hoane M.R. Transplantation of GABAergic neurons but not astrocytes induces recovery of sensorimotor function in the traumatically injured brain. Behav. Brain Res. 2007;179:118–125. doi: 10.1016/j.bbr.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender D. Non-nutritional uses of vitamin B6. Br. J. Nutr. 1999;81:7–20. [PubMed] [Google Scholar]
- Betz A.L. Ren X.D. Ennis S.R. Hultquist D.E. Riboflavin reduces edema in focal cerebral ischemia. Acta Neurochir. (Wien.) 1994;60:314–317. doi: 10.1007/978-3-7091-9334-1_84. [DOI] [PubMed] [Google Scholar]
- Cabrini L. Bergami R. Fiorentini D. Marchetti M. Landi L. Tolomelli B. Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem. Mol. Biol. Int. 1998;46:689–697. doi: 10.1080/15216549800204222. [DOI] [PubMed] [Google Scholar]
- Cernak I. O'Connor C. Vink R. Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp. Brain Res. 2002;147:193–199. doi: 10.1007/s00221-002-1245-z. [DOI] [PubMed] [Google Scholar]
- Chong Z.Z. Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr. Neurovasc. Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R.E. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Cuskelly G. Stacpoole P. Williamson J. Baumgartner T. Gregory J. Deficiencies of folate and vitamin B(6) exert distinct effects on homocysteine, serine and methionine kinetics. Am. J. Physiol. Endocrinol. Metab. 2001;281:182–190. doi: 10.1152/ajpendo.2001.281.6.E1182. [DOI] [PubMed] [Google Scholar]
- Friso S. Girelli D. Martinelli N. Olivieri O. Lotto V. Bozzini C. Pizzolo G. Faccini G. Beltrame F. Corrocher R. Low plasma vitamin B6 concentrations and modulation of coronary artery disease risk. Am. J. Clin. Nutr. 2004;79:992–998. doi: 10.1093/ajcn/79.6.992. [DOI] [PubMed] [Google Scholar]
- Gondusky J.S. Reiter M.P. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil. Med. 2005;170:546–549. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Akstulewicz S.L. Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J. Neurotrauma. 2003;20:1189–1198. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Barth T.M. The behavioral and anatomical effects of MgCl2 therapy in an electrolytic lesion model of cortical injury in the rat. Magnes. Res. 2001;14:51–63. [PubMed] [Google Scholar]
- Hoane M.R. Becerra G.D. Shank J.E. Tatko L. Pak E.S. Smith M. Murashov A.K. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J. Neurotrauma. 2004;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Gilbert D.R. Holland M.A. Pierce J.L. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci. Lett. 2006a;408:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Kaplan S.A. Ellis A.L. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006b;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Magnesium therapy and recovery of function in experimental models of brain injury and neurodegenerative disease. Clin Calcium. 2004;14:65–70. [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Holland M.A. Anderson G.D. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008a;154:861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Holland M.A. Birky N.D. Dang T. Vitek M.P. McKenna S.E. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J. Neurotrauma. 2007;24:1108–1118. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Kaufman N.A. Beare J.E. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid. Med. Cell Longev. 2008b;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Tan A.A. Pierce J.L. Anderson G.D. Smith D.C. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J. Neurotrauma. 2006c;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Hoane M. Wolyniak J. Akstulewicz S. Administration of riboflavin improves behavioral outcome and reduces edema formation and GFAP expression following traumatic brain injury. J. Neurotrauma. 2005;22:1112–1122. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- Holland M.A. Tan A.A. Smith D.C. Hoane M.R. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J. Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Hwang I. Yoo K. Li H. Lee B. Suh H. Kwon Y. Won M. Time course of changes in pyridoxal 5′-phosphate (vitamin B6 active form) and its neuroprotection in experimental ischemic damage. Exp. Neurol. 2007;206:114–125. doi: 10.1016/j.expneurol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Inci S. Ozcan O.E. Kilnic K. Time-level relationship for lipid peroxidation and the protective effect of alpha-tocopherol in experimental mild and severe brain injury. Neurosurgery. 1998;43:330–335. doi: 10.1097/00006123-199808000-00095. [DOI] [PubMed] [Google Scholar]
- Kelly P.F. Shih V.E. Kistler J.P. Barron M. Lee H. Mandell R. Furie K.L. Low vitamin B6 but not homocysteine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke. 2003;34:51–54. doi: 10.1161/01.STR.0000071109.23410.AB. [DOI] [PubMed] [Google Scholar]
- Kozlowski D.A. Nahed B.V. Hovda D.A. Lee S.M. Paradoxical effects of cortical impact injury on enrivonmentally enriched rats. J. Neurotrauma. 2004;21:513–519. doi: 10.1089/089771504774129856. [DOI] [PubMed] [Google Scholar]
- Krinke G. Naylkor D.C. Skorpi V. Pyridoxine megavitaminosis: an analysis of the early changes induced with massive doses of vitamin B6 in rat primary sensory neurons. J. Neuropathol. Exp. Neurol. 1985;44:117–129. [PubMed] [Google Scholar]
- Krinke G. Schaumburg H. Spencer P. Pyridoxine mega-vitaminosis produces degeneration of peripheral sensory neurons (sensory neuropathy) in the dog. Neurotoxicology. 1980;2:13–24. [PubMed] [Google Scholar]
- Kruman I. Bruce-Keller A.J. Bredesen D.E. Waeg G. Mattson M.P. Evidence that 4-hydroxynonenal mediates oxidative stress induced neuronal apoptosis. J. Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S.A. Kim W.K. Choi Y.B. Kumar S. D'emilia D.M. Rayudu P.V. Arnell D.R. Stamler J.S. Neurtoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. Chong Z.Z. Hou J. Shang Y.C. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M. Ishikawa K. Watanabe K. Effect of vitamin B6 deficiency on glycogen metabolism in the skeletal muscle, heart, and liver of rats. J. Nutr. Sci. Vitaminol. 1991;37:349–357. doi: 10.3177/jnsv.37.349. [DOI] [PubMed] [Google Scholar]
- Oka T. Modulation of gene expression by vitamin B6. Nutr. Res. Rev. 2001;14:257–266. doi: 10.1079/NRR200125. [DOI] [PubMed] [Google Scholar]
- Parry G. Bredesen D.E. Sensory neuropathy with low-dose pyridoxine. Neurology. 1985;35:1466–1468. doi: 10.1212/wnl.35.10.1466. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; New York: 2005. [DOI] [PubMed] [Google Scholar]
- Perry T.A. Weerasuriya A. Mouton P.R. Holloway H.W. Greig N.H. Pyridoxine-induced toxicity in rats: a stereological quantification of the sensory neuropathy. Exp. Neurol. 2004;190:133–144. doi: 10.1016/j.expneurol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Quigley A. Tan A.A. Hoane M.R. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–148. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. Wein J. Simonsen D.J. Gamma-aminobutyric acid (GABA), vitamin B6 and neuronal function. Vitam. Horm. 1964;22:503–559. [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T. Woodlee M.T. Whishaw I.Q. Kolb B. The Behavior of the Laboratory Rat. Oxford University Press; New York: 2005. Orienting and Placing; pp. 129–140. [Google Scholar]
- Shen J. Lai C.Q. Mattei J. Ordovas J.M. Tucker K.L. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2010;91:337–342. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler K.D. Jenkins L.W. Dixon C.E. Clark R.S. Marion D.W. Kochanek P.M. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C. Vink R. The role of magnesium in traumatic brain injury. Clin. Calcium. 2004;14:9–14. [PubMed] [Google Scholar]
- Vink R. Nimmo A.J. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6:28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. Sladky J.T. Brown M.J. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]