Abstract
It has been 33 years since I first presented results of genetic experiments that established the gene transposition model as the mechanism of mating-type switching in the budding yeast Saccharomyces cerevisiae at the Cold Spring Harbor Laboratory (CSHL) Yeast Genetics meeting in August 1977. Over two decades ago the Genetics Perspectives editors solicited a perspective on my participation in the studies that deciphered the mechanism of mating-type switching and revealed the phenomenon of gene silencing in yeast. Although flattered at the time, I thought that preparation of such an article called for a more seasoned researcher who had benefitted from seeing his contributions stand the test of time. Now realizing that our discovery of the transposition of a mutation from the HMα locus into the MAT (mating type) locus has provided the genetic evidence that established the gene transposition model, and having witnessed our conclusions confirmed by subsequent molecular studies, I decided that perhaps this is a good time to recount the chronology of events as they unfolded for me decades ago.
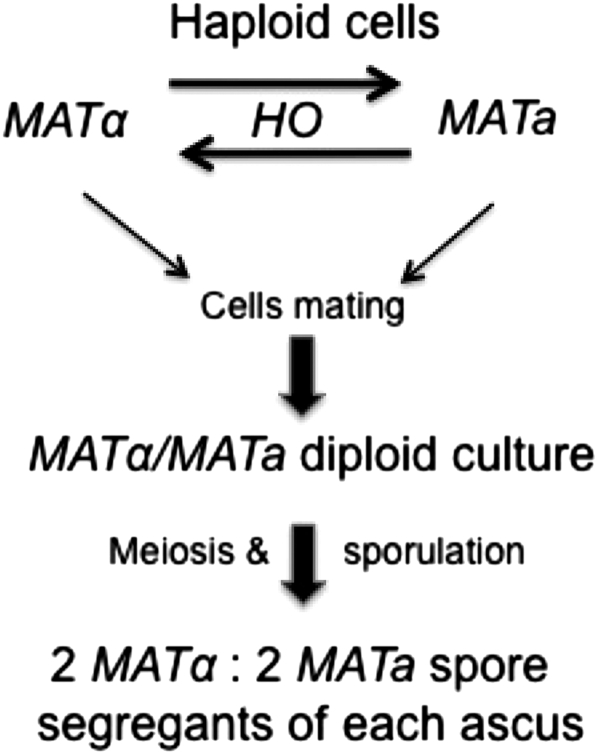
THE sexual cell types of yeast are designated a and α, which are correspondingly conferred by the MATa and MATα alleles of the mating type locus (MAT). Cells of opposite type can mate to establish a cell of the MATα/MATa diploid state (Figure 1). Because both MAT alleles are co-dominant, such diploid cells are sterile but can undergo meiosis and sporulation to form asci, each of which contains two MATa and two MATα haploid spores. In so-called heterothallic strains (those containing the nonfunctional ho gene), the MAT alleles switch rarely (<1 × 10−6), but the unusual homothallic (those containing the functional HO gene) cells switch mating type remarkably efficiently, within a few cell divisions after the spore germinates. The cells in the incipient colony of the opposite type mate to reestablish MATα/MATa diploid cells in which the HO gene and the switching process are shut off (Winge and Roberts 1949). This was an odd and fascinating phenomenon that workers in the field initially cracked open by conventional genetics. This Perspectives is about my postdoctoral training research, during which different aspects of the mystery were cleared up by a series of informative experiments. This is a personal account of the excitement I enjoyed through my own and my colleagues' genetic studies.
Figure 1.—
The yeast mating-type switching homothallism phenomenon (see text for details).
I started my graduate school training in 1969, working on yeast at the University of Wisconsin with Harlyn O. Halvorson, a prominent researcher of the cell cycle of yeast and of sporulation of yeast and Bacillus. His group moved from Madison, Wisconsin, to Brandeis University in Waltham, Massachusetts, in 1971. While conducting my thesis research on two other projects, two unrelated instances were instrumental in igniting my interest in the yeast mating-type switching phenomenon. First, Johanes van der Plaat, a visiting scientist from Gist-Brocades in Holland, related that controlling ploidy of industrial yeast strains was difficult. I proposed a project to test whether cells homozygous for the mating-type allele (MATa/MATa or MATα/MATα) containing the D (for diploidization) gene (also called HO, for homothallism, Figure 1; Winge and Roberts 1949) would switch mating type, as haploid cells of either mating type do. I wondered if the MATa/MATα constitution itself, and not the diploid state per se, turns off the switching process? Second, another visiting scientist in our group, Isamu Takano from Osaka University in Japan, handed me a couple of the Osaka group's articles to read, in which they had implicated two additional genes, HMa and HMα, in MAT switching. In one of these papers, the Osaka group reported that an inefficiently switching MATα-inc (inc for inconvertible, a naturally occurring variant) switches to the readily switchable MATa and MATα (Takano et al. 1973). I was most impressed by this puzzle of repairing a switchability defect in MATα by the switching process. Because the molecular nature of the MATα-inc mutation was unknown, I proposed to conduct a similar switching experiment, but with a matα-ochre mutation, because such a mutation must lie within the gene's coding region. Isamu Takano very much encouraged me to perform this experiment. I requested the mutant from Don Hawthorne of the University of Washington, but unfortunately I did not receive the strain. I later found out that Don Hawthorne knew the answer to this question (see below).
MAT ALLELE HOMOZYGOUS DIPLOID CELLS SWITCH
In the middle of 1975 I took up a postdoctoral position under Seymour Fogel of the University of California at Berkeley. As a side project, I explored whether the HO gene responds to diploidy or to the cell's mating-type constitution. An absolutely clear result was that cells homozygous for the MAT allele switched to establish a mixture of diploid MATa/MATα cells by switching a single MAT locus and that tetraploid MATa/MATa/MATα/MATα cultures were due to switching of both MAT loci and subsequent mating between cells of opposite type. These results clearly showed that: (1) HO directs switching in diploid cells when they are homozygous for the MAT allele; (2) HO action is not influenced by ploidy; (3) HO action is turned off by MATa/MATα heterozygosity; and (4) HO is dominant to the ho allele. I also noted a very interesting paradox in the literature and proceeded to employ our diploid cell's switching analysis to simplify the very confusing genetics of the switching specificity of HM/hm loci.
MATα SWITCHES BY hma
The HM genes and their hm alleles were identified as naturally occurring variants from different stocks (Santa Maria and Vidal 1970; Naumov and Tolstorukov 1973; Harashima and Oshima 1976). The HMa gene is required to switch MATa to MATα, and HMα is required to switch MATα to MATa (see Table 1). By convention, three letters are used in yeast gene nomenclature to designate a gene; uppercase letters are used for the dominant allele; lowercase letters are used for the recessive, usually nonfunctional allele. HMa, HMα strains switch in both directions; hma, HMα switch only from MATα to MATa; HMa, hmα switch only from MATa to MATα. This suggested that the hm genes are defective for the switching function normally directed by the HM loci. A beautiful paradox was that stocks containing hma, hmα switched in both directions, similar to the HMa, HMα strains (Table 1). How could that be explained? HMa maps near the left telomere, HMα near the right telomere, and MAT in the middle of chromosome III (Harashima and Oshima 1976).
TABLE 1.
HM loci nomenclature and MAT switching direction
| Old names | Year 1978 names | Switching direction |
|---|---|---|
| HMa, HMα | HMLα, HMRa | a ↔ α |
| hma, HMα | HMLa, HMRa | a ← α |
| HMa, hmα | HMLα, HMRα | a → α |
| hma, hmα | HMLa, HMRα | a ↔ α |
Naumov and Tolstorukov (1973) theorized that perhaps hma performs the HMα function, and hmα performs the HMa function. To test this conjecture, I made hma/HMa, MATα/MATα, hmα/hmα diploid cells. Note that this strain does not contain HMα. If it switched, hma must have done the job of switching MATα to MATa. The cells indeed switched! This showed that hma functions to switch MATα to MATa, thus solving the paradox posed above. To answer such a question now, a new student would proceed to simply look up the HM gene sequence in the database. But that was not available at the time, so classical genetics tools had to be honed and used.
We submitted the HO dominance and HM/hm function results for publication in Genetics. Apparently, Ira Herskowitz of the University of Oregon at Eugene received our article to review. He contacted Seymor Fogel and requested that he hold up our publication to publish it with an article from his group that addressed switching of MAT homozygous diploid cells. The two articles were published back to back in Genetics (Hicks et al. 1977a; Klar and Fogel 1977).
THE OSHIMA GROUP'S CONTROLLING ELEMENT MODEL
Ever since my Brandeis days I have been very much influenced by the controlling element attachment/transposition model proposed for switching by Yasuji Oshima's group at Osaka University (Takano et al. 1973). By this model, the HMa element “attached” to MAT constitutes the MATα allele, whereas its replacement by the HMα-derived element forms the MATa allele. I had long appreciated the significance of the “healing” of the MATα-inc allele for defining the mechanism of switching. My experiment to test the repair of the matα-ochre mutation that should define the coding region of the MAT locus was borne of my desire to test whether the switching process transposes the structural part of the MAT locus or only some regulatory region of it. David Radin, a graduate student in our group at Berkeley, had isolated a mata− mutation in an ho strain as part of an unrelated project. We set out to test if the switching process could heal a mata− mutation as well. It did (Klar et al. 1979b), a result in accord with the DNA transposition model for switching of both MAT alleles.
THE HERSKOWITZ GROUP'S CASSETTE MODEL
Ira Herskowitz learned the result of matα-ochre mutation healing experiment from Don Hawthorne, and the University of Oregon at Eugene group showed healing of other matα-sterile mutations by switching (Hicks and Herskowitz 1977).Two key insights led the Eugene group to further define the transposition model as a mechanism for cell-type switching (Herskowitz 1988; Satrathern 1992). The first was the insight that a fusion of the MATα and HMα loci, caused by deletion of the interval between these loci called the Hawthorne deletion, caused the MATα-to-MATa switch in ho strains. The second was the knowledge that bacteriophage λ mutations can be repaired by copying DNA from cryptic prophages residing in the bacterial genome.
In their cassette model (Hicks and Herskowitz 1977; Hicks et al. 1977b), the Herskowitz group proposed that HMa contains the library copy of the coding region of MATα, that HMα likewise contains the MATa genetic information, and that HMa and HMα remain unexpressed, possibly because they lack promoter elements. To activate the genetic information harbored at HMa and HMα, a copy (“cassette”) of one or the other “donor locus” is transposed into the MAT locus by a recombination/substitution reaction. I considered the cassette model to be a specific version of the controlling element model, and therefore it was very much in line with my thinking of the switching process. However, two other models were being considered at the time, which raised the possibility that the HM loci could function by reversibly “mutating” through DNA modification or by inverting a shared promoter located between MATa and MATα coding sequences. Indeed, HM loci were sometimes referred to as “mutator” genes (Naumov and Tolstorukov 1973). When Ira Herskowitz visited Berkeley in April 1977, he stopped by our laboratory, presumably because he knew we were working on MAT switching. In a very brief chat made to fewer than four of us standing around the chalkboard, Ira described the cassette model and the result of healing matα mutations, and I related to him our unpublished result of healing the mata− mutation through switching (Klar et al. 1979b).
DISCOVERY OF THE MAR1 GENE AS A REPRESSOR OF THE HM/hm LOCI
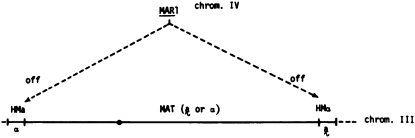
Over a year before Ira's visit to our laboratory, we had been working on a spontaneous mutation that prevented yeast cells from mating with cells of either mating type. The mutant strain (MATa, ho) had misbehaved several times over the years in two different laboratories at Berkeley. In our laboratory a spontaneous diploid arose from this strain during routine culturing. Kathy Macleod, a technician in our laboratory, found that, upon sporulation, each ascus produced four spores that gave rise to non-mating cells that maintained the size of a haploid, but that exhibited behavior expected of MATa/MATα cells: polar budding pattern and insensitivity to the α-factor. In the past, such sterile derivatives were discarded in the laboratory, but since I was working on MAT switching, I entertained the possibility that the ste phenotype might have resulted from the mutation of a gene that represses the MAT cassettes thought to reside at the HM loci. My co-workers argued that my proposal was inconsistent with the cassette model because the absence of promoter regions in HM loci would preclude their expression. I countered that the model could be revised so the entire MAT locus, including its promoter, resides at both HM loci and that the MAR1 (mating type regulator 1) gene that I proposed as the site of this ste mutation might function to keep them repressed.
My hypothesis was that the expression of the HM loci in the mar1-1 mutant causes the ste phenotype by making the cell think that it is a diploid due to the expression of both MATa and MATα information.(Figure 2). In support of this idea, the haploid cells exhibited incipient sporulation, a phenotype possibly resulting from aberrant meiosis due to expression of both MATa and MATα genes in a haploid cell. We occasionally found fully matured meiotic products (asci) in a culture of mar1-1, where each ascus produced four-haploid, sterile meiotic products.
Figure 2.—
Model for MAR1 as the repressor of HM loci. Reproduced with permission from Klar et al. (1979a).
In crosses with wild-type strains, Kathy Macleod found that the mar1 mutation segregated as a single gene in one hybrid strain, but the pattern was much more complicated in other hybrids that had acquired mar1 suppressor mutations. The MAR1 gene was found to be linked to TRP1, located on chromosome IV. Luckily, mar1 mutant spores derived from the MAR1/mar1 heterozygous strain showed mating competence during spore germination. This spore-to-cell mating technique greatly facilitated analysis of the mar1 mutant.
THE NATURAL hma AND hmα ALLELES SUPPRESS STERILITY OF THE mar1-1 MUTANT
The controlling/cassette model predicted that hma and hmα alleles should suppress the mar1 mutant's sterile phenotype in predictable ways. For example, the hma, MATa, HMα (Table 1), and mar1 mutant should exhibit the a-mating type because all cassettes are expected to contain the MATa information. By the same logic, the HMa, MATα, hmα, and mar1 cells should be of the α-mating type. Indeed, these predictions were precisely borne out by our genetic analysis (Klar et al. 1979a). This analysis confirmed that HM and hm loci contain functional MAT information and that their expression is repressed by MAR1 (Figure 2).
HM LOCI MUTATIONS AND SWITCHES DISCOVERED AS SUPPRESSORS OF THE mar1-1 MUTATION
The mar1 mutant showed a very tight sterile phenotype, but its cells could acquire mating capacity due to rare, spontaneous, unlinked suppressor mutations. We found two types of suppressors and, most tellingly, both classes mapped to the HM loci! One class of suppressors was composed of those changing from HM to hm alleles; the other class carried novel mutations of HM cassettes. By employing this “rare mating” selection, we identified several hmα− and hma− mutants. (The entire mar1 mutant analysis was conducted in ho strains in which the MAT locus is highly stable.)
hmα− MUTATIONS TRANSPOSE TO GENERATE ONLY MUTANT mata− SWITCHES
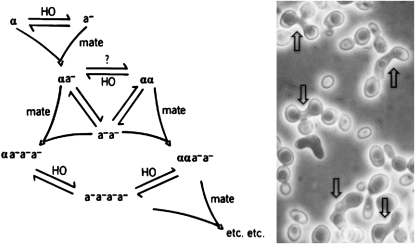
A critical prediction of the transposition model is that cells containing the hmα− mutation should switch MATα to mutant mata− because the donor locus contains the mutant copy. This prediction was satisfyingly realized (Figures 3 and 4). With just one look at the cells, we got the “aha” feeling. Most tellingly, the hmα− mutant culture kept on switching and mating: the switching was not shut off because of the mata− mutation. Consequently, the cells continued to increase in ploidy. Indeed, such cultures produced smaller colonies because of the hmα− mutant's uncontrolled sexual activity (Figure 4). This MAT “wounding” result satisfied a key prediction of the transposition model (Klar and Fogel 1979). This result meant that the mutation from hmα− is transmitted to MAT by the switching mechanism. We tested four such hmα− mutants; all produced similar results.
Figure 3.—
The mating orgy of the hmα−-2 mutant. The HO, HMa, and hmα−-2 mutant switches continuously between MATα and mutant mata− (left). Reproduced from Klar and Fogel (1979). The mata− cells mate as a-mating type. Photograph of promiscuous matings between switched cells show dumbbell-shaped zygotic cells (marked by arrows, right).
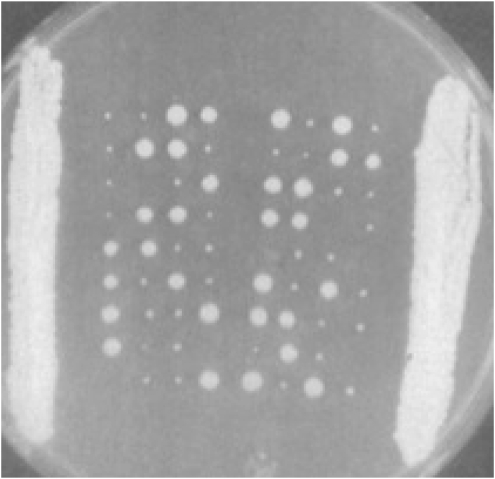
Figure 4.—
Segregant analysis of HO/HO, HMa/HMa, MATa/MATα, and hmα−-2/hmα−-2 strain (Klar and Fogel 1979). Four-spore segregants from each ascus were planted to grow in a horizontal line on solid medium in two parallel columns. In each tetrad, the two MATa spores produced standard-size MATa/MATα diploid segregants due to homothallism, and two MATα spores produced much smaller colonies due to continuous switching and mating.
RESULTS PRESENTED AT THE 1977 CSHL YEAST MEETING
We submitted an abstract to the CSHL yeast genetics conference describing the mata− “healing” by switching. After submitting the abstract, we made the advances described above. I sought Ira Herskowitz's opinion about our findings before my formal presentation, scheduled for the first session of the conference, on the steps of the Blackford Hall cafeteria: Ira Herskowitz, David Kaback, my friend from my graduate school days, and two other people who were unknown to me at the time. I quickly described to them that we had found a mutation that causes derepression of the HM loci, that the mutation maps near the TRP1 marker on chromosome IV, that the mutant is sterile, that the suppressors of the sterile mutation map at HM loci, and so on. One of the two strangers inquired with urgency about what happens when we use the hmα− mutant for switching. After I had described the meager details of how the mutations were isolated and characterized, I answered that the hmα− mutant alternates between MATα and mutant mata− and that it keeps on switching back and forth. The stranger became very excited and stomped the ground a few times while yelling, “A shrine should be made for mating type right there.” Then Ira introduced me to the two strangers: his student Jasper Rine and a former student, Jeffrey N. Strathern (the stranger who stomped). They immediately understood what I had described. Within minutes I was introduced to James B. Hicks, another former student of Ira's who also had worked on MAT switching for his thesis research.
MAT was put on the agenda of the first session of the meeting because the organizers thought that the presentations would be an exciting start to what promised to be a great meeting. Jasper Rine from the Eugene group led off with a talk about a suppressor of a matα-sterile mutant, called ssp1, and I learned for the first time of their proposal for its function in gene silencing of HM loci. At the time Jasper thought that the ssp1 gene was probably allelic to MAR1, as it was linked to TRP1. [I later tested their allelism and found that it was a different gene, which they named SIR1 (silent information regulator 1).] In my turn, I presented an analysis of the mar1 mutant (Figure 2) and of the transposition of a mutation from hmα− to generate the mata− mutant allele (Figures 3 and 4). My talk, embarrassingly, went on past the buzzer, taking me out of the running for a bottle of champagne promised by organizers for the shortest talk of the meeting. However, Jim Hicks called out from the audience, “I'll buy you champagne, keep going.” I finished by concluding that both results genetically support the cassette model.
I felt that only a few people understood my presentation because many people asked me to explain our results during the conference. It must have been difficult for most people to follow the complex analysis of four genes segregating in crosses, one of which switches between two alleles. James Broach from the CSHL characterized my presentation as a “bombshell.” Seymor Fogel was overjoyed for me and remarked, “Amar, your talk was so well received. I don't think such a wonderful reception could happen to you in the future.” I was happy for the pat on the back from my advisor, but luckily this was not to be the single most important day of my career.
MY FIRST TASTE OF COMPETITION
One of the participants who must have understood my talk was James E. Haber of Brandeis University. After hearing my presentation, Anita Hopper (personal communication) from the University of Massachusetts told Haber that the cmt (change of mating type) mutation that she had published in 1975 had properties exactly like those of the mutant that I had presented. She speculated that the CMT gene is probably MAR1. Haber promptly requested the mutant from Hopper, and a manuscript describing a MAR1-related explanation for the cmt mutation was prepared quickly before our results had been published. The findings about MAR1 and CMT in HM loci gene silencing came out in the September issue of Genetics (Haber and George 1979; Klar et al. 1979a). In the acknowledgment of the CMT article, the authors noted that “We began to study the cmt mutation after Amar Klar communicated to us his characterization of the MAR1 mutation, which has a phenotype apparently identical to cmt.” The SIR1 article was published later in the December 1979 issue of Genetics (Rine et al. 1979).
DON HAWTHORNE BELIEVED OUR STORY
A few months after the CSHL meeting, Leland Hartwell of the University of Washington invited me to Seattle, having heard about my talk from his students. There I met Don Hawthorne, a legendary yeast geneticist who favored the hypothesis of MAT switching occurring by directed mutations. Being aware of the Hawthorne deletion, and his unpublished work on the matα-ochre mutation's healing result, I was therefore most interested in Don's opinion for my presentation. He said, “I believe you. It's neat.” I was elated by his response. A few years later, I related to Don that by not publishing the matα-ochre healing result in due time he had held back the field for 10 years. He countered, “Five years.”
JOINING THE CSHL YEAST GROUP IN 1978
Jeff Strathern and James Broach were already working as postdoctoral fellows at CSHL, and Jim Hicks was a postdoctoral fellow at Cornell University. [With several Jim's (Hicks, Broach, and Watson) who figure in this story, hereafter I will identify them by their last names.] Both Hicks and Jeff proposed that I be asked to join the CSHL yeast group that included Jeff, Broach, and Hicks (Hicks 2009). According to Hicks (personal communication), for his job seminar he presented MAT studies to a handful of CSHL senior scientists, which included the director, James D. Watson. All went well for Hicks's appointment, but a question was raised as to why I should be hired into the group. After some silence, one inquisitor asked for a summary of what I had done in research, a summary that Hicks gave. Some silence ensued, and then one committee member said, “I got it. You guys proposed a model, Klar established it. Is that why you want him to join the group?” Hicks agreed.
Jim Watson tracked me down while I was in the middle of delivering a seminar at the University of California at Irvine. Perhaps because of Watson's insistence, my host's secretary walked into the seminar room and politely interrupted my seminar to tell me that Watson wanted to talk to me right away. Immediately after finishing my talk, I got on the phone: “Hello, Dr. Watson; this is Amar Klar.” Abruptly, Watson replied, “Oh, yes, I know you; I know what you do. We are opening a yeast lab to work on mating-type switching. Whatever you need, I will arrange for it. You should realize your appointment has to go through a committee, but I will send you the appointment letter tomorrow. You should sign it and send it back to my assistant director, Mr. Bill Udry. Bye” (Klar 2003).
I joined the yeast group in the summer of 1978. At the yeast meeting held in Rochester, New York, that year, Ira Herskowitz presented his version of the MAT wounding experiment of the hma− mutant in support of the cassette model. Yasuji Oshima stood up after Ira's presentation to ask the first question, “How is your model different from the controlling element model?” Ira replied, “They are similar.” This answer clarified the intellectual linkage between these gene transposition models, and subsequently the MAT controlling elements came to be referred to as cassettes.
My postdoctoral work paved the way for me to join a wonderful group of colleagues at CSHL. Initially, there were four of us. Broach soon moved to Stony Brook, leaving Hicks, Jeff, myself, other colleagues from CSHL, and our postdoctoral colleagues to work on the mating-type story. Our long-term collaboration led to many discoveries in an exciting research environment. This group (1) cloned the MAT gene, molecularly confirming conclusions derived from the genetics studies described above; (2) discovered that MAT switching is initiated by a double-stranded DNA break at MAT; (3) showed that HO encodes the endonuclease that cleaves MAT; (4) established that switching occurs by gene conversion; (5) showed that MAR/SIR-promoted gene silencing prohibits HM loci from switching; and (6) found that the directionality of MAT switching (MATa prefers to switch to α, and vice versa) results from a specific chromosomal location and not from the genetic content of HML and HMR.
This Perspective narrates my participation in the mating-type story from my postdoctoral research. Descriptions of related contributions are presented in other commentaries (Herskowitz 1988, 1992; Klar 1992; Strathern 1992; Oshima 1993; Hicks 2009). Time has flown quickly since the 1977 yeast meeting, but thankfully the people I met along the way have made the intervening time very exciting for me. I am sad that this story has not been shared until this time because several of the personalities who figured prominently in it—Harlyn Halvorson, Seymor Fogel, Ira Herskowitz, and Don Hawthorne—have passed away. I wish I could have refreshed their memory about the story with this Perspective.
Acknowledgments
I very much appreciate the research experience with Jim Hicks, Jeff Strathern, Jim Broach, and numerous postdoctoral associates and collaborators and visiting scientists over the years. I thank David Kaback for his suggestions on the manuscript. The Intramural Research Program of the National Institutes of Health, National Cancer Institute, Frederick, Maryland, has supported my research for the past 22 years.
I dedicate this contribution to my advisors, Harlyn O. Halvorson and Seymour Fogel, for training me to become an independent researcher.
References
- Haber, J. E., and J. P. George, 1979. A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae. Genetics 93 13–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima, S., and Y. Oshima, 1976. Mapping of the homothallic genes, HMα and HMa, in Saccharomyces yeasts. Genetics 84 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz, I., 1988. The Hawthorne deletion twenty-five years later. Genetics 120 857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz, I., 1992. Controlling elements, mutable alleles, and mating type interconversion, pp. 287–297 in The Dynamic Genome: Barbara McClintock's Ideas in the Century of Genetics, edited by N. Federoff and D. Botstein. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hicks, J. B., 2009. Commentary of Strathern et al. 1982 Cell 31: 183–192, pp. 13–17 in Life Illuminated, edited by J. Witkoski, A. Gann and J. Sambrook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hicks, J. B., and I. Herskowitz, 1977. Interconversion of yeast mating types II. Restoration of mating ability to sterile mutants in homothallic and heterothallic strains. Genetics 85 373–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, J. B., J. N. Strathern and I. Herskowitz, 1977. a Interconversion of yeast mating types III. Action of the homothallism (HO) gene in cells homozygous for the mating type locus. Genetics 85 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, J. B., J. N. Strathern and I. Herskowitz, 1977. b The cassette model of mating type interconversion, pp. 457–462 in DNA Insertion Elements, Plasmids and Episomes, edited by A. Bukhari, J. Shapiro and S. Adhya. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Klar, A. J. S., and S. Fogel, 1977. The action of homothallism genes in Saccharomyces diploids during vegetative growth and the equivalence of hma and HMα loci functions. Genetics 85 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J. S., and S. Fogel, 1979. Activation of mating type genes by transposition in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76 4539–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J. S., S. Fogel and K. Macleod, 1979. a MAR1: a regulator of the HMa and HMα loci in Sacharomyces cerevisiae. Genetics 93 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J. S., S. Fogel and D. N. Radin, 1979. b Switching of a mating-type a mutant allele in budding yeast Saccharomyces cerevisiae. Genetics 92 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J. S., 1992. The role of McClintock's controlling element concept in the story of yeast mating type switching, pp. 307–314 in The Dynamic Genome: Barbara McClintock's Ideas in the Century of Genetics, edited by N. Federoff and D. Botstein. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Klar, A. J. S., 2003. From development of yeast cells to human brain hemispheres, pp. 277–284 in Inspiring Science: Jim Watson and the Age of DNA, edited J. R. Inglis, J. Sambrook and J. Witkowski. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Naumov, G. I., and I. I. Tolstorukov, 1973. Comparative genetics of yeast. X. Reidentification of mutators of mating types in Saccharomyces. Genetika 9 81–91. [PubMed] [Google Scholar]
- Oshima, Y., 1993. Homothallism, mating-type switching, and the controlling element model in Saccharomyces cerevisiae, pp. 291–304 In The Early Days of Yeast Genetics, edited by M. N. Hall and P. Linden. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Rine, J., J. N. Strathern, J. B. Hicks and I. Herskowitz, 1979. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93 877–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria, J., and D. Vidal, 1970. Segregacion anormaldel “mating type” en Saccharomyces. Inst. Nac. Invest. Agron. Conf. 30 1–8. [Google Scholar]
- Strathern, J. N., 1992. Thinking about programmed genome rearrangement in a genomic state of mind, pp. 289–305 in The Dynamic Genome: Barbara McClintock's Ideas in the Century of Genetics, edited by N. Federoff and D. Botstein. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Takano, I., T. Kusumi and Y. Oshima, 1973. An α mating-type allele insensitive to the mutagenic action of the homothallic gene system in Saccharomyces diastaticus. Mol. Gen. Genet. 126 19–28. [DOI] [PubMed] [Google Scholar]
- Winge, O., and C. Roberts, 1949. A gene for diploidization of yeast. Cr. Trav. Lab. Carlsberg Ser. Physiol. 24 341–346. [Google Scholar]