Abstract
YKL-40 (chitinase 3-like protein 1) is expressed in a broad spectrum of inflammatory conditions and cancers. We have previously reported that YKL-40 levels are elevated in the cerebrospinal fluid (CSF) of macaques and humans with lentiviral encephalitis, as well as multiple sclerosis (MS). The current study assessed temporal CSF YKL-40 levels in subjects with severe traumatic brain injury (TBI; Glasgow Coma Scale [GCS] score ≤8). We also evaluated temporal expression of YKL-40 after parasagittal controlled cortical impact (CCI) injury over the parietal cortex (2.8 mm deep, 4 m/sec). We demonstrate that CSF YKL-40 levels are elevated after acute TBI, and that YKL-40 levels are higher in patients who died following injury than in patients who survived. YKL-40 levels significantly correlate with CSF levels of inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), as well as the inflammatory marker C-reactive protein (CRP). After CCI, in situ hybridization (ISH) showed that YKL-40 transcription is primarily associated with reactive astrocytes in pericontusional cortex. Tissue YKL-40 transcription time course analysis after CCI showed that YKL40 transcription in astrocytes began 1 day after injury, remained elevated for several days, and then declined by day 12. Similarly to our temporal CSF measurements in humans, YKL-40 induction after CCI is coincident with IL-1β expression. Taken together these findings demonstrate that YKL-40 is induced in astrocytes during acute neuroinflammation, is temporally related to inflammatory mediator expression, and may be a useful biomarker for understanding secondary injury and for patient prognosis.
Key words: chitinase, controlled cortical impact, cytokine, gliosis, neuroinflammation, traumatic brain injury, YKL-40
Introduction
Traumatic brain injury (TBI) initiates acute neuroinflammation that results in astrocyte and microglial activation and increased production of immune mediators. Following TBI there is infiltration of immune cells, in particular leukocytes; however, both astrocytes and microglia participate in mounting neuroinflammation, and are capable of synthesizing cytokines and chemokines (Morganti-Kossmann et al., 2007). A growing body of evidence shows that cytokines like interleukin-1β (IL-1β), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) are elevated in the cerebrospinal fluid (CSF) following TBI (Buttram et al., 2007; Chiaretti et al., 2008a; Hayakata et al., 2004; Kirchhoff et al., 2008). This inflammatory response induces acute cerebral edema and longer-term neuronal damage that can cause cognitive impairment, dementia, epilepsy, depression, and neurodegenerative disease. Viability of surviving neurons and axonal regeneration are known to be affected by the glial scar; however, controversy exists as to the detrimental or beneficial role of reactive astrocytes in the glial scar and the role of astrocyte-derived cytokines (Laird et al., 2008). Although cytokines play a role in the pathophysiology of neuroinflammation in TBI, there are conflicting reports about the relationship between cytokine CSF levels and clinical outcome. Some researchers have reported that elevated concentrations of IL-1β and IL-6 in the CSF of neurotrauma patients are associated with an unfavorable clinical outcome (Hayakata et al., 2004; Singhal et al., 2002; Waje-Andreassen et al., 2005), while others failed to show such an association (Buttram et al., 2007). Thus, finding biomarkers for prognosis and monitoring of patients with TBI are warranted.
In recent studies, we showed that YKL-40 (chitinase 3-like protein 1, or breast regression protein-39 [BRP-39]) transcription is elevated in astrocytes in multiple sclerosis (MS) and acute stroke (in press), as well as in lentiviral encephalitis (Bonneh-Barkay et al., 2008). YKL-40 is a member of the glycosyl hydrolase family 18; however, it does not have hydrolase activity (Johansen, 2006). YKL-40 is expressed and released by chondrocytes, synovial cells, neutrophils, and macrophages during differentiation (Johansen, 2006; Kirkpatrick et al., 1997; Rehli et al., 2003). It is upregulated in inflamed tissue in ulcerative colitis, Crohn's disease, rheumatoid arthritis, osteoarthritis, asthma, chronic obstructive pulmonary disease, and liver cirrhosis, as well as in solid cancers (Johansen, 2006). The physiological role of YKL-40 is not yet known; however, it is hypothesized to be involved with tissue remodeling during inflammation. In a recent study, BRP-39 knockout mice showed a blunted immune response to allergic sensitization, accompanied by reduced peribronchial fibrosis and collagen content, suggesting that YKL-40 potentiates the deleterious effect of inflammation (Lee et al., 2009).
Our previous work showed that YKL-40 is expressed in reactive astrocytes (in press). In addition, YKL-40 inhibited basic fibroblast growth factor (bFGF) signaling through fibroblast growth factor receptor 1 (FGFR1), and inhibited bFGF-induced axonal branching in hippocampal neurons (Bonneh-Barkay et al., 2008). Thus YKL-40 also potentially has the capacity to negatively modulate neurotrophic factor–associated changes in neuronal repair and regeneration.
The current study assesses the utility of YKL-40 as a temporal biomarker for assessing pathophysiology and prognosis in a clinical population with severe TBI, and explores the relationship between YKL-40 and inflammatory cytokines in both human and experimental TBI. We show that CSF YKL-40 levels are elevated in patients with severe TBI, and that they correspond to levels of inflammatory cytokines. YKL-40 transcription is induced in reactive astrocytes, and is associated with local neuroinflammation in an acute animal model of TBI.
Methods
Human subject characteristics and specimens
Twenty persons (14 male and 6 female) with severe TBI (Glasgow Coma Scale [GCS] score ≤8) were prospectively enrolled in a larger study assessing biomarkers and TBI outcome at our Level 1 trauma center between 2005 and 2009. Additionally, 6 healthy control subjects were separately enrolled as controls for CSF collection and biomarker measurement. These human studies were approved by our institutional review board.
TBI patients were enrolled if they were aged between 16 and 70 years, had a severe TBI based on a GCS score ≤8 with positive findings on head compute tomography (CT) scan, and required an extraventricular drainage catheter (EVD) for intracranial pressure (ICP) monitoring and management. Patients with penetrating head injury, as well as prolonged cardiac or respiratory arrest at injury, were excluded from the study.
For subjects with severe TBI, CSF was collected via EVD every 12 h for up to 7 days after TBI. For healthy subjects, CSF was collected via lumbar puncture. Upon collection, each sample was centrifuged (for 5 min at 2500 rpm at 25°C), and tested for hemoglobin using a HemoCue Plasma Low/Hb photometer (Lake Forest, CA). After testing, the samples were aliquoted in polypropylene cryovials and stored at −80°C until the time of assay. Samples from the same corresponding time points post-injury were used to assess both YKL-40 and cytokine levels for each patient.
Demographic, clinical severity, and outcome assessments
Demographics, including age and gender, were collected, and type of injury based on head CT findings were also recorded. Initial hospital GCS scores were recorded for each patient after resuscitation and without the influence of paralytics. TBI subjects were assessed for Glasgow Outcome Scale (GOS) scores at 6 months post-injury. The GOS is a widely utilized measure that classifies outcome into five categories: 5, good recovery; 4, moderate disability; 3, severe disability; 2, persistent vegetative state; and 1, death (Jennett and Bond, 1975).
YKL-40 enzyme-linked immunosorbent assay (ELISA)
YKL-40 levels were determined, in duplicate, for all of the CSF samples using the MicroVue YKL-40 ELISA kit from Quidel Corporation (San Diego, CA), according to the manufacturer's protocol. Absorbance was measured using a microplate reader (BioTek Instruments, Inc., Winooski, VT).
Quantification of CSF inflammation markers
Interleukins (IL-1β, IL-6, and IL-10), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) concentrations in CSF were determined using the open-architecture xMap technology developed by the Luminex Corporation (Austin, TX), and assayed on the MILLIPLEX MAP High Sensitivity Human Cytokine Panel-Premixed 13-Plex and Human Neurodegenerative Disease Panel II (Millipore, Billerica, MA). Quantification of each biomarker was based on the reported fluorescent signal specific for each individual microsphere coded by the specific fluorescent dye on the marker (molecule) of interest. The median fluorescence intensity (MFI) data were evaluated using a five-parameter logistic or spline curve-fitting method for calculating marker concentrations in each sample.
Controlled cortical impact (CCI)
The CCI injury device, originally described in detail by Dixon and associates (1991), consists of a small-bore (1.975 cm) double-acting stroke-constrained pneumatic cylinder with a 5-cm stroke (Dixon et al., 1991). The cylinder was rigidly mounted on a crossbar in an angled position. The lower rod end has an impact tip with a diameter of 6 mm, and the upper rod end is attached to the transducer core of a linear velocity displacement transducer (LVDT; model 500 HR by Macro Sensors; Howard A. Schaevitz, Inc., Pennsauken, NJ). The impactor tip is pneumatically driven at a pre-programmed velocity, depth, and duration of tissue deformation. The velocity of the cylinder is controlled by gas pressure and measured directly by the LVDT.
Controlled cortical impact surgery
Sprague-Dawley rats received isoflurane anesthesia via inhalation of 4% isoflurane and a 2:1 N2:O2 mixture, followed by 1–1.5% isoflurane maintenance anesthesia. While under anesthesia, each animal was secured in a stereotactic frame. After midline incision and reflection of the soft tissues, a craniectomy (approximately 6 mm) was made between the bregma and the lambda in the right hemisphere between the central suture and the coronal ridge. The cortical injury was then delivered such that the impactor was perpendicular to the dural plane. The impact was delivered to a depth of 2.8 mm at 4.0 m/sec. Core body temperature was monitored via rectal probe, and a homeothermic blanket (Harvard Apparatus, Hollison, MA) was used to maintain core body temperature at 37.0 ± 0.5°C.
Rat traumatic brain injury tissues
On days 1, 2, 3, 4, 6, 8, and 12 after CCI or sham injury, animals (n = 3 per time point) were perfused transcardially under deep anesthesia with pentobarbital 100 mg/kg IP (Abbott Laboratories, North Chicago, IL), using 200 mL 0.9% saline and 300 mL 0.1 M phosphate-buffered saline (PBS, pH 7.4). The brains were extracted and post-fixed in 10% buffered formalin for immunohistochemistry. At the same time points, an additional group of animals (n = 3 per time point) were deeply anesthetized with pentobarbital 100 mg/kg, and then underwent rapid decapitation. The brains were quickly removed and the cortices were dissected on a Petri dish over ice. The cortices were flash-frozen in liquid nitrogen and stored at −80°C for RNA extraction and real-time PCR analysis.
All animal studies were approved by our institutional animal care and utilization committee, and were carried out in our American Association for Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities.
In situ hybridization
Antisense YKL-40 DNA templates containing the T7 promoter were generated by PCR from the pUC57 vector (GenScript, Piscataway, NJ) containing the full length YKL40 cDNA. 35S-labeled RNA probes were generated using the MAXIscript in vitro transcription kit (Ambion, Austin, TX). Cortical tissue sections from rats after TBI were processed for in situ hybridization (ISH), and then for immunohistochemistry. For ISH, tissue sections were microwave-oven treated and then incubated in hybridization buffer (1 × HYB buffer; 0.6 M NaCl, 10% dextran, 50 μg/mL tRNA, and 0.1 M DTT) containing radiolabeled YKL-40 probe (50,000 cpm/μL) at 50°C overnight. The following day the tissue sections were washed and processed for immunofluorescence. The tissue sections were exposed to emulsion (Eastman Kodak, Rochester, NY) for 10 days at 4°C, and then ISH signal was visualized using D19 developer (Sigma-Aldrich, St. Louis, MO), and fixed in Rapid Fix (Sigma-Aldrich).
Immunofluorescence
Formalin-fixed, paraffin-embedded, 6-μm-thick tissue sections were deparaffinized in Histoclear (National Diagnostics, Atlanta, GA), and rehydrated for 3 min in 100%, 95%, and 70% alcohol, followed by PBS. Endogenous peroxidase activity was inactivated by immersing the sections in 3% H2O2 for 20 min. Antigen unmasking was performed using antigen retrieval citra solution (BioGenex, San Ramon, CA). The tissue sections were blocked with protein blocking agent (Thermo, Pittsburgh, PA) for 20 min. Glial fibrillary acidic protein (GFAP) staining was performed using polyclonal rabbit anti-human GFAP (1:500; Dako North America, Inc., Carpenteria, CA), followed by goat anti-rabbit Dylight 488 antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA). CD68 staining of macrophages was performed using mouse anti-rat CD68 (1:100; Dako), followed by goat anti-mouse Dylight 488 antibody (1:200; Jackson ImmunoResearch Laboratories).
Quantitation of in situ hybridization
Three fields from the pericontusional area from each rat were captured by confocal microscopy and analyzed for the number of ISH-positive cells per field (LSM 510; Carl Zeiss MicroImaging, Inc., Thornwood, NY). Illumination was provided by argon (458, 477, 488, and 514 nm, 30 mW) and HeNe (543 nm, 1 mW) lasers. Digital images were captured with LSM 510 version 4.2 (Zeiss).
Tissue RNA isolation
Frozen hemispheres were sectioned into 0.5-cm2 sections and placed in RNAlater-ICE (Ambion) for 16 h. The tissues were then removed from the RNAlater-ICE solution and immediately placed in TRIzol lysis solution (Invitrogen, Carlsbad, CA; 1 mL of TRIzol/100 mg tissue). Tissue dissociation was done using the gentleMACS dissociator (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). RNA was extracted using the TRIzol reagent protocol.
IL-1β and TNF-α real-time PCR
cDNA from cortical homogenates was generated using the RETROscript kit two-step protocol (Ambion). IL-1β transcription was measured using the TaqMan Gene Expression Assay for IL-1β (Applied Biosystems, Carlsbad, CA) and 2 × TaqMan Gene Expression Master Mix (Applied Biosystems), and analyzed on an Applied Biosystems StepOne real-time PCR machine.
Statistical analysis
CSF levels were assessed every 12 h for up to 7 days in human subjects with severe TBI, and average levels for each 24-h period after TBI onset were graphed. Exploratory data analysis preceded all formal statistical analyses. We checked for data errors and verified the normal assumption using the Kolmogorov-Smirnov test, and there was no violation of this assumption. In addition to assessing average daily YKL-40 levels over time compared to healthy controls, we also calculated average YKL-40 and inflammatory cytokine levels over the first week post-injury. We compared daily average YKL-40 levels to healthy controls using the Mann-Whitney test. We then performed Pearson correlations to test the associations between CSF YKL-40 levels and the levels of IL-1β, IL-6, IL-10, TNF-α, and CRP. We performed the Mann-Whitney test to compare the levels of YKL-40 between people who died in acute care and those who survived. We performed sample size calculations assuming a two-sided hypothesis, α = 0.05, and 80% power. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC), and SPSS statistical software (SPSS release 17.0.0; SPSS, Inc., Chicago, IL).
Results
CSF YKL-40 levels are elevated in patients with acute traumatic brain injury
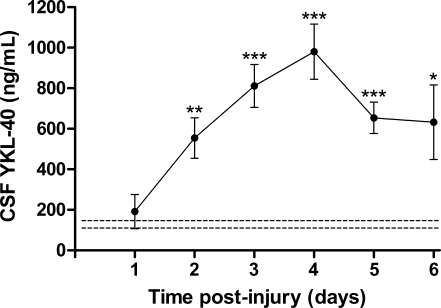
CSF samples from 20 patients with severe TBI were assessed. Clinical manifestations such as type of injury (i.e., subdural or epidural hematoma, or subarachnoid, intraventricular, or intracerebral hemorrhage), as well as gender, age, acute care mortality status, and outcome, were summarized (see Table 1 for patient clinical characteristics). Of 20 patients with TBI, there were 14 males and 6 females, ranging in age from 16–56 years. The median initial GCS score was 6 (range 3–8). Three patients died following trauma. YKL-40 levels were significantly elevated compared to control levels in healthy subjects (129 ± 18.8 ng/mL) after the first day, reaching a peak of 980 ± 136 ng/mL at day 4 post-injury (Fig. 1). Thereafter, YKL-40 levels started declining, to 653 ± 77.5 ng/mL, and 632.5 ± 183.6 ng/mL, at days 5 and 6 post-injury, respectively.
Table 1.
Patient Clinical Characteristics
| No. | Age | Sex | GCS | Mortality | SDH | DAI | EDH | SAH | Contusion | IVH | ICH | GOS6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | M | 6 | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 5 |
| 2 | 40 | F | 6 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 3 | 53 | F | 5 | + | 0 | 0 | 0 | + | + | 0 | 0 | 1 |
| 4 | 21 | F | 7 | 0 | 0 | 0 | 0 | + | 0 | 0 | + | NA |
| 5 | 19 | M | 7 | 0 | + | 0 | 0 | 0 | 0 | 0 | + | 3 |
| 6 | 21 | M | 8 | 0 | + | + | 0 | + | 0 | 0 | 0 | 4 |
| 7 | 31 | M | 7 | 0 | 0 | 0 | + | + | 0 | 0 | + | 4 |
| 8 | 43 | M | 8 | 0 | + | 0 | + | + | 0 | 0 | + | 4 |
| 9 | 25 | M | 7 | 0 | + | 0 | 0 | + | 0 | 0 | + | 3 |
| 10 | 34 | F | 6 | 0 | + | + | 0 | + | 0 | 0 | + | 3 |
| 11 | 42 | M | 8 | 0 | 0 | 0 | 0 | + | 0 | + | 0 | 3 |
| 12 | 26 | M | 6 | 0 | + | 0 | + | + | + | 0 | 0 | 4 |
| 13 | 56 | F | 6 | 0 | + | 0 | 0 | + | + | 0 | 0 | 3 |
| 14 | 48 | M | 6 | 0 | + | 0 | 0 | + | + | 0 | + | 3 |
| 15 | 39 | M | 3 | 0 | + | 0 | 0 | + | 0 | + | + | 3 |
| 16 | 18 | M | 7 | 0 | + | + | 0 | + | 0 | 0 | 0 | 4 |
| 17 | 19 | M | 7 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | 4 |
| 18 | 16 | M | 5 | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 1 |
| 19 | 25 | M | 4 | 0 | 0 | + | + | 0 | 0 | 0 | 0 | NA |
| 20 | 52 | F | 5 | + | + | 0 | 0 | + | 0 | + | + | 1 |
GCS, Glasgow Coma Scale score; SDH, subdural hematoma; DAI, diffuse axonal injury; EDH, epidural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; GOS6, Glasgow Outcome Score 6 months after injury; +, positive; 0, negative; NA, not available.
FIG. 1.
YKL-40 levels are elevated in the cerebrospinal fluid (CSF) of patients after traumatic brain injury (TBI). CSF samples from control and patients following TBI were analyzed for YKL-40 using the MicroVue YKL-40 ELISA kit. The Mann-Whitney test was used to evaluate differences between YKL-40 levels (average ± standard error of the mean) at each day following TBI (solid line), and YKL-40 levels of control healthy subjects (dashed line; *p < 0.05, **p < 0.001, ***p < 0.0001 compared to controls).
CSF YKL-40 levels correlate with those of IL-1β, TNF-α, and C-reactive protein
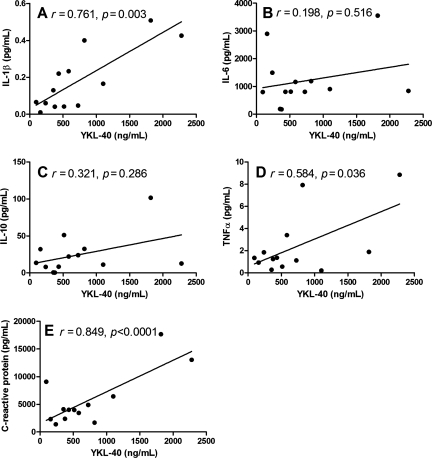
TBI is known to induce neuroinflammation, and previous studies have shown that inflammatory cytokines are elevated in the CSF at early phases in patients with TBI (Chiaretti et al., 2008b; Shiozaki et al., 2005). Consistent with the literature, our data showed significant associations between mean YKL-40 and mean cytokine levels. There was a significant correlation between average CSF YKL-40 levels and IL-1β levels (r = 0.761; p = 0.003; Fig. 2A), TNF-α levels (r = 0.584; p = 0.036; Fig. 2D), and the inflammatory marker CRP (r = 0.849; p < 0.0001; Fig. 2E). However, the associations between IL-6 (r = 0.198; Fig. 2B) and IL-10 levels (r = 0.321; Fig. 2C) were not significant.
FIG. 2.
Cerebrospinal fluid (CSF) YKL-40 correlations with CSF inflammatory mediators. CSF samples from traumatic brain injury (TBI) patients and controls were analyzed for YKL-40 using MicroVue YKL-40 ELISA kits and IL-1β (A), TNF-α (D), CRP (E), IL-6 (B), and IL-10 (C), using Luminex analysis as described in the text (IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; IL-6, interleukin-6; IL-10, interleukin-10; ELISA, enzyme-linked immunosorbent assay).
CSF YKL-40 levels are related to clinical outcome
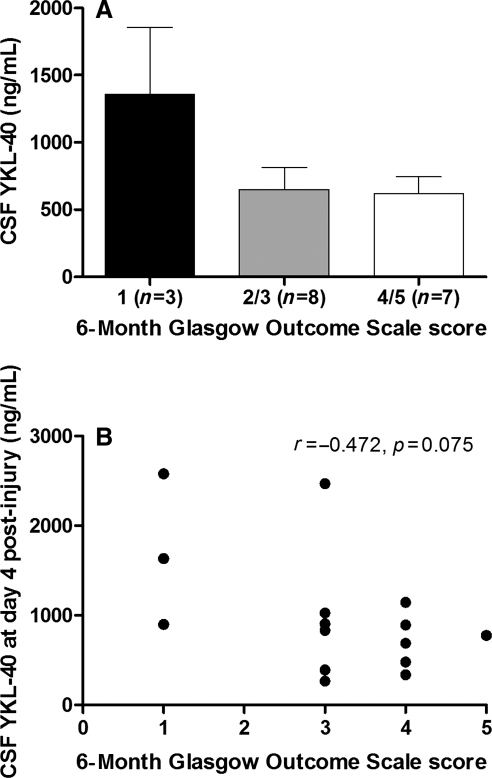
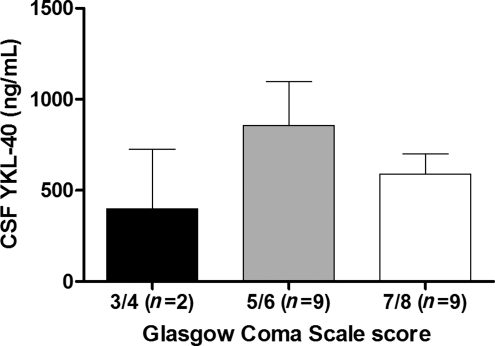
Although the differences were not significantly different, patients that died during their acute care hospitalization for TBI tended to have higher CSF YKL-40 levels than patients who survived their injuries (p = 0.066; Fig. 3A). In addition, there was an inverse correlation between average levels of YKL-40 at day 4 and GOS scores 6 months post-injury (Spearman correlation coefficient = −0.4720; p = 0.075) that trended toward statistical significance (Fig. 3B). This implies that higher levels of YKL-40 after injury might predict unfavorable outcome. Based on these data, we generated a sample size calculation to determine the number of subjects needed to show a significant relationship between CSF YKL-40 levels and GOS scores at 6 months post-TBI. A total sample of 42 subjects is needed to achieve significant differences with 80% power. No differences in YKL-40 levels were detected between patients with different GCS scores (Fig. 4).
FIG. 3.
Cerebrospinal fluid (CSF) YKL-40 associations with outcome. CSF samples from traumatic brain injury (TBI) patients were analyzed for YKL-40. TBI subjects were assessed for Glasgow Outcome Scale (GOS) scores at 6 months post-injury. The Mann-Whitney test was used to evaluate differences in average YKL-40 levels in patients who survived their injuries versus those who did not (A). (B) Scatterplot of CSF YKL-40 concentrations at day 4 post-injury versus GOS score at 6 months post-injury (B). Spearman's correlation coefficient (r) and p values are shown.
FIG. 4.
YKL-40 level associations with Glasgow Coma Scale (GCS) score. Cerebrospinal fluid (CSF) samples from traumatic brain injury (TBI) patients were analyzed for YKL-40. TBI subjects were assessed for GCS score at admission as described in the text. The Mann-Whitney test was used to evaluate differences in YKL-40 levels in patients with different GCS scores.
YKL-40 expression time course after acute traumatic brain injury in rats
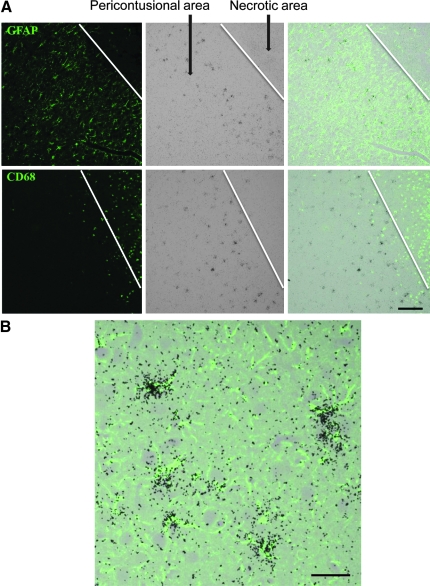
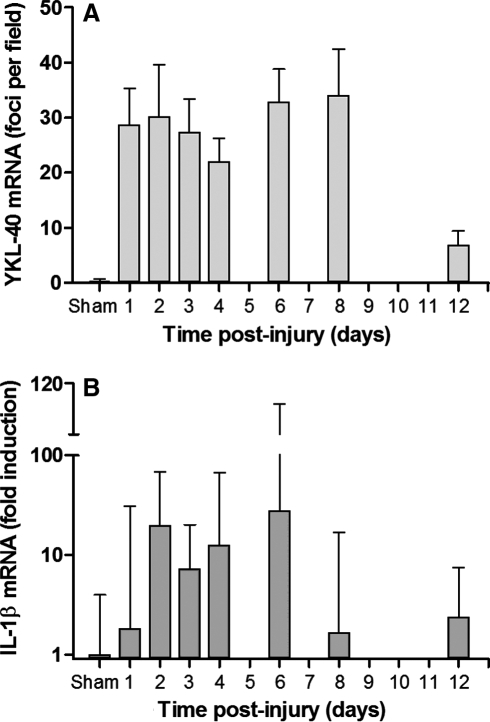
We used a rat model of CCI as a model for acute neuroinflammation in order to further assess the time course of YKL-40 expression, and the CNS cell populations associated with YKL-40 induction. Combined ISH and GFAP staining showed induced YKL-40 expression in astrocytes restricted to the pericontusional region (Fig. 5) on day 3 post-CCI. Interestingly, macrophages that infiltrated the necrotic area did not hybridize for YKL-40 transcripts at day 3 post-injury. In order to follow the time course of YKL-40 expression in TBI, we next assessed brain sections for YKL-40 ISH in the rats on days 1, 2, 3, 4, 6, 8, and 12 after CCI. YKL-40 astrocytic transcription started at day 1 post-injury, stayed elevated for 8 days, and was diminished at 12 days post-injury, as assessed by counting ISH-positive cells (Fig. 6A). In order to further quantify the degree of inflammation, we measured IL-1β and TNF-α transcription levels via quantitative PCR in the injured cortical hemisphere and compared them to sham controls. Here we found induced IL-1β transcription by day 2 post-injury that remained elevated for 5 days, and then diminished by day 8 post-injury (Fig. 6B). Very low levels of IL-1β transcription were found in the contralateral side (data not shown). TNF-α levels were too low to quantify significant differences between hemispheres (data not shown).
FIG. 5.
Focal and temporal expression of YKL-40 in astrocytes on day 3 after controlled cortical impact. Paraffin-embedded sections were hybridized with 35S-labeled RNA probe for YKL-40 (A, middle panels), followed by immunohistochemistry with GFAP or CD68 (A, left panels) as described in the text (scale bar = 0 μm). The right panels show the combined images of the ISH and immunohistochemistry. (B) YKL-40 co-localizes with GFAP staining (scale bar = 5 μm; GFAP, glial fibrillary acidic protein; ISH, in situ hybridization).
FIG. 6.
YKL-40 transcription corresponds to the inflammatory input as measured by IL-1β mRNA. (A) YKL-40 ISH signal in the pericontusional area was counted under the microscope. The data show the average ± standard error of the mean of three fields from three rats for each time point. (B) IL-1β mRNA was measured in cortical homogenates by real-time polymerase chain reaction in the ipsilateral hemispheres, and normalized to sham controls as described in the text. The data show the average ± standard deviation (ISH, in situ hybridization; IL-1β, interleukin-1β).
In summary, YKL-40 is elevated in CSF after severe TBI compared to controls, and is strongly associated with other proinflammatory and cytokine markers. YKL-40 transcription is induced in astrocytes in conjunction with acute neuroinflammation caused by CCI. In this model, YKL-40 expression is associated with IL-1β and is diminished when inflammation is resolved.
Discussion
For the first time in TBI patients, the present study showed that CSF YKL-40 concentrations are significantly elevated, reaching peak concentrations 4 days after injury. Given that YKL-40 expression is observed in several inflammatory diseases and conditions, these findings imply that YKL-40 represents a factor in the acute neuroinflammatory response that occurs as a part of secondary injury. These findings support our previous clinical research showing more pronounced YKL-40 expression in patients with acute infarcts and diminished expression in subacute or older infarcts. In that previous study, combined ISH and GFAP staining showed induced YKL-40 expression in astrocytes that was restricted to the penumbra of the infarct (in press). These findings are consistent with the CCI results presented here, and imply that acute inflammation induces YKL-40 expression in astrocytes proximal to the injury site, and that as inflammation resolves YKL-40 expression is diminished.
TBI-induced neuroinflammation is associated with elevation of numerous cytokines in the CSF in humans (Bell et al., 1997; Shiozaki et al., 2005). In our clinical cohort, we examined candidate cytokines for their overall association with YKL-40 expression. We show that there is a significant correlation between CSF YKL-40 concentrations and CSF IL-1β and TNF-α, suggesting that YKL-40 expression in TBI is associated with the level of neuroinflammation mediated by these cytokines. Interestingly, there was no correlation with CSF IL-6 and IL-10 levels. While it is possible that these cytokines indeed do not affect YKL-40 expression, it is also possible that they are expressed with different kinetics. Some cytokines such as IL-6 are induced rapidly, after 24 h, but they also decline quickly, after 72 h (Buttram et al., 2007; Shore et al., 2004), while it takes days for YKL-40 expression to reach its maximal levels. IL-10 is considered an anti-inflammatory cytokine, and it has been shown in some studies to be neuroprotective (Kline et al., 2002; Zhou et al., 2009a, 2009b). Thus it is not surprising that it does not show the same relationship to YKL-40 transcription that the proinflammatory cytokines do. Another interesting correlation occurred between YKL-40 and C-reactive protein (CRP). CRP is a known inflammatory marker found in the serum during systemic inflammatory conditions, and some reports suggest that there are elevations in serum CRP after TBI (Hergenroeder et al., 2008). CSF CRP measurements in clinical TBI are novel; however, the source of CSF CRP levels is not clear, and may be the result of blood–brain barrier disruption. Future ISH studies would enable analysis of the source of CRP in the brain.
This study shows that YKL-40 potentially could be used as a biomarker for the pathophysiology and prognosis of TBI. The results show that patients that died from injury have higher levels of CSF YKL-40 post-injury than patients with more favorable outcomes. Average YKL-40 concentrations measured during the first week post-TBI were inversely correlated with GOS score at 6 months. This association, along with YKL-40/cytokine associations, implies that the higher YKL-40 levels are associated with a proinflammatory response that may negatively influence clinical outcome. Although the GOS association with YKL-40 was a trend, and the population studied was small, our sample size calculations suggest that definitive associations with GOS score at 6 months could be achieved with a larger cohort. Larger population studies would also provide the opportunity to determine if there are population substructures with uniquely different temporal YKL-40 profiles after TBI, and to appropriately adjust for covariates. Given the associations between YKL-40 and cytokines noted here, and cytokine associations with TBI outcome in the literature, future work with larger sample sizes should concurrently assess the value of YKL-40 levels in combination with other cytokines like IL-1β and TNF-α to help predict outcome after TBI. Finally, future studies may also include serum YKL-40 measurements and associations with outcome, in order to determine the potential use of YKL-40 as a biomarker in other TBI populations in whom EVD placement and intracranial pressure management are not required.
Early increases in various cytokines in the brain have been also reported in rat brain injury models. Cytokines such as IL-1β, TNF-α, and IL-6 peak within few hours after brain injury, and thereafter start to decline (Shohami et al., 1994; Taupin et al., 1993). Using our rat model of TBI, we found focal and temporal increases in YKL-40 expression in astrocytes at the site of injury that temporally corresponded to the acute inflammatory reaction as measured by IL-1β mRNA. As the pericontusional region and inflammation resolved, IL-1β and YKL-40 expression diminished. This could be due to a variety of reasons; however, because it coincides with decreased inflammatory infiltrate, we hypothesize that decreased macrophage stimulation of astrocytes leads to decreased YKL40 expression. Our results in this CCI model may support the notion that cytokines released from macrophages that infiltrate the necrotic area during acute neuroinflammation are responsible for the focal pericontusional astrocytic YKL-40 transcription. Results from the rat TBI model complement human clinical data showing a correlation between CSF IL-1β and YKL-40 levels. Although we found a correlation between TNF-α and YKL-40 in human subjects, TNF-α levels in the animal model were very low, and we could not detect differences in TNF-α mRNA between the injured and non-injured hemispheres. However, regional increases in TNF-α mRNA may have been obscured by evaluation of whole cortical hemisphere changes in mRNA levels.
Neuroinflammation can persist for extended periods after TBI, and the role of glia and neuroinflammatory mediators can evolve with time from processes that perpetuate secondary injury, to processes that facilitate cellular rescue and recovery (Laird et al., 2008; Morganti-Kossmann et al., 2007). The role of YKL-40 in reactive astrogliosis is still obscure, but data from our previous study indicate that it may be involved in growth factor mobilization from the extracellular matrix, and thus may attenuate growth factor biological activities. Our work here suggests that YKL-40 elevations early after TBI are associated with acute mortality and worse global outcome. However, CSF YKL-40 levels also remain significantly elevated in our clinical cohort compared to controls by day 6 post-injury, and our animal studies suggest that YKL-40 tissue levels are elevated for some time after the acute period (at least 12 days post-CCI). Given the sustained increases in YKL-40 noted here, future work should assess YKL-40 at time points even more remote from the time of injury, in order to fully assess the time course for this marker, and its role in both injury and recovery. Further work to understand the role of YKL-40 in human and experimental TBI should also assess YKL-40 relationships with neurotrophins.
In summary, while the precise biological functions of YKL-40 are speculative, its expression is related to inflammation in a variety of disease states, and we show that this is true also in TBI. Further work is required to further evaluate the utility of YKL-40 as a biomarker and its role in neuroinflammation as well as neuroplasticity and recovery. In addition to the future directions discussed above, studies utilizing genetically modified animals not able to express YKL40, and pharmacology studies may help elucidate its role in TBI.
Acknowledgements
We thank Arlene Carbone-Wiley, Jonette Werley, and Mark Stauffer for valuable technical assistance. We thank the Brain Trauma Research Center (National Institutes of Health grant no. 5P01NS030318-16) for assistance with subject recruitment and sample collection. This work was supported by grants DODW81XWH-071-0701 and CDC R49 CCR323155-03 to A.K.W.; K24 MH01717 and RO1 MH071151 to C.A.W.; and Pittsburgh Foundation Emmerling funds to D.B.-B.
Author Disclosure Statement
No competing financial interests exist.
References
- Bell M.J. Kochanek P.M. Doughty L.A. Carcillo J.A. Adelson P.D. Clark R.S. Wisniewski S.R. Whalen M.J. DeKosky S.T. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J. Neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D. Bissel S.J. Wang G. Fish K.N. Nicholl G.C. Darko S.W. Medina-Flores R. Murphey-Corb M. Rajakumar P.A. Nyaundi J. Mellors J.W. Bowser R. Wiley C.A. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am. J. Pathol. 2008;173:130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttram S.D. Wisniewski S.R. Jackson E.K. Adelson P.D. Feldman K. Bayir H. Berger R.P. Clark R.S. Kochanek P.M. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Chiaretti A. Antonelli A. Mastrangelo A. Pezzotti P. Tortorolo L. Tosi F. Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma. 2008a;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- Chiaretti A. Antonelli A. Riccardi R. Genovese O. Pezzotti P. Di Rocco C. Tortorolo L. Piedimonte G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 2008b;12:195–204. doi: 10.1016/j.ejpn.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Hayakata T. Shiozaki T. Tasaki O. Ikegawa H. Inoue Y. Toshiyuki F. Hosotubo H. Kieko F. Yamashita T. Tanaka H. Shimazu T. Sugimoto H. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G. Redell J.B. Moore A.N. Dubinsky W.P. Funk R.T. Crommett J. Clifton G.L. Levine R. Valadka A. Dash P.K. Identification of serum biomarkers in brain-injured adults: potential for predicting elevated intracranial pressure. J. Neurotrauma. 2008;25:79–93. doi: 10.1089/neu.2007.0386. [DOI] [PubMed] [Google Scholar]
- Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Johansen J.S. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- Kirchhoff C. Buhmann S. Bogner V. Stegmaier J. Leidel B.A. Braunstein V. Mutschler W. Biberthaler P. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur. J. Med. Res. 2008;13:464–468. [PubMed] [Google Scholar]
- Kirkpatrick R.B. Emery J.G. Connor J.R. Dodds R. Lysko P.G. Rosenberg M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 1997;237:46–54. doi: 10.1006/excr.1997.3764. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Bolinger B.D. Kochanek P.M. Carlos T.M. Yan H.Q. Jenkins L.W. Marion D.W. Dixon C.E. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002;937:22–31. doi: 10.1016/s0006-8993(02)02458-7. [DOI] [PubMed] [Google Scholar]
- Laird M.D. Vender J.R. Dhandapani K.M. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lee C.G. Hartl D. Lee G.R. Koller B. Matsuura H. Da Silva C.A. Sohn M.H. Cohn L. Homer R.J. Kozhich A.A. Humbles A. Kearley J. Coyle A. Chupp G. Reed J. Flavell R.A. Elias J.A. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Satgunaseelan L. Bye N. Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Rehli M. Niller H.H. Ammon C. Langmann S. Schwarzfischer L. Andreesen R. Krause S.W. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- Shiozaki T. Hayakata T. Tasaki O. Hosotubo H. Fuijita K. Mouri T. Tajima G. Kajino K. Nakae H. Tanaka H. Shimazu T. Sugimoto H. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Shohami E. Novikov M. Bass R. Yamin A. Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J. Cereb. Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Shore P.M. Thomas N.J. Clark R.S. Adelson P.D. Wisniewski S.R. Janesko K.L. Bayir H. Jackson E.K. Kochanek P.M. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J. Neurotrauma. 2004;21:1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- Singhal A. Baker A.J. Hare G.M. Reinders F.X. Schlichter L.C. Moulton R.J. Association between cerebrospinal fluid interleukin-6 concentrations and outcome after severe human traumatic brain injury. J. Neurotrauma. 2002;19:929–937. doi: 10.1089/089771502320317087. [DOI] [PubMed] [Google Scholar]
- Taupin V. Toulmond S. Serrano A. Benavides J. Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J. Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Waje-Andreassen U. Krakenes J. Ulvestad E. Thomassen L. Myhr K.M. Aarseth J. Vedeler C.A. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z. Peng X. Insolera R. Fink D.J. Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp. Neurol. 2009a;220:183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. Peng X. Insolera R. Fink D.J. Mata M. Interleukin-10 provides direct trophic support to neurons. J. Neurochem. 2009b;110:1617–1627. doi: 10.1111/j.1471-4159.2009.06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]