Abstract
In this study we assessed the clinical utility of quantitative assessments of αII-spectrin breakdown products (SBDP145 produced by calpain, and SBDP120 produced by caspase-3) in cerebrospinal fluid (CSF) as markers of brain damage and outcome after severe traumatic brain injury (TBI). We analyzed 40 adult patients with severe TBI (Glasgow Coma Scale [GCS] score ≤8) who underwent ventriculostomy. Patients requiring CSF drainage for other medical reasons served as controls. CSF samples were taken at admission and every 6 h thereafter for a maximum of 7 days and assessed using novel quantitative fragment-specific ELISAs for SBDPs. Outcome was assessed using the 3-month Glasgow Outcome Scale. Mean CSF levels of SBDPs were significantly higher in TBI patients than in controls at all time points examined. Different temporal release patterns of CSF SBDP145 and SBDP120 were observed. SBDP145 provided accurate diagnoses at all time points examined, while SBDP120 release was more accurate 24 h after injury. Within 24 h after injury, SBDP145 CSF concentrations significantly correlated with GCS scores, while SBDP120 levels correlated with age. SBDP levels were significantly higher in patients who died than in those who survived. SBDP145 levels (>6 ng/mL) and SBDP120 levels (>17.55 ng/mL) strongly predicted death (odds ratio 5.9 for SBDP145, and 18.34 for SBDP120). The time course of SBDPs in nonsurvivors also differed from that of survivors. These results suggest that CSF SBDP levels can predict injury severity and mortality after severe TBI, and can be useful complements to clinical assessment.
Key words: biomarkers, brain injury, diagnostic, outcome, neuronal death, spectrin breakdown products
Introduction
Traumatic brain injury (TBI) constitutes a major worldwide health and socioeconomic problem (Cole, 2004; Ghajar et al., 2000). In the United States alone, approximately 2 million injuries occur each year, resulting in 56,000 deaths and 18,000 survivors who suffer from permanent neurological impairment (Bruns and Hauser, 2009; Sosin et al., 1996). The resulting direct and indirect annual costs in the United States are estimated at $56 billion (Thurman et al., 1998).
The early assessment of injury severity and prognosis are major challenges for physicians treating patients suffering from TBI. Commonly used techniques have significant limitations for accurate determination of the extent of brain damage. Functional scores such as the Glasgow Coma Scale (GCS), and classification of CT scans such as with the Marshall Computed Tomographic Classification (MCTC) are well established and investigated, but major concerns over these tools persist (Choi and Barnes, 1996; Marion, 1996; Report on The Traumatic Coma Data Bank, 1991; Stein, 1996; Teasdale and Jennett, 1974; Brenner and Hall, 2007; Bullard et al., 2007; Kmietowicz, 2007; Maas et al., 2005; Marshall et al., 1991; Robertson et al., 2003; Servadei et al., 2000; Jennett and Teasdale, 1977).
The GCS is often confounded by intubation and use of sedatives and muscle relaxants in TBI patients (Balestreri et al., 2004; Stocchetti et al., 2004). Neuroimaging techniques such as CT scanning capture only momentary snapshots of significant lesions, and lesions such as diffuse axonal injury (DAI) cannot be accurately visualized (Levi et al., 1990). MRI scanning has better sensitivity to diffuse brain damage, but is often unavailable and impractical to perform in physiologically unstable patients (Kesler, 2000).
Alternatively, several proteins synthesized in astroglial cells or neurons have been proposed as biomarkers of cell damage in the CNS, but insufficient evidence is currently available to justify their routine clinical use (Ingebrigtsen and Romner, 2002; Lyeth et al., 1993; Papa et al., 2008; Raabe and Seifert, 1999; Raabe et al,. 1999a). The most commonly used biomarkers include neuron-specific enolase (NSE), S-100β protein, glial fibrillary acidic protein, myelin basic protein (Palfreyman et al. 1978; Thomas et al., 1978), and recently, ubiquitin C-terminal hydrolase-L1 (UCH-L1).
NSE is thought to assess damage to the functional cells of the brain (i.e., neurons; McKeating et al., 1998; Woertgen et al., 1997; Marangos and Schmechel, 1987), but slow elimination makes it difficult to assess the amount of primary damage, and impossible to distinguish between primary and secondary injuries (Ross et al., 1996). NSE is also released in the blood by hemolysis, which may be a serious source of error (Johnsson, 1996). Numerous reports in the literature document the usefulness of measuring glial fibrillary acidic protein in CSF as a specific indicator of CNS pathological abnormalities (Eng et al., 1971; Eng 1980; Aurell et al., 1991; Blennow et al., 1996). S-100β, the principal low-affinity calcium-binding protein in astrocytes (Xiong et al., 2000), was reported to consistently correlate with both GCS score and neuroradiological findings at admission (Raabe et al., 1999b, 1998; Romner et al., 2000; Woertgen et al., 1999). Nevertheless S-100β, initially considered unique to the nervous system, is present in other tissues including adipocytes and chondrocytes. High serum S-100β levels have been observed in trauma patients without head injuries (Rothoerl et al., 2001; Anderson et al., 2001; Romner and Ingebritsen, 2001). In a recent study it was reported that levels of UCH-L1 in CSF were significantly increased in severe TBI patients, with a significant association seen between levels of UCH-L1 in CSF and injury severity measures, including GCS, evolving lesions on CT, and 6-week mortality (Papa et al., 2009).
Recently investigators have described αII-spectrin breakdown products (SBDPs) as potential biomarkers for brain injury in rats and humans (Pike et al., 2004, 2001; Pineda et al., 2007; Wang et al., 2005). αII-spectrin is primarily found in neurons, and is abundant in axons and presynaptic terminals (Riederer et al., 1986), and the protein is processed to breakdown products (SBDP) of molecular weights 150 kDa (SBDP150) and 145 kDa (SBDP145) by calpain, and is also cleaved to a 120-kDa product (SBDP120) by caspase-3. Calpain and caspase-3 are major factors causing necrotic and apoptotic cell death, respectively, during ischemia or TBI (Pike et al., 2001; Pineda et al., 2007; Ringger et al., 2004; Wang et al., 1998). Furthermore, there is considerable evidence of the presence of SBDPs in in vitro neuronal cell culture models of injury (Beer et al., 2000), in preclinical studies of TBI in mice (Hall et al., 2005), and in human studies of TBI (Farkas et al., 2005; Cardali and Maugeri, 2006; Pineda et al., 2007), and of subarachnoid hemorrhage (Lewis et al., 2007).
Pineda and colleagues, employing Western blot analyses, examined levels of SBDPs in CSF from adults with severe TBI (Pineda et al., 2007). In this study they demonstrated significant increases of SBDP levels in CSF following TBI. They also observed associations between SBDP values, severity of injury, CT scan findings, and clinical outcome. CSF biokinetic analyses of SBDPs as measured by Western blotting (Brophy et al., 2009) supported these findings, also suggesting that calpain-mediated necrotic oncosis may play a greater role in acute pathological responses to TBI than caspase-3-mediated apoptosis.
Thus SBDPs could provide crucial information not only on severity of brain injury, but also on the underlying pathophysiological injury mechanisms associated with necrotic and apoptotic cell death. Our study is the first to employ quantitative ELISA assays to evaluate levels of SBDP145 and SBDP120 in CSF of severe head-injured patients. Development and clinical validation of such quantitative assays are necessary prerequisites to the development of SBDPs as biomarkers having practical clinical utility. Our aim was to analyze the different release patterns of SBDP145 and SBDP120 in the acute (first 24 h) and subacute (7 days) phases post-injury, in order to assess the accuracy of CSF levels of SBDPs as markers of the extent of brain damage, and predictors of outcome and mortality after TBI.
Methods
Study sites, design, and population
This study was approved by the Institutional Review Board (IRB) of the University of Florida. Adult patients (n = 7) presenting to the University of Florida Trauma System (Shands Hospital in Gainesville and Jacksonville) following a severe head injury as defined by a GCS score of ≤8, and requiring ventricular intracranial pressure (ICP) monitoring, were enrolled in the study and were followed for 3 months after study entry. Consent was obtained within 24 h of enrollment. The clinical data and CSF samples contributed to the study from the University of Pécs site were collected from 33 patients admitted with a GCS score ≤8 as a part of an IRB-approved protocol. Control subjects, also from the University of Pécs, were patients without TBI (n = 24) requiring CSF drainage for other medical reasons with normal pressure hydrocephalus. All patient identifiers were kept confidential. CSF samples from severe TBI subjects were directly collected from the ventriculostomy catheter every 6 h for a maximum of 7 days following TBI (ventriculostomy catheters are placed as routine medical care for patients with severe TBI at these institutions). Since the duration of time between trauma, admission, and ventriculostomy catheter placement varied, CSF sampling for SBDP measurement was not always possible at exactly the same time points after TBI for the first 24 h. Therefore we established 6-h time intervals for sampling of CSF, and documented the time interval of measurement accordingly. Approximately 3–4 mL of CSF was collected from each subject at each sample point. The samples were immediately centrifuged for 10 min at 4000 rpm to separate CSF from blood cells, and were immediately frozen and stored at −70°C until the time of analysis.
βII-Spectrin breakdown product measurements
For SBDP145 and SBDP120 sandwich ELISAs, 96-well plates were coated with 100 μL/well of capture antibody (1000 ng/well of affinity-purified rabbit polyclonal anti-SBDP145 or anti-SBDP120 fragment-specific antibody) overnight at 4°C. After a blocking buffer (Fisher 37539 Startingblock T20-PBS; Thermo Fisher Scientific, Rockford, IL) step, antigen standard (recombinant GST-fusion-αII-spectrin, repeat 13–18[ + 145]) cleaved with calpain (1:50 ratio for 10 min at 4°C, or with caspase-3 4 h at room temperature) were used to establish a standard curve. Stock solution of 0.5–5000 ng/mL of prepared SBDP145 protein (0.005–50 ng in 10 μL) were diluted 1:10 with sample diluent to a final incubation volume of 100 μL/well. Thus the standard curve range was 0.05–500 ng/mL in the wells. Each sample was evaluated in duplicate. The target (10 μL CSF/well and 90 μL PBST blocking buffer) and capture antibody were incubated for 2 h at 27°C with gentle shaking. The plate was washed with TBST washing buffer 5× with an automatic plate washer. Then a 1:3000–1:4000 dilution of HRP-labeled detection antibody (alpha fodrin from Biomol International, Plymouth Meeting, PA) was added to each well, 100 μL per well, and was incubated at room temperature for 1.5 h with gentle shaking. If amplification was needed, biotinyl-tyramide solution (Elast Amplification Kit; PerkinElmer, Waltham, MA) was added for 15 min at room temperature, then washed and followed by 100 μL/well streptavidin-HRP (1:3000) in PBS with 0.02% Tween-20 and 1% BSA for 30 min, followed by 5× washing by an automatic plate washer. Lastly, the wells were developed with 100 μL/well chemiluminescent substrate solution (SuperSignal ELISA Femto #37075; Pierce Protein Research Products, Rockford, IL) with an incubation time of 1 min. The signal was read by a 96-well chemiluminescence microplate reader (GloRunner DXL Luminometer; Turner BioSystems, Inc., Sunnyvale, CA).
Clinical and outcome measures
To examine the relationships between accumulation of SBDPs and clinical variables, severity of injury was assessed using the GCS as previously described (Teasdale and Jennett, 1974, 1976). Patients were classified with the GCS according to IMPACT study criteria (Marmarou et al., 2007). We identified two critical time points: prehospital assessment (post-injury GCS), and the qualifying scores for enrollment in the study (hospital admission GCS). The qualifying score for enrollment was defined as the score obtained post-stabilization, or if this was not available, the score obtained on admission. Patients were classified according to a commonly used dichotomization of the GCS (GCS score 3–5 versus GCS score 6–8; Narayan et al., 1981). Outcome was assessed at 3 months after injury using the Glasgow Outcome Scale (GOS) (Jennett, 1975). Assessment GOS was obtained by direct patient contact or via telephone interview with the patient and/or a family member. For statistical analyses, outcome was dichotomized into mortality and survival.
Statistical analysis
For statistical analyses, biomarker levels were treated as continuous data, measured in nanograms per milliliter and expressed as mean ± SEM. Data were assessed for equality of variance and distribution and examined using the Mann-Whitney U test.
Age was tested for linear relationship with SBDPs by Pearson's correlation coefficient. Results were confirmed by linear regression models with SBDPs as the dependent variable. Statistical significance was set at p < 0.05. Receiver-operating characteristic (ROC) curves were generated to explore the ability of the biomarker to distinguish between TBI patients and uninjured controls at different time points post-injury. Reasonable cutoff values for SBDPs to predict mortality were constructed. The optimal cutoff value for a particular protein was chosen so that the sum of the sensitivity and the specificity to predict mortality was maximal. Crude odds ratios with 95% confidence intervals are presented. These analyses were performed using the statistical software package SigmaPlot version 11.0 (Systat Software, Inc., Richmond, CA).
Results
The study included 40 severe TBI patients (GCS score 3–8), and 24 control patients with normal pressure hydrocephalus who had samples obtained from a ventricular catheter placed as part of routine clinical care (Table 1).
Table 1.
Summary of Demographic and Clinical Data for Severe Traumatic Brain Injury (TBI) Patients and Normal Pressure Hydrocephalus Cases Included in This Study
| Characteristic | TBI patients (n = 40) | Controls (n = 24) |
|---|---|---|
| Age (y) | ||
| Mean ± SEM | 41.5 ± 3.17 | 56.2 ± 4.42 |
| Range | 18–82 | 23–83 |
| Median | 39.5 | 62 |
| Gender male/female | 29/11 | 15/9 |
| Male/female (%) | 71/29 | 62/38 |
| Marshall CT classification | ||
| Diffuse injury II | 1 (2.5%) | |
| Diffuse injury III | 12 (30%) | |
| Diffuse injury IV | 1 (2.5%) | |
| Evacuated mass lesion | 13 (32.5%) | |
| Glasgow Coma Scale (GSC) score | 15 | |
| Median injury GSC | 4.5 | |
| Median ED GSC | 4 | |
SEM, standard error of the mean; ED, emergency department; CT, computed tomography.
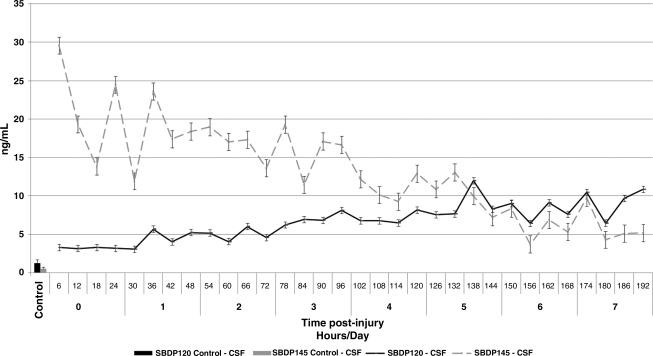
Both SBDP145 (mean ± SEM 14.42 ± 0.91 ng/mL versus 0.52 ± 0.22 ng/mL; p < 0.0001) and SBDP120 (mean ± SEM 6.05 ± 0.28 ng/mL versus 1.21 ± 0.48 ng/mL; p < 0.0001) concentrations were significantly higher in TBI patients than in controls at all time points post-injury (Fig. 1 and Table 2). Longitudinal data from serial SBDP145 and 120 measurements showed that the release pattern of SBDP145 into the CSF was different from that of SBDP120 in patients with severe TBI. SBDP145 mean concentrations peaked earlier (29.56 ng/mL at 6 h on day 0) than SBDP120 following TBI (11.96 ng/mL at 138 h on day 5).
FIG. 1.
Mean ± standard error of the mean concentrations of cerebrospinal fluid (CSF) βII-spectrin breakdown product (SBDP)145 and SBDP 120 over 7 days for severe traumatic brain injury (TBI) patients and for control subjects (single time point only).
Table 2.
Levels of βII-Spectrin Breakdown Products (SPDP) in Controls and in the First 24 Hours after Severe Traumatic Brain Injury
| |
|
Severe TBI |
|||
|---|---|---|---|---|---|
| |
Controls |
≤6 h |
7–12 h |
13–18 h |
19–24 h |
| n = 24 | n = 12 | n = 12 | n = 17 | n = 23 | |
| SBDP120 | 1.21 ± 0.48 | 3.31 ± 1.65 | 3.15 ± 0.63 | 3.32 ± 0.74 | 3.20 ± 0.86 |
| SBDP145 | 0.52 ± 0.22 | 29.56 ± 10.45 | 20.32 ± 7.33 | 13.84 ± 3.61 | 24.46 ± 7.22 |
Mean values ± standard error of the mean for samples obtained at 6, 12, 18, and 24 h post-injury.
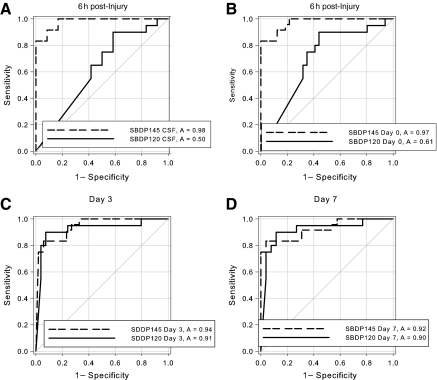
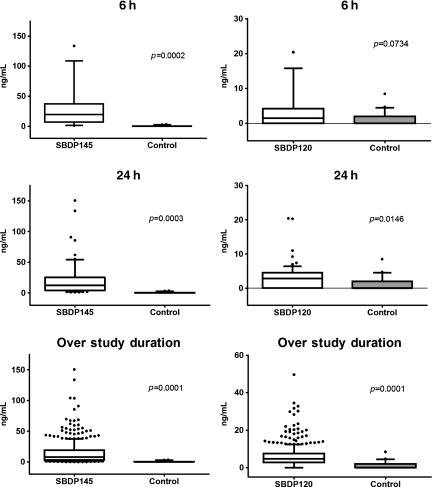
SBDP145 had comparable diagnostic accuracy at all time points (p < 0.0001), with optimal detection as early as 6 h after injury. In contrast, SBDP120 did not reliably detect severe TBI at 6 h after injury, but was comparable to SBDP145 by 7 days after injury. The area under the curve (AUC) is a measure of predictive discrimination: 50% is equivalent to random guessing and 100% is perfect prediction. Thus a higher AUC indicates a better ability to discriminate between injured patients and uninjured controls. Interestingly, diagnostic accuracy as assessed by ROC curves reflected the temporal profile (Fig. 2). Frequency distributions of SBDP145 and SBDP120 at 6 h, 24 h, and over the study duration are shown as box plots in Figure 3. Box plots convey information about the data distribution: location, measured by the median (the line across the middle); spread, measured by the height of the box (lower quartile and upper quartile); range of individual observations; and the presence of outliers.
FIG. 2.
Receiver-operating characteristic (ROC) curves and area under the curve (AUC) values for βII-spectrin breakdown product (SBDP)145 and SBDP120 concentrations in CSF samples obtained at 6 h (A), 24 h (B), and at day 3 (C), and day 7 (D) post-injury. Note that the AUC values for SBDP120 increased over time.
FIG. 3.
Box and whisker graphs showing frequency distributions of value for samples obtained at 6 h and 24 h, and at day 7 post-injury. There were significant differences in βII-spectrin breakdown product (SBDP)145 cerebrospinal fluid (CSF) levels between patients with severe TBI and controls at all time points (6 h: p = 0.0002; 24 h: p = 0.0003, and over study duration p = 0.0001). Significant differences in SBDP120 CSF levels were observed at all time points (24 h: p = 0.0146, and over study duration p = 0.0001), except at 6 h post-injury (p = 0.0734). The bottom and top of the boxes represent the 25th and 75th percentile, respectively. The band near the middle is the median. Lower and upper whiskers represent data within 1.5 of the interquartile range. Outliers are plotted as small circles.
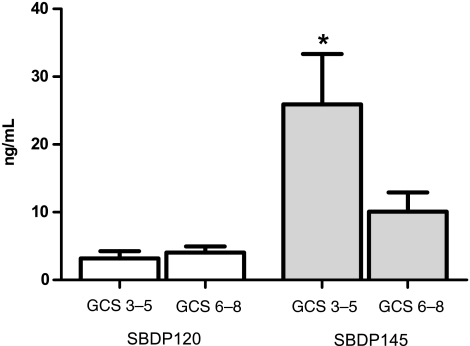
Lower levels of CSF SBDP145 were found in patients with higher hospital admission GCS scores (GCS scores recorded in the emergency department; GCS score 6–8; 10.10 ± 2.81 ng/mL), than in patients with lower GCS scores (GCS score 3–5; 25.93 ± 7.43 ng/mL; p < 0.05; Fig. 4). Initial CSF SBDP120 levels were not significantly related to hospital admission and post-injury GCS (Fig. 4).
FIG. 4.
βII-Spectrin breakdown product (SBDP)145 levels, but not SBDP 120 levels, within the first 24 h were significantly lower in patients with higher hospital admission Glasgow Coma Scale (GCS) scores (6–8; 10.10 ± 2.807 ng/mL), than in patients with lower hospital admission GCS scores (3–5; 25.93 ± 7.428 ng/mL; p = 0.03 by Mann-Whitney U test). *p < 0.05.
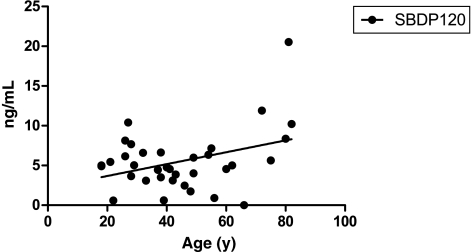
Levels of SBDP120, but not levels of SBDP145, in CSF significantly correlated with age (r = 0.37; p < 0.05; Fig. 5). The median age of patients who died was significantly higher than the age of patients who survived (36.7 versus 67 years; p < 0.005). We did not find statistical correlations between SBDP145 and SBDP120 levels in CSF and gender.
FIG. 5.
Scatterplot and linear regression showing significant relationship between βII-spectrin breakdown product (SBDP)120 average levels in the first 24 h post-injury and age (r = 0.37; p = 0.03) in severe traumatic brain injury patients
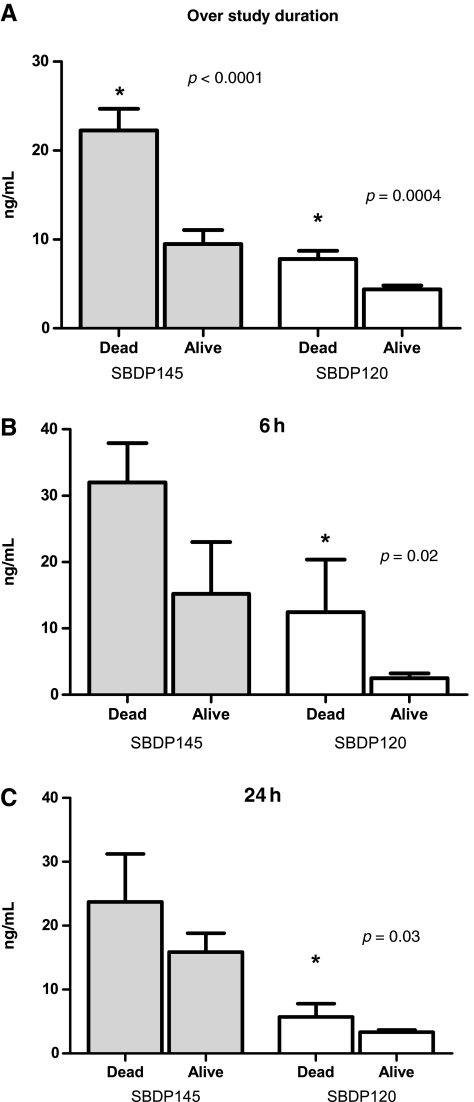
Of the 40 patients included in this study, 8 patients (20%) died, 26 patients (65%) survived, and 6 patients (15%) were lost to follow-up within 3 months. Patients who died had significantly higher SBDP145 and SBDP120 concentrations than surviving patients (over 7 days post-injury SBDP145 mean ± SEM = 22.25 ± 2.43 ng/mL versus 9.49 ± 1.58 ng/mL; p < 0.0001; over 7 days post-injury SBDP120 mean ± SEM = 7.81 ± 0.91 ng/mL versus 4.38 ± 0.43 ng/mL; p < 0.005; Fig. 6A). Moreover, levels of CSF SBDP120 were significantly higher at 6 h (p = 0.02) and at 24 h (p = 0.03) in patients who died (Fig. 6B and C). The temporal profile of SBDP120 was also relatively more sustained in patients who died. To discriminate survivors from nonsurvivors, optimal cutoff values were calculated for the proteins by using the criterion of equal-cost-of-misclassification. The sensitivity in predicting death at 3 months for SBDP145 levels (>6 ng/mL) within 24 h after injury was high (0.92), although the specificity was low (0.51; Table 3). The sensitivity in predicting death at 3 months for SBDP120 levels (>17.55 ng/mL) was low (0.19), but the specificity was very high (0.99; Table 3). The crude odds ratio for CSF SBDP145 levels >6 ng/mL was 5.9 (95% CI 3.26,10.68; p < 0.0001 by Fisher's exact test, dichotomized, using the cutoff values), and for CSF SBDP120 levels >17.55 ng/mL was 18.34 (95% CI 5.77,58.29; p < 0.0001 by Fisher's exact test, dichotomized, using the cutoff values; Table 4). The odds ratio represents a risk measure. Our data show that the odds of mortality among patients with CSF SBDP145 levels above 6 ng/mL and among patients with CSF SBDP120 levels above 17.55 ng/mL were 5.9 and 18.34 times, respectively, greater than the odds of mortality among patients with lower levels. Since this is a crude odds ratio, it was not adjusted for other confounders in the model. Note that the cutoff levels were clearly higher than the reference values in healthy donors (for SBDP145 6 versus 0.52 ng/mL, and for SBDP120 17.55 versus 1.21 ng/mL).
FIG. 6.
(A) Over 7 days after injury βII-spectrin breakdown product (SBDP)145 and SBDP120 levels were significantly higher in patients who died than in those who survived (0.54 ng/mL versus 2.7 ng/mL; *p < 0.0001 and p = 0.0004, respectively). (B) Mean SBDP120 concentrations were statistically significantly higher in patients who died than in surviving patients at 6 h post-injury (p = 0.02), and (C) 24 h post-injury (p = 0.03).
Table 3.
Summary of Predictive Values of βII-Spectrin Breakdown Products (SBDPs) for 3-Month Survival After Severe Traumatic Brain Injury
| |
|
Mortality |
|||
|---|---|---|---|---|---|
| Cutoff | Sensitivity | Specificity | PPV | NPV | |
| SBDP145 within 24 h after injury | 6 ng/mL | 0.92 | 0.51 | 0.65 | 0.86 |
| SBDP120 | 17.55 ng/mL | 0.19 | 0.99 | 0.94 | 0.55 |
Cutoff values were constructed using receiver-operating characteristic (ROC) curves under the assumption of equal costs of misclassification of cases and noncases (i.e., the sum of the sensitivity and the specificity to predict outcome was maximal).
PPV, positive predictive value; NPV, negative predictive value.
Table 4.
Crude Odds Ratio (OR) for Mortality at 3 Months After Severe Traumatic Brain Injury
| |
|
Mortality |
|
|---|---|---|---|
| Cutoff | OR (95% confidence interval) | p | |
| SBDP145 | 6 ng/mL | 5.9 (3.26, 10.68) | <0.0001 |
| SBDP120 | 17.55 ng/mL | 18.34 (5.77–58.27) | <0.0001 |
By Fisher's exact test, dichotomized, using the cutoff values.
Discussion
Several proteins detected in CSF have been proposed as useful indicators of injury magnitude and outcome after brain injury. Detection of these proteins in blood or CSF offers little insight into neurochemical alterations that mediate brain damage after TBI. In contrast, SBDP levels are related to specific cell death processes within cells (necrosis versus apoptosis, and activation of calpain versus caspase-3; Pike et al., 2004; Ringger et al., 2004; Wang et al., 1998). Using ELISA assays, this prospective observational study of 40 patients provides the first quantitative extension of previous findings employing Western blot analyses collected in humans following a severe brain injury (Brophy et al., 2009; Farkas et al., 2005; Pineda et al, 2007;). Data further confirm the significant contribution of calpain- and caspase-3-mediated proteolysis to the pathobiology of human TBI. Moreover, this study demonstrates that elevated and sustained levels can be useful for identification of subjects at risk for death, and that assessments of severity of injury and outcome in severe head-injured patients might be improved via the determination of CSF SBDPs.
Our data are also consistent with CSF biokinetic analyses of SBDPs employing Western blotting data (Brophy et al., 2009). When compared with caspase-3-mediated SBDP120, the median area under the curve and maximum concentration were significantly greater for calpain-mediated SBDP145 and SBDP150. However, the time to reach maximum concentration was longer for SBDP120.
As in previous studies (Farkas et al., 2005; Pineda et al, 2007), we found that levels of SBDPs in CSF were able to distinguish between brain-injured patients and uninjured controls. In addition, levels of SBDPs were significantly elevated in injured subjects across all time points examined. Like Pineda and colleagues (Pineda et al., 2007), we also found that the release pattern of SBDP145 into the CSF differed from that of SBDP120 in patients with severe TBI. In our study, SBDP145 mean concentrations peaked early (at 6 h post-injury), and decreased slowly for at least 7 days post-injury. In contrast, SBDP120 showed a sustained elevation that persisted for at least 7 days post-injury, and mean concentrations peaked on day 5 (Fig. 1). These observations suggest that necrotic/oncotic and apoptotic cell death mechanisms are activated with distinct time patterns in humans after severe TBI. Thus SBDPs can be used to monitor different temporal characteristics of protease activation.
Previous investigations into calpain activation and subsequent spectrin proteolysis in the injured brain (including both contusional and non-contusional domains) have largely associated these proteolytic processes with overt somatic damage and/or necrotic cell death of neurons in the contusional and peri-contusional regions (Hall et al., 2005; Newcomb et al., 1997; Saatman et al., 1996). Our data are in agreement with previous findings; furthermore, our results are consistent with a growing body of experimental literature in which emphasis has been placed on the relationship between nerve fiber involvement and SBDPs (Ai et al., 2007; McGinn et al., 2009; Saatman et al., 1996, 2003; Serbest et al., 2007). However, the current study included patients with varying magnitudes of diffuse and focal lesions, which could potentially influence biomarker levels, and ongoing studies by our group are assessing relationships between biomarkers and CT classification of patients using the Marshall and Rotterdam scores.
Axons are vulnerable to the mechanical forces associated with TBI. McCreacken and colleagues provided evidence in TBI patients that calpain-mediated breakdown of the cytoskeleton may contribute to axonal delayed damage after head injury (McCracken et al., 1999). Although a proportion of axons become severed at the time of injury—the so-called primary axotomy—many axons undergo secondary or delayed axotomy (Graham and Gennarelli, 1997; Maxwell et al., 1997), distinct from the irreversible axonal damage that occurs at the moment of injury (Gennarelli, 1996; Povlishock and Christman, 1995). Other investigators have hypothesized that an alteration in axolemmal permeability can lead to activation of neutral proteases, such as calpains and caspase-3, which in turn causes collapse of the axonal cytoskeleton. Processes leading to secondary axotomy are thought to occur over a period of 12 h and more in humans (Blumbergs et al., 1994, 1995; Gentleman et al., 1993; Povlishock and Christman, 1995). Our data are consistent with these previous studies. The potential use of SBDPs as markers of axonal damage in TBI patients could be particularly relevant in cases of diffuse brain injury, in which conventional imaging techniques often fail to reveal the full extent of diffuse axonal injury (Belanger et al., 2007). ROC analyses demonstrated that SBDP145 has a similar diagnostic accuracy at all time points, with optimal detection as early as 6 h after injury. In contrast, SBDP120 did not reliably detect severe TBI at 6 h after injury, but was comparable to SBDP145 by 24 h after injury (Fig. 2). Thus the accuracy of diagnostic assays of necrotic versus apoptotic cell death may be critically dependent on the time point after injury the sample is taken, and is consistent with the more delayed expression of apoptotic cell death (Pineda et al., 2007).
We found a significant correlation between CSF calpain-related SBDP (SBDP145) levels within the first 24 h after injury and initial severity of injury, as measured by hospital admission GCS scores (the initial clinical assessment of severity of injury in the emergency department). Analysis of the earliest sample collection time point (6 h) was limited by the number of samples available (n = 12). SBDP120, produced by apoptotic-induced caspase proteolysis, did not correlate with hospital admission GCS score and initial severity of injury, consistent with a potentially more prominent role of calpain proteolysis in the acute phase of injury. The GCS score is universally accepted in the assessment of neurological function and level of consciousness of patients with head injury after trauma (Rowley and Fielding, 1991). Despite the limitations of the GCS in assessing severity of injury (Stocchetti et al., 2004), hospital enrollment GCS is recommended by the IMPACT study investigators for prognostic analyses (Marmorou et al., 2007). Pre-hospitalization GCS score and SBDPs, though they had a similar trend, did not show a statistically significant correlation, an observation possibly related to the reduced accuracy of pre-hospitalization GCS.
Levels of SBDP120 were significantly higher in older patients than in younger patients, suggesting increased apoptotic processes in elderly people. We also examined the correlation between SBDP120 levels and age in our control group, and detected a similar but not statistically significant trend. Future studies will need to rigorously examine this issue with a larger and more comprehensive control group, allowing multivariate analyses of other relevant clinical and demographic variables.
SBDPs were significantly associated with mortality within 12 weeks after injury. Patients dying within 3 months after severe TBI presented with higher mean initial SDBP145 and SBDP120 concentrations over the 7-day observation period (Fig. 6). This observation is consistent with the ongoing secondary destructive processes of neural tissue (e.g., secondary insults) during the early stage of severe TBI in patients with poor prognoses. Interestingly, non-survivors showed secondary and repeated elevations of both SBDPs that persisted for at least 7 days post-injury (data not shown).
It is important to know the potential outcome of the patient at the earliest possible stage after TBI, and the prediction of outcome may be improved with the determination of CSF levels of SBDPs (SBDP145 and SBDP120). Cutoff values for the serum markers were determined upon inspection of the data, and were based on giving equal weight to sensitivity and specificity for predicting outcome. Choosing other cutoffs will change the specificity, and consequently the estimate of the independent effect of each marker on outcome prediction. Confirmation by other studies is clearly needed. The sensitivity of SBDP145 CSF levels within 24 h after injury in predicting survival with the chosen cutoffs was 0.92, and the negative predictive value was 0.86, whereas the specificity of SBDP120 CSF levels (over the study duration) was 0.99, with a positive predictive value of 0.94 (Table 3). Thus when CSF levels of SBDP145 within 24 h after injury are below the cutoff level, the patient has an 86% chance of surviving after 3 months. When SBDP120 CSF levels (over the study duration) are above the cutoff level, the patient has a 99% chance of dying within 3 months. The calculated combination of sensitivity and specificity represent a potentially important tool for application in clinical practice.
While prediction of mortality may have limited clinical utility, SBDPs could also provide information about threatening or ongoing neurological complications and outcome in intensive care unit (ICU) patients. Here, since patients often have reduced consciousness and/or are sedated, adequate monitoring of clinical status is challenging and neuroimaging is difficult. Other available techniques, such as ICP monitoring (invasive), microdialysis (invasive and measures only focally), and transcranial doppler (user-dependent), have limitations.
Thus repeated assessments of biomarkers in CSF and serum of ICU patients could provide critical information about the most effective management of patients. Future studies of the biokinetics of biomarkers will be necessary to guide timing of repeated assessments in CSF and blood.
Our data are in accord with previous studies showing a correlation of initial SBDP145 CSF levels with outcome after severe TBI (Pineda et al., 2007), and also suggest that early and sustained expression of apoptotic cell death, as well as necrotic cell death, play a crucial role in poor patient outcome. Further studies with larger samples are needed, including multivariate analyses of other relevant clinical variables that may influence outcome.
Our data are also consistent with the IMPACT study, that showed a strong correlation between age and mortality (poor outcome) after severe TBI (Mushkudiani et al., 2007). In addition, other studies have assessed the effect of age on outcome (Hukkelhoven et al., 2003). This is the first investigation to correlate CSF SBDP120 levels with age and CSF SBDP120 levels with mortality (Figs. 5 and 6). Additional studies of SBDPs, age, and mortality, will further our understanding of the relationships among biomarkers, age, and clinical outcome.
The use of ELISA provides a more rapid, practical, and quantitative approach to CSF detection of proteins than the Western blot used in previous studies (Lewis et al., 2007; Pineda et al., 2007). However, our data are consistent with results of these previous studies. The authors recognize that collection of CSF samples is invasive and not always possible, but the aim of our study was to establish SBDP levels in CSF as a first step of validation of our ELISA assay, and to avoid confounding factors such as the influence of the actions of different proteases on serum SBDP levels. Ongoing studies in our laboratories are focused on developing a serum-based assay to detect these SBDPs in blood. These assays will also allow assessment of these biomarkers in patients experiencing different severities of head injury (e.g., patients with mild or moderate head injuries who do not require ICP monitoring).
It is possible that our data could have been contaminated by SBDPs of extracerebral origin. However, since we studied CSF, contributions from extracerebral sources are unlikely. In addition, because α-spectrin is not found in erythrocytes (Pike et al., 2001), there is little possibility that SBDP values were increased by the frequent hemolysis that occurs as a result of systemic injury. However, ongoing studies employing polytrauma and orthopedic controls are needed to further assess the potential of extracerebral sources of biomarkers.
In conclusion, the ELISA assays used here confirm the occurrence of calpain-mediated proteolysis in CSF after severe TBI. These results strongly support the utility of measurement of α-spectrin proteolytic cleavage products in patients with brain injury to monitor for ongoing secondary proteolytic cell death processes (Wang, 2000). Measurement of SBDPs, each with its own characteristics, could add important prognostic information to the initial evaluation of patients with severe TBI, and be a novel component of an early assessment of outcome and risk.
Acknowledgments
This study was supported in part by Department of Defense Award numbers DAMD17-03-1-0772 and DAMD17-03-1-0066; National Institutes of Health Award numbers R01 NS049175-01, R01-NS052831-01, and R01 NS051431-01; Navy grant number N00014-06-1-1029 (University of Florida); and National Institutes of Health grant #P01-NS38660 (Baylor College of Medicine).
Author Disclosure Statement
Drs. Robicsek, Gabrielli, Brophy and Papa are consultants of Banyan Biomarkers, Inc.; Ms. Scharf, Jixiang, Akinyi and are employees of Banyan Biomarkers, Inc.; Drs. Muller, Wang and Hayes own stock, receive royalties from, and are officers of Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
References
- Ai J. Liu E. Wang J. Chen Y. Yu J. Baker A.J. Calpain inhibitor MDL-28170 reduces the functional and structural deterioration of corpus callosum following fluid percussion injury. J. Neurotrauma. 2007;24:960–978. doi: 10.1089/neu.2006.0224. [DOI] [PubMed] [Google Scholar]
- Anderson R.E.H.L. Nilsson O. Dijai-Merzoug R. Settergen G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;49:1272–1273. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Aurell A. Rosengren L.E. Karlsson B. Olsson J.E. Zbornikowa V. Haglid K.G. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke. 1991;22:1254–1258. doi: 10.1161/01.str.22.10.1254. [DOI] [PubMed] [Google Scholar]
- Balestreri M. Czosnyka M. Hutchinson P. Steiner L.A. Hiler M. Smielewski P. Pickard J.D. Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. J. Neurol. Neurosurg. Psychiatry. 2004;75:161–162. [PMC free article] [PubMed] [Google Scholar]
- Beer R. Franz G. Krajewski S. Pike B.R. Hayes R.L. Reed J.C. Wang K.K. Klimmer C. Schmutzhard E. Poewe W. Kampfl A. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J. Neurochem. 2000;75:1264–1273. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Blennow M. Rosengren L. Jonsson S. Forssberg H. Katz-Salamon M. Hagberg H. Hesser U. Lagercrantz H. Glial fibrillary acidic protein is increased in the cerebrospinal fluid of preterm infants with abnormal neurological findings. Acta Paediatr. 1996;85:485–489. doi: 10.1111/j.1651-2227.1996.tb14068.x. [DOI] [PubMed] [Google Scholar]
- Blumbergs P.C. Scott G. Manavis J. Wainwright H. Simpson D.A. McLean A.J. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Blumbergs P.C. Scott G. Manavis J. Wainwright H. Simpson D.A. McLean A.J. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma. 1995;12:565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- Brenner D.J. Hall E.J. Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 2007;22:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- Brophy G.M. Pineda J.A. Papa L. Lewis S.B. Valadka A.B. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Tepas J.J., 3rd Gabrielli A. Robicsek S. Wang K.K. Robertson C.S. Hayes R.L. alphaII-Spectrin breakdown product cerebrospinal fluid exposure metrics suggest differences in cellular injury mechanisms after severe traumatic brain injury. J. Neurotrauma. 2009;264:471–479. doi: 10.1089/neu.2008.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J., Jr. Hauser W.A. The epidemiology of traumatic brain injury: a review. Epilepsia. 2009;44(Suppl. 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Bullard T. Papa L. Wegst A. Falk J. Estimating the cumulative risk of ionizing radiation exposure from diagnostic testing in an emergency department population: What do we really know? Acad. Emerg. Med. 2007;14:5. (Suppl. 1). [Google Scholar]
- Cardali S. Maugeri R. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. J. Neurosurg. Sci. 2006;50:25–31. [PubMed] [Google Scholar]
- Choi S.C. Barnes T.Y. Predicting outcome in the head-injured patient. In: Narayan R.K., editor; Wilberger E., editor; Povlishock J.T., editor. Neurotrauma. McGraw Hill; New York: 1996. pp. 779–792. [Google Scholar]
- Cole T.B. Global road safety crisis remedy sought: 1.2 million killed, 50 million injured annually. JAMA. 2004;291:531–532. doi: 10.1001/jama.291.21.2531. [DOI] [PubMed] [Google Scholar]
- Eng L.F. The glial fibrillary acidic protein. In: Bradshaw R.A., editor; Schneider D.M., editor. Proteins of the Nervous System. Raven Press; New York: 1980. pp. 85–117. [Google Scholar]
- Eng L.F. Vanderhaeghen J.J. Bignami A. Gersti B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971;28:351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Farkas O. Polgár B. Szekeres-Barthó J. Dóczi T. Povlishock J.T. Büki A. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—preliminary observations. Acta Neurochir. Wien. 2005;1478:855–861. doi: 10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- Gennarelli T.A. The spectrum of traumatic axonal injury. Neuropathol. Appl. Neurobiol. 1996;22:509–513. doi: 10.1111/j.1365-2990.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Gentleman S.M. Nash M.J. Sweeting C.J. Graham D.I. Roberts G.W. ß-Amyloid precursor protein/3-APP as a marker for axonal injury after head injury. Neurosci. Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Gennarelli T.A. Greenfields Neuropathology. 6th. Arnold; London: 1997. Trauma; pp. 197–262. [Google Scholar]
- Hall E.D. Sullivan P.G. Gibson T.R. Pavel K.M. Thompson B.M. Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hukkelhoven C.W. Steyerberg E.W. Rampen A.J. Farace E. Habbema J.D. Marshall L.F. Murray G.D. Maas A.I. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J. Neurosurg. 2003;994:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T. Romner B. Biochemical serum markers of TBI. J. Trauma. 2002;52:798–808. doi: 10.1097/00005373-200204000-00038. [DOI] [PubMed] [Google Scholar]
- Jennett B. Predicting outcome after head injury. J R Coll Physicians Land. 1975;9:231–237. [PMC free article] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Aspects of coma after severe head injury. Lancet. 1977;1:878–881. doi: 10.1016/s0140-6736(77)91201-6. [DOI] [PubMed] [Google Scholar]
- Johnson A.M. Rohlfs E.M. Silverman L.N. Proteins. In: Burtis C.A., editor; Ashwood E.R., editor. Tietz Textbook of Clinical Chemistry. W.B. Saunders Company; Philadelphia: 1999. p. 516. [Google Scholar]
- Johnsson P. Markers of cerebral ischemia after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 1996;10:120–126. doi: 10.1016/s1053-0770(96)80187-x. [DOI] [PubMed] [Google Scholar]
- Kesler E.A. SPECT, MR and quantitative MR imaging: correlates with neuropsychological and psychological outcomes in traumatic brain injury. Brain Inj. 2000;14:851–857. doi: 10.1080/026990500445682. [DOI] [PubMed] [Google Scholar]
- Kmietowicz Z. Better safe than sorry? BMJ. 2007;335:1182–1184. doi: 10.1136/bmj.39415.528623.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi E.A. Diffuse axonal injury: Analysis of 100 patients with radiological signs. Neurosurgery. 1990;27:429–432. [PubMed] [Google Scholar]
- Levin H.S. Saydjari C. Eisenberg H.M. Foulkes M. Marshall L.F. Ruff R.M. Jane J.A. Marmarou A. Vegetative state after closed-head injury. A Traumatic Coma Data Bank Report. Arch Neurol. 1991;48:580–585. doi: 10.1001/archneur.1991.00530180032013. [DOI] [PubMed] [Google Scholar]
- Lewis S.B. Velat G.J. Miralia L. Papa L. Aikman J.M. Wolper R.A. Firment C.S. Liu M.C. Pineda J.A. Wang K.K. Hayes R.L. Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: a novel biomarker of proteolytic injury. J. Neurosurg. 2007;1074:792–796. doi: 10.3171/JNS-07/10/0792. [DOI] [PubMed] [Google Scholar]
- Lyeth B.G. Jiang J.Y. Robinson S.E. Guo H. Jenkins L.W. Hypothermia blunts acetylcholine increase in CSF of traumatically brain injured rats. Mol. Chem. Neuropathol. 1993;18:247–256. doi: 10.1007/BF03160117. [DOI] [PubMed] [Google Scholar]
- Maas A.I. Hukkelhoven C.W. Marshall L.F. Steyerberg E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- Marangos P.J. Schmechel D.E. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Ann. Rev. Neurosci. 1987;10:269–295. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- Marion D.W. Outcome from severe head injury. In: Narayan R.K., editor; Wilberger E., editor; Povlishock J.T., editor. Neurotrauma. McGraw Hill; New York: 1996. pp. 767–777. [Google Scholar]
- Marmarou A. Lu J. Butcher I. McHugh G.S. Murray G.D. Steyerberg E.W. Mushkudiani N.A. Choi S. Maas A.I. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J. Neurotrauma. 2007;242:270–280. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75(Suppl.):S14–S20. [Google Scholar]
- Maxwell W.L. Povlishock J.T. Graham D.I. A mechanistic analysis of non-disruptive axonal injury: a review. J. Neurotrauma. 1997;14:419–439. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- McCracken E. Hunter A.J. Patel S. Graham D.I. Dewar D. Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J. Neurotrauma. 1999;169:749–761. doi: 10.1089/neu.1999.16.749. [DOI] [PubMed] [Google Scholar]
- McGinn M.J. Kelley B.J. Akinyi L. Oli M.W. Liu M.C. Hayes R.L. Wang K.K. Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating E.G. Andrews P.J.D. Mascia L. Relationship of neuron specific enolase and protein S-100 concentrations in systematic and jugular venous serum to injury severity and outcome after traumatic brain injury. Acta Neurochir. Suppl. Wien. 1998;71:117–119. doi: 10.1007/978-3-7091-6475-4_35. [DOI] [PubMed] [Google Scholar]
- Mushkudiani N.A. Engel D.C. Steyerberg E.W. Butcher I. Lu J. Marmarou A. Slieker F. McHugh G.S. Murray G.D. Maas A.I. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;242:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Greenberg R.P. Miller J.D. Enas G.G. Choi S.C. Kishore P.R. Selhorst J.B. Lutz H.A., 3rd Becker D.P. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J. Neurosurg. 1981;54:751–762. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- Newcomb J.K. Kampfl A. Posmantur R.M. Zhao X. Pike B.R. Liu S.J. Clifton G.L. Hayes R.L. Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J. Neurotrauma. 1997;14:369–383. doi: 10.1089/neu.1997.14.369. [DOI] [PubMed] [Google Scholar]
- Palfreyman J.W. Thomas D.G.T. Ratcliffe J.G. Radioimmunoassay of human myelin basic protein in tissue extract, cerebrospinal fluid and serum and its clinical application to patients with head injury. Clin. Chim. Acta. 1978;82:259–270. doi: 10.1016/0009-8981(78)90008-6. [DOI] [PubMed] [Google Scholar]
- Papa L. Akinyi L. Liu M.C. Pineda J.A. Tepas J.J., 3rd Oli M.W. Zheng W. Robinson G. Robicsek S.A. Gabrielli A. Heaton S.C. Hannay H.J. Demery J.A. Brophy G.M. Layon J. Robertson C.S. Hayes R.L. Wang K.K. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care. Med. 2009;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L. Robinson G. Oli M. Pineda J. Demery J. Brophy G. Robicsek S.A. Gabrielli A. Robertson C.S. Wang K.K. Hayes R.L. Use of biomarkers for diagnosis and management of traumatic brain injury. Expert Opin. Med. Diagn. 2008;28:1–9. doi: 10.1517/17530059.2.8.937. [DOI] [PubMed] [Google Scholar]
- Pike B.R. Flint J. Dutta S. Johnson E. Wang K.K. Hayes R.L. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 2001;786:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Pike B.R. Flint J. Dave J.R. Lu X.C. Wang K.K. Tortella F.C. Hayes R.L. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alpha II-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Lewis S.B. Valadka A.B. Papa L. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Aikman J.M. Akle V. Brophy G.M. Tepas J.J. Wang K.K. Robertson C.S. Hayes R.L. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Christman C.W.J. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. Neurotrauma. 1995;124:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Raabe A. Seifert V. Fatal secondary increase in serum S-100B protein after severe head injury. J. Neurosurg. 1999;91:875–877. doi: 10.3171/jns.1999.91.5.0875. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br. J. Neurosurg. 1999a;13:56–59. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- Raabe A. Grolms C. Sorge O. Zimmermann M. Seifert V. Serum S-100B protein in severe head injury. J. Neurosurg. 1999b;45:477–483. doi: 10.1097/00006123-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Raabe A. Menon D.K. Gupta S. Czosnyka M. Pickard J.D. Jugular venous and arterial concentrations of serum S-100B protein in patients with severe head injury: a pilot study. J. Neurol. Neurosurg. Psychiatry. 1998;65:930–932. doi: 10.1136/jnnp.65.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B.M. Zagon I.S. Goodman S.R. Brain spectrin 240/235 and brain spectrin 240/235E: two distinct spectrin subtypes with different locations within mammalian neural cells. J. Cell Biol. 1986;102:2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringger N.C. O'Steen B.E. Brabham J.G. Silver X. Pineda J. Wang K.K. Hayes R.L. Papa L. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J. Neurotrauma. 2004;21:1443–1456. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- Robertson R.L. Robson C.D. Zurakowski D. Antiles S. Strauss K. Mulkern R.V. CT versus MR in neonatal brain imaging at term. Pediatr. Radiol. 2003;337:442–449. doi: 10.1007/s00247-003-0933-6. [DOI] [PubMed] [Google Scholar]
- Romner B. Ingebrigtsen T. High serum S100B levels for trauma patients without head injuries. J. Neurosurg. 2001;49:1490. doi: 10.1097/00006123-200112000-00053. [DOI] [PubMed] [Google Scholar]
- Romner B. Ingebrigtsen T. Kongstad P. Borgesen S.E. Traumatic brain injury: serum S-100 measurements related to neuroradiological findings. J. Neurotrauma. 2000;17:641–647. doi: 10.1089/089771500415391. [DOI] [PubMed] [Google Scholar]
- Ross S.A. Cunningham R.T. Johnston C.F. Rowlands B.J. Neuron-specific enolase as an aid to outcome prediction in head injury. Br. J. Neurosurg. 1996;10:471–476. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- Rothoerl R.D. Woertgen C. High serum S100B levels for trauma patients without head injuries. J. Neurosurg. 2001;49:1490–1491. doi: 10.1097/00006123-200112000-00054. [DOI] [PubMed] [Google Scholar]
- Rowley G. Fielding K. Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users. Lancet. 1991;337:535–538. doi: 10.1016/0140-6736(91)91309-i. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Abai B. Grosvenor A. Vorwerk C.K. Smith D.H. Meaney D.F. Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J. Cereb. Blood Flow Metab. 2003;23:34–42. doi: 10.1097/01.WCB.0000035040.10031.B0. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Bozyczko-Coyne D. Marcy V. Siman R. McIntosh T.K. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J. Neuropathol. Exp. Neurol. 1996;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Serbest G. Burkhardt M.F. Siman R. Raghupathi R. Saatman K.E. Temporal profiles of cytoskeletal protein loss following traumatic axonal injury in mice. Neurochem. Res. 2007;32:2006–2014. doi: 10.1007/s11064-007-9318-9. [DOI] [PubMed] [Google Scholar]
- Servadei F. Murray G.D. Penny K. Teasdale G.M. Dearden M. Iannotti F. Lapierre F. Maas A.J. Karimi A. Ohman J. Persson L. Stocchetti N. Trojanowski T. Unterberg A. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. European Brain Injury Consortium [discussion 75–77] J. Neurosurg. 2000;46:70–75. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sosin D.M. Sniezek J.E. Thurman D.J. Incidence of mild and moderate brain injury in the United States. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- Stein S.C. Classification of head injury. In: Narayan R.K., editor; Wilberger E., editor; Povlishock J.T., editor. Neurotrauma. McGraw-Hill; New York: 1996. pp. 31–41. [Google Scholar]
- Stocchetti N. Pagan F. Calappi E. Canavesi K. Beretta L. Citerio G. Cormio M. Colombo A. Inaccurate early assessment of neurological severity in head injury. J. Neurotrauma. 2004;21:1131–1140. doi: 10.1089/neu.2004.21.1131. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir. Wien. 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thomas D.G. Palfreyman J.W. Ratcliffe J.G. Serum myelin basic protein assay in diagnosis and prognosis of patients with head injury. Lancet. 1978;21:113–115. doi: 10.1016/s0140-6736(78)90415-4. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1998;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Ottens A.K. Liu M.C. Lewis S.B. Meegan C. Oli M.W. Tortella F.C. Hayes R.L. Proteomic identification of biomarkers of traumatic brain injury. Expert Rev. Proteomics. 2005;24:603–614. doi: 10.1586/14789450.2.4.603. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Posmantur R. Nath R. McGinnis K. Whitton M. Talanian R.V. Glantz S.B. Morrow J.S. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 CPP32 in apoptotic cells. J. Biol. Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Wang K.K.W. Calpain and caspase: Can you tell the difference. Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Woertgen C. Rothoerl R.D. Holzschuh M. Metz C. Brawanski A. Comparison of serial S-100 and NSE serum measurements after severe head injury. Acta Neurochir. Wien. 1997;139:1161–1165. doi: 10.1007/BF01410977. [DOI] [PubMed] [Google Scholar]
- Woertgen C. Rothoerl R.D. Metz C. Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J. Trauma. 1999;47:1126–1130. doi: 10.1097/00005373-199912000-00026. [DOI] [PubMed] [Google Scholar]
- Xiong H. Liang W.L. Wu X.R. [Pathophysiological alterations in cultured astrocytes exposed to hypoxia/reoxygenation] Sheng Li Ke Xue Jin Zhan. 2000;31:217–221. [PubMed] [Google Scholar]