Abstract
The present study directly compares the effects of experimental brain injury in two commonly used rat strains: Fisher 344 and Sprague-Dawley. We previously found that Fisher rats have a higher mortality rate and more frequent seizure attacks at the same injury level than Sprague-Dawley rats. Although strain differences in rats are commonly accepted as contributing to variability among studies, there is a paucity of literature addressing strain influence in experimental neurotrauma. Therefore this study compares outcome measures in two rat strains following lateral fluid percussion injury. Fisher 344 and Sprague-Dawley rats were monitored for changes in physiological measurements, intracranial pressure, and electroencephalographic activity. We further analyzed neuronal degeneration and cell death in the injured brain using Fluoro-Jade-B (FJB) histochemistry and caspase-3 immunostaining. Behavioral studies using the beam walk and Morris water maze were conducted to characterize strain differences in both motor and cognitive functional recovery following injury. We found that Fisher rats had significantly higher intracranial pressure, prolonged seizure activity, increased FJB-positive staining in the injured cortex and thalamus, and increased caspase-3 expression than Sprague-Dawley rats. On average, Fisher rats displayed a greater amount of total recording time in seizure activity and had longer ictal durations. The Fisher rats also had increased motor deficits, correlating with the above results. In spite of these results, Fisher rats performed better on cognitive tests following injury. The results demonstrate that different rat strains respond to injury differently, and thus in preclinical neurotrauma studies strain influence is an important consideration when evaluating outcomes.
Key words: epilepsy, Fischer 344 rat, fluid percussion injury, Sprague-Dawley rat, strain
Introduction
The heterogeneity of traumatic brain injury (TBI) has been difficult to replicate in standardized animal models of TBI that strive for less variability in experimental conditions. Several experimental TBI models have been developed to replicate the various pathoanatomical conditions observed clinically, and to test therapeutic interventions. One factor that has been relatively unexplored in the TBI literature is how rat strain (genetic background) may influence outcomes following TBI. Inconsistencies in data across laboratories might be due to the differences between the strains of rats employed in the experiments. Comparing the injury responses between strains may be a useful tool for exploring how strain choice influences outcomes following injury, and may reveal subtle differences that affect the reproducibility of results across different experiments.
Strain differences have been previously reported in a wide variety of studies under both normal and pathological circumstances. For example, significant stain-related differences have been reported with regard to neurotransmitter receptor distributions (Jiao et al., 2003; Lei et al., 2009), rates of neurogenesis in the dentate gyrus (Alahmed and Herbert, 2008), susceptibility to seizure development and behavioral recovery profiles following status epilepticus (Hort et al., 2000; Xu et al., 2004), ischemic lesion volumes following experimental stroke (Walberer et al., 2006), and forebrain activation before and after spinal cord injury (Paulson et al., 2005). Other studies have found strain-related differences in neuroendocrine profiles and behavioral differences regarding addictive behavior and stress (Kearns, 2006; Uchida et al., 2008). Specific to the current study are the results showing that inbred Fisher 344 rats are hyperresponsive to stress (Uchida et al., 2008), more susceptible to ischemic insult (Iwasaki et al., 1995), and have lower electroconvulsive seizure thresholds than wild-type Sprague-Dawley rats (Golden et al., 1995; Statler et al., 2008).
Although strain differences are commonly thought to contribute to variability among results, few studies have addressed how strain influence may affect outcome measures following experimental brain injury. One previous study compared differences in injury response between different mouse strains and found no significant differences in measures of hippocampal neuronal loss (Witgen et al., 2006). However, this study used mice with very subtle genetic differences and only looked at one outcome measure. Another study reported differences in behavioral recovery profiles following experimental brain injury in rats, suggesting that strain differences are an important consideration in experimental design (Tan et al., 2009). In TBI studies, Sprague-Dawley rats have been most commonly used. In contrast, Fisher 344 rats have been less stringently investigated, and their response to injury is not well characterized. Inbred strains such as the Fisher 344 are being used more frequently in TBI studies, especially in aging and transplantation studies, due to their inbred nature, body size, and wide availability. In our preliminary studies with Fisher 344 rats, we observed acute seizures and a higher mortality rate following lateral fluid percussion injury (L-FPI) compared to Sprague-Dawley rats. Therefore this study was designed to directly compare the consequences of L-FPI at the same level of severity in Fisher 344 and Sprague-Dawley rats.
Methods
Subjects
Adult (3-month-old) male Sprague-Dawley (Harlan Sprague-Dawley, Inc., Indianapolis, IN) and Fischer 344 (Harlan Sprague-Dawley) rats weighing 250–350 g were used in all studies. The animals were housed with free access to food and water in a 12-h dark-light cycle at 22°C. All procedures followed the guidelines established in the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services), and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Three studies with separate groups of both Sprague-Dawley and Fischer 344 rats were performed (Table 1). In the first study, adult male Sprague-Dawley and Fischer 344 rats were randomly assigned to be evaluated acutely (0–2.5 h post-injury) following L-FPI for changes in mean arterial blood pressure, blood gases, and intracranial pressure. In the second study, electroencephalographic (EEG) monitoring of acute post-injury seizure activity was performed for a period of 24 h post-injury, followed by histochemical analysis of brain sections to assess injury-induced neuronal degeneration. A third group of animals was examined for motor and cognitive function as described below. Animals that died immediately or before the end of the experiment were used in the calculation of mortality rate, but were not used in the analysis of any of the other experiments.
Table 1.
Study Design
| Strain | Acute study MABP, blood gases, and ICP (0–3 h) | EEG and FJ (24 h) | Behavioral tests (motor and cognitive) | Died immediately or within 2 h of L-FPI | Total animals |
|---|---|---|---|---|---|
| Fischer 344 | Injured n = 4 | Injured | Injured: n = 7 | 16 | Injured n = 35 |
| EEG n = 5 | Sham: n = 10 | Sham n = 10 | |||
| FJ n = 8 | |||||
| Sprague-Dawley | Injured n = 4 | Injured | Injured: n = 8 | 0 | Injured n = 17 |
| EEG n = 5 | Sham: n = 4 | Sham: n = 12 | |||
| FJ n = 5 | Motor n = 9 MWM |
MABP, mean arterial blood pressure; FJ, Fluoro-Jade; ICP, intracranial pressure; L-FPI, lateral fluid percussion injury; MWM, Morris water maze; EEG, electroencephalogram.
Fluid percussion injury
FPI is the most commonly used rodent model of TBI. L-FPI produces a combination of both focal and diffuse injury, and has become one of the most extensively utilized models of injury (Thompson et al., 2005). In all experiments, the animals were anesthetized with 4% isoflurane in a 70% N2O:30% oxygen mixture for 4 min and placed in a stereotaxic frame. The scalp was sagittally incised and a 4.8-mm lateral craniotomy was trephined into the skull to the right of the sagittal suture midway between the bregma and the lambda. A Luer-Lok™ syringe hub 2.6 mm in diameter was secured onto the skull at the site of the craniotomy with cyanoacrylate adhesive. One nickel-plated screw was placed 1 mm caudal to the lambda on the contralateral side. Dental acrylic was applied around the hub and allowed to dry before injury.
The fluid percussion device used to produce experimental brain injury was identical to that used previously on rodents, and was described in detail by Dixon and colleagues (1987). Briefly, the device consists of a Plexiglas cylinder that is 60 cm long and 4.5 cm in diameter with a rubber-covered O-ring-fitted acrylic glass piston at one end. The opposite end of the cylinder has a metal housing 2 cm in length that contains a pressure transducer. At the end of the metal housing is a 5-mm tube with a 2.6-mm inside diameter that connects with the surgically implanted Luer-Loc fitting implanted in the rat's skull. The device was filled with isotonic saline. The injury was produced by a pendulum that strikes the piston of the injury device and injects a small volume of saline into the cranial cavity to produce brief displacement and deformation of brain tissue. The extracranial pressure pulse was expressed in atmospheres (atm), and all animals were injured with 1.65–1.75 atm. Tail and foot pinch reflexes as well as righting reflexes were recorded in all injured groups.
Acute neurological evaluations
Sprague-Dawley (n = 4) and Fischer 344 (n = 4) rats were anesthetized and surgically prepared for L-FPI as described above. Before injury, the femoral artery was cannulated to monitor mean arterial blood pressure (MABP) and to measure arterial blood gases. Blood gas and plasma pH were measured using a blood gas machine (ABL 700; Radiometer A/S, Copenhagen, Denmark) before injury, immediately after injury, and at 30, 60, and 120 min after injury. Intracranial pressure (ICP) was monitored using a previously published method (Clausen and Hillered, 2005; Levasseur, 2000). For the experiment presented here, the high-pressure transducer for recording the fluid pressure injury pulse was placed in the regular housing of the head injury apparatus, and a standard low-pressure transducer, adapted to a 20-cm length of polyethylene 50 tubing, was used to monitor the ICP at a point just above the cranial connector at the base of the volume displacement indicator. During the administration of the injury pulse, the low-pressure transducer was protected by a closed stopcock, which was immediately reopened following injury to monitor changes in ICP. The ICP was recorded before injury, immediately after injury, and at 30, 60, and 120 min post-injury. At 3 h post-injury, all animals were perfused with ice cold saline and their brains and lungs were removed for pathological analysis.
Preparation for electroencephalographic monitoring
After the animals were anesthetized by isoflurane inhalation, four skull electrodes were implanted as routinely performed in our laboratory (Singleton et al., 2005), with modifications made to accommodate L-FPI. Two frontal leads were implanted over the frontal cortex (3.5 mm anterior to the bregma, +2.5 L/R F1/F2). One posterior lead was implanted over the parietal cortex and hippocampus (2.0 mm post-bregma, +2.5 mm P2). The fourth lead (P1) was moved caudally to allow for the implantation of the Luer-Loc syringe hub. A 4.8-mm hole was then trephined into the skull over the sagittal suture midway between the bregma and the lambda (–4.0 mm post-bregma, −3 left of midline). This location approximates an injury to the parietal cortex. A modified female Luer-Loc syringe hub (2.6 mm inside diameter) was placed over the exposed dura and bonded in place with cyanoacrylate adhesive. Dental acrylic was then poured around the syringe hub and screws.
Electroencephalographic monitoring and data analysis
Prior to injury, the animals (n = 5, for both groups) were connected via headset to video-EEG machines (BMSI 5000; Nicolet, Madison,WI), and baseline recordings were obtained using a sampling rate of 420 Hz and the notch filter set at 60 Hz. Following L-FPI, the animals were reconnected to the EEG machines and monitored for EEG activity from 3–24 h post-injury. Visual examination of the EEG was performed to assess the total number of discrete seizures, the average duration of each discrete seizure, and the total time spent in ictal activity. Quantitative EEG analysis (qEEG) was completed using Insight II software (Persyst Corporation, Prescott, AZ), with a sampling rate of 200 samples/sec, the notch filter at 60 Hz, and the reading filters at 20 Hz to minimize background noise. For the initial analysis, fast Fourier transfer (FFT) analysis was performed to identify the major frequencies seen during the discrete seizure phase. Considering amplitude of 2.5 μV2 as baseline, all frequencies were defined as minor frequencies having amplitudes ranging from 2.5–5 μV2, and major frequencies with amplitudes ranging from 6–10 μV2.
Baseline and ictal activity was further studied for quantification of power in specific frequency bins ranging from 0–30 Hz. Five separate frequency bins were defined: a delta frame (0–4.0 Hz), a theta frame (4–8 Hz), an alpha frame (8–13 Hz), a β1 frame (13–18 Hz), and a β2 frame (18–30 Hz). Average amplitudes of all frequencies, measured in μV2 (power), were recorded in each frequency bin using 12-sec epochs, and averaged for each animal and then averaged for each group. Spectral analyses were tested by Mann-Whitney rank sums.
Tissue preparation and histology
At 24 h after L-FPI (1.7 ± 0.05 atm), Sprague-Dawley (n = 5) and Fischer 344 (n = 8) rats were deeply anesthetized with an overdose injection of sodium pentobarbital (100 mg/kg), and transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). The brains were removed and fixed with 4% paraformaldehyde for 48 h. The brains were then sectioned in the coronal plane at 60 μm throughout the entire length of the hippocampus using a vibratome (Leica Microsystems, Wetzlar, Germany), and stored in 24-well plates with PBS and .01% sodium azide. Every fourth section was mounted on a slide (Superfrost Plus; Erie Scientific Company, Portsmouth, NH) and processed for Fluoro-Jade B (FJB) staining.
FJB staining has been reported to specifically stain degenerating neurons and has been proven to be a sensitive and reliable marker of neuronal vulnerability following TBI (Anderson et al., 2004). The method of FJB staining we used was similar to that described by Schmued and Hopkins (2000). Briefly, the sections were first treated with 1% NaOH in 80% ethanol for 5 min and then were hydrated in graded ethanols and distilled water. They were then incubated in .06% potassium permanganate solution for 10 min, followed by a quick rinse and incubation with .0004% FJB (Histo-Chem, Inc., Jefferson, AR) plus .0004% DAPI (Sigma-Aldrich, St. Louis, MO) solution for 20 min. The slides were then dried, immersed in Citra Solv (2 × 5 min; Citra Solv, Danbury, CT), and cover-slipped.
Immunohistochemistry
Adjacent sections were used for immunohistochemistry to examine caspase-3 activity. After several rinses in PBS, the sections were incubated in 3% H2O2 for 1 h, and then rinsed in PBS plus .03% Triton X-100. The sections were blocked in 5 % normal horse serum in PBS plus .03% Triton X-100 for 2 h to block nonspecific binding. The sections were then incubated overnight with monoclonal mouse anti-caspase-3 (1:200; Cell Signaling Technology, Inc., Beverly, MA), with blocking buffer at 4°C. Following several washes with PBS plus .03%Triton X-100, the sections were incubated with Alexa Fluor 568 goat anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA) overnight at 4°C. After three washes with PBS, the sections were counterstained with DAPI (1:1000; Sigma-Aldrich) for 10 min at room temperature, then mounted with Vectashield (Vector Laboratories, Burlingame, CA), cover-slipped, and examined with a fluorescent microscope.
Stereological quantification of FJB-positive cells
The total number of FJB-positive cells in the cortex, thalamus, and hippocampus (dentate gyrus, hilus, CA1, and CA3) was quantified in each section using the Olympus Imaging System NewCAST program (Olympus, Ballerup, Denmark). From each brain, ten 60-μm coronal sections containing the hippocampus were examined using the stereology optical fractionator method. Ten 60-μm-thick sections 240 μm apart through the rostro-caudal extent of the hippocampus at the level from −2.56 mm to −5 mm of the bregma were examined. The number of FJB-positive cells in the ipsilateral cortex, thalamus, and hippocampus (dentate gyrus [DG], CA3, and hilus regions, counted separately) were counted by a blinded observer. We used the design-based stereological method, which is commonly used in neuroscience studies of this nature (Grady et al., 2003; Sun et al., 2009; Tran et al., 2006). Briefly, the region of interest was outlined using a 4 × objective, and a 60 × oil immersion objective was used for cell counting. In the examined region, an optical dissector counting frame was used to count FJB-positive cells at predetermined regular x, y intervals. The area (a) of the counting frame was known relative to the stage-stepping intervals over the section, and yielded the sampling fraction (asf) = a (frame)/a (x,y step). The dissector height (h) was known relative to the section thickness (t). Using these parameters, the number of total cells (n) was estimated as n = (ΣQ)(t/h)(1/asf )(1/ssf ), where ssf was the section-sampling fraction (0.25 in this study), and ΣQ was the number of cells counted.
Behavioral measures
All animals were assessed for motor (post-injury days 1, 3, and 7) and cognitive (post-injury days 11–15 for latency, and day 16 for the probe trial) performance following injury. To assess fine motor coordination, we used a standard beam-walking task, in which the Sprague-Dawley (n = 4 sham and n = 8 injured) and Fischer (n = 10 sham and n = 7 injured) rats were pre-trained prior to injury to escape a bright light and loud white noise by traversing an elevated 100-cm long 2.5-cm wide wooden beam to enter a darkened goal box at the opposite end of the beam. Performance was assessed by recording the latency for the rat to reach the goal box (not to exceed 60 sec). The mean of three trials per day was used for statistical analysis.
The Morris water maze (MWM) was used to assess cognitive function following injury. The MWM is a well-established method for the assessment of cognitive function following TBI (Sun et al., 2009). Sprague-Dawley (n = 9 sham and n = 8 injured) and Fischer 344 (n = 10 sham and n = 7 injured) rats were given four trials per day for five consecutive days. The submerged platform was placed 45 cm from the outside wall and remained constant throughout testing. The rats were given a maximum of 120 sec to find the goal. After this time, the rats were placed on the goal and held there for 30 sec. The latency and path length to reach the submerged platform were recorded, and swim speed was calculated. A 60-sec probe trial was performed 24 h after MWM training, and proximity and time spent in the goal quadrant were analyzed.
Statistical analysis
All data were analyzed using SPSS software (SPSS, Inc., Chicago, IL), and are presented as the mean ± standard error. Acute physiological variables and ICP measurements were analyzed using analysis of variance (ANOVA). The Student's t-test was used to compare strain differences in ICP values at each time point. FJB-positive cell counts were also analyzed with Student's t-test. A repeated-measures ANOVA was used to analyze beam-walk and MWM latencies, and probe trial data were compared using a one-way ANOVA. When necessary, post-hoc comparisons between groups were performed using Tukey's honestly significant difference test. Differences between means were assessed at the probability level of P < 0.05 and 0.01.
Results
Severity of injury
A total of 35 Fisher 344 rats and 17 Sprague-Dawley rats received moderate to mild L-FPI (1.65–1.75 atm). At this injury level, the Fisher rats experienced a much higher mortality rate than the Sprague-Dawley rats. Among the Fisher rats only 55% regained their righting reflex and survived past 2 h, compared to a 100% survival rate for Sprague-Dawley rats. Of the Fisher 344 rats surviving injury, the average righting reflex time was 12.87 ± 1.45 min, compared to an average of 5.58 ± 0.58 min for the Sprague-Dawley rats.
Physiological evaluations
To evaluate injury-induced physiological changes, we measured MABP, Po2, Pco2, plasma pH, and concentration of total hemoglobin (ctHB) acutely following L-FPI in both Fisher and Sprague-Dawley rats (Table 2). We found that in both strains MABP increased immediately after trauma, and then gradually returned to baseline within 2 h. No significant differences were found in any systemic physiological measurements at baseline or at any time point post-injury between the strains.
Table 2.
Physiological Variables
| Variable | Strain | Pretrauma value | Immediate | 30 min | 1 h | 2 h |
|---|---|---|---|---|---|---|
| MABP | Fischer | 86.5 ± 3.6 | 113 ± 33 | 95 ± 5.1 | 99 ± 4.5 | 99 ± 2.1 |
| SD | 80.1 ± 2.8 | 102 ± 21 | 79 ± 9 | 92 ± 3.2 | 91 ± 4.2 | |
| Blood Po2 | Fischer | 115 ± 6.9 | 155 ± 17.4 | 130 ± 16.8 | 129 ± 11.7 | 157 ± 3.4 |
| SD | 118 ± 9.3 | 105 ± 17.2 | 134 ± 20.5 | 147 ± 19.4 | 122 ± 19.1 | |
| Blood Pco2 | Fischer | 41.9 ± 3.2 | 39.9 ± 2.8 | 38.8 ± 2.6 | 45.1 ± 4.2 | 36 ± 7.6 |
| SD | 43.1 ± 3.2 | 25.8 ± 4.1 | 35.4 ± 3.6 | 36 ± 3.7 | 35 ± 2.8 | |
| Plasma pH | Fischer | 7.4 ± 0.02 | 7.4 ± 0.03 | 7.4 ± 0.02 | 7.4 ± 0.03 | 7.3 ± 0.03 |
| SD | 7.4 ± 0.03 | 7.4 ± 0.03 | 7.44 ± 0.03 | 7.5 ± 0.12 | 7.5 ± 0.03 | |
| ctHb | Fischer | 13.1 ± 0.35 | 13.2 ± 0.48 | 13.5 ± 0.56 | 13.4 ± 0.44 | 11.4 ± 1.7 |
| SD | 14.2 ± 0.25 | 10.5 ± 0.7 | 13.5 ± 1.0 | 14.33 ± 0.17 | 13.7 ± 0.76 |
MABP, mean arterial blood pressure; SD, standard deviation.
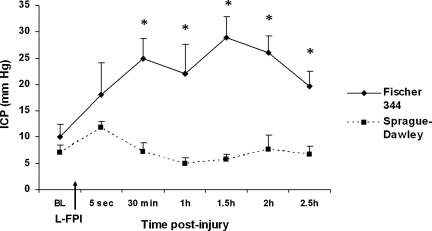
ANOVA found a significant effect on strain (F1,6 = 21.7; p = 0.003). No significant differences between the strains were found at baseline or immediately following injury (p > 0.05; Fig. 1). Beginning at 30 min after L-FPI, the Fisher rats displayed significantly higher ICP values (expressed as mm Hg) than Sprague-Dawley rats (p < 0.05; Fig. 1). The ICP values for Fischer rats remained significantly higher than those of Sprague-Dawley rats at 1 (p < 0.05), 1.5, 2, and 2.5 h (p < 0.01; Fig. 1) post-injury. The lungs were also analyzed, but no pathological differences were found between the two strains (data not shown).
FIG. 1.
Recordings of intracranial pressure (ICP) in Fisher 344 and Sprague-Dawley rats following lateral fluid percussion injury (L-FPI). Fisher rats displayed significantly higher ICP than Sprague-Dawley rats at 30 min (p < 0.05), 1 h (p < 0.001), 1.5 h (p < 0.001), 2 h (p < 0.001), and 2.5 h (p < 0.05) post-injury.
Strain differences on electroencephalographic patterns following L-FPI
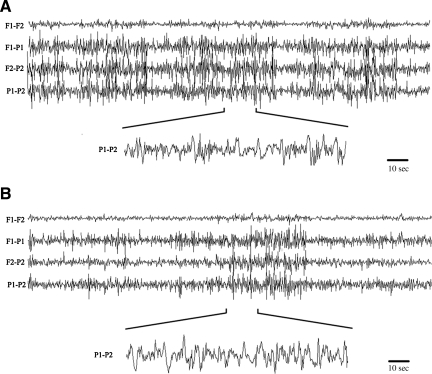
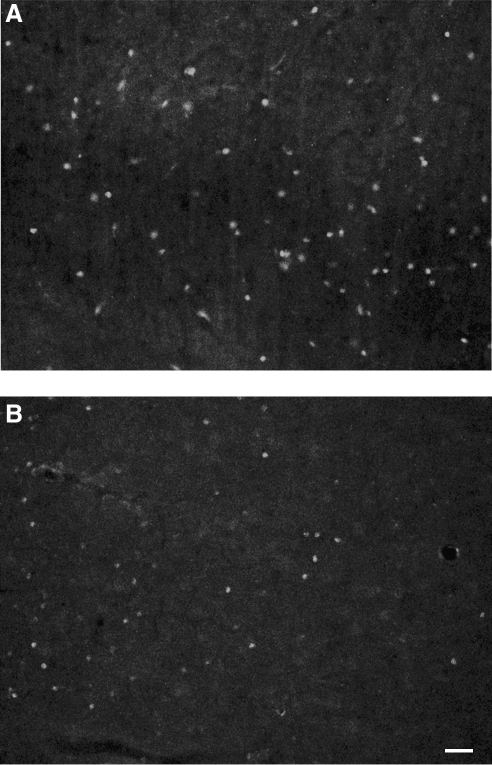
We have previously observed that Fisher 344 rats frequently display acute seizure following L-FPI. To evaluate post-injury seizure activity, we monitored both Fisher and Sprague-Dawley rats with EEG for 24 h post-injury. In both strains, average baseline electrographic activity was nondescript, and FFT analysis demonstrated a flat frequency contribution, suggesting a lack of any dominant frequencies under normal conditions. Following injury, EEG activity was affected in both groups. All Fisher rats displayed significant ictal activity, and 3 of 5 animals displayed status epilepticus-like events (Fig. 2). Overall, Fisher animals averaged over 55% of total recording time in ictal activity (range 27–94.4%). Fisher animals had several ictal events with durations longer than 45 min, and overall average ictal duration was 40 min 50 sec ± 17 min 25 sec. In all animals, the activity tended to be present on the caudal leads, with no ictal activity generalized to the frontal leads (motor and association cortex). As would be predicted by the electroencephalographic data, no overt motor seizures were observed in these animals. This observation suggests that overt clinical seizure activity may not be observed in otherwise seizing animals. Overt ictal activity was observed in 40% of Sprague-Dawley rats; however, the ictal events were usually of shorter duration (average duration 12 min 47 sec). Three Sprague-Dawley rats did not have any overt electroencephalographic seizure activity.
FIG. 2.
Representative electroencephalographic activity in Fisher 344 (A) and Sprague-Dawley (B) rats recorded for 24 h following lateral fluid percussion injury. Injured Fisher rats displayed significantly more ictal activity compared to injured Sprague-Dawley rats, and 60% of Fisher rats had status epilepticus-like seizure activity that was not observed in Sprague-Dawley rats. Overall, electroencephalographic events averaged approximately 40 min in Fisher rats, compared to 12.78 min in Sprague-Dawley rats. No overt motor seizures were observed in either strain.
Seizure activity was further analyzed for frequency distribution and absolute power contribution. Fisher rats displayed significant EEG slowing with a significant increase in all frequency bins analyzed compared to background EEG activity. Average absolute power for all frequency groups are presented in. In animals that displayed ictal activity, Sprague-Dawley rats also showed a significant increase in all frequency bins compared to baseline activity. Sprague-Dawley rats were indistinguishable from Fisher rats when measuring absolute delta power (p = 1.00). However, for all other frequency bins Sprague-Dawley rats were significantly lower than Fisher rats (Table 3). Overall, the Fisher rats displayed more seizure events, tended to have longer ictal duration, and spent a greater amount of total time in seizure activity. In addition, Fisher rats displayed greater EEG slowing during ictal activity than Sprague-Dawley rats.
Table 3.
Electroencephalographic Analysis (Mean ± Standard Deviation)
| Delta | Theta | Alpha | Beta-1 | Beta-2 | |
|---|---|---|---|---|---|
| Fisher | 21.2 ± 2.8 | 11.3 ± 1.6 | 7.9 ± 0.9 | 6.9 ± 1.0 | 3.5 ± 0.4 |
| Sprague-Dawley | 19.9 ± 1.0 | 7.0 ± 0.3 | 3.7 ± 0.2 | 2.7 ± 0.1 | 1.3 ± 0.1 |
| p Value | 1.00 | <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of neuronal damage between strains
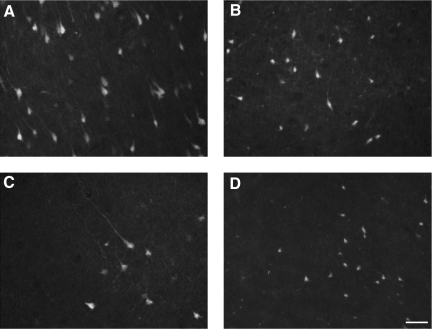
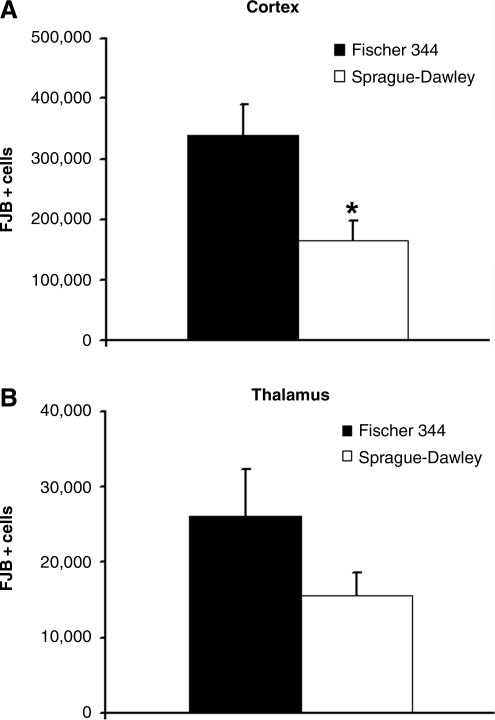
To evaluate the degree of injury-induced neuronal degeneration in association with the injury-induced seizure activity, we examined the number of FJB-positive cells in the injured ipsilateral cortex, hippocampus, and thalamus regions, in both Fisher and Sprague-Dawley rats at 24 h post-injury after completing EEG recording. Prominent FJB staining of the cell body could be identified in the injured ipsilateral cortex in both strains, and a few FJB-positive cells were also seen in the contralateral cortex. Fisher rats displayed more pronounced FJB-positive staining throughout the cortex and thalamus than Sprague-Dawley rats (Fig. 3). Stereological analysis confirmed that there were more FJB-positive neurons in the ipsilateral cortex and thalamus of Fisher rats, reaching statistical significance in the cortex (p < 0.05; Fig. 4). FJB-positive neurons were also observed in the dentate gyrus, hilus, CA3, and CA1 regions of the hippocampus; however, no statistically significant differences between strains were found in any of the hippocampal regions analyzed.
FIG. 3.
Representative micrograph of Fluoro-Jade-B (FJB) staining of degenerating neurons. At 24 h post-injury, abundant FJB-labeled neurons were observed in the injured ipsilateral cortex (A and C) and thalamus (B and D) in both Fisher (A and B) and Sprague-Dawley (C and D) rats. FJB-labeled neurons with irregular shapes of cell bodies and processes were particularly prominent in the injured cortex in Fisher rats (scale bar = 80 μm).
FIG. 4.
Quantitative analysis of the total number of Fluoro-Jade-B (FJB)-positive neurons in the injured cortex and thalamus regions. (A) Fisher rats displayed a significantly higher number of FJB-positive cells in the injured cortex than Sprague-Dawley rats (p < 0.05). (B) Fisher rats also had more FJB-positive cells in the thalamus than Sprague-Dawley rats, although it did not reach statistical significance.
Correlating with the increased FJB-positive staining observed in the cortex, we also observed an increased pattern of caspase-3 staining in the cortex of Fisher rats compared to Sprague-Dawley rats (Fig. 5E and F).
FIG. 5.
Representative micrograph of caspase-3 staining in the injured cortex. At 24 h post-injury, caspase-3-stained cells were observed in the injured cortex in both strains, and were more pronounced in the injured Fisher rats (A) than in the injured Sprague-Dawley rats (B; scale bar = 50 μm).
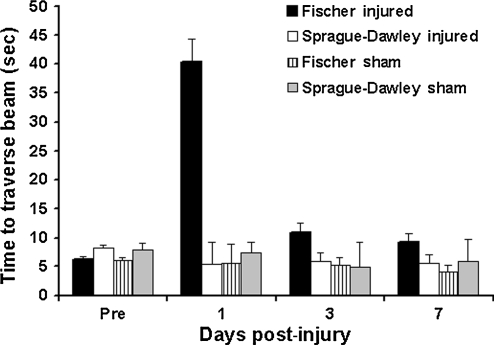
Motor function
Mean time to traverse the beam is presented in Figure 6. Prior to injury, all animals were trained to criteria and no significant differences were found between groups. Following injury, the Fisher injured group had significantly longer latencies to traverse the beam compared to both sham control groups and Sprague-Dawley injured rats (p < 0.001; Fig. 6). The repeated-measures ANOVA found a significant effect of group (F3,25 = 15; p < 0.001). No significant differences were found between injured Sprague-Dawley rats and shams.
FIG. 6.
Motor functional performance in the beam walk task. The graph shows that injured Fischer rats took significantly longer than both sham groups and the Sprague-Dawley injured group to traverse the beam (p < 0.001) No significant differences between any of the groups were found before injury.
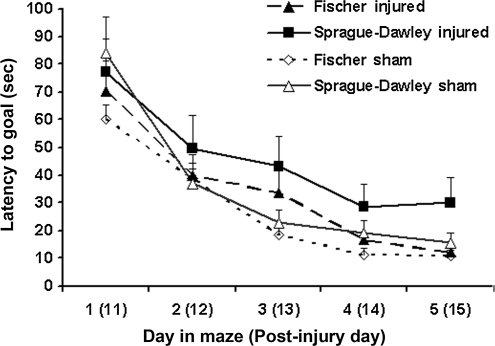
Cognitive function
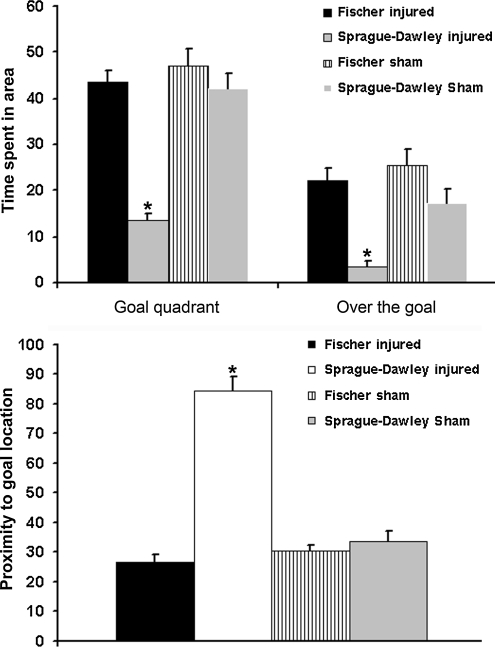
The mean latency to find the goal for each group is presented in Figure 7. A repeated-measures ANOVA over 5 days found no significant effect of group (F3,30 = 2.024; p = 0.132). However, the injured Fischer rats showed a trend toward faster acquisition of the task compared to injured Sprague-Dawley rats. When memory retention in a probe trial was analyzed, the Sprague-Dawley injured group performed significantly worse than both sham groups and the Fischer 344 injured group on measures of time spent in the target quadrant (p < 0.001; Fig. 8), time spent directly over the goal location (p < 0.001; Fig. 8), and proximity to the goal (p < 0.001, Fig. 8). In contrast, the Fischer injured group was not significantly different from either sham group in proximity or time spent in the goal quadrant. On average, the Fisher injured group spent 75% of their probe trial time in the target quadrant, and 38% of the time directly over the goal location, compared to the Sprague-Dawley injured group, that spent just below chance level (23%) in the target quadrant, and only 5% of the time directly over the platform. No significant differences were found between the two sham groups in any of the measurements analyzed, suggesting that the differences are injury-related. Taken together, the results suggest that the Fisher rats perform significantly better in cognitive tests following brain injury than Sprague-Dawley rats.
FIG. 7.
Goal latency of cognitive performance in the Morris water maze (MWM) test. The graph shows the mean latency (sec) to locate a hidden platform in the MWM on days 11–15 post-injury for each group. A repeated-measures analysis of variance found no significant group differences; however, there is a clear trend towards increased acquisition in the injured Fischer rats compared to the injured Sprague-Dawley rats.
FIG. 8.
Probe trial of cognitive performance in the Morris water maze (MWM) test. The top graph shows the average time (sec) out of a total of 60 sec that the animals spent in the goal quadrant, and directly over the goal location. The bottom graph shows proximity to the goal, in which lower scores reflect better performance. The Sprague-Dawley injured group performed significantly worse than all other groups on measures of time spent in the goal quadrant (p < 0.001), time spent over the goal location (p < 0.001), and proximity to the goal (p < 0.001). In contrast, the injured Fisher group did not show any differences in performance from the sham groups.
Discussion
This study is the first to demonstrate acute differences in the outcome measures of different rat strains subjected to the same level of L-FPI. In this study we administered L-FPI at a severity (1.65–1.75 atm) that is traditionally associated with mild brain injury. Among the Sprague-Dawley rats, we observed a 100% survival rate and a shorter duration to regain righting reflexes. In contrast, the Fisher 344 rats exhibited a 45% mortality rate and had significantly longer durations to regain righting reflex. One caveat of this study is that Fischer rats that died shortly after brain injury were not able to be used in subsequent data analyses. The high initial mortality rate in Fischer rats may be related to the increased ICP; however, the exact mechanism is unknown. Of the Fisher rats that survived injury, we observed increased ICP in the initial hours following brain injury, more frequent acute seizure activities as monitored by EEG, and increased neuronal damage in the ipsilateral thalamus and cortex regions, as well as decreased motor performance compared to their Sprague-Dawley counterparts. Although the mechanisms are unknown, our results are in agreement with those of previous studies, indicating that Fisher rats have lower electroconvulsive seizure thresholds than Sprague-Dawley rats (Golden et al., 1995; Statler et al., 2008). Taken together, the results suggest Fisher rats respond differently than Sprague-Dawley rats to L-FPI.
A significant finding in this study is the duration and location of ictal activity in Fischer rats. The nature of the EEG activity seen in the Fisher rats suggests that these animals do not display overt tonic-clonic activity. The EEG activity was not generalized to the more cortical associative and motor cortices. Thus the animals would not be expected to display overt clinical seizure-like activity. In fact, video review did not show overt Racine behavioral seizure activity. Thus a casual observer would not suspect the animals to have ictal activity. Clinically, non-convulsive seizures without overt clinical manifestations have been reported in 22–37% of patients, depending on the type of brain lesion (Claassen et al., 2004; Vespa et al., 1999). Therefore, Fisher rats may be a useful model for studying non-convulsive seizure activity and identifying therapeutic treatments to prevent seizures in the acute period. Adding significance to this observation is that unlike the Sprague-Dawley animals, 60% of the Fisher animals displayed status epilepticus-like ictal activity. Unlike the self-terminating ictal activity observed in both groups, status epilepticus has been shown to alter neuronal signaling cascades (Churn et al., 2000; Kochan et al., 2000; Kurx et al., 2001), neuronal survival (Fujikawa et al., 2000; Wasterlain et al., 2002), and neurological function (Delorenzo and Sun, 2006; Mazarati et al., 2002). In this study, increased ictal activity was correlated with increased ICP and increased FJB-positive staining in the cortex and thalamus in injured Fisher rats. The expression of status epilepticus may contribute to, or exacerbate, many of the TBI-induced cellular processes in the Fisher rats. Therefore electroencephalographic monitoring should be used to reduce any confounding factors of ongoing seizure activity in these animals. Further studies are necessary to distinguish what mechanisms led to the development of status epilepticus activity in some, but not all, of the Fisher rats.
Seizure activity in rats and humans has been linked to long-lasting cognitive and neurological disorders. In order to determine whether the strain differences we observed early after injury would affect behavioral outcome, a second group of animals was tested using the beam-walking and MWM tests. Fisher rats performed significantly worse on day 1 post-injury on the beam-walking test than Sprague-Dawley rats, correlating with the increased FJB-positive staining observed in the cortex at 24 h post-injury. More interesting were the results of the cognitive testing, in which we found that Sprague-Dawley rats actually performed significantly worse than Fisher rats on day 5 of MWM training and on the probe trial test performed 24 h later. Although the mechanism is unknown, there is a controversial hypothesis suggesting that increased activation of cells after brain injury may improve recovery of function. For example, in rodents, acute post-injury activation and seizure activity (Feenry et al., 1987; Hovda et al., 1989), as well as post-injury pentylenetetrazol-induced seizures (Hamm et al., 1995), led to improved behavioral outcomes. Therefore one conclusion may be that the early ictal activity observed in Fisher rats led to better performance on the MWM. However, several of the Fisher rats exhibited status epilepticus during the first 24-h post-injury period, and this has been previously associated with maladaptive behavior (Schallert et al., 1986). Therefore it was surprising that the Fisher rats did not display any cognitive deficits compared to Fischer sham animals. One explanation could be that the thalamo-cortical circuits in Fischer rats, rather than the hippocampal circuits, are more susceptible to neuronal damage, and therefore no cognitive differences were observed in the MWM. Alternatively, Fisher 344 rats may have different neurochemical profiles that aid in plasticity and recovery from injury. Further studies are needed to understand why Fisher rats displayed no cognitive deficits following injury, in spite of the observed increases in ictal activity and FJB-positive staining.
The current study clearly demonstrates rat stain differences following brain injury, and supports the findings that strain influence (genetic background) is an important consideration in preclinical neurotrauma studies (Tan et al., 2009). Strain differences between Fisher 344 and Sprague-Dawley rats may contribute to the different pathologies seen following experimental TBI, thereby affecting outcome measures and the results of therapeutic interventions. We hope that these studies will increase awareness of strain differences and provide useful information regarding the secondary mechanisms of injury. Results of the present study suggest that Fisher rats may be a good model for studying post-traumatic epilepsy and the mechanisms that lead to the development of status epilepticus and non-convulsive seizures in some rats. Further studies will investigate potential mechanisms, characterize the long-term recovery profiles of Fischer rats, and delineate similarities and differences among strains that may lead to improved long-term outcomes. Additionally, other studies using different injury models and rat strains should be performed to increase our awareness of how genetic differences within a species may affect outcome following neurotrauma.
Acknowledgments
This study was funded by the National Institutes of Health grant no. NS055086 (to D.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- Alahmed S. Herbert J. Strain differences in proliferation of progenitor cells in the dentate gyrus of the adult rat and the response to fluoxetine are dependent on corticosterone. Neuroscience. 2008;157:677–682. doi: 10.1016/j.neuroscience.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.J. Fugaccia I. Scheff S.W. Fluoro-jade B stains quiescent and reactive astrocytes in the rodent spinal cord. J. Neurotrauma. 2003;20:1223–1231. doi: 10.1089/089771503770802899. [DOI] [PubMed] [Google Scholar]
- Churn S.B. Kochan L.D. Delorenzo R.J. Chronic inhibition of Ca(2+)/calmodulin kinase II activity in the pilocarpine model of epilepsy. Brain Res. 2000;875:66–77. doi: 10.1016/s0006-8993(00)02623-8. [DOI] [PubMed] [Google Scholar]
- Claassen J. Mayer S.A. Kowalski R.G. Emerson R.G. Hirsch L.J. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- Clausen F. Hillered L. Intracranial pressure changes during fluid percussion, controlled cortical impact and weight drop injury in rats. Acta Neurochir. 2005;147:775–780. doi: 10.1007/s00701-005-0550-2. [DOI] [PubMed] [Google Scholar]
- Delorenzo R.J. Sun D.A. Basic mechanisms in status epilepticus: role of calcium in neuronal injury and the induction of epileptogenesis. Adv. Neurol. 2006;97:187–197. [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Feenry D.M. Bailey B.Y. Boyeson M.G. Hovda D.A. Sutton R.L. The effect of seizures on recovery of function following cortical contusion in the rat. Brain Inj. 1987;1:27–32. doi: 10.3109/02699058709034441. [DOI] [PubMed] [Google Scholar]
- Fujikawa D.G. Itabashi H.H. Wu A. Shinmei S. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–991. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Golden G.T. Smith G.G. Ferraro T.N. Reyes P.F. Rat strain and age differences in kainic acid induced seizures. Epilepsy Res. 1995;20:151–159. doi: 10.1016/0920-1211(94)00079-c. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Pike B.R. Temple M.D. O'Dell D.M. Lyeth B.G. The effect of postinjury kindled seizures on cognitive performance of traumatically brain-injured rats. Exp. Neurol. 1995;136:143–148. doi: 10.1006/exnr.1995.1091. [DOI] [PubMed] [Google Scholar]
- Hort J. Brozek G. Komarek V. Landmeier M. Mares P. Interstrain differences in cognitive functions in rats in relation to status epilepticus. Behav. Brain Res. 2000;112:77–83. doi: 10.1016/s0166-4328(00)00163-7. [DOI] [PubMed] [Google Scholar]
- Hovda A.D.A. Sutton R.L. Feeney D.M. Amphetamine-induced recovery of visual cliff performance after bilateral visual cortex ablation in cats: measurements of depth perception thresholds. Behav. Neurosci. 1989;103:574–584. doi: 10.1037//0735-7044.103.3.574. [DOI] [PubMed] [Google Scholar]
- Iwasaki H. Ohmachi Y. Kume E. Krieglstein J. Strain differences in vulnerability of hippocampal neurons to transient cerebral ischaemia in the rat. Int. J. Exp. Pathol. 1995;76:171–178. [PMC free article] [PubMed] [Google Scholar]
- Jiao X. Pare W.P. Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Kearns D.N. Gomez-Serrano M.A. Weiss S.J. Riley A.L. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative auto-maintenance. Behav. Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kochan L.D. Churn S.B. Omojokun O. Rice A. Delorenzo R.J. Status epilepticus results in an N-methyl-D-aspartate receptor-dependent inhibition of Ca2+/calmodulin-dependent kinase II activity in the rat. Neuroscience. 2000;95:735–743. doi: 10.1016/s0306-4522(99)00462-5. [DOI] [PubMed] [Google Scholar]
- Kurx J.E. Hamm R.J. Singleton R.H. Povlishock J.T. Churn S.B. A persistent change in subcellular distribution of calcineurin following fluid percussion injury in the rat. Brain Res. 2005;1048:153–160. doi: 10.1016/j.brainres.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Lei Y. Yaroslavsky I. Tejani-Butt S.M. Strain differences in the distribution of N-methyl-d-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain. Life Sci. 2009;85:794–799. doi: 10.1016/j.lfs.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur J.E. Alessandri B. Reinert M. Bullock R. Kontos H.A. Fluid percussion injury transiently increases then decreases brain oxygen consumption in the rat. J. Neurotrauma. 2000;17:101–112. doi: 10.1089/neu.2000.17.101. [DOI] [PubMed] [Google Scholar]
- Mazarati A. Bragin A. Baldwin R. Shin D. Wilson C. Sankar R. Naylor D. Engel J. Wasterlain C.G. Epileptogenesis after self-sustaining status epilepticus. Epilepsia 43 Suppl. 2002;5:74–80. doi: 10.1046/j.1528-1157.43.s.5.25.x. [DOI] [PubMed] [Google Scholar]
- Paulson P.E. Gorman A.L. Yezierski R.P. Casey K.L. Morrow T.J. Differences in forebrain activation in two strains of rat at rest and after spinal cord injury. Exp. Neurol. 2005;196:413–421. doi: 10.1016/j.expneurol.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T. Hernandez T.D. Barth T.M. Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain Res. 1986;379:104–111. doi: 10.1016/0006-8993(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Hopkins K.J. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Singleton M.W. Holbert W.H. Lee A.T. Bracey J.M. Churn S.B. Modulation of CaM kinase II activity is coincident with induction of status epilepticus in the rat pilocarpine model. Epilepsia. 2005;46:1389–1400. doi: 10.1111/j.1528-1167.2005.19205.x. [DOI] [PubMed] [Google Scholar]
- Singleton M.W. Holbert W.H. Ryan M.L. Lee A.T. Kurz J.E. Churn S.B. Age dependence of pilocarpine-induced status epilepticus and inhibition of CaM kinase II activity in the rat. Brain Res. Dev. Brain Res. 2005;156:67–77. doi: 10.1016/j.devbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Swank S. White H.S. Strain and age affect electroconvulsive seizure testing in rats. Epilepsy Res. 2008;78:232–234. doi: 10.1016/j.eplepsyres.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. Bullock M.R. McGinn M.J. Zhou Z. Altememi N. Hagood S. Hamm R. Colello R.J. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.A. Quigley A. Smith D.C. Hoane M.R. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J. Neurotrauma. 2009;26:539–548. doi: 10.1089/neu.2008.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Tran L.D. Lifshitz J. Witgen B.M. Schwarzbach E. Cohem A.S. Grady M.S. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Uchida S. Nishida A. Hara K. Kanemoto T. Suetsugi M. Fujimoto M. Watanuki T. Wakabayashi Y. Otsuki K. McEwen B.S. Watanabe Y. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur. J. Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. Nuwer M.R. Nenov V. Ronne-Engstrom E. Hovda D.A. Bergsneider M. Kelly D.F. Martin N.A. Becker D.P. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberer M. Stolz E. Muller C. Friedrich C. Rottger C. Blaes F. Kaps M. Fisher M. Bachmann G. Gerriets T. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI) Lab. Anim. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- Wasterlain C.G. Niquet J. Thompson K.W. Baldwin R. Liu H. Sankar R. Mazarati A.M. Naylor D. Katsumori H. Suchomelova L. Shirasaka Y. Seizure-induced neuronal death in the immature brain. Prog. Brain Res. 2002;135:335–353. doi: 10.1016/S0079-6123(02)35031-3. [DOI] [PubMed] [Google Scholar]
- Witgen B.M. Lifshitz J. Grady S.M. Inbred mouse strains as a tool to analyze hippocampus neuronal loss after brain injury: A stereological study. J. Neurotrauma. 2006;23:1320–1329. doi: 10.1089/neu.2006.23.1320. [DOI] [PubMed] [Google Scholar]
- Xu B. McIntyre D.C. Fahnestock M. Racine R.J. Strain differences affect the induction of status epilepticus and seizure-induced morphological changes. Eur. J. Neurosci. 2004;20:403–418. doi: 10.1111/j.1460-9568.2004.03489.x. [DOI] [PubMed] [Google Scholar]