Abstract
Certain forms of hexavalent chromium [Cr(VI)] are known respiratory carcinogens that induce a broad spectrum of DNA damage. Cr(VI)-carcinogenesis may be initiated or promoted through several mechanistic processes including, the intracellular metabolic reduction of Cr(VI) producing chromium species capable of interacting with DNA to yield genotoxic and mutagenic effects, Cr(VI)-induced inflammatory/immunological responses, and alteration of survival signaling pathways. Cr(VI) enters the cell through nonspecific anion channels, and is metabolically reduced by agents including ascorbate, glutathione, and cysteine to Cr(V), Cr(IV), and Cr(III). Cr(III) has a weak membrane permeability capacity and is unable to cross the cell membrane, thereby trapping it within the cell where it can bind to DNA and produce genetic damage leading to genomic instability. Structural genetic lesions produced by the intracellular reduction of Cr(VI) include DNA adducts, DNA strand breaks, DNA-protein crosslinks, oxidized bases, abasic sites, and DNA inter- and intrastrand crosslinks. The damage induced by Cr(VI) can lead to dysfunctional DNA replication and transcription, aberrant cell cycle checkpoints, dysregulated DNA repair mechanisms, microsatelite instability, inflammatory responses, and the disruption of key regulatory gene networks responsible for the balance of cell survival and cell death, which may all play an important role in Cr(VI) carcinogenesis. Several lines of evidence have indicated that neoplastic progression is a result of consecutive genetic/epigenetic changes that provide cellular survival advantages, and ultimately lead to the conversion of normal human cells to malignant cancer cells. This review is based on studies that provide a glimpse into Cr(VI) carcinogenicity via mechanisms including Cr(VI)-induced death-resistance, the involvement of DNA repair mechanisms in survival after chromium exposure, and the activation of survival signaling cascades in response to Cr(VI) genotoxicity.
Keywords: Hexavalent chromium, carcinogenesis, genotoxicity, survival signaling, DNA damage response
1. Introduction
Certain hexavalent chromium [Cr(VI)] compounds are well established environmental contaminants and human occupational respiratory carcinogens, primarily generated from industrial processes [1]. The primary route of exposure is inhalation; however, additional potential modes include oral ingestion of contaminated water, or by direct dermal contact with products manufactured using chromium such as pressure treated wood [2]. Worldwide chromium production is concentrated in a few regions. Of the 16,400,000 tons produced worldwide, 15,376,000 tons are produced in seven countries: South Africa, India, Kazakstan, Zimbabwe, Finland, Brazil and Turkey. Although the United States is not a significant primary producer of chromium, it is one of the world’s leading importers and producers of chromium compounds with approximately 3,400 facilities producing 170,000 metric tons of chromium-containing compounds. These are used in wood preservation (52%), leather tanning (13%), metal finishing (13%), paint pigments (12%) and other applications [3].
Environmental Cr(VI) enters the air through burning of fossil fuels and waste incineration, and can be discharged into water from chromium-containing waste. In some instances chromium slag has been used for landfill. At least 386 of the hazardous waste sites on the National Priority List contain high levels of Cr(VI). This environmental release is estimated at over 90,000,000 pounds annually [4]. In addition, the burning of one cigarette produces up to 0.5 μg of chromium. It is estimated that the atmospheric concentration of particulate chromates in rural or residential areas of the U.S. ranges from 0.2 to 9 ng/m [4]. In urban areas, especially in areas with a ferrochrome industry presence, those concentrations can easily be 10–100 fold higher [5]. If one assumes that an urban dweller in a city with a ferrochrome production facility receives an inhalation exposure of 20 m3 per day of 90 ng/m3, then the daily lung intake is 1.8 μg/day. This dose of particulates does not distribute evenly throughout the lung but concentrates at major bifurcations; however, epidemiological studies assessing lung cancer incidence in ferrochrome workers are inconclusive [2,5].
Epidemiological studies carried out in the U.K., Europe, Japan and the U.S. have consistently shown that workers in occupations where particulate chromates are generated or used have an elevated risk of respiratory disease, fibrosis, perforation of the nasal septum, development of nasal polyps, and lung cancer [6,7]. Chromate workers have been found to have marked lung burdens of chromium [5,8]. Lung concentrations up to and exceeding 10,000 μg chromium per 100 gm wet weight (1.0–3.0 mM) have been reported [8,9]. Ishikawa et al. have identified “hot spots” of particulate chromium accumulation at the bifurcations of the bronchi of chromate workers more than 15 years after cessation of employment [5]. These workers show pathological evidence of lung tissue injury, fibrosis and in some cases nasal ulceration and perforation. Taken together, extensive studies and data collection on the industrial and environmental exposure to chromium compounds have led the National Institute of Occupational Safety and Health (NIOSH) to list chromate compounds as one of the major causes of occupational lung cancer, and the U.S. Environmental Protection Agency (U.S. EPA) and the International Agency for Research on Cancer (IARC) to classify chromium as a human carcinogen, currently making Cr(VI) one of 33 compounds listed to pose the greatest potential health threat in urban areas [2,10–12].
More recently the potential risks of Cr(VI) exposure by ingestion in drinking water have come under increased scrutiny. A number of short-term in vivo studies definitively showed that Cr(VI)’s potential as a genotoxicant is markedly dose dependent with evidence of a strong threshold effect due to extracellular detoxification (by reduction to Cr(III)) prior to absorption by peripheral organs and tissues [13]. Several studies in mice and rats have shown chromosomal aberrations in bone marrow, DNA-protein crosslinks and DNA single strand breaks in the liver and brain, as well as single and double stranded DNA breaks in leukocytes, but only following high dose acute and chronic oral administration of Cr(VI) at levels sufficient to overwhelm the reductive capacity of the extracellular environment [14–20]. Thus, it has been generally accepted that low or moderate doses of orally ingested Cr(VI) are non-carcinogenic.
In 2004, the California Department of Health Services reported that 38% of the state’s drinking water sources contained detectible levels of Cr(VI) [20–22], and requested that the National Toxicology Program conduct a large scale in vivo carcinogenicity bioassay of Cr(VI) in drinking water. Chronic, long-term administration of Cr(VI)-treated water induced a low incidence of oral and intestinal tumors [23], but only at very high doses at which both body weight and water consumption were affected. The small increase in intestinal tumors is difficult to explain mechanistically since there was no increase in forestomach or stomach tumors. Nevertheless, similar to the animal genotoxicity studies, these data suggest that chronic ingestion of very high doses of Cr(VI) may ultimately saturate the extensive extracellular protective mechanisms in local microenviroments (point of ingestion), thereby enabling its potential as a genotoxicant and carcinogen.
As early as 1951, epidemiologists noted that the relatively insoluble Cr(VI) compounds presented the greatest toxic and carcinogenic risk [reviewed in [24,25]. Numerous studies have been conducted using inhaled soluble sodium chromate and calcium chromate administered after complete dissolution. The vast majority of these studies did not yield any increase in tumor response, except occasionally at extraordinarily high doses, given repeatedly (fives times weekly for life). In contrast, tumors were produced in nearly every study utilizing the slightly soluble to highly insoluble particulates such as zinc, lead, strontium and sintered calcium chromate (administered as a particle suspension). A large number of published reports show that particulate chromates embody the highest risk because of adhesion to the cell surface followed by slow but chronic dissolution in the immediate microenvironment of the cell surface allowing released chromate oxyanions to escape extracellular reduction and be absorbed into the cell [9,26–37].
Although many researchers agree that Cr(VI)-induced carcinogenesis results only from extensive long-term respiratory exposure, a linear extrapolation (non-threshold) application of epidemiological studies [38,39] suggests that there may be a 25% risk of lung cancer morbidity resulting from occupational exposure to Cr(VI)-containing dusts and mists under 52 μg/m3 Cr(VI), which was the exposure limit deemed permissible by the Occupational Safety and Health Administration (OSHA) in 1971 [20]. These levels were readjusted in 2006 to 5 μg/m3, highlighting the fact that environmental and occupational exposure to chromate continues to loom large as a major public health issue. It should be noted that a few review articles have suggested that chromium exposure can lead to non-respiratory cancers, such as bone cancer and leukemia [2,40]. This conclusion is not supported by application of rigorous epidemiological and statistical methodology.
2. Mode of action of chromium carcinogenesis
The specific mechanism of chromium carcinogenicity remains unclear, however, there exists an abundance of data supporting the genotoxicity and mutagenicity of Cr(VI) in vivo and in vitro. Here, we will summarize the potential molecular mechanisms of Cr(VI) carcinogenesis, highlighting the importance of intracellular Cr(VI) metabolism, Cr(VI)-induced oxidative stress, Cr(VI)-induced DNA damage and mutagenesis, Cr(VI)-induced inflammation, and tumor formation.
2.1 Cr(VI) metabolism
Chromium is a transition-group metal on the periodic chart that has several oxidation states [2]. Under physiological pH and temperature, Cr(VI) is unable to interact with DNA and lacks genotoxic activity [41]. Prior to cellular entry and reduction, Cr(VI) is considered to be a pro-carcinogen. The structural similarity of chromates to physiological sulfates and phosphate ions, enables molecular mimicry and easy entry of Cr(VI) into cells through nonspecific anion channels [21,42]. Cr(VI) enters the cell as an oxyanion followed by its metabolic reduction to Cr(V), Cr(IV), and to the final reduced trivalent (III) form. These reduced forms have been shown to induce a wide range of genomic DNA damage, which may lead to DNA replication inhibition [43]. At physiological pH, intracellular reduction is facilitated by several molecules/compounds such as ascorbate, glutathione (GSH), cysteine, lipoic acid, hydrogen peroxide, NAD(P)H, fructose and ribose [13,20,21,41,44]. The respective cellular availability and reaction rate with Cr(VI) determines which is the primary reducing agent. There are studies supporting the significance of both ascorbate and GSH in Cr(VI) reduction, however most data show that ascorbate is the dominant kinetically favored biological reductant responsible for around 90% of in vivo Cr(VI) metabolism [21,44]. Interestingly, evidence suggests that in vitro studies may overestimate the genotoxic and mutagenic effects of Cr(VI) due to lack of biologically relevant levels of ascorbate, which are at millimolar concentrations in vivo and micromolar concentrations in vitro [21]. Moreover, GSH, which is highly abundant intracellularly and found at millimolar concentrations, has been identified as a modulator of cell stress induced by Cr(VI) cytotoxicity. Upon GSH depletion, Cr(VI) induces the overexpression of heme-oxygenase 1 gene expression in human dermal fibroblasts, which can be used as a marker for Cr(VI)-induced cell stress and cytotoxicity [45].
The final product of chromate reduction, Cr(III), is biologically stable and has a greater DNA binding efficiency than that of Cr(VI) [13]. Notably, extracellular reduction of Cr(VI) to Cr(III) detoxifies the molecule due to the inability of Cr(III) to cross the cellular membrane. However, when Cr(VI) is reduced in the cell to Cr(III), the weak membrane permeability of the trivalent form intracellularly traps the molecule, enabling the formation of stable Cr(III) complexes with proteins and nucleic acids leading to a large spectrum of DNA damage [21].
2.2 Cr(VI)-induced oxidative stress
It is hypothesized that the process of carcinogenesis can be triggered by the dysregulation of gene expression, cellular redox state, and DNA damage as a result of oxidative stress. Oxidative stress, or the favoring of cellular oxidant production over that of antioxidants, leads to the formation of reactive oxygen species (ROS), and damage to cellular RNA, DNA, proteins, and lipids [46]. ROS are derived from endogenous sources such as the mitochondrial oxidative phosphorylation, cytochrome P450 metabolism, peroxisomes, and inflammatory cells; as well as, exogenous sources such as radiation, redox cycling components, and metals [46]. Enzymatic and nonenzymatic antioxidants are intracellularly produced to counteract the effects of oxidant production, they include, superoxide dismutase, glutathione, catalase, thioredoxin, and vitamin C and E [46].
The reduction of Cr(VI) has been shown to yield reactive species such as free radicals, superoxide anions and hydroxyl radicals, possibly through Fenton-like reactions of Cr(V) and Cr(IV) with hydrogen peroxide [20,47]. Several electron spin resonance (ESR) spin trapping studies have shown the generation of hydroxyl radicals in conjunction with long-lived Cr(V) species, following the reduction of Cr(VI) by NADPH and glutathione [47–57]. While the direct relationship between DNA-reactive oxygen species and chromium-induced DNA damage is heavily debated and unclear, there have been several studies supporting the role of ROS in Cr(VI)-induced genotoxicity, cytotoxicity, and oxidative stress [17,57–61]. An example of this debate is evident in the controversy between the identity of the specific Cr(V) species responsible for the generation of hydroxyl radicals. Kawanishi et al. presented a “tetraperoxochromate(V) theory of carcinogenesis from chromate,” with data suggesting that the reduction of Cr(VI) by hydrogen peroxide forms tetraperoxochromate(V), and that this intermediate species leads to hydroxyl radical and singlet oxygen formation causing DNA damage [48]. In contrast, Shi et al. reported data supporting, “the Cr(V)-complexation-Fenton reaction model of carcinogenesis from chromate” theory. In this study, the authors showed by ESR, that significant levels of hydroxyl radicals were not formed by the reaction between tetraperoxochromate(V) and hydrogen peroxide, and that significant levels of tetraperoxochromate(V) species were not formed from chromate reduction by glutathione, glutathione reductase, ascorbate, NADPH, or vitamin B2 [49].
While the formation of chromate intermediates and modes of reactive species production is controversial, many studies have attempted to investigate the complexities of Cr(VI)-mediated oxidative stress and damage. Sugiyama et al. have shown Cr(VI) can be reduced to Cr(V) in the presence of vitamin B2 (riboflavin) in Chinese hamster V79 cells, leading to an increase in hydroxyl radicals, chromosomal aberrations and mutations at the HGPRT locus [59,62]. Moreover, when pretreated with flavin adenine dinucleotide or vitamin E (a free radical scavenger), there was a marked decrease in Cr(VI)-induced single-strand breaks and cytotoxicity [63]. Interestingly, there was no difference in Cr(VI) uptake, Cr(VI)-induced DNA-protein crosslinks, the levels of glutathione and glutathione reductase activity, or the formation of Cr(III) species when pretreated with either vitamin B2 or vitamin E. Other in vitro studies have shown that antioxidant regulation may be dependent on the concentration of Cr(VI). Asatiani et al. demonstrated in human epithelial like L-41 cells and fetal human lung fibroblasts, that toxic Cr(VI) concentrations (20 μM) lead to an increase in ROS, and a significant reduction in catalase, glutathione, and cytosolic Cu/Zn superoxide dismutase activity [64]. In contrast, there was a marked increase in catalase and glutathione peroxidase activity following non-toxic doses of Cr(VI) (2 μM), demonstrating the intricacies of Cr(VI)-induced oxidative stress. Interestingly, a recent report from this laboratory, has suggested that the most important antioxidant system responsible for cell fate is between the balance of superoxide dismutase and the hydrogen peroxide degrading enzymes (glutathione peroxidase and catalase) [64,65].
Furthermore, many reports studying Cr(VI)-mediated oxidative stress investigate its effect on cell death, or apoptosis. A study by Shi et al. suggests that ROS contributes to the early effects of Cr(VI)-induced apoptosis via a p53-independent mechanism, whereas the apoptotic effects could be blocked in the presence of ROS scavengers, such as catalase, aspirin and N-acetyl-L-cysteine [57]. Bagchi and colleagues have also shown an increase in ROS production that correlates with apoptotic death in a Cr(VI) concentration and dose-dependent fashion [66]. Additionally, a recent in vitro study by Azad et al. has suggested that Cr(VI)-induced apoptosis is mediated by the production of superoxide anions and subsequent proteosomal degradation of the anti-apoptotic regulatory protein, Bcl-2 [60].
Moreover, there have been several studies focusing on the effect of Cr(VI) treatment on the regulation of oxidative stress-related gene expression. An early report by Dubrovskaya et al. assessed the steady state mRNA expression levels of several oxidative stress genes on Cr(VI)-treated normal human lung LL 24 cells, and human lung adenocarcinoma A549 cells, by RT-PCR and northern blot analysis [67]. Out of the genes analyzed, including, catalase, glutathione s-transferase, glutathione reductase, Cu/Zn-superoxide dismutase (CuZnSOD), Mn-superoxide dismutase (MnSOD), and NAD(P)H:quinone oxidoreductase and heme oxygenase 1 (HO1), HO1 was the only gene significantly upregulated in the presence of both low and high doses of Cr(VI) (5–200 μM). This increased gene expression was seen only in the LL 24 cells, and did not correlate with intracellular glutathione levels. The Cr(VI)-induced expression of the HO1 gene has also been shown in other studies [45], however a report from O’Hara and colleagues contradicts these data [68]. The authors show no effect of Cr(VI) on basal HO1 mRNA or protein expression levels in human bronchial epithelial BEAS-2B cells, as well as an inhibition of HO1 gene expression in lung cells of C57BL/6 mice intranasally exposed to Cr(VI). While a role for HO1 gene expression has been defined in the cellular response to Cr(VI)-mediated oxidative stress, other genetic profiles have been acquired using the global microarray approach. Gene expression analysis of Cr(VI)-treated A549 cells showed increases in glutathione peroxidase, CuZnSOD, and MT-II (protects cells from toxicity and oxidative stress) gene expression [69]; while NADH-ubiquinone oxidoreductase B18 subunit (a subunit of the mitochondrial respiratory chain complex I), glutathione peroxidase, and the sodium/potassium-transporting ATPase alpha 1 subunit (an ROS regulator) were upregulated in Cr(VI)-treated BEAS-2B cells [70].
Although there are many studies reporting on the relationship between oxidative stress and Cr(VI)-mediated cytotoxicity, genotoxicity, and potential carcinogenesis, due to nonuniform treatment protocols, conflicting data and controversial methods used for detection of Cr(VI)-induced oxidative stress, this relationship remains unclear. Martin et al. demonstrated that the high valence chromium species, bis(2-ethyl-2-hydroxybutyrato)oxochromate(V) [Cr(V)-EHBA], was able to independently induce the fluorescence of two dyes commonly used to detect ROS (2′,7′-dichlorofluorescin and dihydrorhodamine) in A549 cells, which was not affected by treatment with radical scavengers [71]. These data suggest that 2′,7′-dichlorofluorescin and dihydrorhodamine are more suitable for the qualitative detection of Cr(V), rather than ROS production in the presence of Cr(VI).
Moreover, it is likely that any discussion on the potential role of Cr(VI)-induced oxidative stress needs to include careful consideration of total dose over time and dose rate, as well as the relative ratios and availability of intracellular reductants and anti-oxidants. Given the intricate and unclear relationship between apoptosis and oxidative stress, it will be very difficult to determine whether Cr(VI)-associated oxidative stress is caused by, or causes, the initiation of apoptosis.
2.3 Cr(VI)-induced DNA damage and mutagenesis
Chromium-induced DNA damage is thought to be the primary mechanism of chromate genotoxicity and mutagenicity, but it is only clearly observed at doses that are also capable of producing cell death [72]. Recently, data has been presented to the EPA’s Cancer Assessment Review Committee (CARC) to support mutagenicity as the initiating step in Cr(VI)-induced carcinogenesis [73]. Structural genetic lesions produced by Cr(VI) include DNA adducts, DNA strand breaks, DNA-protein crosslinks, oxidized bases, abasic sites, and DNA inter- and intrastrand crosslinks [41,74]. The association of chromium with the phosphodiester backbone of DNA (chromium-DNA adduct) is one of the most abundant genetic lesions induced by chromium in mammalian cells and is thought to be a primary cause of Cr(VI) mutagenicity [21]. Cr(III)-ligand-DNA complexes form binary and ternary adducts, the latter of which are predominant and more toxicologically relevant [21,41]. There are four types of ternary adducts that primarily persist following Cr(VI) exposure and intracellular reduction; they are Cr(III)-ascorbate-, Cr(III)-cysteine-, Cr(III)-histadine-, and Cr(III)-glutathione-DNA adducts, respectively [21]. Mutational analysis studies using SV-40 immortalized human fibroblasts transfected with plasmids extracellularly treated with Cr(VI), showed that the primary and most mutagenic adduct formed was ternary in nature, and that the Cr(III)-cysteine-DNA and Cr(III)-ascorbate-DNA adducts were more mutagenic than the binary adducts [75,76]. O’Brien et al. showed significant DNA damage in the form of DNA-chromium-DNA crosslinks and DNA-chromium-GSH crosslinks following treatment of DNA with Cr(III) and Cr(VI) with ascorbate and GSH, respectively [43].
The damage induced by Cr(VI) can lead to dysfunctional DNA replication and transcription causing base substitutions and deletions serving as substrates for base excision repair (BER) and nucleotide excision repair (NER), and promoting genomic instability [77]. Genomic instability, which can be caused by aberrant cell cycle checkpoints and dysregulated DNA repair mechanisms via microsatellite instability (MIN), plays an important role in Cr(VI) carcinogenesis [75]. There are several lines of evidence suggesting the importance of base substitutions in Cr(VI) mutagenicity, with G:C substitutions shown to be the primary target in vitro and in vivo; however, studies supporting the relevance of specific Cr(VI)-induced base deletions are inconsistent [75].
2.3.1 DNA damage response to Cr(VI)
An understanding of the role of DNA repair mechanisms in Cr(VI) carcinogenesis is important, but fairly limited. Studies from our laboratory and others, have shown the significance of respective DNA repair mechanisms paradoxically in the protection against, and promotion of Cr(VI)-induced mutagenesis. These data revealed the importance of DNA double strand break repair by homologous recombination in the promotion of cell survival following Cr(VI) exposure in yeast [78]; the role of Mus81, an enzyme involved in replication fork repair, in the repair of replication-associated Cr(VI)-induced DNA damage [79]; and the protection of cells from Cr(VI)-induced DNA damage and mutagenesis by BER and NER [77,80,81]. Interestingly, recent work has also shown that both a proficient NER-dependent mechanism and DNA polymerase ζ-mediated translesion synthesis (TLS) may mediate Cr(VI)-induced mutagenesis [77,81]. Moreover, as MIN is synonymous with complete loss of a functional mismatch repair (MMR) mechanism, in vivo studies by Takahashi et al. found a strong correlation in chromate-exposed lung cancers with an increase in MIN and a decrease in the mismatch repair genes, hMLH1 and hMSH2, which was significant in hMLH1 expression [21,82]. In vitro work by Peterson-Roth et al., also support the role of MMR regulation of Cr(VI)-induced toxicity and carcinogenesis [83]. In keeping with these data, a recent review by Holmes et al. concluded that the mutagenic capacity of Cr(VI) is not potent enough to induce sufficient levels of mutations to critical tumor suppressor and oncogenes, such as p53 and ras, that would lead to Cr(VI)-induced multistage carcinogenesis, but supported the hypothesis that genomic instability could potentiate Cr(VI) carcinogenesis [21,75]. Conversely, a recent in vitro study showed that chronic exposure to Cr(VI) induced malignant transformation of human bronchial epithelial cells, which displayed no MIN, up-regulated expression of hMLH1, and a functional MMR mechanism [84].
2.3.2 Cr(VI)-associated epigenetic alterations
While it is critical to understand the mutagenic effects of Cr(VI) on cellular DNA, it is also important to understand and recognize the role of nonmutagenic, but heritable, epigenetic alterations on chromate-mediated carcinogenicity. Several forms of epigenetic modifications have been identified, which include, microRNAs, histone modification, and DNA methylation [85]. One of the earliest reports of Cr(VI)-induced epigenetic alterations came from Klein et al. The authors demonstrated the ability of soluble sodium chromate to induce aberrant DNA methylation of the gpt reporter gene, expressed in mammalian cell lines derived from G12 cells containing the gpt gene [86]. This methylation was correlated with silencing of the gpt gene expression. Interestingly, when the cells were treated with insoluble barium chromate, there was no alteration in DNA methylation. Studies on chromate exposed lung cancer patients have also correlated with DNA methylation with altered expression of DNA repair and tumor suppressor genes. Takahashi et al. reported that 62.5% of chromate lung cancer samples had methylation of the hMLH1 gene, which was correlated to a repression of hMLH1 protein expression in 80% of the samples studied [82]. Additionally, Kondo et al. showed methylation and a decrease in protein expression in the p16 tumor suppressor gene in chromate lung cancer samples [87]. Moreover, an in vitro study using mouse hepatoma Hepa-1c1c7 cells showed, that upon coexposure to Cr(VI) and bezo[a]pyrene (an environmental procarcinogen that activates Cyp1a1), Cr(VI) caused crosslinking of the histone deacetylase 1-DNA methyltransferase 1 complex with chromatin, leading to the inhibition of histone marks such as, acetylation of histones H3 and H4, phosphorylation of histone H3 Ser-10, and trimethylation of H3 Lys-4, which all contributed to the inhibition of aryl hydrocarbon receptor-mediated gene transactivation [88]. Finally, a very recent study from Sun et al. suggested that Cr(VI) is able to affect global and gene promoter specific histone methylation, which leads to tumor suppressor gene silencing events [89]. The authors demonstrated that human lung carcinoma A549 cells treated with Cr(VI) exhibited an increase in the di- and tri-methylation of histone H3 Lys-9 and Lys-4. Interestingly, there was an enrichment of histone H3 Lys-9 dimethylation in the promoter region of the hMLH1 gene, which correlated to a decrease in mRNA expression [89].
While all of these data are interesting and shed light on the role of Cr(VI) in epigenetic modifications, the mechanisms by which these alterations occur are still unclear; and due to the potential carcinogenic effects of epigenetic changes, it is critical that more data be gathered pertaining to the area. Moreover, it should be noted that data collected in studies using A549 cells may not be indicative of carcinogenesis-related events, as they are already tumorigenic, not normal cells.
2.4 Chromium- induced inflammation
A growing body of evidence suggests that the development of lung cancer could be attributed to pro-inflammatory conditions in the context of chronic pulmonary inflammation, particularly in non-smokers [90–92]. Chromium exposure produces a wide range of immunological reactions depending on the dose, route of exposure, and nature of the chromium compound [93]. Generally, inhalation of particulate chromium compounds results in lung injury and a marked inflammatory response in the lung [94–96]. Recently published studies from our laboratory show a pronounced inflammatory response in the lung to intranasal (IN) particulate zinc chromate administration [97,98]. Analysis of cells collected by bronchoalveolar lavage showed an increase in viable cells in the lung airways of BALB/cJ mice as early as 6 h after Cr(VI) exposure, which persisted for up to 24 h. Flow cytometric and cytospin studies determined that there was a significant 64.8% increase in neutrophils present after 6 h Cr(VI) exposure as compared to 9.6% in the saline-treated control mouse; while at the same time point, there was a significant decrease in the presence of macrophages from 90.4% in control mice, to 35.2% in Cr(VI)-treated animals [97]. Moreover, protein analysis by ELISA demonstrated that there was a significant increase in the chemokine IL-8 homolog, GRO-α, as well as an increase in the cytokine IL-6 as early as 2 h after Cr(VI) treatment. The single Cr(VI) exposure administered to the animals induced lung injury and bronchiolar cell apoptosis, which led to interstitial and alveolar pneumonitis. Further studies of chronic IN zinc chromate exposure of BALB/c mice by Beaver et al. found that Cr(VI) treatment induced chronic peribronchial inflammation with alveolar and interstitial pneumonitis, increased pro-matrix metalloprotease-9, and caused a reactive proliferative response of airway epithelial cells [99]. Another study exposing the lungs of tumor susceptible a/j mice, by pharyngeal aspiration, to welding fume particles containing chromium resulted in significant lung injury, infiltration of neutrophils and histiocytic peribronchiolar inflammation [94]. The exposure and subsequent airway damage in the latter study ultimately resulted in fibrosis, bronchiolar epithelial hyperplasia with associated cellular atypia and broncho-alveolar hyperplasia. Intratracheal exposure of Sprague-Dawley rats to chromium-containing welding fumes or other particulate air pollutants resulted in an influx of macrophages, neutrophils and eosinophils to the lungs, which was coincident with cytotoxic damage to the alveolar capillary barrier [100]. Furthermore, in vitro exposure of differentiated human epithelial cells of the respiratory tract to mixed particulates that contained chromium among other chemicals, resulted in the stimulation of IL-8 and amphiregulin, an EGFR ligand [101]. Chronic exposure of the lung to sodium dichromate alone resulted in the detection of cellular inflammatory foci containing alveolar macrophages and chronic inflammatory thickening of alveolar septa. The lungs were further characterized as having fibrotic regions containing residual distorted bronchiolar lumens and proliferated epithelium [102]. The role of the immune system and inflammation in cancer development is paradoxical in that it can be both protective and pathological. For instance, the immune system is thought to be necessary for inhibiting tumor development and progression; conversely, it has been shown that many cancers arise from sites of chronic inflammation and that inflammatory microenvironments can be critical for initial neoplastic processes, along with subsequent tumor survival and/or metastasis [103].
2.5 Tumor formation
Several studies have shown that workers exposed to specific forms of Cr(VI) exhibit a significant increase in lung cancer risk, and in vivo and cell culture studies have demonstrated an increased incidence of neoplastic transformation and tumor formation [1,104,105]. Interestingly, not all Cr(VI) compounds are carcinogenic at toxicologically relevant exposure conditions [106]. Several studies have noted that the relatively insoluble, particulate Cr(VI) compounds yield the greatest toxicity leading to transformation in mouse embryo cells and tumor formation in animals [24,25,72,106]. Animal studies using an inhalation model of soluble sodium and calcium chromate did not yield increased tumor response, unless toxicologically irrelevant chromium concentrations were chronically administered. In contrast, tumors were produced in nearly every study utilizing the slightly soluble to highly insoluble particulates such as zinc, lead, strontium and sintered calcium chromate. By using intratracheal, intrabronchial, intrapleural and subcutaneous routes of administration to mice and rats of particulate zinc chromate, lead chromate and strontium chromate, tumor responses (tumors/animal) were produced in the range of 6/136, 6/80, 25/100, 12/34, 18/24, 8/35, 7/40, 22/33, 3/61, 31/62, 26/40, 31/47, and 17/28 [102,105,107–115]. It has been presumed that this is likely due to adherence of the particles to the lining of the respiratory tract and slow in situ particle dissolution delivering a chronic genotoxic insult to the surrounding tissue leading to tumor formation.
Reported characteristics of cells from human lung tumors associated with Cr(VI) exposure include aberrant p16INK4A methylation, low p53 mutation rate, loss of MLH1 expression and consequently, increased MSI [82,84,87,116]. Moreover, chromosomal damage leading to aneuploidy, triploidy and tetraploidy, is found in 70–80% of human lung tumors [117]. Notably, studies from the Wise laboratory, employing both soluble and particulate Cr(VI) in human lung epithelial cells show a concentration-dependent increase in chromosomal aberrations, consistent with neoplastic transformation [106]. These studies also demonstrate that particulate Cr(VI) is able to transform human bronchial epithelial cells exhibiting a concentration-dependent increase in aneuploidy [117]. Due to the lack of access and availability of chromate-induced lung cancer tissue samples, more studies both in vivo and in vitro will need to be conducted for a more comprehensive analysis on tumor formation in chromate-exposed humans.
3. Chromium genotoxicity: a double-edged sword
An initial consequence of genetic injury is cell cycle checkpoint arrest but genotoxicity may also activate cell death pathways of apoptosis or “terminal” growth arrest. Cellular survival responses to genotoxic insult may produce intrinsic death-resistance; such a selective growth advantage may allow for the emergence of a transformed phenotype. The hallmarks of cancer, as reviewed by Hanahan and Weinberg, are characterized by a multistage process of transformation and tumorigenesis, and include evasion of apoptosis, self-sufficiency in growth signals, insensitivity to antigrowth signals, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis [118]. Several lines of evidence have indicated that carcinogenesis evolves from consecutive genetic/epigenetic changes that provide cellular survival advantages, and ultimately lead to the conversion of normal human cells to malignant cancer cells. The sequence by which these events occur is unknown, and many of these processes are mechanistically related. The “pre-malignant” process of carcinogenesis begins with an “initiation” step, wherein exposure to a genotoxin or genotoxic stress can result in the selection of cells with a specific growth advantage. The response of a cell to this compromised state is largely epigenetic, involving de- and dys-regulation of gene networks that govern cell cycle, cell survival and cell death. Here, we will summarize the results of studies that investigate dysregulation of critical gene networks that may contribute to mechanisms of chromium-induced genotoxicity and carcinogenicity.
3.1 Death resistance after chromium exposure
Following exposure to a DNA damaging agent, such as Cr(VI), a cell cycle checkpoint arrest will be induced. As a consequence of irreparable genetic damage, the injured cell will be eliminated from the proliferating population by the activation of death mechanisms, either apoptosis or terminal growth arrest. Resistance to death leading to long term survival is common in most, if not all cancers, and a number of reports have shown the critical role apoptosis plays in metal-induced disorders such as neurotoxicity, autoimmunity, renal toxicity, hepatoxicity, and carcinogenesis [119,120]. It has previously been established that induction of apoptosis is the primary mode of Cr(VI)-induced cell death [29,121], and that the events leading to apoptosis may be either p53-dependent or p53-independent [41,119,122]. Escape from or resistance to apoptosis or terminal growth arrest seems to be a requirement for cells with limitless replicative potential [27]. Moreover, the selection of cells with the ability to survive after exposure to apoptogenic levels of a DNA damaging agent, may yield a precursor pool of cells from which neoplastic variants may emerge [27]. With respect to chromium, there are several studies reporting cellular resistance to Cr(VI) exposure in human, mouse, and bacterial cells. While the studies of enhanced survival and Cr(VI)-resistance in bacteria are interesting and yield pertinent information regarding the bioremediation of chromium contaminated areas, we will focus on studies in mammalian cells in this review (Table 1) [123,124].
Table 1.
Death resistance after Cr(VI) exposure
| Mode of inducing Cr(VI) resistance | Model system | Cr(VI) transport status | Resistant phenotype | Reference |
|---|---|---|---|---|
| Single exposure selection | Chinese hamster mutant cells | Dependent | Reduced uptake of labeled sulfate | [100] |
| Chronic exposure | Chinese hamster ovary cells | Dependent | Enhanced long term survival Cr(VI)-specific resistance |
[102] |
| Chronic exposure | 293 embryonic kidney cells | Dependent | Sensitive to density-dependent growth inhibition Grew slower than WT cells Cr(VI)-specific resistance |
[103] |
| Single exposure selection | BJ-hTERT human foreskin fibroblasts | Independent | Enhanced clonogenic survival p53 independent Increased Bcl-X gene expression Increased XPF gene expression Phenotype not Cr(VI)-specific, Cells also display resistance to H2O2 |
[27] |
| Genetic deletion of ATM | Human dermal fibroblasts | Independent | Resistant to Cr(VI)-induced apoptosis Display increased sensitivity to Cr(VI)- induced clonogenic lethality |
[104] |
| Genetic deletion of MMR genes | Human colon HCT116 and DLD1 cells, and mouse embryonic fibroblasts | Independent | Enhanced clonogenic survival Resistant to Cr(VI)-induced apoptosis p53 independent Cr(VI)-specific resistance |
[62] |
| Chronic exposure | Human bronchial epithelial cell line, BEAS-2B | Data not provided | Decreased clonogenic efficiency Altered cellular morphology and growth pattern Upregulated expression of DNA repair genes Upregulated expression of survival genes Page 51 of 52 Aneuploidy |
[63] |
3.1.1 Cr(VI) transport-dependent death resistance
One of the earliest studies of Cr(VI) resistance was reported in 1981. Campbell et al. showed that Chinese hamster mutant cells made resistant to chromate toxicity by single-exposure selection, exhibited a 10-fold reduction rate in the uptake of radiolabeled sulfate, indicating that a potential mechanism of chromate resistance is an insufficient sulfate transport system [125]. These data correlate with the ability of chromate molecules to gain cellular entry via non-specific anion channels [126]. Another study employed a Chinese hamster ovary (CHO) cell line, CHO-K1, which was made resistant to Cr(VI) through chronic exposure to progressively increasing concentrations of chromium trioxide (CrO3) [127]. In this study, the authors showed enhanced long term survival after 24 h Cr(VI) treatment in the Cr(VI)-resistant cells as measured by a clonogenicity assay, which is an indicator of long-term cell survival and replicative potential. Toxicity analysis demonstrated that the LD50 of the resistant cells to CrO3 was greater than 25-fold higher than that of the treated parental control cells. Interestingly, as compared to the parental control cells, the resistant cells showed no substantial differences in growth rate, chromosomal number, HPRT mutation frequency, or the expression/activity of the antioxidants glutathione, glutathione S-transferase, and catalase [127]. These authors concluded that the acquired resistance was due to alterations in cellular Cr(VI) uptake, which was attenuated in the resistant subclones, as assessed by measuring sulfate anion uptake. Moreover, the reduced clonogenic lethality was shown to be Cr(VI)-specific, as the resistant subclonal populations displayed death sensitivity to hydrogen peroxide, methanesulfonate methyl ester, and nickel chloride. A more recent study, also using a model of Cr(VI) resistance by chronic exposure to CrO3, corroborates the potential involvement of a decreased sulfate anion transport system as a mechanism of death resistance. Using human 293 embryonic kidney cells made resistant to Cr(VI) (as assessed by toxicity assays), the authors also showed no difference in cellular morphology between the Cr(VI)-resistant and -sensitive cell lines. These authors reported a significant reduction in Cr(VI) uptake in the resistant cells as measured by the uptake of radiolabeled 51Cr [128]. The Cr(VI)- resistant cells displayed a greater sensitivity to density-dependent growth inhibition and were found to grow at a slower rate than the wild-type sensitive cells, in the absence of Cr(VI). Moreover, these studies revealed that the observed resistant phenotype was also Cr(VI)-specific, as the Cr(VI)-resistant cells were sensitive to treatment with nickel sulfate. Notably, one draw back of this study of Cr(VI) resistance is that the wild-type cells used to create the resistant subclones, were tumorigenic when injected into nude mice [128]. This implies that these cells may already harbor a genetic predisposition to dysregulation of DNA damage response pathways, leading to Cr(VI) resistance.
3.1.2 Cr(VI) transport-independent death resistance
One notable similarity among the previously reported models of Cr(VI) resistance is the specificity of the resistance to Cr(VI). While Cr(VI) itself is a well known carcinogen, there is evidence that it may also act as a cocarcinogen [21]. In a study by Pritchard et al., Cr(VI)-resistant sub-populations of telomerase-transfected human foreskin fibroblasts (BJ-hTERT), were derived, which displayed apoptotic resistance not only to apoptogenic doses of Cr(VI), but also to hydrogen peroxide, as assessed by phosphatidylserine translocation [27]. In these studies, BJ-hTERT cells, seeded at low densities, were exposed to a single dose of Cr(VI) (5 μM) for 24 h, which led to 99% clonogenic lethality. The surviving cells were clonogenically expanded into a viable cell line (B-5Cr) that exhibited marked resistance to Cr(VI)-induced clonogenic lethality and apoptosis [27]. Notably, there was no difference in Cr(VI) uptake in the B-5Cr death-resistant cells as compared to control BJ-hTERT cells, while the stability of p53 protein was maintained following Cr(VI) treatment. This latter point is consistent with reports that Cr(VI)-induced apoptosis can be mediated via p53-independent mechanisms [27,82–84,87,116]. Moreover, gene expression analysis following Cr(VI) treatment of the BJ-hTERT and B-5Cr cells, showed a lack of induction in the expression of cell death/growth arrest pathway regulators such as, GADD45 and caspase 3, in the B-5Cr cells. Additionally, there was an observed upregulation of the gene expression of the anti-apoptotic protein, Bcl-X, and altered expression levels of DNA damage response genes, XPF and UV-RAG, after Cr(VI) exposure, which may be involved in the promotion of cell survival in the B-5Cr cells [27].
Recent studies in our laboratory were carried out in clonally expanded cell lines of the aforementioned B-5Cr cells, as compared to a clonogenic control (untreated but clonally expanded). Of note, the death resistant phenotype was maintained and shown to be dependent on resistance to mitochondrial-mediated apoptosis. Intriguingly, data from these studies suggest that death resistance after a single exposure to Cr(VI) may involve deregulation of mitochondrial respiration and bioenergetics (Nickens, unpublished data).
3.2 The involvement of DNA repair mechanisms in survival after chromium exposure
Alterations in the expression of key DNA repair proteins have been shown to be critical in the promotion of tumorigenesis. Ha et al. showed that normal dermal fibroblasts exposed to Cr(VI) trigger the activation of the DNA repair protein, ataxia telangiectasia mutated (ATM) [129]. Moreover, the downstream targets of ATM, i.e., p53 and Chk2, were phosphorylated after Cr(VI) exposure. The ability of Cr(VI) to activate the ATM pathway was also reported by other laboratories [130]. In the study by Ha et al., the effect of Cr(VI) on cells deficient in ATM (ATM−/−) was explored [129]. The ATM−/− cells, as compared to wild-type control cells, displayed significant resistance to Cr(VI)-induced apoptosis, as determined by decreased annexin V staining and the lack of caspase 3 cleavage. Paradoxically, the ATM−/− cells exhibited markedly increased sensitivity to Cr(VI)-induced terminal growth arrest as compared to wild-type cells, indicating that ATM is required for recovery from Cr(VI)-induced cellular growth arrest.
The role of mismatch repair proteins in Cr(VI) resistance was highlighted in a study by Peterson-Roth et al., using the human colon cell lines, HCT116 and DLD1, as well as mouse embryonic fibroblasts (MEFs), which were all deficient in MMR (MMR−). The MMR− cells showed significant resistance to Cr(VI)-induced apoptosis when compared to control cells as assessed by DNA fragmentation, annexin V staining, caspase activation, and PARP cleavage [83]. Cells lacking the MMR genes MLH1, MSH6, and PMS2, exhibited increased survival when treated with Cr(VI) as compared to MMR control cells (MMR+). As shown in previous studies, MMR− cells appear to be relatively uniquely resistant to Cr(VI)-induced toxicity, as there was no difference in toxicity between the MMR− and MMR+ cells after treatment with hydrogen peroxide, formaldehyde, and mitomycin C. Also consistent with other reports, there was no difference in p53 stability or phosphorylation upon Cr(VI) treatment between the MMR− and MMR+ cells. These data were supported by siRNA silencing of the MMR genes in human lung epithelial (H460) and primary human lung (IMR90) cells followed by a Cr(VI) challenge in the presence or absence of the critical reducing agent, ascorbate [131]. The results of this study showed that Cr(VI) exposure in the presence of ascorbate potentiated cell death, however MMR− cells exhibited increased survival after Cr(VI) treatment, while the effect of ascorbate to increase Cr(VI)-mediated cell death was abrogated. Taken together, these studies suggest a role of MMR in Cr(VI) resistance. A hypothesis was proposed in light of these data suggesting that chronic exposure to Cr(VI) may select cells lacking proficient MMR, yielding high levels of spontaneous mutations and microsatellite instability (MSI), which are characteristic of chromate-induced lung cancer [21,82]. Interestingly, a recently published study by Rodrigues et al., investigated whether the malignant phenotype of the Cr(VI)-transformed BEAS-2B human bronchial epithelial cell line (RenG2) was due to the selection of MMR deficient-, Cr(VI) resistant-genomically instable cell populations [84]. However, in contrast to the study by Peterson-Roth, the RenG2 cells showed consistently upregulated expression of crucial DNA repair genes after Cr(VI) treatment, such as RAD51, XRCC3, OGG1, XRCC1, XRCC5, and MLH1. Due to the finding of increased expression of the MMR gene, MLH1, in this latter study, RenG2 cells were analyzed for MSI, however there was no difference in MSI when compared to the control cells. Notably, the RenG2 cells were found to be tumorigenic when injected into nude mice. Moreover, consistent with other studies, the RenG2 cells showed upregulation of key survival genes such as, c-MYC, HIF1α,, LDH-A, EGFR, JNK, ERK, and p38, as well as marked karyotypic alterations in the form of aneuploidy. While this study did not support a role for MSI in the transformation of this human bronchial epithelial cell line, the importance of the appropriate microenvironment for suitable selection and propagation of resistant cells was highlighted. The authors demonstrated that very low density culture conditions in the presence of Cr(VI) were required for the observed phenotypic alterations resembling malignant transformation, such as foci formation and decreased cell-cell interaction. These alterations were not observed in control cells cultured at very low density in the absence of Cr(VI), concluding that proper microenvironmental conditions play a key role in death resistance acquired after chromium exposure; which in turn may affect the incidence of chromate-induced lung cancer. Interestingly, these results prompt speculation that normal, intact (dense) cellular tissue architecture acts as a strong anti-carcinogen which needs to be overcome by doses of chromate (or other toxic agents) high enough to produce cell death and tissue damage, before carcinogenic progression can ensue.
3.3 Survival signaling after chromium exposure
Dysregulation of the complex balance between cell growth and cell death is a determinant for neoplastic progression. Thus, inappropriate activation/inactivation of key signals that control cell survival after genotoxic insult can contribute to autonomous growth and neoplastic transformation.
3.3.1 Cr(VI) effects on gene expression
Studies have shown that positive and negative regulation of gene expression by metals such as chromium is dependent on the structures of cellular DNA and proteins [132–135]. Ye et al., performed an extensive examination of gene regulation following 2 h exposure to 300 μM Cr(VI) in human alveolar basal epithelial carcinoma A549 cells [69]. The authors found altered gene expression in genes involved in redox stress mechanisms, energy metabolism, protein synthesis, cell cycle and carcinogenesis. The Src-related genes cytoplasmic tyrosine kinase (Cy1) and HYL tyrosine kinases were upregulated, as well as MAPKAP kinase, Jun-B, and the Raf oncogene. Cell cycle and cell growth related genes that were upregulated upon Cr(VI) exposure included, the cdc42 interacting protein 4 (CIP4), proliferation-associated gene, retinoblastoma binding protein 2 (RBP2), Cdk5 activator isoform p39, and the INK4p19 gene. Downregulated cell growth and cell cycle related genes, in response to Cr(VI) exposure, included p34Cdc2, Cdc25b, Cdc47, and casein kinase II alpha subunit. Notably, the concentrations of Cr(VI) used in this study are very high and likely exceed the threshold for Cr(VI) toxicity in vitro [13]. Moreover, many years of experimentation in our laboratory has led us to observe that acute, short-term exposure (2–3 h) to high Cr(VI) doses results in different modes of cell death, and therefore different mechanisms of cell death; as compared to chronic exposure (12–48 h) to lower doses that ultimately produce the same degree of clonogenic death [13]. This strongly implies an intracellular threshold that affects the response of the cell to Cr(VI) depending on the nature of the initial insult. Doses between 1–10 μM, administered over 24 h are likely to be more relevant to human exposure conditions yielding between 50–90% cell survival in vitro [28,74,78,129,136].
Also using A549 cells, Castorina and colleagues showed that exposure to 10 μM Cr(VI) at 1 and 4 h, significantly reduced the expression of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (Her2/ErbB2) [137]. Interestingly, following 24 h exposure the ErbB2 receptor was significantly upregulated and the EGFR receptor expression returned to basal levels. These data suggest that Cr(VI) transiently and selectively regulates gene expression. Another global analysis investigating the effects of Cr(VI)-induced toxicity on gene expression was reported using BEAS-2B transformed human bronchial epithelial cells and primary smooth muscle cells [70]. These data showed that after 4 h exposure to 10 μM Cr(VI), both cell types downregulated the expression of c-myc, cyclin K, cyp1b1, MAPKNPK-2, PP1A, FGFR1, HSP90, and Akt1. Kondo et al. conducted an in vivo study on the involvement of cell cycle regulatory gene expression in human lung cancers associate with chromate exposure [87]. These authors found that 86% of chromate lung cancers presented with the methylation of the cell cycle inhibitor gene p16, which was directly correlated with a decrease in p16 protein expression. Conversely, in vitro data from our laboratory using normal human lung fibroblasts exposed to Cr(VI) showed no alteration in the gene expression pattern of p16 [74], suggesting that perhaps the epigenetic changes observed by Kondo et al. are seen at a later stage of the carcinogenic process. Although there were no changes in p16 or p27 in our study, Cr(VI) exposure of human lung fibroblasts induced various transiently altered gene expression patterns. Of note, 9 μM Cr(VI) exposure lead to an increase in gene expression of p21, GADD45, and p15; while cyclin A gene expression was decreased. Moreover, there was a decrease in the pro-survival Bcl-2 proteins, Bcl-W, Bcl-xL, and Bcl-2 [74].
Studies investigating the gene regulatory networks after Cr(VI) exposure are critical to the elucidation of the molecular mechanisms that enable survival pathway activation. Activation of these pathways can promote cellular survival, potentially in the presence of genomic instability resulting from DNA damage, and offer significant insight into the field of molecular carcinogenesis. Signaling pathways of interest include the mitogen activated protein kinase (MAPK) family, Src family of proto-oncogenic tyrosine kinases, and the Akt family of protein kinases.
3.3.2 Cr(VI) effects on survival signaling pathways
Several studies have found that MAPK signaling is activated following exposure to Cr(VI); however, the activation seems to be concentration-dependent [138]. For example, in human airway epithelial cells exposed to concentrations of Cr(VI) exceeding 10 μM, the MAPKs, extracellular signal-regulated kinase (ERK) and the p38 MAPK, are activated [138,139]. It is critical to contextualize this study, due to the use of highly toxic Cr(VI) concentrations. These high Cr(VI) doses not only increase reactive oxygen species production, but most likely trigger a toxic mechanism leading to altered gene regulation and activation. Evidence supporting these data was found in a recent report in which mouse embryonic stem cells were exposed to 50 μM Cr(VI), resulting in the activation of JNK1/2, p38, and ERK1/2 [140]. At non-toxic concentrations, Cr(VI) exhibited selective activation of the MAPK kinase, c-Jun N-terminal kinase (JNK), in A549 cells [138]. Conversely, studies from our lab have shown that after exposure to 6 μM Cr(VI), human lung fibroblasts exhibited ERK activation [74]. However, after inhibition of ERK, it was revealed that this MAPK kinase is not involved in Cr(VI) toxicity, as indicated by its lack of effect on gene expression and on clonogenicity following Cr(VI) exposure. These data are supported by other studies that have shown that ERK activation is not a mediator of Cr(VI)-induced cytotoxicity [74,140–142].
Nemec et al. further studied the effects of Cr(VI) and nickel (Ni) on signaling activation in BEAS-2B cells [143]. They found that Ni stimulated both ERK and phosphoinositide 3 kinase (PI3K), leading to downstream activation of Src kinase. This latter kinase transactivates AP-1 and the signal transducer and activator of transcription 3 (STAT3), followed by downstream activation of the hypoxia inducible factor 1 alpha (HIF1α) and the Sp1 human transcription factor, culminating in the increased transcription of the vascular endothelial growth factor (VEGF). Interestingly, Cr(VI) was able to inhibit the induction of this signaling cascade [143]. VEGF is a major mediator of angiogenesis and plays a role in a number of respiratory disorders such as asthma, fibrosis and cancer (for review, see [144,145]). It is overexpressed in lung cancers and its presence in serum is prognostic and correlates with decreased survival [146–148]. In contrast, a study in a DU145 human prostate carcinoma cell line found that Cr(VI) alone was able to stimulate VEGF expression and secretion [149]. Importantly, Nemec et al. showed that Cr(VI)-induced expression of the signal transducer and activator of transcription 1 (STAT1) is necessary to suppress both SP1 and VEGF-A transcription; concluding that STAT1 is the rate limiting factor in Cr(VI)-mediated gene regulation [143].
The mammalian Src family of kinases includes, Src, Yes, Fyn, Fgr, Lck, Hck, Blk, Lyn, and Frk [150]. These signal transducers have been shown to be involved in the progression of malignant cancer [150]. O’Hara et al. found that Cr(VI) treatment of A549 cells selectively activates Fyn and Lck [142]. Moreover, in vitro studies using purified Fyn, demonstrated that Cr(VI)-induced direct activation of this Src kinase was not dependent on reactive oxygen species generation. Furthermore, a recent study has uncovered a Cr(VI)-specific mechanism leading to the selective activation of Fyn and the initiation of an interferon-like signaling mechanism leading to STAT1-dependent gene transactivation [151]. The authors show that Cr(VI) exposure of BEAS-2B cells leads to a cascade of selective transactivation beginning with the activation of the STAT-response interferon-stimulated response element (ISRE), which further transactivates the interferon regulatory factor 7 (IRF7), an innate immune response gene. Interferon signaling has been shown to be regulated by STAT1, which is required for the activation of innate immune responses [151–154]. Moreover, knockdown of the Src-related gene, Fyn, in the BEAS-2B cells, inhibited the Cr(VI)-induced activation of STAT1-mediated IRF7 transactivation [151].
Akt is a serine/threonine kinase, and is the mediator of the PI3-kinase survival signal [155], for review, see [156]. There is a growing body of evidence that underlines the central role of the Akt pathway in death resistance as related to the pathogenesis of both pulmonary fibrosis and lung cancer [157–161]. Additionally, efforts have been made to exploit this pathway for therapeutic purposes in proliferative diseases of the lung [161–163]. Indeed, a recent report has shown that activated Akt can overcome a G2/M checkpoint arrest induced by DNA damage [164]. Lal et al. has recently reported that Akt-1 activation was sufficient to override the Cr(VI)-induced G1/S cell cycle checkpoint in human lung fibroblasts [165]. Moreover, the involvement of Akt in Cr(VI)-induced genotoxicity was confirmed in in vivo studies where BALB/cJ mice were intranasally administered a single dose of particulate basic zinc chromate [97]. Interestingly, in vivo chromate exposure in mice induced a rapid (by 1 h) and persistent increase in the number of lung airways immunoreactive for active Akt (phosphorylated at Ser-473).
3.3.3 Tyrosine phosphorylation-regulated survival signaling and cellular response to Cr(VI) exposure
The chromium-induced dysregulation of both pro-and anti-survival genes illustrates the complexity of the effect of Cr(VI) on the transcriptional regulation of cell cycle-inhibiting and apoptosis-inducing genes. Moreover, studies have shown that the phosphorylation of proteins that regulate these processes provides a critical molecular switch for rapid control of signaling pathways that includes, but is not limited to, proliferative protein tyrosine kinase cascades, cell death/cell growth-arresting tyrosine phosphatases, and serine/threonine protein kinases that sense DNA damage and activate p53. Reports have shown that dysregulated protein tyrosine phosphatase (PTP) activity is responsible for the maintenance of survival signals in the early stages of neoplastic progression [26]. A recent study by Bae et al., has demonstrated that Cr(VI)-induced growth arrest can be bypassed by the inhibition of PTPs [26]. PTP inhibition by the broad range inhibitor, sodium orthovanadate (SOV), in human lung fibroblasts in the presence of Cr(VI) led to enhanced clonogenic survival, decreased expression of cell cycle inhibiting genes, increased expression of cell cycle promoting genes, and an increase in forward mutations at the HPRT locus. Moreover, with further examination, the authors found that the increase in clonogenic survival potentiated by PTP inhibition in the presence of Cr(VI), was mediated by a MEK/ERK-independent and a Ras/c-Raf-dependent signaling pathway [166]. This is consistent with the previously discussed study showing that Erk-mediated signaling is not involved in the molecular mechanisms regulating the effects of Cr(VI) on human lung fibroblasts.
4. Conclusion
This review has focused on studies which address the various mechanisms by which chromium can alter the critical homeostatic balance of cellular death and survival. The spectrum of cellular effects of Cr(VI) begins with its intracellular metabolism leading to the formation of Cr-DNA adducts, genomic damage and mutagenesis, reactive oxygen species production, and alteration of survival signaling pathways. The resulting biological responses in turn may lead to the selection of cells with the ability to survive in the face of genotoxic stress. We hypothesize that following exposure to levels of Cr(VI) capable of eliciting some manifestation of toxicity, a cell will undergo a transient checkpoint arrest, and repair of any damage will ensue. If the damage is irreparable, the cell will undergo terminal growth arrest or apoptosis; however, a small population of cells may survive.
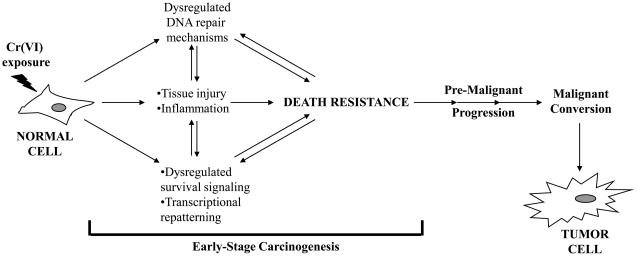
Death resistance may be provoked by the micro-environmental milieu generated by tissue damage and architectural disruption caused by a Cr(VI)-stimulated inflammatory response. The exact mechanism by which cells become resistant to Cr(VI)-induced death is unknown, however, data suggests the involvement of aberrant DNA repair mechanisms and the dysregulation of critical survival signaling pathways and transcriptional repatterning (Figure 1). Studies have shown that even in the presence of proficient DNA repair mechanisms, genomic instability may develop, and that cells lacking repair genes such as ATM, MLH1, MSH6, and PMS2, exhibit significant resistance to Cr(VI) exposure (Table 1) [43,81,83,129]. Moreover, along with altered DNA repair gene expression in Cr(VI)-resistant cells, the same cells have been shown to exhibit alterations in key survival genes, such as ERK, c-MYC, VEGF, and AKT [27,83]. The transcriptional regulation of survival genes and the signaling pathways they control have proven to be critical in the survival of Cr(VI)-exposed cells. Taken together, survivors of Cr(VI) exposure harboring altered repair and survival signaling mechanisms may form the basis for the development of a population of neoplastic precursor cells, which may lead to tumor cell formation.
Figure 1.
Cellular resistance to Cr(VI)-induced death and early stage carcinogenesis. Upon exposure to relevant doses of Cr(VI), a normal cell will undergo a transient checkpoint arrest in an attempt to repair damaged DNA. Cells that are unable to repair the damage will undergo apoptosis or terminal growth arrest (replicative death); however a small population of cells may survive. These survivors may have acquired an intrinsic mechanism(s) of death resistance through dysregulated DNA repair mechanisms and/or dysregulated survival signaling and transcriptional repatterning. Direct damage to the cells from Cr(VI) exposure can led to tissue injury and severe inflammatory responses, which may further contribute to the micro-environmental milieu potentiating the death resistant phenotype. Cells that are phenotypically resistant to Cr(VI)-induced death may be predisposed to pre-malignant progression thereby propagating early stage carcinogenesis, which may over time lead to the malignant conversion of the predisposed precursor cells to tumor cells.
The process by which chromium is able to induce neoplastic progression is both complex and fairly elusive. Data can be found on each end of the spectrum, either supporting or refuting the many mechanisms proposed in the involvement of Cr(VI) carcinogenesis, thereby arguing the need for further research on the subject. Further research on the mechanisms of Cr(VI)-induced carcinogenesis may provide a more complete understanding by which not only human exposure to Cr(VI), but also other metals, can lead to neoplastic progression.
Acknowledgments
The authors acknowledge recent and present members of the Patierno and Ceryak laboratories, whose studies have contributed to this review, including: Dr. Daryl Pritchard, Dr. Linan Ha, Dr. Tura Camilli, Dr. Bradford Brooks, Dr. Laura Beaver, Dr. Gina Chun, Dr. Madhu Lal-Nag, and Dr. Dongsoon Bae. The authors thank Dr. Travis O’Brien for helpful discussions. Our laboratory is supported by grants NIH R01ES05304 and NIH R01ES09961 to Dr. Patierno, and NIH R01CA107972 to Dr. Ceryak.
Abbreviations
- Cr(VI)
hexavalent chromium
- GSH
glutathione
- ROS
reactive oxygen species
- IN
intranasal
- CHO
Chinese hamster ovary cells
- Ni
nickel
- ATM
ataxia telangiectasia mutated
- BER
base excision repair
- NER
nucleotide excision repair
- MMR
mismatch repair
- MSI
microsatellite instability
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinase
- PI3K
phosphoinositide 3 kinase
- STAT
signal transducer and activator of transcription
- HIF1α
hypoxia inducible factor 1 alpha
- VEGF
vascular endothelial growth factor
- PTP
protein tyrosine phosphatase
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- siRNA
small-interfering ribonucleic acid
- ESR
electron spin resonance
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chromium, nickel and welding, IARC Monogr. Eval Carcinog Risks Hum. 1990;49:1–648. [PMC free article] [PubMed] [Google Scholar]
- 2.National Toxicology Program. NTP 11th Report on Carcinogens. Rep Carcinog. 2005;(11):1-A32. [PubMed] [Google Scholar]
- 3.U.S. Department of the Interior and U.S. Geological Survey. Mineral Commodity Summaries. 2004. [Google Scholar]
- 4.National Safety Council. National Overview of 1998 Toxics Release Inventory. 1998. [Google Scholar]
- 5.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Res. 1994;54:2342–2346. [PubMed] [Google Scholar]
- 6.Plunkett ER. Handbook of Industrial Toxicology. Chemical Publishing; New York, NY: 1976. [Google Scholar]
- 7.Amdur MO, Doull J. Cassarett and Doull’s Toxicology. 4. Maxwell-MacMillan-Pergamon; New York, NY: 1991. [Google Scholar]
- 8.Uddin AN, Burns FJ, Rossman TG, Chen H, Kluz T, Costa M. Dietary chromium and nickel enhance UV-carcinogenesis in skin of hairless mice. Toxicol Appl Pharmacol. 2007;221:329–338. doi: 10.1016/j.taap.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Singh J, Pritchard DE, Carlisle DL, Mclean JA, Montaser A, Orenstein JM, Patierno SR. Internalization of carcinogenic lead chromate particles by cultured normal human lung epithelial cells: formation of intracellular lead-inclusion bodies and induction of apoptosis. Toxicol Appl Pharmacol. 1999;161:240–248. doi: 10.1006/taap.1999.8816. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Polychlorinated Biphenyls (PCBs) 2000 [PubMed] [Google Scholar]
- 11.U.S. Department of Labor. Occupational Safety & Health Administration, Contract No. J-9-F-0030. 2006. [Google Scholar]
- 12.U.S. Environmental Protection Agency. Integrated Risk Information System on Chromium. VI. 1999. [Google Scholar]
- 13.De Flora S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- 14.Acharya S, Mehta K, Krishnan S, Rao CV. A subtoxic interactive toxicity study of ethanol and chromium in male Wistar rats. Alcohol. 2001;23:99–108. doi: 10.1016/s0741-8329(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 15.Izzotti A, Bagnasco M, Camoirano A, Orlando M, De Flora S. DNA fragmentation, DNA-protein crosslinks, postlabeled nucleotidic modifications, and 8-hydroxy-2′-deoxyguanosine in the lung but not in the liver of rats receiving intratracheal instillations of chromium(VI). Chemoprevention by oral N-acetylcysteine. Mutat Res. 1998;400:233–244. doi: 10.1016/s0027-5107(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Shindo Y, Toyoda Y, Kawamura K, Kurebe M, Shimada H, Hattori C, Satake S. Micronucleus test with potassium chromate(VI) administered intraperitoneally and orally to mice. Mutat Res. 1989;223:403–406. doi: 10.1016/0165-1218(89)90096-7. [DOI] [PubMed] [Google Scholar]
- 17.Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ. Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic Biol Med. 1997;22:471–478. doi: 10.1016/s0891-5849(96)00352-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuykendall JR, Kerger BD, Jarvi EJ, Corbett GE, Paustenbach DJ. Measurement of DNA-protein cross-links in human leukocytes following acute ingestion of chromium in drinking water. Carcinogenesis. 1996;17:1971–1977. doi: 10.1093/carcin/17.9.1971. [DOI] [PubMed] [Google Scholar]
- 19.Mirsalis JC, Hamilton CM, O’Loughlin KG, Paustenbach DJ, Kerger BD, Patierno S. Chromium (VI) at plausible drinking water concentrations is not genotoxic in the in vivo bone marrow micronucleus or liver unscheduled DNA synthesis assays. Environ Mol Mutagen. 1996;28:60–63. doi: 10.1002/(SICI)1098-2280(1996)28:1<60::AID-EM9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Sedman RM, Beaumont J, McDonald TA, Reynolds S, Krowech G, Howd R. Review of the evidence regarding the carcinogenicity of hexavalent chromium in drinking water. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:155–182. doi: 10.1080/10590500600614337. [DOI] [PubMed] [Google Scholar]
- 21.Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.California Department of Health Services (DHS) Chromium-6 in Drinking Water: An Overview of Sampling Results. [Accessed November 2004]. [Google Scholar]
- 23.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Sodium Dichromate Dihydrate (CAS No. 7789–12–0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies) Natl Toxicol Program Tech Rep Ser. 2008;(546):1–192. [PubMed] [Google Scholar]
- 24.Doll R. Strategy for detection of cancer hazards to man. Nature. 1977;265:589–596. doi: 10.1038/265589a0. [DOI] [PubMed] [Google Scholar]
- 25.Role of metals in carcinogenesis. Environ Health Perspect. Vol. 40. 1981. Problems of epidemiological evidence; pp. 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae D, Camilli TC, Chun G, Lal M, Wright K, O’Brien TJ, Patierno SR, Ceryak S. Bypass of hexavalent chromium-induced growth arrest by a protein tyrosine phosphatase inhibitor: enhanced survival and mutagenesis. Mutat Res. 2009;660:40–46. doi: 10.1016/j.mrfmmm.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard DE, Ceryak S, Ramsey KE, O’Brien TJ, Ha L, Fornsaglio JL, Stephan DA, Patierno SR. Resistance to apoptosis, increased growth potential, and altered gene expression in cells that survived genotoxic hexavalent chromium [Cr(VI)] exposure. Mol Cell Biochem. 2005;279:169–181. doi: 10.1007/s11010-005-8292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilcheck SK, O’Brien TJ, Pritchard DE, Ha L, Ceryak S, Fornsaglio JL, Patierno SR. Fanconi anemia complementation group A cells are hypersensitive to chromium(VI)-induced toxicity. Environ Health Perspect. 2002;110(Suppl 5):773–777. doi: 10.1289/ehp.02110s5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blankenship LJ, Carlisle DL, Wise JP, Orenstein JM, Dye LE, 3rd, Patierno SR. Induction of apoptotic cell death by particulate lead chromate: differential effects of vitamins C and E on genotoxicity and survival. Toxicol Appl Pharmacol. 1997;146:270–280. doi: 10.1006/taap.1997.8237. [DOI] [PubMed] [Google Scholar]
- 30.Wise JP, Orenstein JM, Patierno SR. Inhibition of lead chromate clastogenesis by ascorbate: relationship to particle dissolution and uptake. Carcinogenesis. 1993;14:429–434. doi: 10.1093/carcin/14.3.429. [DOI] [PubMed] [Google Scholar]
- 31.Wise SJP, Stearns DM, Wetterhahn KE, Patierno SR. Cell-enhanced dissolution of carcinogenic lead chromate particles: the role of individual dissolution products in clastogenesis. Carcinogenesis. 1994;15:2249–2254. doi: 10.1093/carcin/15.10.2249. [DOI] [PubMed] [Google Scholar]
- 32.Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise SJP. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol Appl Pharmacol. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Wise SS, Holmes AL, Qin Q, Xie H, Katsifis SP, Thompson WD, Wise JP. Comparative Genotoxicity and Cytotoxicity of Four Hexavalent Chromium Compounds in Human Bronchial Cells. Chem Res Toxicol. 2009 doi: 10.1021/tx900363j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Chen T, Wise SS, Holmes A, Shaffiey F, Wise JP, Jr, Thompson WD, Kraus S, Wise SJP. Cytotoxicity and genotoxicity of hexavalent chromium in human and North Atlantic right whale (Eubalaena glacialis) lung cells. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:487–494. doi: 10.1016/j.cbpc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camyre E, Wise SS, Milligan P, Gordon N, Goodale B, Stackpole M, Patzlaff N, Aboueissa AM, Wise SJP. Ku80 deficiency does not affect particulate chromate-induced chromosome damage and cytotoxicity in Chinese hamster ovary cells. Toxicol Sci. 2007;97:348–354. doi: 10.1093/toxsci/kfm045. [DOI] [PubMed] [Google Scholar]
- 36.Xie H, Holmes AL, Wise SS, Gordon N, Wise SJP. Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chem Res Toxicol. 2004;17:1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]
- 37.Wise SS, Schuler JH, Holmes AL, Katsifis SP, Ketterer ME, Hartsock WJ, Zheng T, Wise SJP. Comparison of two particulate hexavalent chromium compounds: Barium chromate is more genotoxic than lead chromate in human lung cells. Environ Mol Mutagen. 2004;44:156–162. doi: 10.1002/em.20044. [DOI] [PubMed] [Google Scholar]
- 38.Gibb HJ, Lees PS, Pinsky PF, Rooney BC. Lung cancer among workers in chromium chemical production. Am J Ind Med. 2000;38:115–126. doi: 10.1002/1097-0274(200008)38:2<115::aid-ajim1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 39.Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal. 2004;24:1099–1108. doi: 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 40.Costa M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit Rev Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 1995;120:931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien T, Xu J, Patierno SR. Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol Cell Biochem. 2001;222:173–182. [PubMed] [Google Scholar]
- 44.Ding M, Shi X. Molecular mechanisms of Cr(VI)-induced carcinogenesis. Mol Cell Biochem. 2002;234–235:293–300. [PubMed] [Google Scholar]
- 45.Joseph P, He Q, Umbright C. Heme-oxygenase 1 gene expression is a marker for hexavalent chromium-induced stress and toxicity in human dermal fibroblasts. Toxicol Sci. 2008;103:325–334. doi: 10.1093/toxsci/kfn048. [DOI] [PubMed] [Google Scholar]
- 46.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 47.Jennette KW. Microsomal Reduction of the Carcinogen Chromate Produces Chromium (V) J Am Chem Soc. 1982:874. [Google Scholar]
- 48.Kawanishi S, Inoue S, Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986;261:5952–5958. [PubMed] [Google Scholar]
- 49.Shi X, Dalal NS. Generation of hydroxyl radical by chromate in biologically relevant systems: role of Cr(V) complexes versus tetraperoxochromate(V) Environ Health Perspect. 1994;102(Suppl 3):231–236. doi: 10.1289/ehp.94102s3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi X, Dong Z, Dalal NS, Gannett PM. Chromate-mediated free radical generation from cysteine, penicillamine, hydrogen peroxide, and lipid hydroperoxides. Biochim Biophys Acta. 1994;1226:65–72. doi: 10.1016/0925-4439(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 51.Shi XL, Dalal NS. The role of superoxide radical in chromium (VI)-generated hydroxyl radical: the Cr(VI) Haber-Weiss cycle. Arch Biochem Biophys. 1992;292:323–327. doi: 10.1016/0003-9861(92)90085-b. [DOI] [PubMed] [Google Scholar]
- 52.Shi XL, Dalal NS. ESR spin trapping detection of hydroxyl radicals in the reactions of Cr(V) complexes with hydrogen peroxide. Free Radic Res Commun. 1990;10:17–26. doi: 10.3109/10715769009145929. [DOI] [PubMed] [Google Scholar]
- 53.Shi XL, Dalal NS. Evidence for a Fenton-type mechanism for the generation of .OH radicals in the reduction of Cr(VI) in cellular media. Arch Biochem Biophys. 1990;281:90–95. doi: 10.1016/0003-9861(90)90417-w. [DOI] [PubMed] [Google Scholar]
- 54.Shi XL, Dalal NS. One-electron reduction of chromate by NADPH-dependent glutathione reductase. J Inorg Biochem. 1990;40:1–12. doi: 10.1016/0162-0134(90)80034-u. [DOI] [PubMed] [Google Scholar]
- 55.Shi XL, Dalal NS. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI) Biochem Biophys Res Commun. 1989;163:627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- 56.Shi XL, Dalal NS, Vallyathan V. One-electron reduction of carcinogen chromate by microsomes, mitochondria, and Escherichia coli: identification of Cr(V) and .OH radical. Arch Biochem Biophys. 1991;290:381–386. doi: 10.1016/0003-9861(91)90555-w. [DOI] [PubMed] [Google Scholar]
- 57.Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J Toxicol Environ Health B Crit Rev. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- 58.Patlolla AK, Barnes C, Hackett D, Tchounwou PB. Potassium dichromate induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health. 2009;6:643–653. doi: 10.3390/ijerph6020643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugiyama M, Tsuzuki K, Lin X, Costa M. Potentiation of sodium chromate(VI)-induced chromosomal aberrations and mutation by vitamin B2 in Chinese hamster V79 cells. Mutat Res. 1992;283:211–214. doi: 10.1016/0165-7992(92)90109-u. [DOI] [PubMed] [Google Scholar]
- 60.Azad N, Iyer AK, Manosroi A, Wang L, Rojanasakul Y. Superoxide-mediated proteasomal degradation of Bcl-2 determines cell susceptibility to Cr(VI)-induced apoptosis. Carcinogenesis. 2008;29:1538–1545. doi: 10.1093/carcin/bgn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azad N, Iyer AK, Wang L, Lu Y, Medan D, Castranova V, Rojanasakul Y. Nitric Oxide-Mediated Bcl-2 Stabilization Potentiates Malignant Transformation of Human Lung Epithelial Cells. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sugiyama M. Effects of vitamins on chromium(VI)-induced damage. Environ Health Perspect. 1991;92:63–70. doi: 10.1289/ehp.919263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugiyama M. Effects of vitamin E and vitamin B2 on chromate-induced DNA lesions. Biol Trace Elem Res. 1989;21:399–404. doi: 10.1007/BF02917281. [DOI] [PubMed] [Google Scholar]
- 64.Asatiani N, Sapojnikova N, Abuladze M, Kartvelishvili T, Kulikova N, Kiziria E, Namchevadze E, Holman HY. Effects of Cr(VI) long-term and low-dose action on mammalian antioxidant enzymes (an in vitro study) J Inorg Biochem. 2004;98:490–496. doi: 10.1016/j.jinorgbio.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Asatiani N, Abuladze M, Kartvelishvili T, Kulikova N, Asanishvili L, Holman HY, Sapojnikova N. Response of antioxidant defense system to chromium (VI)-induced cytotoxicity in human diploid cells. Biometals. 2010;23:161–172. doi: 10.1007/s10534-009-9276-6. [DOI] [PubMed] [Google Scholar]
- 66.Bagchi D, Bagchi M, Stohs SJ. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol Cell Biochem. 2001;222:149–158. [PubMed] [Google Scholar]
- 67.Dubrovskaya VA, Wetterhahn KE. Effects of Cr(VI) on the expression of the oxidative stress genes in human lung cells. Carcinogenesis. 1998;19:1401–1407. doi: 10.1093/carcin/19.8.1401. [DOI] [PubMed] [Google Scholar]
- 68.O’Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium (VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J Cell Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye J, Shi X. Gene expression profile in response to chromium-induced cell stress in A549 cells. Mol Cell Biochem. 2001;222:189–197. [PubMed] [Google Scholar]
- 70.Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O’Hara KA, Hamilton JW. Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ Health Perspect. 2003;111:825–835. doi: 10.1289/ehp.111-1241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin BD, Schoenhard JA, Sugden KD. Hypervalent chromium mimics reactive oxygen species as measured by the oxidant-sensitive dyes 2′,7′-dichlorofluorescin and dihydrorhodamine. Chem Res Toxicol. 1998;11:1402–1410. doi: 10.1021/tx9801559. [DOI] [PubMed] [Google Scholar]
- 72.Patierno SR, Banh D, Landolph JR. Transformation of C3H/10T1/2 mouse embryo cells to focus formation and anchorage independence by insoluble lead chromate but not soluble calcium chromate: relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988;48:5280–5288. [PubMed] [Google Scholar]
- 73.McCarroll N, Keshava N, Chen J, Akerman G, Kligerman A, Rinde E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: Chromium (VI) Environ Mol Mutagen. 2009 doi: 10.1002/em.20525. [DOI] [PubMed] [Google Scholar]
- 74.Ceryak S, Zingariello C, O’Brien T, Patierno SR. Induction of pro-apoptotic and cell cycle-inhibiting genes in chromium (VI)-treated human lung fibroblasts: lack of effect of ERK. Mol Cell Biochem. 2004;255:139–149. doi: 10.1023/b:mcbi.0000007270.82431.3e. [DOI] [PubMed] [Google Scholar]
- 75.Holmes AL, Wise SS, Wise SJP. Carcinogenicity of hexavalent chromium. Indian J Med Res. 2008;128:353–372. [PubMed] [Google Scholar]
- 76.Zhitkovich A, Song Y, Quievryn G, Voitkun V. Non-oxidative mechanisms are responsible for the induction of mutagenesis by reduction of Cr(VI) with cysteine: role of ternary DNA adducts in Cr(III)-dependent mutagenesis. Biochemistry. 2001;40:549–560. doi: 10.1021/bi0015459. [DOI] [PubMed] [Google Scholar]
- 77.O’Brien TJ, Witcher P, Brooks B, Patierno SR. DNA polymerase zeta is essential for hexavalent chromium-induced mutagenesis. Mutat Res. 2009;663:77–83. doi: 10.1016/j.mrfmmm.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Brien TJ, Fornsaglio JL, Ceryak S, Patierno SR. Effects of hexavalent chromium on the survival and cell cycle distribution of DNA repair-deficient S. cerevisiae. DNA Repair (Amst) 2002;1:617–627. doi: 10.1016/s1568-7864(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 79.Tamblyn L, Li E, Sarras H, Srikanth P, Hande MP, McPherson JP. A role for Mus81 in the repair of chromium-induced DNA damage. Mutat Res. 2009;660:57–65. doi: 10.1016/j.mrfmmm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 80.Reynolds M, Peterson E, Quievryn G, Zhitkovich A. Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J Biol Chem. 2004;279:30419–30424. doi: 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- 81.Brooks B, O’Brien TJ, Ceryak S, Wise SJP, Wise SS, Wise JP, Jr, Defabo E, Patierno SR. Excision repair is required for genotoxin-induced mutagenesis in mammalian cells. Carcinogenesis. 2008;29:1064–1069. doi: 10.1093/carcin/bgn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi Y, Kondo K, Hirose T, Nakagawa H, Tsuyuguchi M, Hashimoto M, Sano T, Ochiai A, Monden Y. Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol Carcinog. 2005;42:150–158. doi: 10.1002/mc.20073. [DOI] [PubMed] [Google Scholar]
- 83.Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol Cell Biol. 2005;25:3596–3607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodrigues CF, Urbano AM, Matoso E, Carreira I, Almeida A, Santos P, Botelho F, Carvalho L, Alves M, Monteiro C, Costa AN, Moreno V, Alpoim MC. Human bronchial epithelial cells malignantly transformed by hexavalent chromium exhibit an aneuploid phenotype but no microsatellite instability. Mutat Res. 2009;670:42–52. doi: 10.1016/j.mrfmmm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein CB, Su L, Bowser D, Leszczynska J. Chromate-induced epimutations in mammalian cells. Environ Health Perspect. 2002;110(Suppl 5):739–743. doi: 10.1289/ehp.02110s5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kondo K, Takahashi Y, Hirose Y, Nagao T, Tsuyuguchi M, Hashimoto M, Ochiai A, Monden Y, Tangoku A. The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer. 2006;53:295–302. doi: 10.1016/j.lungcan.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 88.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun H, Zhou X, Chen H, Li Q, Costa M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol Appl Pharmacol. 2009;237:258–266. doi: 10.1016/j.taap.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10:4266s–4269s. doi: 10.1158/1078-0432.CCR-040014. [DOI] [PubMed] [Google Scholar]
- 91.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med. 2005;11:431–437. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 92.Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp Lung Res. 2005;31:57–82. doi: 10.1080/01902140490495020. [DOI] [PubMed] [Google Scholar]
- 93.Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC. Effects of chromium on the immune system. FEMS Immunol Med Microbiol. 2002;34:1–7. doi: 10.1111/j.1574-695X.2002.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 94.Solano-Lopez C, Zeidler-Erdely PC, Hubbs AF, Reynolds SH, Roberts JR, Taylor MD, Young SH, Castranova V, Antonini JM. Welding fume exposure and associated inflammatory and hyperplastic changes in the lungs of tumor susceptible a/j mice. Toxicol Pathol. 2006;34:364–372. doi: 10.1080/01926230600815122. [DOI] [PubMed] [Google Scholar]
- 95.Glaser U, Hochrainer D, Kloppel H, Kuhnen H. Low level chromium (VI) inhalation effects on alveolar macrophages and immune functions in Wistar rats. Arch Toxicol. 1985;57:250–256. doi: 10.1007/BF00324787. [DOI] [PubMed] [Google Scholar]
- 96.Kim HY, Lee SB, Jang BS. Subchronic inhalation toxicity of soluble hexavalent chromium trioxide in rats. Arch Toxicol. 2004;78:363–368. doi: 10.1007/s00204-004-0553-4. [DOI] [PubMed] [Google Scholar]
- 97.Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol Appl Pharmacol. 2009;235:47–56. doi: 10.1016/j.taap.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bridgewater LC, Manning FC, Patierno SR. Arrest of replication by mammalian DNA polymerases alpha and beta caused by chromium-DNA lesions. Mol Carcinog. 1998;23:201–206. [PubMed] [Google Scholar]
- 99.Beaver LM, Stemmy EJ, Schwartz A, Damsker JM, Constant SL, Ceryak S, Patierno SR. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ Health Perspect. 2009;117 doi: 10.1289/ehp.0900715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor MD, Roberts JR, Leonard SS, Shi X, Antonini JM. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol Sci. 2003;75:181–191. doi: 10.1093/toxsci/kfg173. [DOI] [PubMed] [Google Scholar]
- 101.Auger F, Gendron MC, Chamot C, Marano F, Dazy AC. Responses of well-differentiated nasal epithelial cells exposed to particles: role of the epithelium in airway inflammation. Toxicol Appl Pharmacol. 2006;215:285–294. doi: 10.1016/j.taap.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Steinhoff D, Gad SC, Hatfield GK, Mohr U. Carcinogenicity study with sodium dichromate in rats. Exp Pathol. 1986;30:129–141. doi: 10.1016/s0232-1513(86)80085-8. [DOI] [PubMed] [Google Scholar]
- 103.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leonard A, Lauwerys RR. Carcinogenicity and mutagenicity of chromium. Mutat Res. 1980;76:227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- 105.Levy LS, Venitt S. Carcinogenicity and mutagenicity of chromium compounds: the association between bronchial metaplasia and neoplasia. Carcinogenesis. 1986;7:831–835. doi: 10.1093/carcin/7.5.831. [DOI] [PubMed] [Google Scholar]
- 106.Wise SS, Holmes AL, Wise SJP. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutat Res. 2006;610:2–7. doi: 10.1016/j.mrgentox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 107.Roe FJ, Carter RL. Chromium carcinogenesis: calcium chromate as a potent carcinogen for the subcutaneous tissues of the rat. Br J Cancer. 1969;23:172–176. doi: 10.1038/bjc.1969.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nettesheim P, Hanna MG, Jr, Doherty DG, Newell RF, Hellman A. Effect of calcium chromate dust, influenza virus, and 100 R whole-body × radiation on lung tumor incidence in mice. J Natl Cancer Inst. 1971;47:1129–1144. [PubMed] [Google Scholar]
- 109.Hueper WC, Payne WW. Experimental cancers in rats produced by chromium compounds and their significance to industry and public health. Am Ind Hyg Assoc J. 1959;20:274–280. doi: 10.1080/00028895909343716. [DOI] [PubMed] [Google Scholar]
- 110.Hueper WC, Payne WW. Experimental studies in metal carcinogenesis. Chromium, nickel, iron, arsenic. Arch Environ Health. 1962;5:445–462. doi: 10.1080/00039896.1962.10663311. [DOI] [PubMed] [Google Scholar]
- 111.Baetjer AM, Lowney JF, Steffee H, Budacz V. Effect of chromium on incidence of lung tumors in mice and rats. AMA Arch Ind Health. 1959;20:124–135. [PubMed] [Google Scholar]
- 112.Levy LS, Martin PA, Bidstrup PL. Investigation of the potential carcinogenicity of a range of chromium containing materials on rat lung. Br J Ind Med. 1986;43:243–256. doi: 10.1136/oem.43.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hueper WC. Experimental studies in metal cancerigenesis. X. Cancerigenic effects of chromite ore roast deposited in muscle tissue and pleural cavity of rats. AMA Arch Ind Health. 1958;18:284–291. [PubMed] [Google Scholar]
- 114.Maltoni C, Minardi F, Morisi L. The relevance of the experimental approach in the assessment of the oncogenic risks from fibrous and non-fibrous particles. The oncology project of the Bologna Institute of Oncology. Med Lav. 1982;73:394–407. [PubMed] [Google Scholar]
- 115.Furst A, Schlauder M, Sasmore DP. Tumorigenic activity of lead chromate. Cancer Res. 1976;36:1779–1783. [PubMed] [Google Scholar]
- 116.Kondo K, Hino N, Sasa M, Kamamura Y, Sakiyama S, Tsuyuguchi M, Hashimoto M, Uyama T, Monden Y. Mutations of the p53 gene in human lung cancer from chromate-exposed workers. Biochem Biophys Res Commun. 1997;239:95–100. doi: 10.1006/bbrc.1997.7425. [DOI] [PubMed] [Google Scholar]
- 117.Xie H, Holmes AL, Wise SS, Huang S, Peng C, Wise SJP. Neoplastic transformation of human bronchial cells by lead chromate particles. Am J Respir Cell Mol Biol. 2007;37:544–552. doi: 10.1165/rcmb.2007-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 119.Rana SV. Metals and apoptosis: recent developments. J Trace Elem Med Biol. 2008;22:262–284. doi: 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 120.Manning FC, Patierno SR. The role of apoptosis in carcinogenesis. Current Topics in Mol Pharmacol. 1993;1:123. [Google Scholar]
- 121.Blankenship LJ, Manning FC, Orenstein JM, Patierno SR. Apoptosis is the mode of cell death caused by carcinogenic chromium. Toxicol Appl Pharmacol. 1994;126:75–83. doi: 10.1006/taap.1994.1092. [DOI] [PubMed] [Google Scholar]
- 122.Quinteros FA, Machiavelli LI, Miler EA, Cabilla JP, Duvilanski BH. Mechanisms of chromium (VI)-induced apoptosis in anterior pituitary cells. Toxicology. 2008;249:109–115. doi: 10.1016/j.tox.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 123.Henne KL, Nakatsu CH, Thompson DK, Konopka AE. High-level chromate resistance in Arthrobacter sp. strain FB24 requires previously uncharacterized accessory genes. BMC Microbiol. 2009;9:199. doi: 10.1186/1471-2180-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verma T, Garg SK, Ramteke PW. Genetic correlation between chromium resistance and reduction in Bacillus brevis isolated from tannery effluent. J Appl Microbiol. 2009;107:1425–1432. doi: 10.1111/j.1365-2672.2009.04326.x. [DOI] [PubMed] [Google Scholar]
- 125.Campbell CE, Gravel RA, Worton RG. Isolation and characterization of Chinese hamster cell mutants resistant to the cytotoxic effects of chromate. Somatic Cell Genet. 1981;7:535–546. doi: 10.1007/BF01549657. [DOI] [PubMed] [Google Scholar]
- 126.Esko JD, Elgavish A, Prasthofer T, Taylor WH, Weinke JL. Sulfate transport-deficient mutants of Chinese hamster ovary cells. Sulfation of glycosaminoglycans dependent on cysteine. J Biol Chem. 1986;261:15725–15733. [PubMed] [Google Scholar]
- 127.Lu YY, Yang JL. Long-term exposure to chromium(VI) oxide leads to defects in sulfate transport system in Chinese hamster ovary cells. J Cell Biochem. 1995;57:655–665. doi: 10.1002/jcb.240570410. [DOI] [PubMed] [Google Scholar]
- 128.Son KH, Zhang M, Rucobo E, Nwaigwe D, Montgomery F, Leffert H. Derivation and study of human epithelial cell lines resistant to killing by chromium trioxide. J Toxicol Environ Health A. 2004;67:1027–1049. doi: 10.1080/15287390490447304. [DOI] [PubMed] [Google Scholar]
- 129.Ha L, Ceryak S, Patierno SR. Chromium (VI) activates ataxia telangiectasia mutated (ATM) protein. Requirement of ATM for both apoptosis and recovery from terminal growth arrest. J Biol Chem. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 130.Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise SJP. Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat Res. 2005;586:160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reynolds M, Zhitkovich A. Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis. 2007;28:1613–1620. doi: 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- 132.Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor-kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J Biol Chem. 1999;274:36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- 133.Hamilton JW, Wetterhahn KE. Differential effects of chromium(VI) on constitutive and inducible gene expression in chick embryo liver in vivo and correlation with chromium(VI)-induced DNA damage. Mol Carcinog. 1989;2:274–286. doi: 10.1002/mc.2940020508. [DOI] [PubMed] [Google Scholar]
- 134.Shumilla JA, Wetterhahn KE, Barchowsky A. Inhibition of NF-kappa B binding to DNA by chromium, cadmium, mercury, zinc, and arsenite in vitro: evidence of a thiol mechanism. Arch Biochem Biophys. 1998;349:356–362. doi: 10.1006/abbi.1997.0470. [DOI] [PubMed] [Google Scholar]
- 135.Wetterhahn KE, Hamilton JW, Aiyar J, Borges KM, Floyd R. Mechanism of chromium(VI) carcinogenesis. Reactive intermediates and effect on gene expression. Biol Trace Elem Res. 1989;21:405–411. doi: 10.1007/BF02917282. [DOI] [PubMed] [Google Scholar]
- 136.Vilcheck SK, Ceryak S, O’Brien TJ, Patierno SR. FANCD2 monoubiquitination and activation by hexavalent chromium [Cr(VI)] exposure: activation is not required for repair of Cr(VI)-induced DSBs. Mutat Res. 2006;610:21–30. doi: 10.1016/j.mrgentox.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Castorina A, Tiralongo A, Cavallo D, Loreto C, Carnazza ML, Iavicoli S, D’Agata V. Expression profile of ErbB receptor’s family in human alveolar type 2-like cell line A549 exposed to hexavalent chromium. Toxicol In Vitro. 2008;22:541–547. doi: 10.1016/j.tiv.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 138.Barchowsky A, O’Hara KA. Metal-induced cell signaling and gene activation in lung diseases. Free Radic Biol Med. 2003;34:1130–1135. doi: 10.1016/s0891-5849(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 139.Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am J Respir Cell Mol Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 140.Chen L, Ovesen JL, Puga A, Xia Y. Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ Health Perspect. 2009;117:1124–1130. doi: 10.1289/ehp.0800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- 142.O’Hara KA, Klei LR, Barchowsky A. Selective activation of Src family kinases and JNK by low levels of chromium(VI) Toxicol Appl Pharmacol. 2003;190:214–223. doi: 10.1016/s0041-008x(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 143.Nemec AA, Barchowsky A. Signal transducer and activator of transcription 1 (STAT1) is essential for chromium silencing of gene induction in human airway epithelial cells. Toxicol Sci. 2009;110:212–223. doi: 10.1093/toxsci/kfp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Papaioannou AI, Kostikas K, Kollia P, Gourgoulianis KI. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res. 2006;7:128. doi: 10.1186/1465-9921-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 146.Kishiro I, Kato S, Fuse D, Yoshida T, Machida S, Kaneko N. Clinical significance of vascular endothelial growth factor in patients with primary lung cancer. Respirology. 2002;7:93–98. doi: 10.1046/j.1440-1843.2002.00376.x. [DOI] [PubMed] [Google Scholar]
- 147.Kaya A, Ciledag A, Gulbay BE, Poyraz BM, Celik G, Sen E, Savas H, Savas I. The prognostic significance of vascular endothelial growth factor levels in sera of non-small cell lung cancer patients. Respir Med. 2004;98:632–636. doi: 10.1016/j.rmed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 148.Cressey R, Wattananupong O, Lertprasertsuke N, Vinitketkumnuen U. Alteration of protein expression pattern of vascular endothelial growth factor (VEGF) from soluble to cell-associated isoform during tumourigenesis. BMC Cancer. 2005;5:128. doi: 10.1186/1471-2407-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao N, Jiang BH, Leonard SS, Corum L, Zhang Z, Roberts JR, Antonini J, Zheng JZ, Flynn DC, Castranova V, Shi X. p38 Signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J Biol Chem. 2002;277:45041–45048. doi: 10.1074/jbc.M202775200. [DOI] [PubMed] [Google Scholar]
- 150.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 151.Nemec AA, Zubritsky LM, Barchowsky A. Chromium(VI) Stimulates Fyn to Initiate Innate Immune Gene Induction in Human Airway Epithelial Cells. Chem Res Toxicol. 2009 doi: 10.1021/tx900365u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 153.Gimeno R, Lee CK, Schindler C, Levy DE. Stat1 and Stat2 but not Stat3 arbitrate contradictory growth signals elicited by alpha/beta interferon in T lymphocytes. Mol Cell Biol. 2005;25:5456–5465. doi: 10.1128/MCB.25.13.5456-5465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanabe Y, Nishibori T, Su L, Arduini RM, Baker DP, David M. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174:609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- 155.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 156.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 157.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 158.Crowell JA, Steele VE. AKT and the phosphatidylinositol 3-kinase/AKT pathway: important molecular targets for lung cancer prevention and treatment. J Natl Cancer Inst. 2003;95:252–253. doi: 10.1093/jnci/95.4.252. [DOI] [PubMed] [Google Scholar]
- 159.Lee SH, Kim HS, Park WS, Kim SY, Lee KY, Kim SH, Lee JY, Yoo NJ. Non-small cell lung cancers frequently express phosphorylated Akt; an immunohistochemical study. APMIS. 2002;110:587–592. doi: 10.1034/j.1600-0463.2002.11007811.x. [DOI] [PubMed] [Google Scholar]
- 160.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 163.Chen YL, Law PY, Loh HH. Inhibition of akt/protein kinase B signaling by naltrindole in small cell lung cancer cells. Cancer Res. 2004;64:8723–8730. doi: 10.1158/0008-5472.CAN-03-3091. [DOI] [PubMed] [Google Scholar]
- 164.Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lal MA, Bae D, Camilli TC, Patierno SR, Ceryak S. AKT1 mediates bypass of the G1/S checkpoint after genotoxic stress in normal human cells. Cell Cycle. 2009;8:1589–1602. doi: 10.4161/cc.8.10.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bae D, Camilli TC, Ha NT, Ceryak S. Enhanced clonogenic survival induced by protein tyrosine phosphatase (PTP) inhibition after Cr(VI) exposure is mediated by c-Raf and Ras activity. Cell Signal. 2009;21:727–736. doi: 10.1016/j.cellsig.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]