Abstract
TRIM family proteins are involved in a broad range of biological processes, and their alteration results in many diverse pathological conditions found in genetic diseases, viral infections, and cancers. However, the spatial and temporal expression and function of TRIM9, one of TRIM family proteins, remain obscure. Our results here showed that TRIM9 protein is mainly expressed in the cerebral cortex, and functions as an E3 ubiquitin ligase collaborating with an E2 ubiquitin conjugating enzyme UbcH5b. Immunohistochemical examination revealed that TRIM9 is localized to the neurons in the normal mouse and human brain and that TRIM9 immunoreactivity is severely decreased in the affected brain areas in Parkinson’s disease and dementia with Lewy bodies. This repressed level of TRIM9 protein was supported by immunoblotting analysis. Intriguingly, cortical and brainstem-type Lewy bodies were immunopositive for TRIM9. These results suggest that TRIM9 plays an important role in the regulation of neuronal functions and participates in pathological process of Lewy body disease through its ligase activity.
Keywords: α-Synuclein, Dementia with Lewy bodies, Parkinson’s disease, Tripartite motif protein 9 (TRIM9), Ubiquitin
Introduction
Ubiquitination is a post-translational modification of protein substrates by ubiquitin, which is a highly conserved 76-amino acid polypeptide. Ubiquitination is essential for regulating the turnover of proteins for biological events such as inflammation, cell proliferation, and apoptosis (Hershko and Ciechanover, 1998; Kim et al., 2008; Zuccato et al., 2007). This system is initiated by an ubiquitin-activating enzyme (E1), which activates and transfers ubiquitin to ubiquitin-conjugating enzymes (E2). Conjugation of target proteins with ubiquitin is then mediated by ubiquitin ligases (E3) (Hershko and Ciechanover, 1998). In contrast to E1 and E2 enzymes, E3s are highly diverse, and there are two types: HECT and RING finger types. The latter has the RING finger motif characterized as the cysteine- and histidine-rich domain (Freemont, 2000).
Tripartite motif (TRIM) proteins are composed of RING finger, B-box, and coiled-coil domains, and have been defined as a subgroup of RING finger proteins based on their conserved structure (Reymond et al., 2001). Recently, some members of TRIM proteins have been identified as an E3 enzyme, and play important roles in various cellular processes such as cell proliferation, tumorigenesis, and immune modification (Balint et al., 2004; Kong et al., 2007; Miyajima et al., 2009; Stremlau et al., 2004). However, the function of most TRIM proteins has not yet been defined.
TRIM9 belongs to TRIM family and its function remains unclear. In situ hybridization demonstrated that the expression of mouse TRIM9 mRNA is restricted to the central nervous system during the development from the embryo to the adult, but the distribution of TRIM9 protein in the nervous and non-nervous tissues remains unknown (Berti et al., 2002). We speculated that TRIM9 protein is predominantly expressed in the brain, and is a brain-specific E3 ligase. To address this possibility, in vitro ubiquitination assays, and biochemical and immunohistochemical analyses were performed. The results in our studies showed that TRIM9 has an E3 ligase activity and is highly expressed in the cerebral cortex. Based on the spatial expression of TRIM9, we further hypothesized that alterations of TRIM9 protein occur in pathological conditions affecting the cerebral cortex. Indeed, TRIM9 immunoreactivity was severely decreased in the affected brain areas in Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). Immunoblot analysis further revealed the reduction of TRIM9 expression in DLB brain. Intriguingly, cortical and brainstem-type Lewy bodies found in PD and DLB were immunopositive for TRIM9. This is the first demonstration of the role of TRIM9 involving the neurodegenerative disorders.
Materials and methods
Antibodies and reagents
Rabbit polyclonal antibodies against TRIM9 (ProteinTec Group, Inc., Chicago, IL), and actin (Sigma, Saint Louis, MO), and mouse monoclonal antibodies against hemagglutinin (HA)-epitope (Covance, Richmond, CA), Arginine-Glycine-Serine-polyHistidine (RH) (Qiagen, Santa Clara, CA), and ubiquitin (MBL, Woburn, MA) were used. The commercial anti-TRIM9 antibody was raised against a N-terminal peptide of human TRIM9 (1–350) and was designated TRIM9-N. In addition, we generated anti-human TRIM9 antiserum by immunizing rabbits with a GST-fused TRIM9 (corresponding to amino acids 440–665 of the C terminal of human TRIM9) and named it TRIM9-C. In order to demonstrate the specificity of TRIM9-C, the rabbit antiserum against GST-TRIM9 was preabsorbed with either GST or GST-TRIM9, and used for immunoblot and immunohistochemical analyses as a primary antibody. For this preabsorption, GST-TRIM9-coated beads were incubated with rabbit antiserum against TRIM9. After centrifugation, the supernatant was filtered and used for analyses with 1:500 dilutions.
Preparation of recombinant TRIM9
“Human TRIM9 cDNA” was purchased from Origene Company (Rockville, MD). Using this cDNA as a template, PCR-based, site-directed mutagenesis was applied to get a cDNA of human TRIM9α (GeneBank accession number: AF220036). Human TPIM9 isoform 2 cDNA was amplified from human fetal brain cDNA library using polymerase chain reaction, followed by DNA sequencing. Each TRIM9 cDNA was subcloned into the pcDNA3 vector (Invitogen, Carlsbad, CA) tagged with RGSHHHHHH at C-terminus. A plasmid was transfected into human embryonic kidney (HEK) 293T cells using Fugene 6 (BD Biosciences, San Jose, CA). TPIM9proteins were precipitated by TALON-beads system as described below, and used as recombinant proteins.
In vitro ubiquitination
We first expressed recombinant proteins in bacteria using pMAL-c4E or pGEX-5X1 vector (Amersham Pharmacia Biotech, Piscataway, NJ) as previously described (Yamauchi et al., 2008; Zhang et al., 2008). Amylose resin beads-immobilized MBP-TRIM9 or glutathione-sepharose beads-immobilized glutathione-S-transferase (GST)-TRIM9 was incubated with HA-ubiquitin, an E1 ubiquitin-activating enzyme (Boston Biochem, Cambridge, MA), and a poly-His-tagged E2 ubiquitin-conjugating enzyme in reaction buffer (50 mM Tris-HCl, pH 7.5, 2 mM ATP, 4 mM MgCl2, 2 mM dithiothreitol) for 30 min at 37 . After this reaction, the beads were washed with washing buffer (25 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.5% Nonidet P-40) and treated for 30 min at 50 in sample treatment solution containing 2% SDS and 5% β-mercaptoethanol. Finally the solubilized MBP-TRIM9 or GST-TRIM9 was analyzed by Western blotting, using antibody against HA-epitope to detect ubiquitinated TRIM9, and an antibody against MBP or GST to detect TRIM9.
Immunohistochemistry
Four-micrometer-thick, formalin-fixed, paraffin-embedded sections from the midbrain and pons of patients with PD (n=3) and multiple system atrophy (MSA) (n=3), and the temporal cortex and hippocampus of patients with DLB (n=3) and Alzheimer’s disease (AD) (n=3) were processed for immunohistochemistry. We also examined the midbrain, pons, temporal cortex and hippocampus from neurologically normal individuals (n=3). The sections were dehydrated, and pretreated with heat retrieval using an autoclave for 10 min in 10 mM citrate buffer, pH 6.0. The sections were then subjected to immunohistochemical processing using the avidin-biotin-peroxidase complex method with diaminobenzidine as the chromogen. TRIM9-N (diluted 1:100), TRIM9-C (diluted 1:100) and anti-phosphorylated α-synuclein (WAKO, Osaka, Japan; diluted 1:5,000) were used as a primary antibody. The sections were counterstained with hematoxylin.
Cell cultures, transfection and treatment
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and antibiotics. Plasmids were transfected into HEK293T cells using Fugene 6 (BD Biosciences). For inhibition of proteasomal activity, MG132 and epoxomicin were used (Calbiochem, San Diego, CA). (Meng et al., 1999; Vabulas and Hartl, 2005). After 24 h of transfection, the cells were treated with epoxomicin (1 μM) or MG132 (20 μM) for 12 h or 8 h, respectively.
Total tissue lysates from rodents and human subjects
The brain and various visceral organs were taken from the rats and mice at the indicated ages (embryonic day 16, and postnatal 1-, 2-, 3- and 12-week-old). All animal experiments were carried out in accordance with the Guidelines for Animal Experimentation, Hirosaki University Graduate School of Medicine, Japan. Human brain tissues were obtained from Brain Research Institute, University of Niigata, Japan. Brain tissues were dissected at autopsy and frozen rapidly at −70 . Middle temporal cortex from patients with DLB (n=3) and normal control subjects (n=3) were used in this study. Each tissue was weighted and homogenized with 20 volume of sample treatment solution containing 4% SDS and 5% β-mercaptoethanol.
Fractionation of brain extracts
Frozen tissues from the temporal cortex of patients with DLB (n=3) and control subjects (n=3) were weighted, and sequentially extracted with buffers of increasing strength using a protocol, which was previously described (Arai et al., 2006) but modified by us. In detail, samples were homogenized with 10 volumes of buffer A (10 mM Tris-HCl, pH 7.5, 1 mM EGTA, 10% sucrose, 0.8 M NaCl) and centrifuged (Fraction I). Afterwards, another equal volume of buffer A containing 2% Triton X-100 was added. It was then incubated for 30 min at 37 and spun at 100,000g for 30 min at 4 (Fraction II). The resultant pellet was homogenized in 5 volumes of buffer A, followed by an incubation for 30 min at 37 with 1% sarkosyl. The homogenate was then spun at 100,000g for 30 min at room temperature (Fraction III). The sarkosyl-insoluble pellet was homogenized in 4 volumes of buffer A containing 1% CHAPS and spun at 100,000g for 20 min at room temperature (Fraction IV). The pellet was sonicated in 0.2 volumes of 8 M urea buffer (fraction V).
TALON precipitation
In order to solubilize all derivatives of TRIM9-RH for biochemical analyses, we performed TALON-beads precipitation of TRIM9-RH using 6 M guanidine HCl as previously described (Tanji et al., 2006).
Western blot analysis
After SDS-polyacrylamide gel electrophoresis (SDS-PAGE), Western blot analysis was performed as previously described (Zhang et al., 2008). Transfer and detection were carried out according to the protocol provided with the ECL detection system (Amersham Pharmacia Biotech). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG or anti-rabbit IgG antibody (Santa Cruz Biotechnology) was used as a secondary antibody.
Statistics
All values were represented as means + standard deviation (SD). Statistical significance of the data was evaluated using analysis of variance, followed by post hoc test using the Fisher’s adjustment or the Student’s t-test when comparing two conditions. Probability values less than 0.05 (p < 0.05) were considered significant and less than 0.01 (p < 0.01) were highly significant.
Results
TRIM9 is an E3 ligase for itself
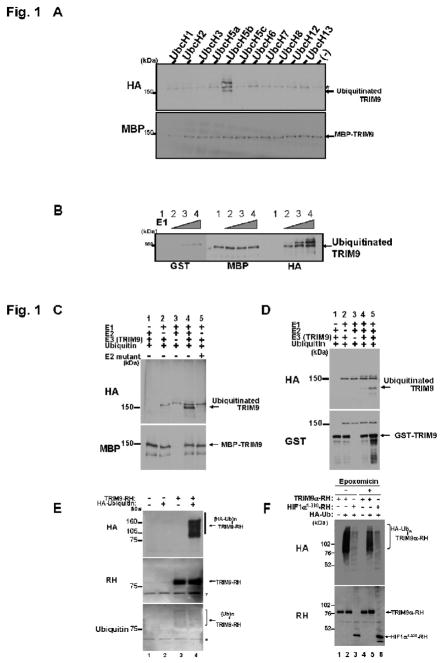
Because in vitro ubiquitination assay is free from possible contamination of other ubiquitin ligases, this allowed us to study the E3 activity of intrinsic TRIM9. Therefore we first performed this assay. Briefly human TRIM9 was fused with MBP purified with MBP-beads from E. Coli., and then incubated together with HA-ubiquitin, E1 ubiquitin-activating enzyme, and one of the E2 ubiquitin-conjugating enzymes in reaction buffer for 30 min at 37 . As shown in Fig. 1A, TRIM9 was ubiquitinated by itself when incubated with UbcH5b. In contrast, no ubiquitination was observed when incubated with other E2 enzymes. These results suggest that TRIM9 cooperates with UbcH5b for its self-ubiquitination. Next, we investigated E1 enzyme using the same assay. As shown in Fig. 1B, TRIM9 was ubiquitinated in accordance with increasing amount of E1 enzyme, suggesting that E1 enzyme is also involved in this self-ubiquitination. We further investigated E2 enzyme using an UbcH5b mutant (C85A), which has no activity due to substitution of Ala at active Cys-85. As shown in Fig. 1C, ubiquitination of TRIM9 was not detected in the presence of UbcH5b mutant (lane 5), indicating that active UbcH5b is required for this ubiquitination. To confirm that TRIM9 has an E3 ligase activity, we used GST-TRIM9 instead of MBP-TRIM9. GST-TRIM9 was ubiquitinated only in the presence of E1, E2 (ubcH5b) and E3 enzymes (Fig. 1D, lanes 4 and 5). This ubiquitination was enhanced with increasing amount of E3. Although ubiquitinated TRIM9 should be theoretically observed using anti-MBP or anti-GST antibody, we failed to detect it (Fig. 1C, lane 4; Fig. 1D, lanes 4 and 5). This may be due to limited percentage of ubiquitinated TRIM9 or low activity of ubiquitination in our in vitro ubiquitination assay. Subsequently, in vivo experiment was performed in cultured HEK293T cells. RH-tagged TRIM9 was expressed with or without HA-tagged ubiquitin. The cells were harvested and lysed under harsh conditions using 6 M guanidium chloride. Thereafter TRIM9-RH was precipitated by TALON beads, and applied to SDS-PAGE. Immunoblot analyses clearly showed that TRIM9 is poly-ubiquitinated in the presence of HA-ubiquitin (Fig. 1E, lane 4). Immunoblotting using anti-ubiquitin antibody showed a smear pattern of TRIM9-RH, suggesting that TRIM9 is ubiquitinated in mammalian cells. To determine whether ubiquitination of TRIM9 leads to degradation by the proteasome, proteasomal inhibitors were used. As a positive control for proteasomal degradation of ubiquitinated proteins, we used truncated HIF1α (corresponding to amino acids 1–330), because we had previously detected a clear effect of MG132 on the proteasomal degradation of this protein (Wada and Kamitani, 2006). As shown in Fig. 1F, treatment with the most selective proteasomal inhibitor, epoxomicin, increased the expression level of TRIM9 as well as of HIF1α (Fig. 1F, lane 5 versus lane 2, lane 6 versus lane 3). Another proteasomal inhibitor, MG132, similarly increased the level of TRIM9 expression (data not shown). Collectively, these results indicated that TRIM9 is an E3 ligase for its self-ubiquitination and that the ubiquitination of TRIM9 likely serves as a signal for proteasomal degradation.
Fig. 1.
In vitro ubiquitination assay. (A) Determination of E2 enzyme for ubiquitination of TRIM9. MBP-TRIM9 was incubated for 30 min at 37 together with HA-ubiquitin (400 μM), E1 enzyme and the indicated E2 enzymes (100 nM). After incubation, MBP-TRIM9 was solubilized and analyzed by Western blotting using anti-MBP to detect MBP-TRIM9 derivatives and anti-HA to detect ubiquitinated TRIM9. Ubiquitinated TRIM9 is seen only in the presence of UbcH5b as E2 enzyme. An asterisk represents a non-specific band. (B) Ubiquitination of TRIM9 in accordance with increasing amount of E1 enzyme. MBP-TRIM9 was incubated with various amount of E1 enzyme (lane 1, 0 nM; lane 2, 50 nM; lane 3, 100 nM; lane 4, 200 nM). Western blotting using anti-HA antibody shows that ubiquitinated TRIM9 is observed only in the presence of E1 enzyme (lanes 2, 3 and 4). (C) Inability of E2 mutant C85A for ubiquitination of TRIM9. Immunoblot using anti-HA antibody shows that TRIM9 is ubiquitinated in the presence of wild-type UbcH5b (lane 4), but not its mutant C85A (lane 5). (D) Ubiquitination of GST-TRIM9 protein. The complete reaction mixture (lanes 4 and 5) contains HA-ubiquitin, E1 enzyme, UbcH5b as an E2 enzyme, and GST-TRIM9 as a substrate and an E3 enzyme. After incubation, GST-TRIM9 was solubilized and analyzed by Western blotting using anti-GST to detect GST-TRIM9 derivatives and anti-HA to detect ubiquitinated TRIM9. Ubiquitinated TRIM9 is seen only in the presence of E1, E2 and E3 (lanes 4 and 5). Ubiquitination efficiency is enhanced in larger amount of E3 (500 nM in lane 5) than in smaller amount (200 nM in lane 4). (E) E3 activity of TRIM9 in mammalian cells. RH-tagged TRIM9 was expressed with or without HA-tagged ubiquitin in HEK293T cells by plasmid transfection. Twenty-four hours after transfection, the cells were harvested and lysed under denaturing conditions. TRIM9-RH in the lysate was precipitated with cobalt-coated TALON beads and analyzed by immunoblotting using anti-RH antibody to detect both non-ubiquitinated and ubiquitinated TRIM9-RH (middle panel), and anti-HA antibody to detect ubiquitinated TRIM9-RH (top panel). Anti-ubiquitin immunoblot also shows that TRIM9 is ubiquitinated by endogenous ubiquitin (bottom panel, lane 3). Asterisks represent non-specific bands. (HA-Ub)n or (Ub)n indicates poly-ubiquitination. (F) The effect of proteasome inhibitor on the level of TRIM9 protein. Anti-RH immunoblot shows that TRIM9 expression is slightly increased after treatment with 1 μM proteasome inhibitor epoxomicin (lane 5 versus lane 2). HIF1α is used as a positive control for proteasomal degradation. The level of HIF1α is also increased after treatment with a proteasome inhibitor (lane 6 versus lane 3).
TRIM9 localizes to neurons in the brain
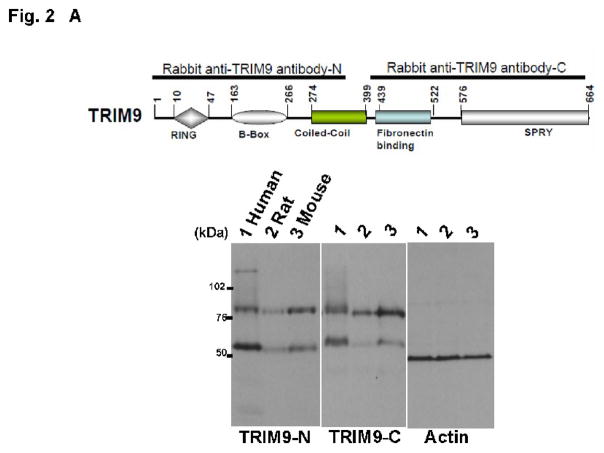
We used two polyclonal antibodies against TRIM9 (TRIM9-N and TRIM9-C) to explore the temporal and spatial expression of TRIM9 protein. TRIM9-N antibody recognized two positive bands in brain homogenates from human, mouse and rat (Fig. 2A). Like TRIM9-N, TRIM9-C also detected two positive bands in human, mouse and rat brains. Antigen-absorbed antibody diminished two positive bands in the same samples (data not shown). Thus, two positive bands were specifically recognized with TRIM9-N and TRIM9-C antibodies. A higher band of human TRIM9 (Fig. 2B, lane 3) migrated slower than human recombinant TRIM9α (Fig. 2B, lane 1). A lower band corresponded to human recombinant TRIM9 isoform 2 protein (Fig. 2B, lanes 2 and 3). Since similar results were obtained using both antibodies, TRIM9-C antibody was used for the following biochemical analyses.
Fig. 2.
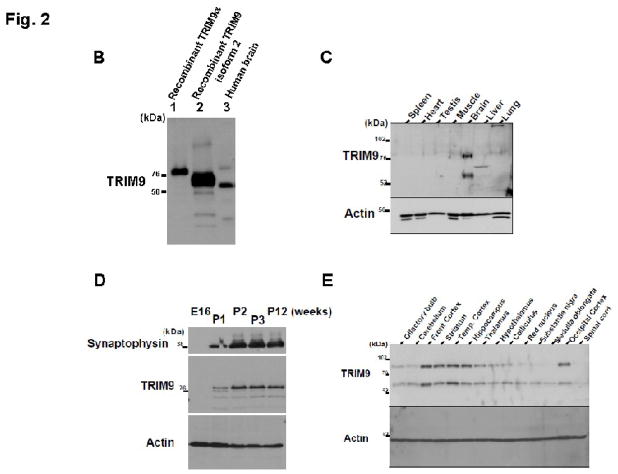
(A) A schematic representation of antigens of anti-human TRIM9α antibodies. TRIM9 possesses RING finger, B-box, coiled-coil, fibronectin-binding, and SPRY domains. Black bars indicate amino acids corresponding to antigens for polyclonal antibodies against TRIM9 protein. Lower panels show Western blotting using TRIM9-N and TRIM9-C antibodies. Both antibodies react with TRIM9 in human (lane 1), rat (lane 2) and mouse brain (lane 3). Note that two bands are seen in each lane. Actin is used as control for loading amount. (B) Comparison of recombinant and endogenous TRIM9 isoforms. Recombinant TRIM9α and isoform 2 were purified by TALON precipitation method from lysates of HEK293T cells after transfection. Immunoblot analysis shows that endogenous TRIM9 consists of two isoforms. Larger one migrates slower than recombinant human TRIM9α , whereas smaller one corresponds to isoform 2. (C) Spatial expression of TRIM9 protein in rodent tissues. Equal amounts of homogenates from the indicated rat tissues were analyzed by immunoblotting using TRIM9-C antibody. Specific signals are observed only in the brain tissue. Actin is used as control for loading amount. (D) Developmental expression of TRIM9 in the mouse brain. Mouse brain homogenates were prepared and equal amounts were analyzed by immunoblotting. Expression of TRIM9 is observed in the mouse brain at postnatal 1-week (P1) and older, but not at embryonic day 16 (E16). Synaptophysin is used as a marker of presynaptic protein. (E) Regional distribution of TRIM9 in the mouse brain. Equal amounts of homogenates from the indicated regions were analyzed by immunoblotting using TRIM9-C antibody. Besides the striatum and hippocampus, the highest expression of TRIM9 protein is seen in the frontal, temporal and occipital cortices.
The amino acid homology of human TRIM9 is higher than 99% among the primates. The amino acid sequence of human TRIM9 is even highly homologous to that of rodents (97.9%). Consistent with previous results (Li et al., 2001), we confirmed that the expression of rat TRIM9 is specific to the brain (Fig. 2C). Furthermore, we prepared mouse brain homogenates (embryonic day 16, and postnatal 1-, 2-, 3- and 12-week-old) to examine the developmental expression of TRIM9. Immunoblot analysis revealed that TRIM9 is detected in the mouse brain from 1-week-old after birth, whereas no positive signal was detected in the embryonic brain (Fig. 2D). Interestingly, a synaptic vesicle protein, synaptophysin, was also detected in the brain from 1-week-old after birth, suggesting that TRIM9 is expressed in accordance with synapse maturation.
To further examine the spatial expression of TRIM9, homogenates were prepared from several regions of the mouse brain and analyzed by immunoblot analysis. As shown in Fig. 2E, TRIM9 was strongly expressed in the frontal, temporal and occipital cortices, hippocampus, and striatum. In contrast, TRIM9 was weakly expressed in the thalamus, hypothalamus, colliculus, olfactory bulb and cerebellum. The expression level of TRIM9 was very low in the red nucleus, substantia nigra, medulla oblongata and spinal cord. These results were similar to previous reports, in which Berti et al. described the distribution of TRIM9 mRNA in the adult mice using in situ hybridization (Berti et al., 2002). Furthermore, immunohistochemical examination was performed to explore the localization of TRIM9 in the rodent and human brain. The cytoplasm and proximal dendrites of pyramidal neurons in the cerebral cortex and hippocampus, and Purkinje cells in the cerebellum of the mice (Fig. 3A–C) and human (Fig. 3D–F) were immunolabelled with both TRIM9-N and TRIM9-C antibodies. Neuronal nuclei were unstained or only weakly immunolabeled.
Fig. 3.
Immunohistochemical localization of TRIM9 in the mouse (A–C) and human brain (D–F). TRIM9 immunoreactivity is found in the neuronal cytoplasm and proximal dendrites of pyramidal neurons in the cerebral cortex (A, D) and hippocampus (B, E), and Purkinje cells in the cerebellum (C, F). Bars = 50 μm.
TRIM9 immunoreactivity in DLB and PD brain
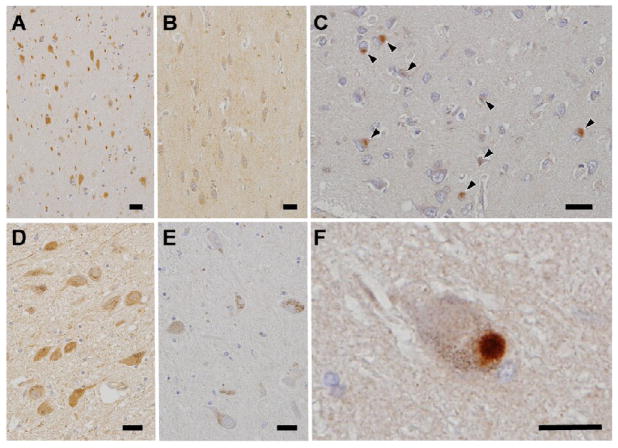
As previously described, the highest level of TRIM9 expression was observed in the cerebral cortex and hippocampus, which are the essential areas for the cognition, learning and memory (Squire, 1987). Based on this finding, we hypothesized that alterations of TRIM9 protein are found in neurodegenerative disorders affecting the cerebral cortex and hippocampus, i.e. neurodegenerative dementia. Since AD is the most common and DLB is the second most frequent dementia in the elderly, we examined the brain from these diseases. In addition to cerebral cortical atrophy, the occurrence of neurofibrillary tangles and senile plaques is a pathological feature of AD, whereas neuronal cytoplasmic inclusions called LBs are generated in DLB brain (Greenfield et al., 2002). To test our hypothesis described above, immunohistochemical examination was performed using brain sections from patients with AD and DLB, and control subjects. TRIM9 immunoreactivity in the neuronal cytoplasm was severely decreased in the temporal cortex and hippocampus in DLB, but not in AD, compared with controls (Fig. 4A, B). Intriguingly, the majority of cortical LBs were positive for TRIM9 (Fig. 4C), whereas neurofibrillary tangles and senile plaques were negative (data not shown). It is known that α-synuclein is a major component of LBs in LB disease (PD and DLB) as well as glial cytoplasmic inclusions in MSA. Thus, LB disease and MSA comprise a new disease concept, namely that of α-synucleinopathy (Spillantini et al., 1997; Wakabayashi et al., 1998). Therefore, TRIM9 immunohistochemistry was also applied to the brain sections from patients with PD and MSA. Although TRIM9 immunoreactivity in the neuronal cytoplasm in the substantia nigra was weaker than that in the cerebral cortex in controls (Fig. 4D), the immunoreactivity in the substantia nigra was markedly diminished in PD (Fig. 4D, E). In contrast, TRIM9 was localized to the brainstem-type LBs in PD (Fig. 4F). Serial sections stained with anti-TRIM9 and anti-α-synuclein antibodies revealed that approximately 80–90% of cortical and brainstem-type LBs were positive for TRIM9. In MSA, TRIM9 immunoreactivity was preserved in the brainstem and no positive signal was observed in glial cytoplasmic inclusions (data not shown).
Fig. 4.
Immunoreactivity of TRIM9 in the brain of controls (A, D) and patients with dementia with Lewy bodies (DLB) (B, C) and Parkinson’s disease (E, F). TRIM9 immunoreactivity in the neuronal cytoplasm is severely decreased in DLB temporal cortex (B) compared with controls (A). Decrease of TRIM9 immunoreactivity in the neuronal cytoplasm in PD substantia nigra (E) compared with controls (D). TRIM9 immunoreactivity in cortical Lewy bodies in the temporal lobe (C, arrowheads) and brainstem-type Lewy bodies in the substantia nigra (F). Bars = 20 μm.
The level of TRIM9 protein is repressed in DLB brain
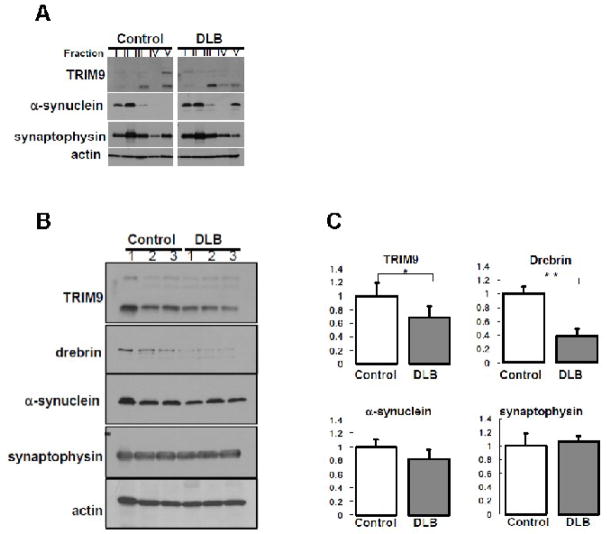
Previous report showed that α-synuclein is modified by ubiquitination, resulting in insoluble α-synuclein in DLB brain (Lippa et al., 1998). To examine the solubility of TRIM9, we fractionated the brain samples from DLB (n=3) and controls (n=3) by increasing the strength of detergent. As shown in Fig. 5A, immunoblotting showed that the solubility of α-synuclein in fraction V was changed in DLB brain, consistent with previous reports (Lippa et al., 1998). Importantly, the solubility of two isoforms (higher and lower bands) of TRIM9 protein in fraction V was also changed. In contrast to α-synuclein, the amount of higher band of insoluble TRIM9 was decreased in DLB, and the amount of lower band was also slightly decreased.
Fig. 5.
(A) Fractionation of human brain samples. Frozen tissues were obtained from the temporal cortex of patients with DLB (n=3) and control subjects (n=3). Sequential biochemical fractionation reveals that TRIM9 protein is composed of soluble and insoluble forms in terms of detergent solubility. The amount of higher band of insoluble TRIM9 and synaptophysin proteins is decreased in a patient with DLB compared with a control subject, whereas insoluble α-synuclein is up-regulated. The amount of lower band of insoluble TRIM9 seems to be decreased in DLB compared with control. Similar results were observed in other cases of DLB and control subjects. Actin is used as control for loading amount. (B) Comparison of expression level of TRIM9 protein in the brain of DLB and controls. Equal amounts of homogenates from human brain were analyzed by immunoblotting using antibodies against TRIM9-C (top), drebrin (second top), α-synuclein (middle), synaptophysin (second bottom), and actin (bottom). The amount of higher and lower bands of TRIM9, drebrin and α-synuclein is decreased in DLB compared with controls. In contrast, the level of synaptophysin is not affected in DLB. Actin is used as control for loading amount. (C) Quantification of the amount of lower band of TRIM9 protein in the brain of DLB and controls. Data are normalized by actin protein level in each sample and indicated by mean + SD. Quantitative analysis shows that the amount of lower band of TRIM9 or drebrin is significantly repressed up to 70% or 40%, respectively, whereas the level of synaptophysin remains stable. * p<0.05, ** p<0.01.
Finally, total amount of TRIM9 protein was assessed. Immunoblot analysis revealed that the level of TRIM9 protein in DLB brain was significantly decreased up to 70% compared with controls (Fig. 5B and C). Synaptophysin known as a marker of presynapse remained stable in the protein level. Consistent with previous reports (Kramer and Schulz-Schaeffer, 2007), the level of postsynaptic marker protein (drebrin), and α-synuclein was decreased in DLB brain. These findings suggest that biochemical alterations of TRIM9 protein occur in the brain of patients with DLB.
Discussion
In order to uncover the function of TRIM9 protein, we initially paid attention to the amino acid structure of TRIM9. In addition to the conserved domains such as RING finger, B-box, and coiled-coil structures in all TRIM family proteins, TRIM9 possesses fibronectin-binding and SPRY domains. Based on the conserved structures and recent evidences that several TRIM proteins have been identified as E3 ubiquitin ligase (Chen et al., 2007; Eldin et al., 2009; Gack et al., 2007; Kallijarvi et al., 2005; Wada and Kamitani, 2006; Yamauchi et al., 2008), we hypothesized that TRIM9 also functions as an E3 ubiquitin ligase. To test the hypothesis, we performed in vitro ubiquitination assay and clearly showed that TRIM9 acts as an E3 ligase and ubiquitinates itself in cooperation with an E2 enzyme UbcH5b. In addition, treatment of proteasomal inhibitor up-regulated the level of TRIM9 protein, suggesting that the ubiquitination of TRIM9 likely serves as a targeting signal for proteasomal degradation. Intriguingly, the ubiquitination pattern of TRIM9 differed between in vivo and in vitro systems; TRIM9 protein was poly-ubiquitinated in cultured cells, whereas it was mono-ubiquitinated in vitro. Immunoblot analysis using anti-TRIM9 antibodies demonstrated the presence of endogenous TRIM9 with a smear pattern in the human brain (Fig. 2A) and anti-ubiquitin antibody detected TRIM9 with high-molecular weight in cultured cells (Figs. 1E and 1D), implying that endogenous TRIM9 is poly-ubiquitinated. It is known that several E3 ligases have an activity of ubiquitin assembly, which is called as ubiquitin elongase or E4 enzyme (Koegl et al., 1999). Similar to TRIM9, parkin protein is also poly-ubiquitinated in cultured cells (Chung et al., 2004; Shimura et al., 2000; Sriram et al., 2005; Staropoli et al., 2003) and is mono-ubiquitinated in vitro (Hampe et al., 2006; Matsuda et al., 2006). In case of incubation together with CHIP or Mdm2 in vitro, poly-ubiquitination occurred in parkin protein (Hampe et al., 2006). Thus, these reports raise the possibility that TRIM9 protein requires additional enzymes to be poly-ubiquitinated. Identification of interactive proteins will help to gain insight into the unveiled mechanism underlying the ubiquitination of TRIM9 protein.
Immunoblot analysis of the mouse brain showed that the amount of TRIM9 protein is higher in the cerebral cortex and hippocampus, which are the areas responsible for cognitive, learning, and memory functions. Based on the distribution of TRIM9, we hypothesized that pathological changes of TRIM9 are found in the brain of patients with dementia. In this study, we investigated whether TRIM9 immunoreactivity was altered in the brain of neurodegenerative dementia (AD and DLB). Immunohistochemical examinations revealed that TRIM9 immunoreactivity in the neuronal cytoplasm was severely decreased in the temporal cortex and hippocampus in DLB compared with controls. TRIM9 immunoreactivity was also decreased in the substantia nigra in PD patients. Thus, TRIM9 immunoreactivity is decreased in the affected brain areas in LB disease (PD and DLB). Furthermore, the majority of cortical and brainstem-type LBs were TRIM9 immunoreactive, but neurofibrillary tangles and senile plaques in AD and glial cytoplasmic inclusions in MSA were negative for TRIM9. These findings suggest that TRIM9 has some roles in the formation or degradation of LBs in PD and DLB. LBs are composed of ubiquitinated proteins, including α-synuclein, parkin, and synphilin-1 (Schlossmacher et al., 2002; Spillantini et al., 1997; Wakabayashi et al., 2000). Considering that TRIM9 has an activity of E3 ligase, it is conceivable that TRIM9 may ubiquitinate several proteins in the brain of patients with PD and DLB. Further studies are needed to address this possibility.
Given the fact that rat TRIM9 was identified as an interactive protein with presynaptic protein, SNAP25, it has been believed that TRIM9 is a presynaptic protein. Here, we report for the first time that TRIM9 is predominantly expressed in the neuronal cytoplasm and proximal dendrites in the brain of human and rodents. Golgi staining clearly demonstrated that the dendritic spines are affected in the cerebral cortex of patients with DLB (Kramer and Schulz-Schaeffer, 2007). Indeed, our immunoblotting analysis confirmed that the level of drebrin, a marker of dendritic spine, was significantly repressed in DLB brain. It is noteworthy that the level of total and insoluble TRIM9 protein is also decreased in DLB brain. This is supported by the immunohistochemical finding that TRIM9 immunoreactivity is decreased in DLB brain. However, this phenomenon does not seem to be specific to DLB, since recent proteomic technique demonstrated that the repressed level of TRIM9 is also observed in the brain infected with rabies virus (Dhingra et al., 2007). Infection of rabies virus in the brain likely causes degeneration of the dendrites and presynapses, resulting in severe clinical features with a fatal outcome (Scott et al., 2008). Collectively, these findings imply that disturbance of the expression level and solubility of TRIM9 leads to the onset of neurological symptoms in certain pathological conditions.
In conclusion, our findings offer the evidence that TRIM9 protein is a brain-specific E3 ubiquitin ligase. Importantly, TRIM9 is present in LBs found in DLB and PD, suggesting that TRIM9 not only plays roles in the regulation of neuronal functions, but also participates in the formation or breakdown of abnormal inclusions through its ligase activity.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.T., F.M. and K.W.), a Grant for Hirosaki University Institutional Research (to K.W.), a Grant-in-Aid for Studies on the Development of Diagnostic Technique and Therapies for Lewy Body Disease, the Ministry of Health, Labour and Welfare, Japan (to K.W.), and a National Institutes of Health Grant R01AG024497 (to T.K.). The authors wish to express their gratitude to M. Nakata for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Balint I, Muller A, Nagy A, Kovacs G. Cloning and characterisation of the RBCC728/TRIM36 zinc-binding protein from the tumor suppressor gene region at chromosome 5q22.3. Gene. 2004;332:45–50. doi: 10.1016/j.gene.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Berti C, Messali S, Ballabio A, Reymond A, Meroni G. TRIM9 is specifically expressed in the embryonic and adult nervous system. Mech Dev. 2002;113:159–162. doi: 10.1016/s0925-4773(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Chen D, Gould C, Garza R, Gao T, Hampton RY, Newton AC. Amplitude control of protein kinase C by RINCK, a novel E3 ubiquitin ligase. J Biol Chem. 2007;282:33776–33787. doi: 10.1074/jbc.M703320200. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Dhingra V, Li X, Liu Y, Fu ZF. Proteomic profiling reveals that rabies virus infection results in differential expression of host proteins involved in ion homeostasis and synaptic physiology in the central nervous system. J Neurovirol. 2007;13:107–117. doi: 10.1080/13550280601178226. [DOI] [PubMed] [Google Scholar]
- Eldin P, Papon L, Oteiza A, Brocchi E, Lawson TG, Mechti N. TRIM22 E3 ubiquitin ligase activity is required to mediate antiviral activity against encephalomyocarditis virus. J Gen Virol. 2009;90:536–545. doi: 10.1099/vir.0.006288-0. [DOI] [PubMed] [Google Scholar]
- Freemont PS. RING for destruction? Curr Biol. 2000;10:R84–87. doi: 10.1016/s0960-9822(00)00287-6. [DOI] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Greenfield JG, Graham DI, Lantos PL. Greenfield’s neuropathology. Arnold; London: 2002. pp. 196–271. [Google Scholar]
- Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O. Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet. 2006;15:2059–2075. doi: 10.1093/hmg/ddl131. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Kallijarvi J, Lahtinen U, Hamalainen R, Lipsanen-Nyman M, Palvimo JJ, Lehesjoki AE. TRIM37 defective in mulibrey nanism is a novel RING finger ubiquitin E3 ligase. Exp Cell Res. 2005;308:146–155. doi: 10.1016/j.yexcr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Anderson DE, Lee CH, Jang MK, Tamura T, Tailor P, Cho HK, Cheong J, Xiong H, Morse HC, 3rd, Ozato K. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF-8 and enhances cytokine expression in macrophages. J Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chin LS, Weigel C, Li L. Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J Biol Chem. 2001;276:40824–40833. doi: 10.1074/jbc.M106141200. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O'Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Kitami T, Suzuki T, Mizuno Y, Hattori N, Tanaka K. Diverse effects of pathogenic mutations of Parkin that catalyze multiple monoubiquitylation in vitro. J Biol Chem. 2006;281:3204–3209. doi: 10.1074/jbc.M510393200. [DOI] [PubMed] [Google Scholar]
- Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N, Maruyama S, Nonomura K, Hatakeyama S. TRIM36 interacts with the kinetochore protein CENP-H and delays cell cycle progression. Biochem Biophys Res Commun. 2009;381:383–387. doi: 10.1016/j.bbrc.2009.02.059. [DOI] [PubMed] [Google Scholar]
- Perry RH, Irving D, Tomlinson BE. Lewy body prevalence in the aging brain: relationship to neuropsychiatric disorders, Alzheimer-type pathology and catecholaminergic nuclei. J Neurol Sci. 1990;100:223–233. doi: 10.1016/0022-510x(90)90037-n. [DOI] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher MG, Frosch MP, Gai WP, Medina M, Sharma N, Forno L, Ochiishi T, Shimura H, Sharon R, Hattori N, Langston JW, Mizuno Y, Hyman BT, Selkoe DJ, Kosik KS. Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am J Pathol. 2002;160:1655–1667. doi: 10.1016/S0002-9440(10)61113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CA, Rossiter JP, Andrew RD, Jackson AC. Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol. 2008;82:513–521. doi: 10.1128/JVI.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and brain. Oxford University Press; New York: 1987. [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, Dawson VL, Dawson TM. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Tanji K, Tanaka T, Mori F, Kito K, Takahashi H, Wakabayashi K, Kamitani T. NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1. Am J Pathol. 2006;169:553–565. doi: 10.2353/ajpath.2006.051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- Wada K, Kamitani T. Autoantigen Ro52 is an E3 ubiquitin ligase. Biochem Biophys Res Commun. 2006;339:415–421. doi: 10.1016/j.bbrc.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H. Synphilin-1 is present in Lewy bodies in Parkinson’s disease. Ann Neurol. 2000;47:521–523. [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Wada K, Tanji K, Tanaka M, Kamitani T. Ubiquitination of E3 ubiquitin ligase TRIM5 alpha and its potential role. Febs J. 2008;275:1540–1555. doi: 10.1111/j.1742-4658.2008.06313.x. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Tanji K, Mori F, Wakabayashi K. Epitope mapping of 2E2–D3, a monoclonal antibody directed against human TDP-43. Neurosci Lett. 2008;434:170–174. doi: 10.1016/j.neulet.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Zuccato E, Blott EJ, Holt O, Sigismund S, Shaw M, Bossi G, Griffiths GM. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci. 2007;120:191–199. doi: 10.1242/jcs.03315. [DOI] [PubMed] [Google Scholar]