Abstract
Neural stem cells (NSCs) in the adult brain continuously supply new neurons to the hippocampal dentate gyrus (DG) and the olfactory bulb (OB). Recent studies indicate that the progression from neural precursor cells (NPCs) to mature neurons is tightly controlled by coordinate cell-intrinsic programs and external signals within the neurogenic niche. In this review, we summarize both classes of regulatory factors involved in distinct stages of adult neurogenesis, including proliferation and lineage differentiation of NSCs, migration of neuroblasts and integration of newborn neurons. A full understanding of the wide variety of signaling pathways will ultimately provide precise targets for therapeutic applications.
Introduction
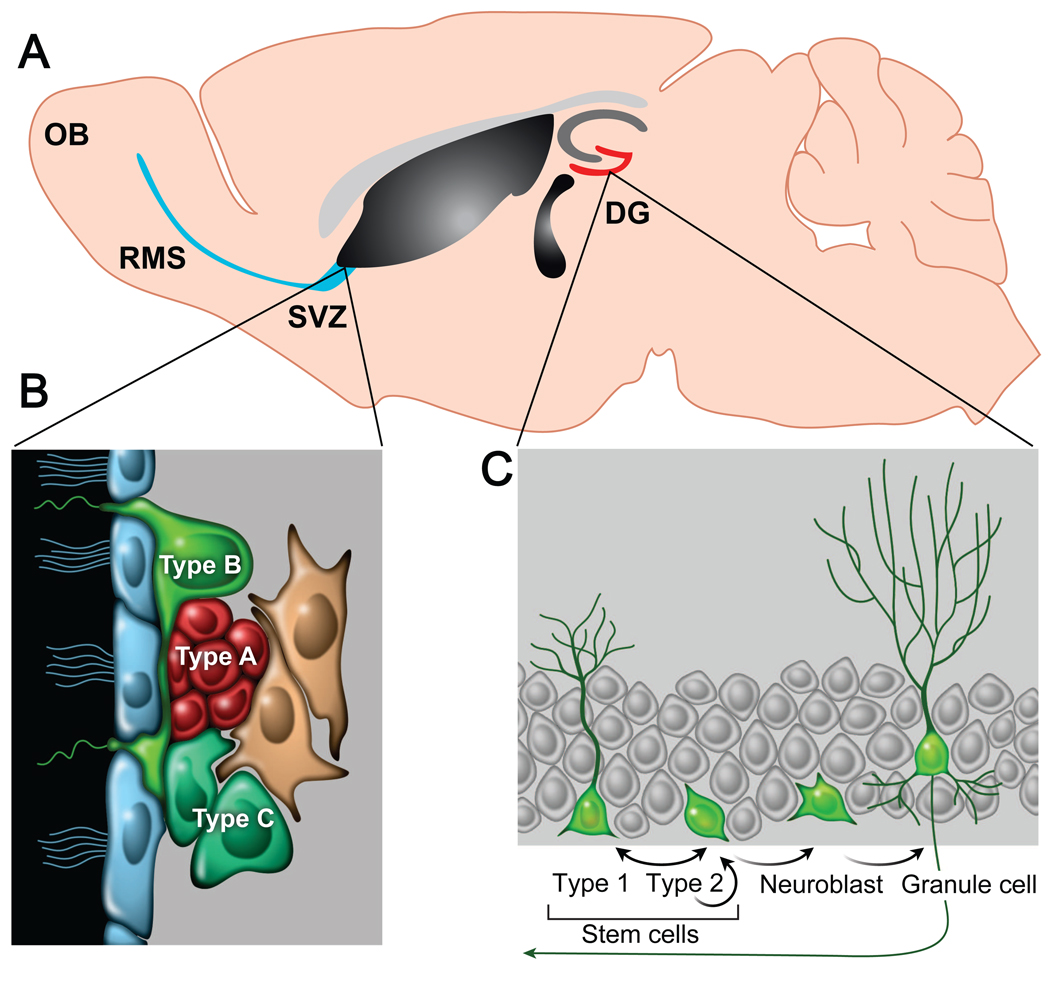
Over the last two decades, it has become apparent that persistent neurogenesis throughout life occurs in two specific brain areas of adult mammals: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the DG (Figure 1a) [1–4]. The newborn neuronal cells originate from adult NSCs in the germinal zones, which are defined by their ability to self-replicate and differentiate into multiple neural lineages, including neurons, astrocytes, and oligodendrocytes [5]. Two types of NSCs have been identified based on their morphology, proliferative behavior and marker expression, although their origin and identity remain to be defined (Figure 1b and c) [6–8]. In the SVZ, slowly-dividing, radial glia-like progenitors (type B cells) that express GFAP and CD133 have been hypothesized to be the primary NSCs in vivo. They are hypothesized to generate rapidly dividing, transit-amplifying progenitors (type C cells) that typically have either no or a very short process and are characterized as positive for Dlx2, Mash1, and EGFR. The majority of these intermediate progenitors subsequently give rise to DCX+ PSA-NCAM+ neuroblasts (type A cells) that migrate into the OB through the rostral migratory stream (RMS) and differentiate into GABA- and dopamine-producing interneurons. In parallel, a population of GFAP+ Sox2+ Nestin+ radial cells (type 1 cells) is found to act as quiescent NSCs in the SGZ. They may generate actively self-renewing nonradial progenitors (type 2 cells) expressing Sox2 and Nestin but not GFAP, and type 2 cells in turn give rise to DCX+ neuroblasts that predominantly differentiate into local glutamatergic dentate granule cells (DGCs). It was recently found that a subset of type 2 Sox2+ cells has the potential to self-renew and generate both neurons and astrocytes, indicating a possible reciprocal lineage relationship between type 1 and 2 cells in the SGZ [9].
Figure 1.
Neurogenesis in the adult brain. (a) Adult NSCs are primarily located in two germinal zones of the brain: the SVZ of the lateral ventricles and the SGZ of hippocampal DG. (b) A subset of relatively quiescent GFAP+ radial cells (type B cells) in the SVZ has the potential to serve as adult NSCs and generate rapidly dividing, transit-amplifying nonradial NSCs (type C cells), which in turn give rise to neuroblasts (type A cells) that migrate through the RMS toward the OB. (c) In the adult SGZ, a population of GFAP+ Sox2+ radial cells corresponds to quiescent NSCs (type 1 cells). They coexist with actively proliferating, GFAP- Sox2+ nonradial NSCs (type 2 cells) that generate both astrocytes and neuroblasts. Neuroblasts then migrate into the granule cell layer and mature into neurons.
Although NSCs have been derived from a variety of adult brain areas, active neurogenesis seems to be restricted to SVZ and SGZ under physiological conditions in vivo. Committed NPCs from SVZ differentiate into glia when grafted outside their normal neurogenic environment [10], whereas glial progenitors derived from spinal cord generate neurons when transplanted into the DG [11]. Furthermore, adult hippocampal NPCs grafted into RMS differentiate appropriately into neurons of non-hippocampus phenotype, whereas those grafted into nonneurogenic sites showed no neuronal differentiation [12]. These transplantation experiments clearly demonstrate that the microenvironment or neurogenic niche plays a crucial role in determining where and how neurogenesis can occur. However, it is also important to recognize that, even within the germinal zones of the adult brain, only a subset of marker-expressing cells are capable of generating neurons. Besides, the populations of adult NSCs are inherently diverse in their nature, as evidenced by the fact that NSCs from different regions of SVZ produce different neuronal subtypes, even when heterotopically grafted or grown in culture [13]. Thus, the progression from NSCs to mature neurons is subject to a tightly coordinated control by a multitude of cell-intrinsic and extracellular factors. Here we review recent progress in our understanding of the molecular mechanisms regulating the developmental steps of neurogenesis in the adult hippocampus and forebrain, including proliferation and fate specification of NPCs, their subsequent migration, and functional maturation.
Signals directing proliferation and fate commitment of NSCs
The extracellular signaling mechanisms that are present in the microenvironments of the SVZ and SGZ provide them with the unique ability to support and promote neurogenesis. (See Table 1) Signaling molecules that are critical during embryonic development of the nervous system are conserved and continue to modulate NSC activity and adult neurogenesis. The Wnt signaling pathway influences NSC proliferation and differentiation during embryonic development. Recent studies have also identified the Wnt signaling pathway as a key regulator of adult hippocampal neurogenesis. NSCs in the adult hippocampus express several Wnts as well as corresponding receptors, and therefore receive Wnt signals produced not only from astrocytes but also from themselves [14,15]. The autocrine signaling of Wnt supports the proliferation and multipotency of NSCs through the canonical pathway involving GSK3β/β-catenin [15]. Activation of the Wnt/β-catenin pathway increases expression of NeuroD1, which is known to promote neuronal differentiation in NSCs. Additionally, in response to Wnt, NSCs increase expression of LINE-1, a retrotransposon important in NSC survival [16]. These findings demonstrate the involvement of the Wnt signaling pathway in all aspects of adult neurogenesis.
Table 1.
| Summary of signaling in adult neurogenesis | References | |
|---|---|---|
| Proliferation | ||
| miR-124 | decreases proliferation | 28 |
| Shh | increases proliferation | 17–18 |
| Sox2 | increases proliferation | 24–25 |
| Tlx | increases proliferation | 19–23 |
| Wnt | increases proliferation | 14–15 |
| Differentiation | ||
| Transcription factors | ||
| Ascl1 | overexpression generates oligodendrocytes | 34 |
| expressed in NSCs to produce GABAergic interneurons in OB | 30 | |
| expressed in NSCs to produce glutamatergic neurons in hippocampus | 30 | |
| Neurog2 | expressed in NSCs to produce glutamatergic neurons in hippocampus | 33 |
| expressed in NSCs to produce glutamatergic neurons in svz | 31 | |
| Tbr2 | expressed in NSCs to produce glutamatergic neurons in svz | 31 |
| Epigenetic mechanisms | ||
| Gadd45b | necessary for dendritic arborization | 37 |
| MBD1 | necessary for neuronal differentiation | 35 |
| MeCP2 | necessary for neuronal maturation | 37 |
| Mll1 | necessary for neuronal differentiation in svz | 38 |
| Migration | ||
| IGF-1 | necessary for neuroblast migration | 45 |
| Shh | necessary for neuroblast migration | 43 |
| Integration | ||
| Extrinsic factors | ||
| BDNF | increases neuronal survival and dendritic arborization | 57–58 |
| FGF-2 | necessary for synaptic plasticity | 59 |
| GABA | necessary for dendritic arborization and synapse formation | 55–56 |
| Glutamate | necessary for neuronal survival and synaptic plasticity | 48,50,–51 |
| NT-3 | necessary for synaptic plasticity | 60 |
| Intrinsic factors | ||
| Cdk | necessary for neuronal survival and dendritic arborization | 63–64 |
| CREB | increases neuronal survival and dendritic arborization | 61–62 |
| DISC1 | decreases synaptic integration | 65 |
| Klf-9 | increases synaptic integration | 66 |
| NeuroDI | necessary for neuronal survival and maturation | 67 |
Sonic hedgehog (Shh) has been studied extensively for its role in the developing nervous system, and it is now known to also be important in regulating the activity of adult NSCs, specifically acting as a mitogen and increasing proliferation [17]. The effects of Shh are mediated by primary cilia found on the NSCs and thus dependent on Kif3a, an essential motor for assembling primary cilia. Mice with a conditional loss of primary cilia on NSCs exhibit significantly reduced levels of proliferation in the DG, which is accompanied by a loss of Shh signaling. Disruption of Shh signaling by a conditional loss of Smoothened (Smo), a downstream target of Shh, results in a similar phenotype with reduced NSC proliferation [18].
In addition to the extracellular cues within the microenvironment of SVZ and SGZ, cell-intrinsic mechanisms comprise another major component of control over the process of adult neurogenesis. An orphan nuclear receptor, Tlx, plays an essential role in maintenance and self-renewal of adult NSCs [19–21], presumably by complexing with histone deacetylases (HDACs) to repress cell cycle genes p21 and PTEN [22]. It was recently revealed that Tlx can activate the expression of Wnt7a and the canonical Wnt/β-catenin pathway, suggesting the potential of NSCs to regulate their own self-renewal by an autocrine-signaling loop [23].
The Sox family of genes, which encode for transcription factors, plays a prominent role in NSC activity, as demonstrated by the expression of Sox2 in NSCs. Additional functions of Sox2 include the regulation of NSC maintenance or proliferation, possibly by repressing GFAP transcription [24]. Adult mice bearing a Sox2 regulatory mutant allele exhibit a substantial decrease in NPC proliferation and generation of new neurons in both SVZ and SGZ [25]. The absence of Sox2 in the NSCs leads to abnormal neuronal differentiation, with the development of shorter and fewer processes and reduced expression of mature neuronal genes. Interestingly, Sox2 is necessary for neuronal differentiation only in the early stages, as expression of Sox2 during this stage rescues the arborizations and maturation of the neurons [24].
Furthermore, a member of the Sox family has been recently implicated in regulating neurogenesis through its interaction with microRNAs (miRNAs). miRNAs are endogenous, non-coding RNAs that regulate gene expression [26]. The microRNA miR-124, found abundantly in the brain [27], and its target gene Sox9 regulate neurogenesis in the SVZ. Expression of miR-124 is found in low levels in the SVZ, but miR-124 is elevated strictly in neuroblasts isolated from this region and increases with neuronal differentiation. The generation of neuroblasts from type C cells and neuronal differentiation in the adult SVZ requires downregulation of the transcription factor Sox9 by miR-124 [28]. In contrast, miR-124 overexpression or knockdown in the chick neural tube model has no effect on NPC proliferation and neuronal differentiation [29], suggesting that the role of miR-124 may be different depending on the developing and adult nervous system. miRNAs represent an exciting new member of the intrinsic factors that modulate adult neurogenesis.
The fate commitment of adult NSCs is sequentially controlled by a family of proneural proteins called basic helix-loop-helix (bHLH) transcription factors, whose functions are largely conserved during embryonic and adult neurogenesis. In the SVZ, Ascl1 (Mash1) is expressed in type C cells destined to become GABAergic interneurons in the OB [30], whereas Neurog2 and Tbr2 are found in a subset of dorsal SVZ progenitors that produce glutamatergic juxtaglomerular neurons [31]. In agreement with these in vivo analyses, all progeny of in vitro expanded adult NSCs of SVZ acquire a glutamatergic identity when forced to express Neurog2, whereas only GABAergic neurons are generated upon expression of Mash1 [32]. Similarly, Ascl1 and Neurog2 are transiently expressed by adult neural progenitors in the hippocampal neurogenic niche that mature into glutamatergic DGCs [30,33]. Interestingly, retrovirus-mediated overexpression of Ascl1 in the proliferating adult hippocampal NSCs causes them to exclusively generate cells of oligodendrocytic lineage instead of neurons [34], suggesting that expression of a single gene may direct fate choice of adult NSCs in their in vivo niche.
There is also increasing evidence that epigenetic mechanisms, such as DNA methylation, chromatin remodeling, histone modification and non-coding RNA expression, are closely associated with multiple aspects of adult neurogenesis. Adult mice lacking MBD1, a member of the methylated DNA-binding protein family, have decreased neurogenesis in the DG [35], whereas those with MeCP2 deletion have normal production but deficient maturation of newborn neurons [36]. Knockout of Gadd45b, a protein required for activity-induced DNA demethylation, is found to compromise both activity-induced proliferation of neural progenitors and dendritic growth of newborn neurons in the adult hippocampus [37]. Similarly, a chromatin remodeling factor Mll1 is required for neuronal differentiation of adult NSCs in the SVZ [38]. Furthermore, inhibition of HDACs induces neuronal differentiation of adult hippocampal neural progenitors [39], and a deficiency in the MYST family of histone acetyltransferases reduces the ability of SVZ NSCs to self-renew and differentiate [40]. Taken together, these findings highlight the complexity and specificity of the control over the process of adult neurogenesis.
Neuroblast migration from the SVZ to the OB
Neuroblasts generated in the SVZ migrate along the RMS by chain migration to the OB [41], where they differentiate into GABAergic neurons that integrate into the preexisting circuits of the granule cell layer and contribute to olfactory learning [42]. The proper migration of the neuroblast from the germinal zone to their target destination underlies the ability of the newborn neurons to populate the OB. Recent studies have identified Shh as playing a crucial role in neuroblast migration along the RMS. In conditional Smoothened-null mice, where Hedgehog signaling has been disrupted, neuroblasts fail to migrate to the OB, which is accompanied by a thin RMS and reduced population of these cells. Interestingly, when the neuroblasts isolated from the conditional Smo-null mice are transplanted into the SVZ of wildtype mice, the mutant neuroblasts migrate along the RMS to the OB, suggesting that the migratory defect of the mutant neuroblasts can be rescued when they are placed in a permissive environment with proper Hedgehog signaling [43]. Specifically, Shh activity modulates neuroblast migration both in vitro and in vivo by serving as a chemoattractant for neuroblasts. Injection of Shh-producing cells into the dorsal telencephalon is sufficient to redirect migration of neuroblasts from the RMS, demonstrating that these cells respond and migrate towards Shh [44]. Insulin-like growth factor-1 (IGF-1) is important in proliferation and differentiation of neural progenitors and their survival as neurons. Additionally, IGF-1 signaling is necessary for neuroblast migration from the SVZ, as is evident in IGF-1−/− mice that exhibit neuroblast accumulation in the SVZ and improper migration to the OB [45]. Once the neuroblasts have reached the OB, they migrate radially to their final destination toward the periphery, utilizing blood vessels as their migrating scaffold. In the OB, neuroblasts are found along the blood vessels and associated with astrocytic endfeet [46]. The interactions between the neuroblasts and astrocytes from the RMS and OB are essential in the process of migration, as astrocytes from other regions such as the cortex do not support neuroblast migration [47].
Regulation of neuronal integration in the adult brain
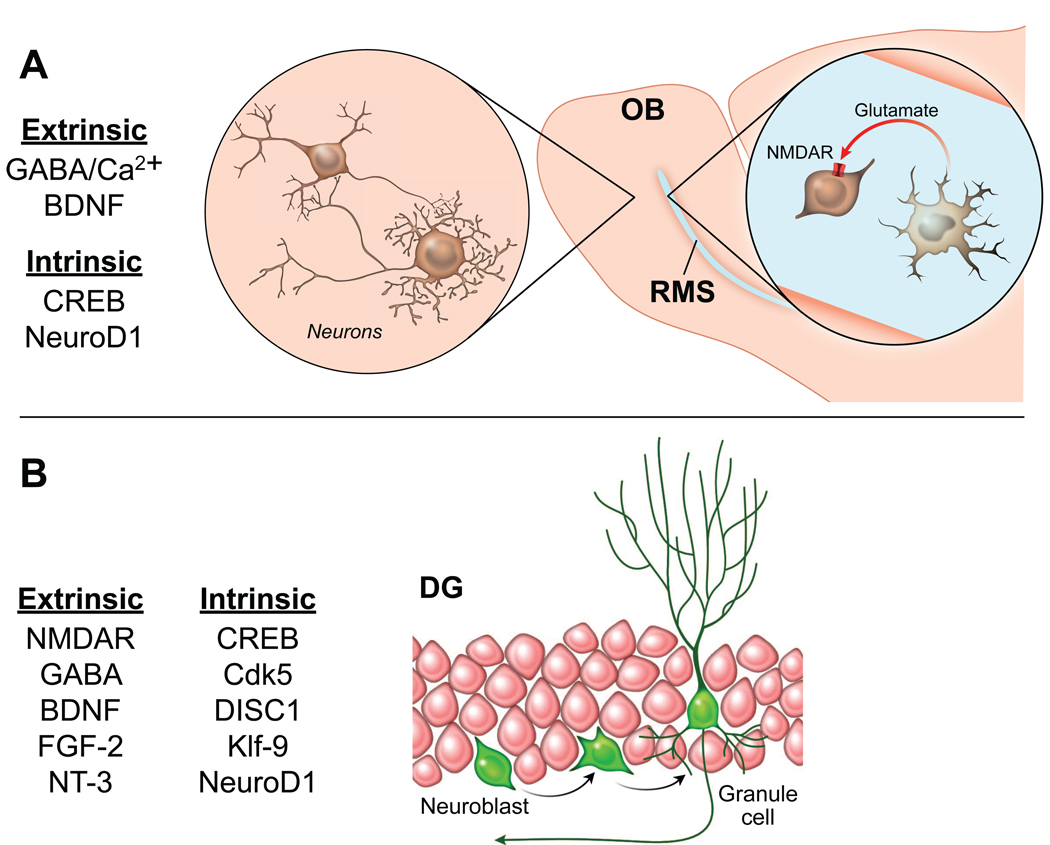
A large portion of newborn neurons dies within 4 weeks after birth. Their survival is subject to regulation by diverse mechanisms, and so is their morphological/physiological development before integration into the existing neural circuitry. (See Fig. 2) Neuroblasts born in the postnatal SVZ express NMDA receptors (NMDARs) during migration to the OB. The NMDAR activity is regulated by glutamate released from astrocyte-like cells that ensheathe the neuroblasts. Single-cell NMDAR knockout leads to neuroblast apoptosis along the migratory path and therefore reduced neurogenesis, suggesting that astrocytic glutamate signaling through NMDARs plays a critical role in controlling the number of adult-born neurons that integrate into the synaptic network [48]. Interestingly, lack of electrical input to the OB does not affect the generation, migration, and early differentiation of olfactory granule cells, but significantly reduces the number of young granule cells in the OB soon after they develop spines and presumably become synaptically connected, which is between 15 and 45 days after their birth [49]. In parallel, knockout of the NR1 subunit of NMDARs in hippocampal progenitors reduces neuronal survival 2–3 weeks after their birth, and this reduction can be partially rescued by global inhibition of neuronal activity [50], suggesting a glutamate-dependent critical period for survival of newborn DGCs. Owing to expression of NR2B-containing NMDARs, immature DGCs between 4 and 6 week old have enhanced capacity for synaptic plasticity, as shown by reduced induction threshold and increased magnitude of long-term potentiation (LTP), indicating a second glutamate-dependent critical period [51]. Although the role of NMDARs in synaptic plasticity in the OB remains to be elucidated, LTP expression is exclusively found in 2–8 week old olfactory granule cells but not in those mature ones [52]. These results indicate that newborn neurons may provide a unique substrate for certain types of learning and memory. GABA, a major inhibitory neurotransmitter in the mature CNS, initially depolarizes newborn DGCs and tangentially migrating SVZ progenitors due to their high intracellular concentration of chloride ions [53,54]. Ambient GABA-induced depolarization and Ca2+-influx is required for dendritic initiation and elongation in postnatally generated olfactory interneurons [55]. Genetically switching GABAergic depolarization to hyperpolarization by knocking down the Cl− importer NKCC1 in hippocampal progenitors reduces dendritic arborization and delays synapse formation [56]. In addition to neurotransmitters, the survival and synaptic integration of newly born cells are subject to regulation by neurotrophic factors. Knock-in of a variant form of BDNF (Val66Met) that results in reduced activity-dependent BDNF secretion impairs survival of SVZ neuroblasts and spontaneous olfactory discrimination [57]. Likewise, deletion of TrkB receptors in hippocampal progenitors reduces the growth of dendrites and spines in adult-born DGCs and compromises cell survival. It also leads to impaired LTP and remarkably increased anxiety-like behavior [58]. In conditional knockout mice that lack expression of FGFR1, a major receptor for FGF-2, decreased neurogenesis is accompanied by a severe impairment of LTP at medial perforant path-DGC synapses and deficits in memory consolidation but not spatial learning [59]. In contrast, conditional knockout of NT-3 gene results in decreased LTP at lateral perforant path-DGC synapses and impaired performance on spatial memory tasks [60]. Furthermore, some molecules playing a cell-intrinsic role become identified, too. In both adult OB and DG, CREB activity positively modulates dendritic development and survival of nascent neurons [61,62]. Cdk5 is required for survival and dendritic growth of adult-generated hippocampal neurons [63,64]. The cell-intrinsic proteins, DISC1 and Klf-9, act as the negative and the positive regulator of synaptic integration in the adult DG, respectively [65,66]. NeuroD1 plays an essential role in the survival and maturation of newborn neurons in both hippocampus and OB [67]. It was recently found that expression of NeuroD1 in the periventricular region in vivo leads to the appearance of mature, neuron-like cells in the SVZ and RMS, where normally do not show addition of new neurons [68]. Taken together, various mechanisms coordinate to link adult neurogenesis and network activity, thereby allowing for experience-dependent construction of neural circuits, which in turn contributes to learning and memory. The evidence in support of the reciprocal relationship between learning and adult neurogenesis has been extensively reviewed elsewhere [8,69,70]. Interestingly, two recent findings respectively suggest that newborn neurons may be required for pattern separation function in the DG [71] and disruption of old memories encoded in the hippocampus [72].
Figure 2.
Factors regulating the functional integration of newborn neurons. (a) Critical factors in the OB modulate survival, dendritic arborization, and maturation of newborn neurons. Migrating neuroblasts express NMDAR and receive glutamate signaling from astrocytes, which is crucial to neuroblast survival. (b) Critical factors in the SGZ influence the survival, dendritic arborization, and synaptic plasticity and integration of newborn neurons.
Conclusions
These recent studies highlight the broad range of signaling mechanisms involved in the regulation of adult neurogenesis. A number of the signaling pathways, such as Wnt and Shh, are conserved and function prominently in both the developing nervous system and the germinal zones of the adult brain, supporting the neurogenic niche. Additionally, intrinsic factors such as miRNAs and transcription factors are increasingly demonstrating the cell-autonomous characteristics that provide the NSCs and NPCs with the potential to proliferate, differentiate, and survive as newborn neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 5.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 7.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 9. Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. Using Sox2-GFP transgenic mice and virus-mediated fate mapping analyses, these authors demonstrated that a subpopulation of Sox2-expressing cells in the SGZ appears to display NSC behaviors in vivo, including self-renewal and multilineage differentiation.
- 10.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 13. Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. By lineage tracing and transplantation experiments, the authors showed that NSCs in the SVZ produce different subtypes of olfactory neurons depending on their location, suggesting that NSC populations are heterogeneous and that their potency is intrinsically specified.
- 14.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 15.Wexler EM, Paucer A, Kornblum HI, Plamer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. The authors provide a mechanism by which Wnt regulates adult neurogenesis. They demonstrate that downstream of the canonical Wnt signaling pathway in NSCs involves both the transcription factor NeuroD1 and retrotransposon LINE-1.
- 17.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 18. Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. The authors show that functional primary cilia found on granule neuron precursors mediate Shh signaling and are necessary for neurogenesis.
- 19.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 21.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 22.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12 sup:31–39. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 25.Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 28. Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. Using both loss-of-function and gain-of-function manipulations in vitro and in vivo, the authors provide the first evidence that activity of microRNA miR-124 modulates adult neurogenesis, which is mediated by the downregulation of its target gene Sox9.
- 29.Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berninger B, Guillemot F, Gotz M. Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur J Neurosci. 2007;25:2581–2590. doi: 10.1111/j.1460-9568.2007.05509.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozen I, Galichet C, Watts C, Parras C, Guillemot F, Raineteau O. Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur J Neurosci. 2007;25:2591–2603. doi: 10.1111/j.1460-9568.2007.05541.x. [DOI] [PubMed] [Google Scholar]
- 34. Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. The authors for the first time demonstrated that adult hippocampal progenitors, which generally produce excitatory neurons, could be instructed by overexpression of a single transcription factor to differentiate into oligodendrocytes in their in vivo niche, suggesting the fate plasticity of NPCs in the adult brain.
- 35.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merson TD, Dixon MP, Collin C, Rietze RL, Bartlett PF, Thomas T, Voss AK. The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci. 2006;26:11359–11370. doi: 10.1523/JNEUROSCI.2247-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 42.Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25:10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells. 2008;26:2311–2320. doi: 10.1634/stemcells.2008-0297. [DOI] [PubMed] [Google Scholar]
- 45.Hurtado-Chong A, Yusta-Boyo MJ, Vergano-Vera E, Bulfone A, de Pablo F, Vicario-Abejon C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci. 2009;30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 46.Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Marques J, De Carlos JA, Greer CA, Lopez-Mascaraque L. Different astroglia permissivity controls the migration of olfactory bulb interneuron precursors. Glia. 2010;58:218–230. doi: 10.1002/glia.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 51.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 53.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 54.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 55.Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jessberger S, Aigner S, Clemenson GD, Jr, Toni N, Lie DC, Karalay O, Overall R, Kempermann G, Gage FH. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boutin C, Hardt O, de Chevigny A, Core N, Goebbels S, Seidenfaden R, Bosio A, Cremer H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A. 2010;107:1201–1206. doi: 10.1073/pnas.0909015107. The authors showed that expression of a single transcription factor, NeuroD1, in SVZ NPCs in vivo is sufficient to induce their neuronal differentiation in regions that normally do not show addition of new neurons, suggesting NeuroD1 is one of the key factors involved in terminal neuronal differentiation.
- 69.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 70.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010 doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]