Abstract
Although acupuncture has been widely and routinely used in healthcare in the USA, its use has been based more on empirical observation than on scientific knowledge. Therefore, there is a great need for better understanding the underlying mechanism(s) of action. A great body of evidence supports that nonhuman primates are a candidate for studying human diseases. However, the use of nonhuman primates in neurophysiological, neuroimaging and neurochemical studies is extremely challenging, especially under fully conscious, alert conditions. In the present study, we developed a protocol for safely performing acupuncture, electro-acupuncture (EA) and electromyography (EMG) in both normal nonhuman primates and animals with parkinsonian-like symptoms. Four normal and four hemiparkinsonian middle-aged rhesus monkeys were extensively trained, behaviorally monitored, and received both EA and EMG for several months. The results demonstrated that (1) all rhesus monkeys used in the study could be trained for procedures including EA and EMG; (2) all animals tolerated the procedures involving needle/electrode insertion; (3) EA procedures used in the study did not adversely alter the animal’s locomotor activities; rather, MPTP-treated animals showed a significant improvement in movement speed; and (4) EMG detected significant differences in muscle activity between the arms with and without MPTP-induced rigidity. Our results support that rhesus monkeys can be used as an experimental animal model to study EA and that EMG has the potential to be used to objectively assess the effects of antiparkinsonian therapies. The results also indicate that animals, especially those with parkinsonian-like symptoms, could benefit from long-term EA stimulations.
Keywords: acupuncture, electroacupuncture, electromyography, rhesus monkeys, rigidity, Parkinson
Research Highlights.
Although acupuncture has been widely and routinely used in healthcare in the USA, its use has been based more on empirical observation than on scientific knowledge. The present study demonstrated that (1) rhesus monkeys used in the study could be trained for procedures including electroacupuncture (EA) and electromyography (EMG); (2) rhesus monkeys used in the present study tolerated the procedures involving needle/electrode insertion; (3) EA procedures used in the present study did not adversely alter the animal’s locomotor activities; rather, parkinsonian animals showed a significant improvement in movement speed; and (4) EMG detected significant differences in muscle activity between the arms with and without MPTP-induced rigidity. Our results support that rhesus monkeys can be used as an experimental animal model to study EA and that EMG has the potential to be used to objectively assess the effects of antiparkinsonian therapies. The results also indicate that animals, especially those with parkinsonian-like symptoms, could benefit from long-term EA stimulations.
1. Introduction
Since President Nixon’s visit to China in 1972, there has been a growing interest in integrating acupuncture into Western medical practice. Acupuncture has been both widely and routinely used for controlling chronic pain, nausea, and vomiting (for details see NIH consensus development panel on acupuncture, JAMA 1998). In addition, acupuncture has also been utilized for treatment of other diseases and disorders including, diabetes, depression and drug abuse (for reviews see Wang et al., 2008, Samuels et al., 2008, Cho and Whang 2009, Cui et al., 2008). Moreover, acupuncture along with electroacupuncture is also used for treatment of Parkinson’s disease (for reviews see Lam et al., 2008; Zesiewicz and Evatt, 2009). Although the use of acupuncture in healthcare is increasingly becoming widespread, the mechanism (s) by which acupuncture induces biological effects in the treatment of various disorders remains unclear.
Developing a suitable animal model for the experimental study of acupuncture is a key component in this stage of research. Most recent acupuncture studies have involved rodents, whereas a few studies have also involved rabbits (for a review see Wang et al., 2008). Most of these experimental acupuncture studies were conducted in anesthethized animals. Due to the effects of anesthesia on neuronal activity in the brain, experimental acupuncture should be studied in conscious animals more closely related to humans (Zhang et al., 2000a, for a review see Ramani and Wardhan 2008). Nonhuman primates provide unique research models to study the effects of acupuncture. Rhesus monkeys are phylogenetically close to humans and have complex social and cognitive behavior. They also live in a controlled environment and have a relatively long lifespan. These unique qualities provide an excellent model for monitoring behavioral effects of acupuncture. In addition, physiological, behavioral, and neuroanatomical similarities of rhesus monkeys to humans facilitate translation of findings in these animal models to the human condition. Therefore, the use of rhesus macaques as animal models of human physiology can greatly enhance our knowledge of human diseases. However, there is a challenge involved in bridging the gap between the ideal use of nonhuman primates as a model to study acupuncture and the ease in successfully using this approach.
The primary goal of this study was to develop methods to conduct acupuncture and/or electroacupuncture experiments in rhesus monkeys under fully conscious, alert conditions. In the past two decades, our group has extensively used rhesus monkeys for neuroimaging studies (Chen et al., 1999; Andersen et al., 2002; Hardy et al., 2005; Zhang et al. 2000a, 2001a, 2006, Luan et al., 2008); biology of aging studies (Andersen et al., 1999; Zhang et al., 2000b; Gerhardt et al., 2002; Maswood et al., 2002; Grondin et al., 2003; Ai et al., 2003; Cass et al., 2007); and Parkinson’s disease related research (Gash et al., 1996; Miyoshi et al., 1997; Zhang et al., 1997, 1999; Gerhardt et al., 1999; Grondin et al., 2002, 2008; Maswood et al., 2004; Xin et al., 2008; Ding et al., 2008). Thus, we have accumulated extensive experience in conducting translational research using nonhuman primates under both anesthetized and awake conditions. Based on our previous experience, we hypothesized that rhesus monkeys could be trained for this acupuncture research with a carefully designed approach.
Another goal for this study was to test whether electromyography (EMG) could be used to objectively measure rigidity to lay down the foundation for employing this technique to determine the effect of therapeutic treatments such as acupuncture on muscle rigidity. Rigidity is an abnormality of muscle tone resulting from dopamine deficiency in the nigrostriatal system, which can be seen in patients with Parkinson’s disease (PD). Clinicians utilize passive flexion and extension movements of major joints to detect such muscle tone abnormalities (Walker, 2007; Okuno et al., 2009; Robichaud et al., 2009). However, it can be difficult to successfully perform a similar physical examination on a nonhuman primate. In this study, EMG was used to quantitatively differentiate arm rigidity while performing a simple motor task in hemiparkinsonian rhesus monkeys with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced rigidity. Prior studies have demonstrated that EMG can be used to objectively and noninvasively monitor PD patients (Meigal et al., 2009; Hogrel 2005). For example, Robichaud and colleagues (2009) reported that the EMG pattern during rapid point-to-point movements can be used to objectively distinguish PD patients from healthy control subjects. In addition, significant correlations were shown between EMG measures and disease severity (i.e. motor UPDRS scores).
In the present study, an experimental protocol was developed and tested in rhesus monkeys for acupuncture as well as electroacupuncture (EA) treatment and electromyography (EMG) recordings. The main objectives of our study were to determine 1) whether rhesus monkeys can be trained for acupuncture and EA studies; 2) whether acupuncture and EA can be safely performed; and 3) whether EMG recordings could be conducted in both normal and hemiparkinsonian rhesus monkeys. This study contributes information on how to train rhesus monkeys in a reasonable time frame for the experimental study of acupuncture, and how to perform EA and EMG techniques in the same animals.
2. Materials and Methods
2.1 Animals
Eight female rhesus monkeys (Macaca mulatta) ranging in age from 17–21 years old and weighing 6.2–8.6 kg were used in the study. All animals were obtained from a commercial supplier (Covance, Alice, TX) and were housed in individual cages with water available ad libitum. Standard primate biscuits were supplemented daily with fresh fruit and vegetables. Four of eight animals received unilateral administration of 0.12mg/kg MPTP at least two years prior to the study via the right carotid artery based on previously described procedures (Ding et al., 2008). All procedures were conducted in the Laboratory Animal Facilities of the University of Kentucky, which are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental protocols were approved by the University of Kentucky Animal Care and Use Committee.
2.2 Animal training and restraint apparatus
All eight animals were first acclimated to the trainer’s presence while in the familiar environment of their home cage. Once the animal was accustomed to trainer interaction, nonhuman primate collars were placed around their neck. These collars were used in conjunction with a pole to manage the monkeys while outside of their home cage (Andersen et al., 2002). Animals were guided out of their cage and were able to move around fairly unrestricted. After 3–5 training sessions in this fashion, the monkeys demonstrated a more relaxed disposition. Once the monkeys were comfortable with the trainers and with the pole and collar system, they were introduced to our newly designed vertical chair used to help control the animal’s movement.
Food was removed from the animal’s home cage prior to each training session. This decreased excreta while in the training session, and increased interest of positive reinforcement from food (ie. grapes and other fresh fruit). The restraint chair (overall width = 25 cm, height = 69 cm, depth = 28 cm) was made primarily of acrylic and constructed in a vertical upright manner (Fig. 1A). The trainers guided the animal into the custom-designed chair by sliding the collar into tracks on the upper portion of the chair (Fig. 1A & B). The collar was locked into place, securing the animal in the chair (Fig. 1). The monkey was able to sit upright comfortably on adjustable platforms while the trainers attached a pair of arm restraints to help control arm movements (Fig. 1). The chair was also equipped with a tray table where preferred food was served, and a leg divider (Fig. 1). These limb restraints also kept the animal from turning around within the chair and have been designed to ensure the greatest comfort possible for the animal. A tray lined with absorbent surgical pads to catch excretion was placed under the sitting platforms to ensure a clean and comfortable environment for the animal (Fig. 1). The animals were provided with various means of enrichment while in the chair, including nature based television programs and preferred foods such as peanut to minimize feelings of anxiety. All training sessions occurred in the same room and were repeated several times per week until the animal was completely comfortable with the entire process and was able to relax in the chair for 90 min at a time.
Figure 1.
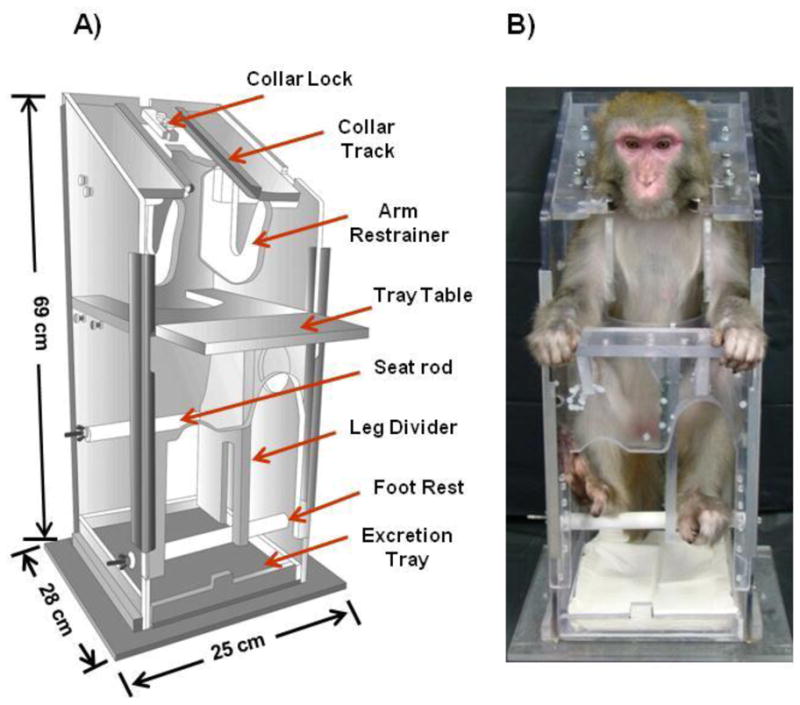
Custom acrylic restraint chair. The newly designed vertical chair was used to hold the animals during EA and EMG sessions. As seen in panel A, it has an overall width of 25 cm, height of 69 cm and depth of 28 cm. A rhesus macaque comfortably sitting in the restraint chair is shown in panel B. A collar, inserted into the collar track, is used to safely anchor the animal in the chair. This chair is designed with arm restraints to prevent unnecessary movement with a tray table to serve food items to the animal, and rods for sitting and foot rest, which can be adjusted based on the size of each individual animal, to provide added comfort while sitting in the chair during a training or treatment session. A tray to catch excretion is placed in the bottom of the chair to ensure the utmost comfort for the animal.
2.3 Electroacupuncture (EA) Procedures
After all eight animals were trained to sit in the restraint chair at the training center for approximately 90 minutes, they were introduced to acupuncture. To familiarize the animal with the procedures, acupuncture needles were administered for 2–3 trials, each lasting approximately 30 minutes. These initial trials not only allowed the monkey to become accustomed to the procedure, but also allowed the trainers to positively reinforce the animal and adjust the restraint chair to ensure the animals comfort. The acupuncture areas were shaven and cleaned with alcohol before the needle was inserted. The stainless-steel acupuncture needles used in the study were 0.18mm in diameter and 20mm in length. The acupoints selected for the non-human primates in this study were based on traditional human acupoints (Color Atlas of acupuncture, Thieme NY) and were accessible while the animal was in the restraint chair. Most acupoints were located in the front arm, hand and leg including: 1) HeGu (LI4), which lies halfway between the middle of metacarpal bone I with the middle of metacarpal bone II, i.e., on the dorsum of the hand, between the 1st and 2nd metacarpal bones and on the midpoint of the radial of the 2nd metacarpal bone in the hand; and 2) Zu San Li (ST36), which lies on the anteriolateral side of the leg, one finger breadth (middle finger) from the anterior crest of the tibia in the leg (Fig. 2).
Figure 2.
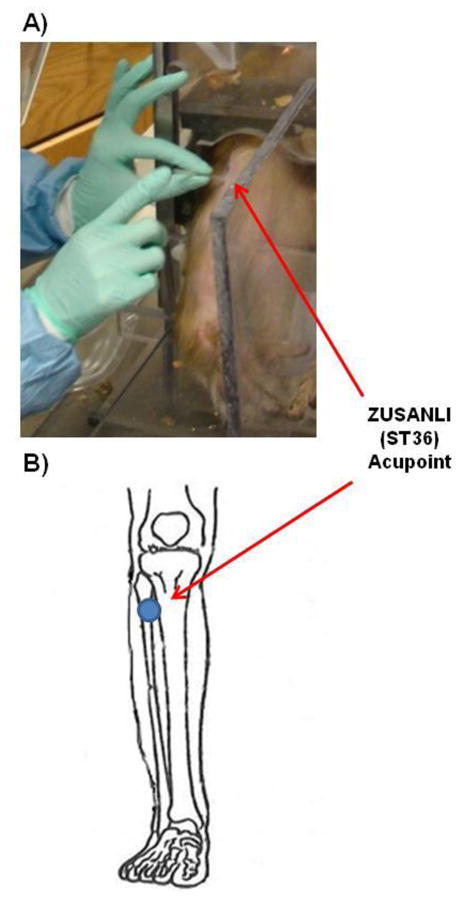
Insertion of acupuncture needles. Panel A shows an sterile acupuncture needle being inserted in the acupoint of ZUSANLI (ST36) of a rhesus macaque. Panel B shows the ZUSANLI (ST36) acupoint being stimulated with an acupuncture needle in the same animal. The image in panel B was modified from http://www.itmonline.org/arts/zusanli.htm.
Upon completing the initial training trials, EA treatment was administered to all animals. Based on experience accumulated from rodent studies (Liang et al., 2002, 2003; Jia et al., 2009), a bidirectional square wave electrical pulse (0.2 ms duration, 2 or 100 Hz) was administered for approximately 30 min per each EA treatment session. The amplitude level was raised until discomfort was observed in the animal and then lowered to a comfortable level. Discomfort was expressed by raising eyebrows or by a withdraw reflex. All animals received 3 EA treatment sessions per week.
2.4 Electromyography (EMG) Procedures
Similar procedures used for EA were also employed for EMG measurements. Positive reinforcement was used to elicit a simple upper limb action of eating preferred food items after the animal was comfortable with sitting in the upright chair. This action involved the animal retrieving food from the serving table of the chair and placing it in its mouth. When the tested rhesus could repeatedly accomplish this standard arm movement, the bicep area was shaved and the skin surface was cleaned before inserting the electrodes (Fig. 5A).
Figure 5.
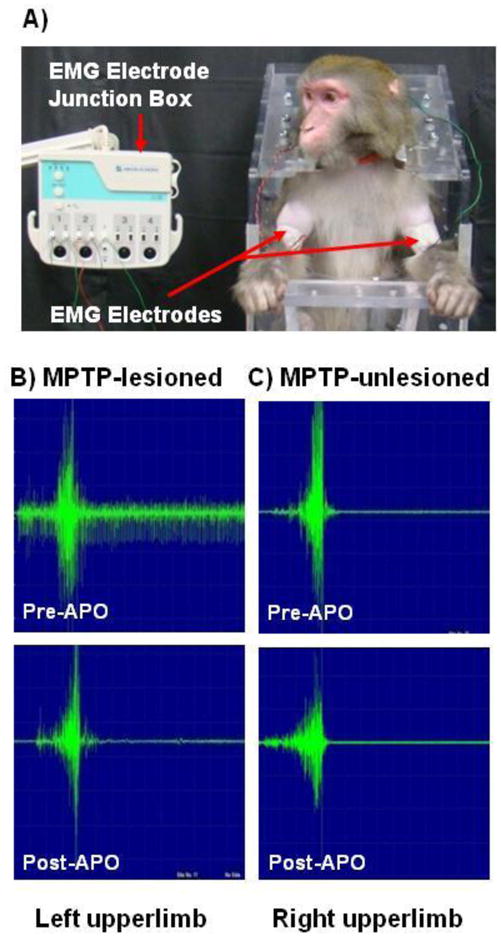
EMG studies in MPTP-treated monkeys. As seen in panel A, the electrodes were attached to the animal’s biceps brachia on both the left and right arms. A representative comparison of EMG spectra between the MPTP-lesioned (panel B) and unlesioned (panel C) sides of one hemiparkinsonian animal is shown pre- and post-apomorphine (APO) administration. As seen in panel B, the pattern of EMG on the MPTP-lesioned side (lower left panel in Panel B) was virtually identical to that seen on the MPTP-unlesioned side (lower right panel in Panel C) after apomorphine (0.15mg/kg) administration.
Ambu® Neuroline single patient twisted pair subdermal (12×0.40mm) electrodes were inserted into the monkey’s biceps brachia muscle (Fig. 5A). The electrical potential from contractions of the biceps brachia was measured as effort was exerted to move the food to the mouth of the monkey. EMG spectra from the MPTP side of the monkey were compared to the spectra from the unlesioned side of that same monkey. A Nihon Kohden EMG measuring system (model MEB-9400A) was used to collect the data and its accompanying Neuropack Manager (version 8.14) software was used to analyze the data. To determine if EMG could be used to monitor the effects of novel treatment strategies in animals with parkinsonism, an apomorphine challenge was conducted on the animals. All four hemiparkinsonian rhesus monkeys received a single dose of apomorphine (0.15mg/kg, s.c.) after an EMG baseline was collected. The dose of apomorphine used in this study has been used in other published studies (Zhang et al., 2000, 2001, 2006; Luan et al., 2008).
2.5 Videotaping Procedures
As per previously described procedures (Ding et al., 2008), all monkeys were videotaped before and after multiple, weekly EA treatments. The monkeys were transferred into a customized-videotaping cage in the morning on the day of testing. All animals were videotaped for 60 min with water available. The videotapes were analyzed for distance moved (cm) and movement speed (cm/sec) using the video-tracking software, EthoVision (Noldus Information Technologies, Asheville, NC).
2.6 EMG data analysis and statistics
When analyzing the EMG data, the following parameters were measured: duration (ms); Amplitude (mv); and area (mv/ms). The duration is the time of one “burst” of electrical activity from skeletal muscle. A “burst” begins when the electrical potential leaves the baseline and ends when it returns to the same baseline. As the duration of the exercise increased, the muscle contractions became slower. The amplitude is the difference between the minimum and maximum peek in an EMG wave. The larger the amplitude, the more muscle activity is needed to accomplish the task. The area bounded by the wave formation and its baseline is also measured. The larger the area, the more energy is used for the measured movement.
The data was analyzed using Prism software, version 4. A paired two-tailed t-Test was used to determine if there were significant differences in locomotor activity before and after EA in the MPTP-lesioned animals. Also, an unpaired two-tailed t-Test was used to determine if there were significant differences between the EMG data collected from the MPTP-lesioned versus unlesioned side in the same animal. P-values ≤0.05 were considered significant. The results are presented as the mean ± the standard error of the mean.
3. Results
3.1 General Conditions
All four MPTP-treated animals had moderate but stable hemiparkinsonian symptoms including: bradykinesia (slowness of movement); stooped posture; mild postural instability; and rigidity of both upper and lower limbs on the contralateral side to MPTP administration. The average total parkinsonian score of these animals was 5.25±0.44 points as measured using our previously published nonhuman primate parkinsonian rating scale (Ovadia et al., 1995; Miyoshi et al., 1997, Ding et al., 2008).
3.2 Animal Training
All eight rhesus monkeys used in the study readily adapted to our newly designed vertical chair. The animals were gradually introduced to the vertical chair in 20–30 min increments until they were able to comfortably sit in the restraint chair at the training center for approximately 90 minutes (Fig. 1B). This process was successfully completed within 4–6 weeks in all eight animals. There were noticeable differences in task acquisition between the normal and MPTP-treated animals. Normal animals required 8.33±1.20 training sessions (6–10 sessions), while the hemiparkinsonian rhesus monkeys required 14.75± 0.85 training sessions (13–17 sessions), on average (p value = 0.006, unpaired two-tailed t-Test). At this point, signs of discomfort including twisting of the body, open mouth threats and limb movements were virtually absent. In addition, all animals were interested in quickly retrieving food items that were placed on the tray table by members of the training team, allowing for reliable, repeated arm movements suitable for testing EMG recordings.
3.3 Acupuncture and EA
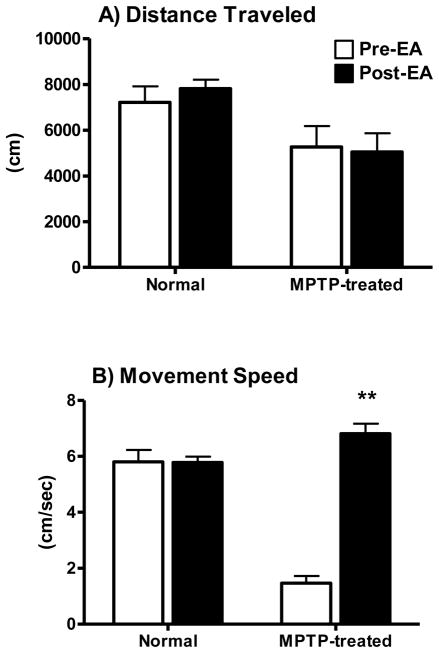
After the initial chair training, all eight animals received acupuncture or EA 3 times per week for 2 weeks. Very few signs of discomfort or anxiety, such as the raising of eyebrows, were observed. The animals would occasionally sleep for short periods of time during EA treatment. No abnormalities at the EA site were observed. No skin irritation or redness was noticed and the animals exhibited no signs of irritation such as abnormal, excessive scratching or grooming. All of the animal’s body weights were maintained throughout the study and remained within baseline range: 7.93 ± 0.14 kg baseline weight versus 7.66 ± 0.22 kg post-training/recording procedures. The animal’s general response to daily stimuli also remained unchanged. There were no behavioral abnormalities noted by the veterinary, husbandry or research staff, such as automutilation or aggressive behavior. In normal animals, there was no change in locomotor activity when comparing total distance traveled and movement speed data collected before and after 3 months of EA treatment (left columns in Fig 3A&B). In parkinsonian monkeys, a significant greater than 3-fold increase in movement speed was measured after EA, although the total distance traveled remained virtually unchanged (right columns in Fig 3A&B). The movement speed measured in MPTP-treated animals post-EA was comparable to that seen in normal controls.
Figure 3.
EthoVision analysis of locomotor activity for normal and MPTP-lesioned animals from pre-recorded DVDs. No differences in distance traveled inside the video-cage were found in both normal and MPTP-lesioned animals between pre- and post EA treatment (panel A) during the 60-min recording period. In contrast, a greater than 3-fold increase in movement speed was seen in the MPTP-lesioned animals but not in normal animals (panel B). The movement speed of the animals with parkinsonian-like symptoms measured post EA was virtually the same as that of normal animals. *P ≤ 0.05; pre vs. post EA in MPTP-treated animals.
3.4 Electromyography (EMG)
No differences in EMG were seen in the four normal animals between the right and left upperlimbs (Fig. 4A). However, as shown in Figure 4 (panel B), the EMG results for the four hemiparkinsonian monkeys with long-term moderate parkinsonian-like symptoms exhibited significant differences between the MPTP-lesioned (left) and unlesioned (right) upperlimb in all three measured parameters of duration (ms), amplitude (mv, and area (mv/ms). When comparing the individual spectra, the unlesioned side shows that the motor unit recruitment was less than that seen in the MPTP-lesioned side, and that the relax phase of the unlesioned side had very little motor unit discharge (Fig. 5B). Specifically as shown in Figure 4B, the MPTP-lesioned side required 179% more time to complete the simple motor task of retrieving food from the tray table (duration), required 34% more motor recruitment (amplitude), and the motor unit discharge was 172% higher than the unlesioned side (area). In addition, apomorphine significantly improved upperlimb rigidity in all four hemiparkinsonian monkeys (Fig. 4C). During the apomorphine trials, EMG data collected on the MPTP-lesioned side were comparable to that collected on the unlesioned side indicating improvement in motor unit recruitment and discharge allowing for the food retrieving task to be completed in less time (Fig. 5B&C).
Figure 4.
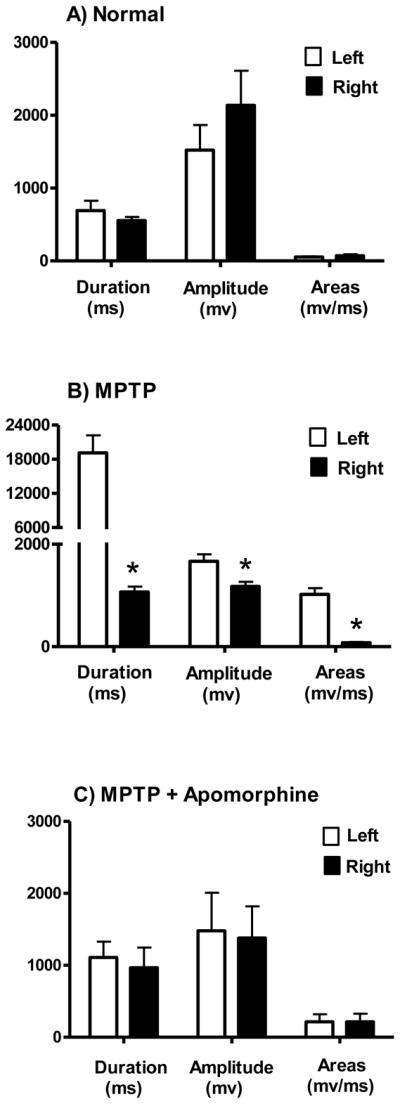
EMG spectra analysis for normal and MPTP-lesioned rhesus macaques. No differences in EMG were seen in the four normal animals between the right and left upper limbs (panel A). For the MPTP-lesioned animals, the data indicates that movements on the MPTP-lesioned side (left) were significantly slower and required more muscle activity (panel B). Apomorphine (0.15mg/kg) administration significantly improved upper limb rigidity on the MPTP-affected side of the body in all four hemiparkinsonian animals (panel C). *P ≤ 0.05; MPTP-lesioned (left side) versus unlesioned (right side).
4. Discussion
The present study demonstrates that 1) both normal and hemiparkinsonian animals can be adapted in a relatively short period of time to a custom-designed vertical chair encouraging a comfortable sitting position suitable for EA and EMG acquisition; 2) acupuncture/EA and EMG can be safely conducted in the rhesus monkey under fully conscious, alert conditions; 3) EA and EMG procedures did not adversely alter the animal’s locomotor function in either normal animals or animals with parkinsonian-like symptoms; instead, movement speed was significantly improved in MPTP-lesioned monkeys; 4) EMG can be used to objectively measure upperlimb muscle rigidity in hemiparkinsonian animals and to detect functional improvements in rigidity on the MPTP-affected side of the body post dopamine agonist administration.
In general, one of the most critical components of working with nonhuman primates is building a relationship of trust between the animals and the training team. This requires a lot of interaction with the animals, beginning at their home cage. Slow controlled movements and interactions are important for the animal to acclimate to the trainer’s voice and disposition. Throughout these early interactions, positive reinforcements should be given so that the trainer can gain the animal’s trust and confidence. When introducing the animals to foreign equipment and surroundings, sufficient time is needed for the animals to become familiar to the new environment. Procedural steps necessary for the conduct of the study should be introduced one training session at a time and the sessions should be consistent in all respects. Here, we used similar training techniques that successfully adapted nonhuman primates into an MRI environment using a horizontally-designed restraint chair for functional and pharmacological MRI studies (Zhang et al., 2000, 2001, 2006, Luan et al., 2008). For the present study, two trainers were needed to affix materials, such as EMG electrodes and acupuncture needles, directly to the animal. One trainer occupied the animal with external stimuli while the other attached the equipment. We used the same training room for the entire study and each training session was limited to 30 min initially. The duration of the training session was gradually increased until the animal was able to relax in the chair for 90 min at a time.
Results from the present study support that electroacupuncture can be safely conducted in both normal nonhuman primates and animals with parkinsonian-like symptoms and also demonstrates its potential to be used as a therapeutic tool for PD. However, more studies are needed due to the challenge of accurately locating acupoints in rhesus monkeys. It has been difficult for the experienced acupuncturist to accurately locate a specific acupoint in a monkey without a proper acupoint atlas, which is currently not available. The acupoints used in our study were located based on the traditional human acupoint atlas and experience in human acupuncture techniques. Lab technicians were trained by an experienced acupuncturist; EA was conducted by a Chinese scientist with animal acupuncture experience. The methods used to locate the key acupoints chosen in this study were as follows: first, the acupoints were selected based on the accessibility of their anatomical locations while the animal was restrained in the vertical chair; and second, some of these acupoints had been used in rodent models of PD (Liang et al., 2002, 2003; Jia et al., 2009).
It is important to point out that several precautions should be taken during training for EA. We observed that the sensitivity to electro-stimulation was variable among animals. The most important signs of overstimulation were facial expression, vocalization and whole body movement. At least one trainer should constantly monitor the animal’s response to the acupuncture needle insertion and electro-stimulation application. In addition, to minimize any possible irritation, sterile acupuncture needles must be used, and the animal’s skin surface must be cleaned before needle entry. While inserting the acupuncture needle, any major blood vessels must be avoided to prevent the possibility of a subcutaneous hematoma. In this study, each acupuncture needle was used only one time, and there was no detection of skin irritation or infection throughout the study.
Although, significant improvement in movement speed was measured in MPTP-treated animals post EA treatment (Fig. 3B), other factors may have contributed to this effect and need to be taken into consideration The improvements in movement speed may have also resulted in part from frequent human interactions as well as from increased physical activity due to animal training and handling, and directly from the benefit of EA. Studies have demonstrated that physical activity can improve parkinsonian-like symptoms in rhesus monkeys (Cameron et al., 2009) and patients with PD can receive benefit from EA (Shulman et al., 2002; ENG et al, 2006). Therefore, the future direction of acupuncture/EA studies should focus on using an unbiased behavioral testing battery to objectively evaluate the efficacy of acupuncture/EA, and use available technology such as EMG to study the underlying mechanisms of acupuncture/EA in rhesus monkeys.
A key symptom seen in post MPTP treatment of rhesus monkeys is the development of rigidity on the affected side of the body (Ding et al., 2008, Mera et al., 2009). Rigidity is defined as increased resistance to the passive movement of a limb persisting throughout its range and is characterized by a continued and uniform increase in resistance to muscle elongation throughout the range of passive movement. Rigidity is one of the major symptoms associated with PD (Poewe, 2009; Boonstra et al., 2008). Our data supports that EMG techniques can objectively measure upper limb muscle rigidity in rhesus macaques. The results from the present study showed that EMG activity differed significantly between the MPTP-lesioned and nonlesioned arm in all four hemiparkinsonian rhesus monkeys.
The EMG results from the present study are in line with recent EMG studies conducted in patients with PD. Endo and colleagues (2009) performed EMG measurements from bilateral bicep muscles on 27 PD patients and 24 health elderly subjects and found that EMG positively correlated with the UPDRS score on arm rigidity (Endo et al., 2009). In another study, Xia and collaborators (2009) examined the correlation between rigidity and the interactions of stretch reflex and shortening reaction during a passive movement of the wrist. They found that the objective scores of rigidity strongly correlated with EMG estimated by calculating a ratio of normalized EMG in stretched to shortened muscle for wrist flexion and extension (Xia et al., 2009). Taken together, the results from both nonhuman primate and human studies support the idea that EMG could be useful to monitor PD patients.
Apomorphine, a non-selective dopamine D1/D2 receptor agonist, has been shown to stimulate supersensitive postsynaptic dopamine receptors in the denervated striatum and has been used in the clinic for treating PD (Schwarting and Huston, 1996; Stacy 2004). In rhesus monkeys, previous studies have demonstrated the marked effects of apomorphine on the central nervous system through BOLD (blood oxygen level-dependent) imaging levels and relative cerebral blood volume in parkinsonian animals receiving apomorphine administrations (Nguyen et al., 2000; Zhang et al 2000, 2001, 2006, 2008). Consequently, in this study we used apomorphine to determine if EMG could be used to monitor changes in upper limb motor function in hemiparkinsonian rhesus monkeys. Our results showed that EMG detected an improvement in rigidity post apomorphine administration. Thus, it is predicted that EMG measurements could also be used for monitoring the therapeutic effects of acupuncture as seen in this nonhuman primate study with the antiparkinsonian drug, apomorphine. We are engaged in further studies to refine our system for detecting acupuncture/EA induced functional changes in rhesus monkeys. Such research will help in further elucidating new insight to a mechanistic description of acupuncture and rigidity, and have implications for evaluating the general therapeutic effects of acupuncture/EA.
Acknowledgments
This study was supported by UPPHS NIH grants NS39787 and NS50242. We also thank Dr. Li Sun, a neurologist from Peking University, for her assistance in EMG throughout this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461(2):250–61. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Andersen AH, Zhang Z, Zhang M, Gash DM, Avison MJ. Age-associated changes in rhesus CNS composition identified by MRI. Brain Res. 1999;829(1–2):90–8. doi: 10.1016/s0006-8993(99)01343-8. [DOI] [PubMed] [Google Scholar]
- Andersen AH, Zhang Z, Barber T, Rayens WS, Zhang J, Grondin R, Hardy P, Gerhardt GA, Gash DM. Functional MRI studies in awake rhesus monkeys: methodological and analytical strategies. J Neurosci Methods. 2002;118(2):141–52. doi: 10.1016/s0165-0270(02)00123-1. [DOI] [PubMed] [Google Scholar]
- Boonstra TA, Van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neuro. 2008;21(4):461–71. doi: 10.1097/WCO.0b013e328305bdaf. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Rockcastle N, Zigmond MJ, Leak RK, Smith A, Lopresti BJ, Mathis CA, Mirnics K, Williams NI, Zhang Z. Exercise protects the striatum against MPTP damage in nonhuman primates. Program 430.7/13. 2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- Cass WA, Grondin R, Andersen AH, Zhang Z, Hardy PA, Hussey-Andersen LK, Rayens WS, Gerhardt GA, Gash DM. Iron accumulation in the striatum predicts aging-related decline in motor function in rhesus monkeys. Neurobiol Aging. 2007;28(2):258–71. doi: 10.1016/j.neurobiolaging.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Chen Q, Andersen AH, Zhang Z, Ovadia A, Cass WA, Gash DM, Avison MJ. Functional MRI of basal ganglia responsiveness to levodopa in parkinsonian rhesus monkeys. Exp Neurol. 1999;158(1):63–75. doi: 10.1006/exnr.1999.7089. [DOI] [PubMed] [Google Scholar]
- Cho SH, Whang WW. Acupuncture for alcohol dependence: a systematic review. Alcohol Clin Exp Res. 2009;33(8):1305–13. doi: 10.1111/j.1530-0277.2009.00959.x. Epub 2009 Apr 30. Review. [DOI] [PubMed] [Google Scholar]
- Cui CL, Wu LZ, Luo F. Acupuncture for the treatment of drug addiction. Neurochem Res. 2008;33(10):2013–22. doi: 10.1007/s11064-008-9784-8. Epub 2008 Jul 10. Review. [DOI] [PubMed] [Google Scholar]
- Ding F, Luan L, Ai Y, Walton A, Gerhardt GA, Gash DM, Grondin R, Zhang Z. Development of a stable, early stage unilateral model of Parkinson’s disease in middle-aged rhesus monkeys. Exp Neurol. 2008;212(2):431–9. doi: 10.1016/j.expneurol.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Okuno R, Yokoe M, Akazawa K, Sakoda S. A novel method for systematic analysis of rigidity in Parkinson’s disease. Mov Disord. 2009;24(15):2218–24. doi: 10.1002/mds.22752. [DOI] [PubMed] [Google Scholar]
- Eng ML, Lyons KE, Greene MS, Pahwa R. Open-label trial regarding the use of acupuncture and yin tui na in Parkinson’s disease outpatients: a pilot study on efficacy, tolerability, and quality of life. J Altern Complement Med. 2006;12(4):395–9. doi: 10.1089/acm.2006.12.395. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–5. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999;817(1–2):163–71. doi: 10.1016/s0006-8993(98)01244-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80(1):168–77. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125(Pt 10):2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23(5):1974–80. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Ding F, Walton AA, Surgener SP, Gerhardt GA, Gash DM. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of MPTP-lesioned rhesus monkeys. Cell Transplant. 2008;17(4):373–81. [PMC free article] [PubMed] [Google Scholar]
- Hardy PA, Gash D, Yokel R, Andersen A, Ai Y, Zhang Z. Correlation of R2 with total iron concentration in the brains of rhesus monkeys. J Magn Reson Imaging. 2005;21(2):118–27. doi: 10.1002/jmri.20244. [DOI] [PubMed] [Google Scholar]
- Hogrel JY. Clinical applications of surface electromyography in neuromuscular disorders. Neurophysiol Clin. 2005;35(2–3):59–71. doi: 10.1016/j.neucli.2005.03.001. Review. [DOI] [PubMed] [Google Scholar]
- Jia J, Sun Z, Li B, Pan Y, Wang H, Wang X, Yu F, Liu L, Zhang L, Wang X. Electro-acupuncture stimulation improves motor disorders in Parkinsonian rats. Behav Brain Res. 2009;205(1):214–8. doi: 10.1016/j.bbr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Lam YC, Kum WF, Durairajan SS, Lu JH, Man SC, Xu M, Zhang XF, Huang XZ, Li M. Efficacy and safety of acupuncture for idiopathic Parkinson’s disease: a systematic review. J Altern Complement Med. 2008;14(6):663–71. doi: 10.1089/acm.2007.0011. [DOI] [PubMed] [Google Scholar]
- Liang XB, Liu XY, Li FQ, Luo Y, Lu J, Zhang WM, Wang XM, Han JS. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res Mol Brain Res. 2002;108(1–2):51–9. doi: 10.1016/s0169-328x(02)00513-2. [DOI] [PubMed] [Google Scholar]
- Liang XB, Luo Y, Liu XY, Lu J, Li FQ, Wang Q, Wang XM, Han JS. Electro-acupuncture improves behavior and upregulates GDNF mRNA in MFB transected rats. Neuroreport. 2003;14(8):1177–81. doi: 10.1097/00001756-200306110-00015. [DOI] [PubMed] [Google Scholar]
- Luan L, Ding F, Ai Y, Andersen A, Hardy P, Forman E, Gerhardt GA, Gash DM, Grondin R, Zhang Z. Pharmacological MRI (phMRI) monitoring of treatment in hemiparkinsonian rhesus monkeys. Cell Transplant. 2008;17(4):417–25. [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, Gerhardt GA. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol Aging. 2002;23(5):881–9. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101(52):18171–6. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigal AI, Rissanen S, Tarvainen MP, Karjalainen PA, Iudina-Vassel IA, Airaksinen O, Kankaanpää M. Novel parameters of surface EMG in patients with Parkinson’s disease and healthy young and old controls. J Electromyogr Kinesiol. 2009 Jun;19(3):e206–13. doi: 10.1016/j.jelekin.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Mera TO, Johnson MD, Rothe D, Zhang J, Xu W, Ghosh D, Vitek J, Alberts JL. Objective quantification of arm rigidity in MPTP-treated primates. J Neurosci Methods. 2009;177(1):20–9. doi: 10.1016/j.jneumeth.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Zhang Z, Ovadia A, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM. Glial cell line-derived neurotrophic factor-levodopa interactions and reduction of side effects in parkinsonian monkeys. Ann Neurol. 1997;42(2):208–14. doi: 10.1002/ana.410420212. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Brownell AL, Iris Chen YC, Livni E, Coyle JT, Rosen BR, Cavagna F, Jenkins BG. Detection of the effects of dopamine receptor supersensitivity using pharmacological MRI and correlations with PET. Synapse. 2000;36(1):57–65. doi: 10.1002/(SICI)1098-2396(200004)36:1<57::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- NIH consensus development pane on acupuncture. JAMA. 1998 Nov 17;:1518–1524. [PubMed] [Google Scholar]
- Okuno R, Fujimoto S, Akazawa J, Yokoe M, Sakoda S, Akazawa K. Analysis of spatial temporal plantar pressure pattern during gait in Parkinson’s disease. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1765–8. doi: 10.1109/IEMBS.2008.4649519. [DOI] [PubMed] [Google Scholar]
- Ovadia A, Zhang Z, Gash DM. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiol Aging. 1995;16(6):931–7. doi: 10.1016/0197-4580(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Poewe W. Clinical measures of progression in Parkinson’s disease. Mov Disord. 2009;24(Suppl 2):S671–6. doi: 10.1002/mds.22600. Review. [DOI] [PubMed] [Google Scholar]
- Ramani R, Wardhan R. Understanding anesthesia through functional imaging. Curr Opin Anaesthesiol. 2008;21(5):530–6. doi: 10.1097/ACO.0b013e32830edbf3. Review. [DOI] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Leurgans S, Vaillancourt DE, Comella CL, Corcos DM. Variability of EMG patterns: a potential neurophysiological marker of Parkinson’s disease. Clin Neurophysiol. 2009;120(2):390–7. doi: 10.1016/j.clinph.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels N, Gropp C, Singer SR, Oberbaum M. Acupuncture for psychiatric illness: a literature review. Behav Med. 2008;34(2):55–64. doi: 10.3200/BMED.34.2.55-64. Review. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50(2–3):275–331. doi: 10.1016/s0301-0082(96)00040-8. Review. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Wen X, Weiner WJ, Bateman D, Minagar A, Duncan R, Konefal J. Acupuncture therapy for the symptoms of Parkinson’s disease. Mov Disord. 2002;17(4):799–802. doi: 10.1002/mds.10134. [DOI] [PubMed] [Google Scholar]
- Stacy M. Apomorphine: North American clinical experience. Neurology. 2004;62(6 Suppl 4):S18–21. doi: 10.1212/wnl.62.6_suppl_4.s18. Review. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet. 2007;369(9557):218–28. doi: 10.1016/S0140-6736(07)60111-1. Review. [DOI] [PubMed] [Google Scholar]
- Wang SM, Kain ZN, White P. Acupuncture analgesia: I. The scientific basis. Anesth Analg. 2008;106(2):602–10. doi: 10.1213/01.ane.0000277493.42335.7b. Review. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi H, Wang BS, Cui YY, Zhu L, Rong ZX, Chen HZ. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2–3):125–34. doi: 10.1016/j.jad.2008.04.020. Review. [DOI] [PubMed] [Google Scholar]
- Xia R, Sun J, Threlkeld AJ. Analysis of interactive effect of stretch reflex and shortening reaction on rigidity in Parkinson’s disease. Clin Neurophysiol. 2009;120(7):1400–7. doi: 10.1016/j.clinph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Xin T, Ai Y, Gerhardt G, Gash D, Zhang Z. Globus pallidus plays a critical role in neurotrophic factor induced functional improvements in hemiparkinsonian monkeys. Biochem Biophys Res Commun. 2008;370(3):434–9. doi: 10.1016/j.bbrc.2008.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zesiewicz TA, Evatt ML. Potential influences of complementary therapy on motor and non-motor complications in Parkinson’s disease. CNS Drugs. 2009;23(10):817–35. doi: 10.2165/11310860-000000000-00000. Review. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Miyoshi Y, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharmacol Exp Ther. 1997;282(3):1396–401. [PubMed] [Google Scholar]
- Zhang Z, Zhang M, Ai Y, Avison C, Gash DM. MPTP-Induced pallidal lesions in rhesus monkeys. Exp Neurol. 1999;155(1):140–9. doi: 10.1006/exnr.1998.6976. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen AH, Avison MJ, Gerhardt GA, Gash DM. Functional MRI of apomorphine activation of the basal ganglia in awake rhesus monkeys. Brain Res. 2000;852(2):290–6. doi: 10.1016/s0006-8993(99)02243-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol A Biol Sci Med Sci. 2000;55(10):B473–80. doi: 10.1093/gerona/55.10.b473. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Grondin R, Barber T, Avison R, Gerhardt G, Gash D. Pharmacological MRI mapping of age-associated changes in basal ganglia circuitry of awake rhesus monkeys. Neuroimage. 2001;14(5):1159–67. doi: 10.1006/nimg.2001.0902. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen AH, Ai Y, Loveland A, Hardy PA, Gerhardt GA, Gash DM. Assessing nigrostriatal dysfunctions by pharmacological MRI in parkinsonian rhesus macaques. Neuroimage. 2006;33(2):636–43. doi: 10.1016/j.neuroimage.2006.07.004. [DOI] [PubMed] [Google Scholar]