Introduction
Despite intensive laboratory and clinical investigation, there are no proven disease-modifying therapies for osteoarthritis (OA). Since the disease may progress inexorably to joint destruction, with attendant pain and functional loss, orthopedic surgical procedures have an important role in the management of osteoarthritis. Surgery is deployed both early in the course of disease as well as later when joint destruction occurs. Surgery may also have a preventive role prior to the onset of osteoarthritis.
Accordingly, this chapter has three sections addressing current surgical treatments. The first covers arthroscopic approaches to osteoarthritis, including the management of lesions commonly associated with OA, such as meniscal tears. The advent of arthroscopic surgical approaches has permitted less invasive access to joints and the opportunity to intervene earlier in the course of joint destruction, potentially to delay and/or prevent what is otherwise a predictably progressive degenerative pathway. The second section of this chapter addresses osteotomies, which are typically done to restore a more anatomic biomechanical environment and prevent or delay the onset of OA or slow its progression. The third section discusses orthopedic procedures for advanced joint destruction including total joint replacement and arthrodesis (joint fusion).
Finally, remarkable advances in tissue engineering and biologic therapies herald an era of collaborative intervention in which surgeons gain access to joints with minimally invasive approaches and apply biologic or tissue engineering therapies. Some examples of these hybrid therapies already exist including autologous cartilage implantation and meniscal allograft. The chapter closes with a brief discussion of tissue engineering and biologic therapies for OA and a glimpse of the role of orthopedic surgery in OA prevention and management over the coming decades.
We recognize that the topic of surgical management of osteoarthritis is large enough to merit a full text. The goal of this review is not to summarize comprehensively all that is known about surgical management of OA but rather to provide readers with evidence and perspectives pertaining to the most salient surgical treatments for OA and to summarize the evidence base supporting the use of these surgical strategies.
Arthroscopic procedures for osteoarthritis
As arthroscopic surgery proliferated in the 1980s it became natural for physicians to consider arthroscopic debridement and lavage for management of osteoarthritis. However, studies of the outcome of arthroscopic procedures for osteoarthritis were methodologically weak and yielded inconsistent results. Two pivotal studies changed the outlook for arthroscopic debridement and lavage for OA. Moseley and colleagues randomized 180 subjects with symptomatic osteoarthritis to receive one of three therapies: arthroscopic debridement, arthroscopic lavage or a sham procedure. After two years of follow up, the outcomes were virtually identical in each group.1 Kirkely and colleagues randomized 170 patients with symptomatic OA to receive either arthroscopic lavage or a standard nonoperative regimen. In this study as well, there was no difference between the two randomized groups after two years of follow-up2. On the basis of these studies there is now general agreement that arthroscopic lavage and debridement are not useful for the management of osteoarthritis per se, in the absence of a superimposed structural lesion such as meniscal tear.3, 4
However, the most common indication for arthroscopic surgery in patients with osteoarthritis of the knee is, indeed, concomitant meniscal tear. The efficacy of arthroscopic partial meniscectomy (APM) in patients with concomitant OA has received relatively little study, especially given that APM is performed in over 500,000 patients with concomitant OA per year in the US.5 Effects of meniscal surgery documented in observational studies may be complicated by confounding by indication, with more active patients electing meniscal surgery and also at greater risk of OA developmet or progression on the basis of their increased activity levels and injury rates. Herrlin and colleagues performed the only RCT addressing this question, comparing the combination of APM with exercise against an exercise program alone6. The trial showed no clinically meaningful or statistically significant differences between the two strategies. A larger multicenter trial of APM with exercise vs. exercise alone, the MeTeOR Trial (Clinicaltrials.gov NCT00597012), is underway in the US, but results are not expected until 2012. Thus, the trials of Moseley and colleagues and Kirkely and colleagues provide strong evidence that patients with symptoms attributable to knee OA per se, and not meniscal tear, do not improve following arthroscopic lavage and debridement. Whether arthroscopic partial meniscectomy is useful in patients with symptomatic meniscal tear and concomitant OA is unclear and an area of active investigation.
Beyond the question of symptomatic relief, there is also concern about whether arthroscopic partial meniscectomy may increase the rate of progression of osteoarthritis. Evidence from cohort studies suggests that patients who have had APM have earlier and more severe OA than age matched patients who have not had APM.7–12 In addition, patients who have not had surgery but who have a meniscal tear on MRI are known to have higher rates of incident OA and of OA progression.13 However, it remains unknown whether the increased OA incidence and progression seen in patients with APM is due to the tear or the surgery or both. Only a trial design can disentangle these potential explanations.
Procedures to alter mechanical environment
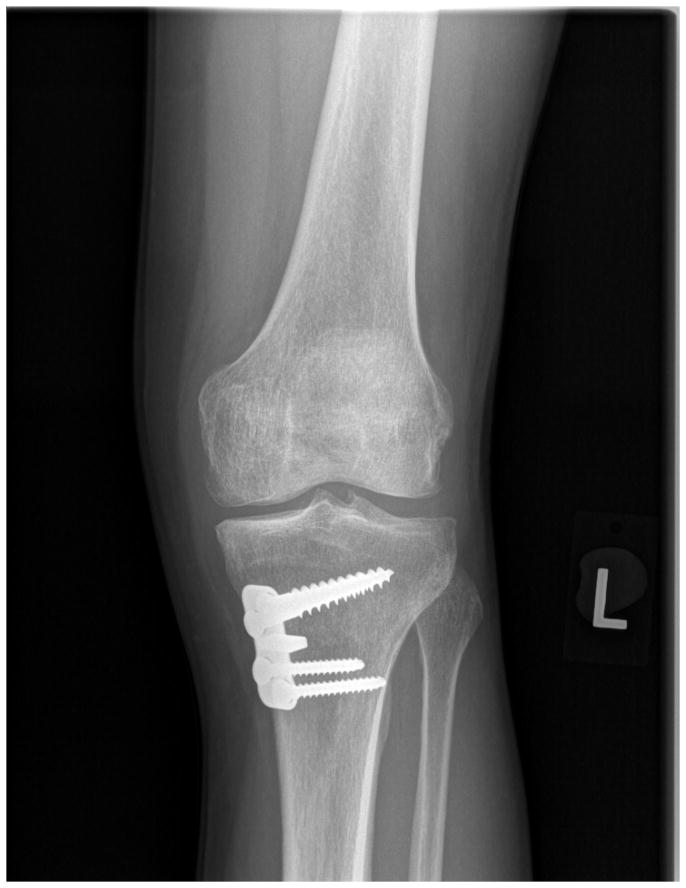
Malalignment is a well recognized risk factor for osteoarthritis incidence and progression.14 Osteotomy is performed to realign joints with the goals of relieving pain (in symptomatic patients) and delaying OA onset or progression. In patients with primarily medial compartment knee OA and varus deformity, high tibial osteotomy is performed either by removing a wedge of bone from the lateral proximal tibia or, more commonly, by opening a wedge space in the medial proximal tibia (Figure 1). This procedure permits the knee to adapt a more valgus alignment, transferring load from the damaged medial compartment to the more normal cartilage of the lateral compartment. Similarly, in patients with lateral compartment knee OA and associated valgus deformity, a distal femoral osteotomy, in which a wedge of bone is removed from the medial distal femur, or a wedge is opened in the lateral aspect, shifts the load to the healthier medial compartment.
Figure 1.
Radiograph of medial opening wedge high tibial osteotomy performed for medial compartment osteoarthritis.
Osteotomy is not as effective as total knee replacement in relieving pain and osteotomy fails sooner than TKA on average, prompting additional surgery. The risk of subsequent surgery following osteotomy in one series was 25% after five years of follow up, with total knee replacement accounting for about 40% of these additional surgeries. 15, 16 Thus, osteotomy is used primarily in younger patients with predominantly unicompartmental OA. This might occur, for example, in the setting of prior trauma or sub-total meniscectomy. A second indication is as an adjunct treatment for cartilage repair procedures to normalize the biomechanical environment. While it might be expected that osteotomies would delay the onset or progression of knee OA, there have been no trials of studies with concurrent controls. A literature synthesis revealed that after ten years one quarter of tibial osteotomies failed, leading to further surgery.16 In the absence of controls, it is difficult to quantify the benefit of this procedure for patients.
In the hip, dysplasia leads to inadequate acetabular coverage of the femur and overloading and premature OA of the articulating portion of the acetabulum. Acetabular osteotomy is typically performed in younger patients with hip dysplasia, to reorient the acetabulum so that healthy acetabular cartilage comes into apposition with the articular surface of the femoral head. There are no controlled studies of whether acetabular osteotomy delays onset or progression of OA. Case series with > 20 year follow-up document THR rates of about 30–40%.17, 18
Femoro-acetabular impingement (FAI) is another precursor lesion that poses a potential risk of hip OA.19 It is not known whether FAI is inherited or acquired. Some propose two parallel etiologies: a primary, congenital deformity that can result in OA, and a secondary deformity from an ongoing arthritic process due to osteophyte formation. This disorder is characterized by decreased congruence between the femoral head and acetabulum. Patients with cam deformity have an eccentric femoral head and head-neck junction. When this eccentric head articulates with the spherical acetabulum, jamming or impingement may occur, potentially damaging the articular cartilage and labrum. In pincer deformity, the anterior edge of the acetabulum is too prominent, resulting in repeated jamming of the acetabular rim with the femoral head during movement. The consequences may include both labral tear and cartilage wear. Cam and pincer lesions may occur in isolation or may be combined. These anatomic abnormalities may be addressed surgically using either open or arthroscopic procedures to remove excess bone and restore congruence. Frequently, repair or debridement of a torn labrum is performed concurrently. Open treatment of FAI was originally pioneered in Europe by Prof. Ganz, but requires significant surgical dissection and dislocation of the hip.20 Due to the morbidity and potential for avascular necrosis of the femoral head with the open procedure, current techniques to address FAI generally utilize an arthroscopic approach that minimizes disruption of soft tissues and allows for outpatient surgery.21 There have been no trials evaluating the efficacy of surgery for FAI in terms of pain relief or OA prevention. Early case series suggest considerable short term improvement in functional status and pain scores following surgical management.22, 23 As more surgeons train in the surgical management of these disorders we are likely to witness rapid growth of surgery for FAI. Defining the appropriate indications for this procedure is an important research priority.
Total joint replacement for advanced OA
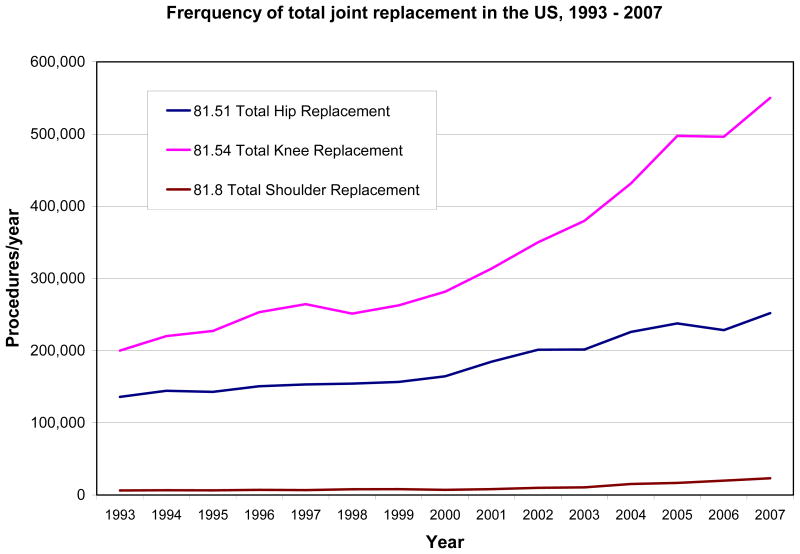
Total hip replacement surgery was introduced by Charnley in the 1960s, and by the 1970s, surgeons in major orthopedic centers in the US were learning hip replacement techniques. The introduction of total knee replacement soon followed. By 2007, over 550,000 total knee, 250,000 total hip and 23,000 total shoulder replacements were performed in the US24 (Figure 2). Some estimates suggest that over 3 million total joint replacements will be performed annually in the US by 2030.25 While total shoulder replacement is the least frequently performed of these three procedures, it is the fastest growing (Figure 2). Ankle and wrist replacement surgeries are performed on just 1100 and 500 patients/year respectively in the US and thus are not represented on the figure.24
Figure 2.
Annual frequency of total knee, hip and shoulder replacement in the US, 1993–2007.
Total ankle, elbow and wrist replacements are performed less frequently for several reasons. Principally, OA occurs less commonly at these sites than at the hip or knee. Secondly, arthrodeses of the ankle and wrist joints are reliable, reproducible, and effective alternatives to total joint replacement and restore a reasonable level of function with good relief of pain. A fused elbow, on the other hand, is quite functionally limiting and as a result elbow arthrodesis has a limited role.
Indications for total hip or knee replacement
The indications for total joint replacement are evolving. When total hip and knee replacement were originally introduced and popularized in the 1970s (THR) and 1980s (TKR), they were deployed as interventions of last resort for patients with substantial interference with activities of daily living. However, the risks of complications and early revisions have diminished in the last two decades. In addition, studies have demonstrated that patients operated upon later in the course of functional decline have worse functional outcomes than patients operated upon earlier26, 27. Thus, the indications are expanding and the option of total joint replacement is now typically presented to patients earlier in the course of arthritic progression and its associated functional decline.
Outcomes of total hip or knee replacement
The risks of complications and death following total hip and knee replacement are similar and most risks are higher following revision then following primary replacement (Table 1).28–30 As shown in Table 1, it is reasonable to inform patients that less than 1% of patients die and fewer than 5% have a serious complication following primary total hip or knee replacement. The risks following revision surgery are generally higher. Naturally, the patient’s exact risk depends upon their age, comorbid conditions and the experience of the hospital and surgeon in performing joint replacement.29–34
Table 1.
| Complication | Primary THR | Revision THR | Primary TKR | Revision TKR |
|---|---|---|---|---|
| Mortality | 1.0% | 2.5% | 0.7% | 1.1% |
| Pulmonary embolus | 0.9% | 0.8% | 0.8% | 0.5% |
| Deep joint infection | 0.2% | 1% | 0.4% | 1.8% |
| Dislocation | 3% | 8% | -- | -- |
Length of inpatient stay for total hip and knee replacement has diminished to a median of three days in the US, largely because capitated reimbursement incents hospitals to shift rehabilitation costs to the outpatient sector or to inpatient rehabilitation facilities.24 Approximately half of TJR recipients in the US are discharged to inpatient rehabilitation facilities.24 Patients who are older, have more comorbid conditions and who have less social support at home are more likely to be discharged to an inpatient facility.35 Over 80% of patients have sustained relief of pain following total hip and knee replacement. Risk factors for continued pain following total knee or hip replacement include preoperative presence of depression and worse preoperative functional status.26, 27, 36, 37
Recipients of total hip and knee replacement can anticipate that the risk of implant failure leading to revision surgery is about 1% per year.38, 39 For both procedures, the risk of failure is higher in younger and more active patients. The risk is also higher if the procedure is performed by a low volume surgeon or center.40, 41 While short term failures generally are due to technical problems, longer term failure of the implant is typically arises from symptomatic loosening due to osteolysis. This is an inflammatory process in which wear debris incites an inflammatory foreign body reaction resulting in bone loss.42
Numerous authors have examined preoperative predictors of outcome of total hip and knee arthroplasty. Worse functional outcomes are predicted by worse preoperative functional status, greater medical comorbidity, lower educational attainment and worse mental health status.26, 27, 37 Higher hospital and surgeon volume of total hip and knee replacement are associated with lower rates of mortality, perioperative complications and subsequent revision.32, 33, 40, 41
Innovative joint arthroplasty technologies
Innovation continues to characterize the total joint replacement field. A population that has prompted considerable technical innovation includes younger (e.g. < 55 years old), active patients, who will likely need at least one revision of their THR or TKR in their lifetimes. This clinical dilemma has stimulated a search for biomaterials that produce less wear debris and, in turn, cause less osteolysis and attendant bone loss and implant failure. This is the rationale for several developments, including highly cross linked polyethylene and ceramic-on-ceramic and metal-on-metal bearing surfaces. The long-term results of implants using these newer biomaterials are not yet known. There has been concern about untoward effects of these new technologies including circulating metal ions in metal-on-metal prostheses43–46 and about squeaking noises arising from ceramic on ceramic hips47. The frequency and impact of these phenomena are subjects of intense investigation. The longer term performance of these biomaterials will not be known for another decade.
Another area of innovation is hip resurfacing. This procedure does not require complete resection of the femoral head. The femoral component of a hip resurfacing implant has a cap that covers the femoral head, replacing the cartilage surface, and a much shorter stem that anchors the implant in the femoral neck. Early results from studies of hip resurfacing suggest that functional and pain outcomes are similar to those achieved with traditional THR.48 However, it appears from early reports that hip resurfacing has a higher rate of revision than traditional total hip replacement, although in certain subgroups such as young males revision rates are similar.49 Thus, the indications for hip resurfacing are evolving.
Gender-specific and patient-specific components have been developed. While it is recognized that women have different knee geometries than men, there is no evidence to date that gender specific prostheses improve pain relief, functional outcomes or implant survival.50 Patient specific implants are now offered, in which preoperative imaging studies are used to develop implants sized specifically to the patient’s anatomic details. There is no evidence to date that this expensive technology offers any advantages.
There have also been several innovations in the process of TJR care. Navigation techniques are used by some investigators to improve the placement of components, putatively with lower rates of failure due to malposition of components. While several studies support the role of navigation in optimizing component positioning, there is no evidence to date that navigation is associated with lower revision rates or improved relief of pain or restoration of functional status over the long term.51–53 Follow up of the studies done to date may clarify whether improvements in component placement translate to greater prosthesis longevity.
Minimally invasive total joint replacement is another area of innovation in the process of TJR. “Minimally invasive” implies a smaller incision with less soft tissue disruption than occurs in the usual procedure. Clinical investigators do not agree fully on the criteria for minimally invasive hip or knee replacement. Minimally invasive protocols tend to incorporate other changes in the process of care such as aggressive use of pain medications and earlier mobilization. Thus, it can be difficult to separate the technical aspects of minimally invasive surgery from other elements of the treatment protocol. Research to date suggests that patients receiving minimally invasive TJR have shorter lengths of inpatient stay than those managed with the traditional approach.54, 55 There is no evidence of superior functional benefit.
While most of this section has addressed total hip and knee replacement, one of the most important innovations in total joint replacement has occurred with total shoulder replacement. A major limitation of total shoulder replacement is that the implant requires an intact rotator cuff for shoulder abduction. This presents a problem for patients who have advanced rotator cuff degeneration, which is a major cause of osteoarthritis in the shoulder.56 Reverse total shoulder replacement changes the biomechanics such that the humeral component serves as the new socket and the glenoid component as the ball. This reversal permits the deltoid to be used as the shoulder abductor, restoring active elevation of the shoulder. Despite the major advance in treating patients who previously had no good surgical option, this technique is not without complication. Studies show that the complication rate for reverse total shoulder arthoplasty is about three times that of conventional total shoulder replacement. In addition, radiographic and clinical results have been shown to deteriorate after six years.57–60 Future studies will allow for a better understanding of how changes that have been made in implant design and technique will affect long term outcomes.
Hand and Foot Procedures for Advanced OA
While the knee and hip are arguably the most disabling sites of osteoarthritis, hand and foot OA are the most prevalent. Advanced osteoarthritis in these sites can lead to considerable pain and functional limitation. We will briefly discuss orthopedic approaches to OA of the first carpal-metacarpal joint (basal joint OA of the thumb), wrist and ankle OA, and OA of the first tarsal-metatarsal joint of the foot.
Advanced destruction of the trapezio-metacarpal joint is a frequent source of pain, deformity and functional limitation. When conservative measures including activity modification, splinting, NSAIDs and injections fail to control symptoms, surgery is a reasonable alternative. The standard procedure is a trapeziectomy, with or without the interposition of a tendon or other graft, and with or without ligamentous reconstruction. This surgery can be performed on an outpatient basis and has high rates of success with few complications. Over 90% of surgically treated patients experience pain relief. 61, 62 Multiple techniques exist which demonstrate good alleviation of pain and improvement of function. These serve to address degenerative changes and instability at the thumb carpometacarpal joint, the scaphotrapezial joint, and the scaphotrapezoidal joint, as necessary. There is no clear “best” technique based on extensive literature, thus surgeon training and preference play a large role in deciding which technique to perform.
Idiopathic osteoarthritis rarely involves the wrists, but these joints can develop OA following significant trauma, such as intra-articular fractures or intercarpal ligament disruptions, which alter joint kinematics. The procedure of choice for advanced, diffuse, painful wrist OA is an arthrodesis (fusion). Wrist arthrodesis provides consistent pain relief but permanent immobilization of wrist flexion and extension and radial and ulnar deviation has functional consequences.63 Patients have to rely upon their shoulders and elbows for positioning the hand during simple activities such as eating and typing.
Limited salvage wrist procedures attempt to alleviate pain by addressing the arthritic joints, while preserving motion at those joints not affected by obvious degenerative change. These procedures must be applied appropriately to each individual’s clinical situation and include scaphotrapezio-trapezoid fusion, radioscapholunate fusion, four corner fusion, and proximal row carpectomy, amongst many others.64, 65 Varying degrees of motion preservation, pain relief, and future arthritic progression have been noted.
Total wrist arthroplasty allows for alleviation of pain and preservation of some motion, and may be appropriate for certain patients with osteoarthritis. However, concerns about the high incidence of implant failure due to loosening and instability have led many surgeons to place postoperative activity restrictions on their patients, thus limiting the ideal patient population to lower demand individuals.66 Interestingly, patients with bilateral wrist arthritis with a total wrist fusion on one side and an athroplasty on the other typically prefer the motion-preserving procedure.67
The most common site of OA in the foot is the first metatarsal phalangeal joint (MTP). This lesion causes hallux rigidus (loss of MTP motion due to osteophytes and joint space loss). This problem is typically responsive to changes in activity and footwear. However, in advanced cases surgery is useful. The least invasive surgical approach is a cheilectomy, in which the anterior joint osteophytes are removed. If this procedure fails, or if joint destruction is particularly severe, the surgeon may proceed directly with an arthrodesis. Arthroplasty procedures have not been successful at this site.
Ankle fusion is also the procedure most frequently performed for advanced ankle arthritis. Here too, the procedure consistently relieves pain but immobilization of the joint precludes certain activities that require ankle motion such as cross country skiing. Patients who wish to retain ankle motion may elect total ankle replacement. A total ankle implant is biomechanically demanding. The procedure is performed routinely, but not frequently due to the generally excellent results of arthrodesis and the historically high rates of infection and technical failure of total ankle replacement.
Surgical and biologic procedures
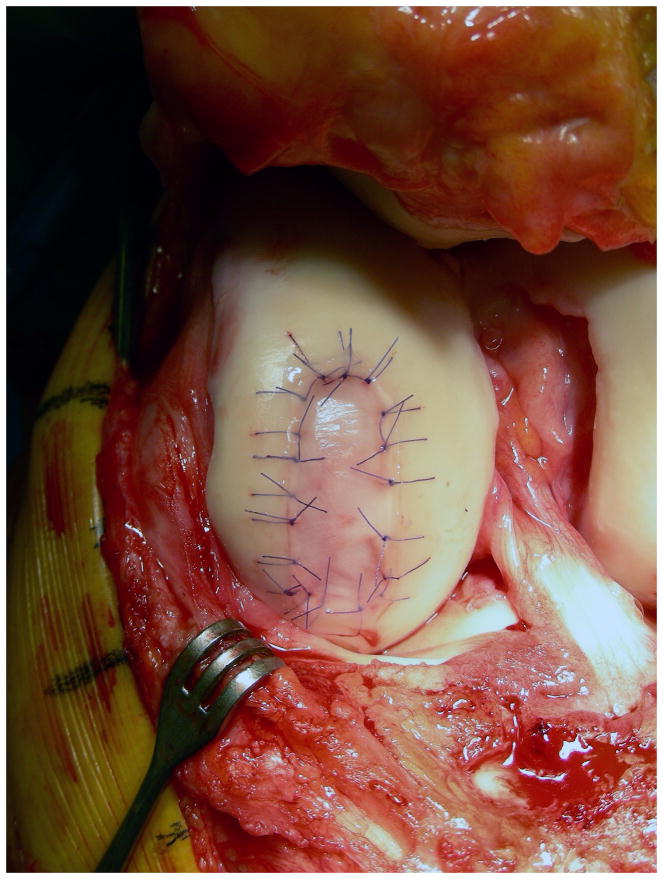
The tissue engineering and biologic therapy communities have worked intensively in osteoarthritis given the prevalence, cost and disability associated with the condition. To date there are a few limited successes. Perhaps the most notable is autologous chondrocyte implantation (ACI).. Performed in the US since 1995, ACI is FDA approved for the treatment of cartilage defects in the knee. Initially seen as an investigative cell-based therapy, it is now considered an accepted treatment option. Due to the complexity of the procedure and the frequent need for concomitant interventions such as osteotomy and meniscal transplantation, the majority are performed at larger specialized centers. This procedure attempts to repair a symptomatic cartilage defect (Figure 3a) through implantation of chondrocytes grown ex vivo from a small cartilage biopsy obtained from the patient in a staging arthroscopy. Appropriate patients for this procedure are younger individuals, typically < 50 years old, with isolated cartilage defects typically greater than 3 cm2 in size. Though not approved for patients with established osteoarthritis, ACI has been shown to be beneficial in early OA in an observational cohort.68 The patient undergoes an initial arthroscopic procedure in which normal cartilage is harvested from a non-weight bearing site. The 200,000–300,000 chondrocytes contained within the biopsy tissue are released enzymatically and are expanded in cell culture to approximately 12 – 48 million, depending on the number and size of defects that have to be implanted. After debridement of any degenerated tissue in the defect, a patch material – either periosteum from the patient or a synthetic collagen membrane – is sutured over the defect to create a watertight chamber into which the chondrocyte suspension is injected (Figure 3b). The chondrocytes attach to the subchondral bone and produce cartilage matrix, eventually filling the defect with hyaline-like cartilage.
Figure 3.
Figure 3a. Cartilage defect on femoral condyle
Figure 3b. Cartilage defect treated with autologous cartilage implantation
Rehabilitation following ACI is demanding; patients cannot be fully weight bearing for two months, and return to athletic activities is delayed for 12 to 18 months until the cartilage has fully matured. Osteotomies or meniscal transplantation are frequently performed concurrently to normalize the commonly abnormal biomechanical joint environment, thereby protecting the implant.
There have been several controlled trials comparing ACI and other approaches to cartilage defects. One RCT compared ACI with osteochondral autograft transfer (OATS or mosaicplasty procedure), where a cylinder of bone and cartilage is transferred into the defect from a lesser weightbearing area of the knee. The authors concluded that ACI demonstrated better outcomes at 19 months.69 Another RCT compared ACI with microfracture, which aims to induce a reparative process by perforating the subchondral plate, thus allowing bone marrow elements and blood to fill the defect and transform into a fibrocartilaginous scar. It showed that overall microfracture and ACI had similar outcomes five years after randomization, with better outcomes in younger patients. Microfracture, however, had significantly worse outcomes in defects larger than 4cm2, while ACI showed no size correlation; the authors therefore concluded that ACI should be recommended for the treatment of these large lesions. 70, 71 Two more recent RCTs of microfracture and ACI demonstrated significantly better repair tissue and clinical outcomes with ACI.72, 73, 74 Based on these trials, ACI appears as a useful treatment option in defects larger than 3 to 4cm2. Further research will be useful to further define the precise role of ACI in the management of cartilage defects.
Meniscal transplantation is a related intervention that has received increased attention in recent years for the treatment of the painful meniscectomized knee. While orthopaedic surgeons have largely abandoned total meniscectomy occasionally, sub-total meniscectomy is still required, for example in complex tears of a discoid lateral meniscus that cannot be salvaged by meniscal repair. Patients frequently report a symptom-free interval of a few months or years after sub-total meniscectomy, followed by increasing weightbearing pain and swelling due to progressive chondral degeneration. Meniscal transplantation can be performed in such patients before significant chondral disease develops. Meniscal transplantation is not performed in patients with established osteoarthritis. Prophylactic meniscal transplantation in asymptomatic patients after subtotal meniscectomy has been proposed by some to decrease the risk of developing chondral disease; however, no data are available to evaluate the efficacy of this intervention.
Meniscal transplantation is performed in an open or arthroscopic-assisted procedure with an overnight hospital admission, or, with the arthroscopic variant, on an outpatient basis. The transplanted meniscal tissue is ordered specifically for the individual patient and is side- and size-matched in order to fit the anatomy of the recipient knee. The grafts are harvested from organ donors with intact knee joints and processed sterilely to reduce the risk of contamination, then frozen for storage until matching recipients can be found.
Meniscal transplantation remains controversial, since there are no trials directly comparing transplanted patients with untreated controls. Cohort studies with over 10-year follow-up have demonstrated good symptomatic relief after meniscal transplantation and rates of osteoarthritis that appear lower than those reported in natural history studies of meniscectomized knees.75, 76 There are trials underway of artificial implants to reduce the reliance on human cadaveric tissue with its associated (albeit low) risk of disease transmission, and limited availability.77
Concluding remarks
In the absence of disease modifying therapy, many patients with osteoarthritis progress to advanced joint destruction. Thus, surgery plays an important role in the management of OA. Advances in biomaterials and tissue engineering will continue to create exciting new opportunities to integrate surgical approaches in OA care. The evidence supporting the use of many surgical approaches is limited by weak study designs and small samples. Advances in the field will need to couple scientific insights with rigorous studies of the efficacy and cost effectiveness of surgical approaches to OA management.
Acknowledgments
NIH K24 AR 02123; NIH P60 AR 47782;
References
- 1.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–8. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 2.Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097–107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 3.Marx RG. Arthroscopic surgery for osteoarthritis of the knee? N Engl J Med. 2008;359:1169–70. doi: 10.1056/NEJMe0804450. [DOI] [PubMed] [Google Scholar]
- 4.Richmond J, Hunter D, Irrgang J, et al. Treatment of osteoarthritis of the knee (nonarthroplasty) J Am Acad Orthop Surg. 2009;17:591–600. doi: 10.5435/00124635-200909000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen KAHM, Golosinskiy A. Statistics NCfH. Ambulatory Surgery in the United States, 2006. Hyattsville; 2009. [PubMed] [Google Scholar]
- 6.Herrlin S, Hallander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393–401. doi: 10.1007/s00167-006-0243-2. [DOI] [PubMed] [Google Scholar]
- 7.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 8.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 9.Englund M, Roos EM, Roos HP, Lohmander LS. Patient-relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology (Oxford) 2001;40:631–9. doi: 10.1093/rheumatology/40.6.631. [DOI] [PubMed] [Google Scholar]
- 10.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9:316–24. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 11.Roos EM, Roos HP, Ryd L, Lohmander LS. Substantial disability 3 months after arthroscopic partial meniscectomy: A prospective study of patient-relevant outcomes. Arthroscopy. 2000;16:619–26. doi: 10.1053/jars.2000.4818. [DOI] [PubMed] [Google Scholar]
- 12.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 15.Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2009 doi: 10.3109/17453670903413186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virolainen P, Aro HT. High tibial osteotomy for the treatment of osteoarthritis of the knee: a review of the literature and a meta-analysis of follow-up studies. Arch Orthop Trauma Surg. 2004;124:258–61. doi: 10.1007/s00402-003-0545-5. [DOI] [PubMed] [Google Scholar]
- 17.Schramm M, Hohmann D, Radespiel-Troger M, Pitto RP. Treatment of the dysplastic acetabulum with Wagner spherical osteotomy. A study of patients followed for a minimum of twenty years. J Bone Joint Surg Am. 2003;85-A:808–14. doi: 10.2106/00004623-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Steppacher SD, Tannast M, Ganz R, Siebenrock KA. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res. 2008;466:1633–44. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaule PE, Allen DJ, Clohisy JC, Schoenecker PL, Leunig M. The young adult with hip impingement: deciding on the optimal intervention. Instr Course Lect. 2009;58:213–22. [PubMed] [Google Scholar]
- 20.Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004:67–73. [PubMed] [Google Scholar]
- 21.Philippon MJ, Stubbs AJ, Schenker ML, Maxwell RB, Ganz R, Leunig M. Arthroscopic management of femoroacetabular impingement: osteoplasty technique and literature review. Am J Sports Med. 2007;35:1571–80. doi: 10.1177/0363546507300258. [DOI] [PubMed] [Google Scholar]
- 22.Graves ML, Mast JW. Femoroacetabular impingement: do outcomes reliably improve with surgical dislocations? Clin Orthop Relat Res. 2009;467:717–23. doi: 10.1007/s11999-008-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 24.AHRQ. HCUPnet, Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 25.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hipand knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 26.Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46:3327–30. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
- 27.Lingard EA, Katz JN, Wright EA, Sledge CB. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179–86. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Felson DT. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res. 2004:S16–21. doi: 10.1097/01.blo.0000144971.12731.a2. [DOI] [PubMed] [Google Scholar]
- 29.Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87:1222–8. doi: 10.2106/JBJS.D.02546. [DOI] [PubMed] [Google Scholar]
- 30.Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85-A:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41:431–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83-A:1622–9. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–16. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 35.de Pablo P, Losina E, Phillips CB, et al. Determinants of discharge destination following elective total hip replacement. Arthritis Rheum. 2004;51:1009–17. doi: 10.1002/art.20818. [DOI] [PubMed] [Google Scholar]
- 36.Hawker G, Wright J, Coyte P, et al. Health-related quality of life after knee replacement. J Bone Joint Surg Am. 1998;80:163–73. doi: 10.2106/00004623-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Rolfson O, Dahlberg LE, Nilsson JA, Malchau H, Garellick G. Variables determining outcome in total hip replacement surgery. J Bone Joint Surg Br. 2009;91:157–61. doi: 10.1302/0301-620X.91B2.20765. [DOI] [PubMed] [Google Scholar]
- 38.NIH Consensus Development Conference on Total Knee Replacement. Bethesda, MD: National Institutes of Arthritis, Musculoskeletal and Skin Diseases; 2003. [Google Scholar]
- 39.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 40.Manley M, Ong K, Lau E, Kurtz SM. Effect of volume on total hip arthroplasty revision rates in the United States Medicare population. J Bone Joint Surg Am. 2008;90:2446–51. doi: 10.2106/JBJS.G.01300. [DOI] [PubMed] [Google Scholar]
- 41.Losina E, Barrett J, Mahomed NN, Baron JA, Katz JN. Early failures of total hip replacement: effect of surgeon volume. Arthritis Rheum. 2004;50:1338–43. doi: 10.1002/art.20148. [DOI] [PubMed] [Google Scholar]
- 42.Archibeck MJ, Jacobs JJ, Roebuck KA, Glant TT. The basic science of periprosthetic osteolysis. Instr Course Lect. 2001;50:185–95. [PubMed] [Google Scholar]
- 43.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. J Am Acad Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Langton DJ, Sprowson AP, Joyce TJ, et al. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–95. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 45.Lazennec JY, Boyer P, Poupon J, et al. Outcome and serum ion determination up to 11 years after implantation of a cemented metal-on-metal hip prosthesis. Acta Orthop. 2009;80:168–73. doi: 10.3109/17453670902947408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quesada MJ, Marker DR, Mont MA. Metal-on-metal hip resurfacing: advantages and disadvantages. J Arthroplasty. 2008;23:69–73. doi: 10.1016/j.arth.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Restrepo C, Parvizi J, Kurtz SM, Sharkey PF, Hozack WJ, Rothman RH. The noisy ceramic hip: is component malpositioning the cause? J Arthroplasty. 2008;23:643–9. doi: 10.1016/j.arth.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Stulberg BN, Trier KK, Naughton M, Zadzilka JD. Results and lessons learned from a United States hip resurfacing investigational device exemption trial. J Bone Joint Surg Am. 2008;90 (Suppl 3):21–6. doi: 10.2106/JBJS.H.00718. [DOI] [PubMed] [Google Scholar]
- 49.Corten K, Macdonald SJ. Hip Resurfacing Data from National Joint Registries: What Do They Tell Us? What Do They Not Tell Us? Clin Orthop Relat Res. 2009 doi: 10.1007/s11999-009-1157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merchant AC, Arendt EA, Dye SF, et al. The female knee: anatomic variations and the female-specific total knee design. Clin Orthop Relat Res. 2008;466:3059–65. doi: 10.1007/s11999-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutzner J, Krummenauer F, Wolf C, Gunther KP, Kirschner S. Computer-assisted and conventional total knee replacement: a comparative, prospective, randomised study with radiological and CT evaluation. J Bone Joint Surg Br. 2008;90:1039–44. doi: 10.1302/0301-620X.90B8.20553. [DOI] [PubMed] [Google Scholar]
- 52.Weng YJ, Hsu RW, Hsu WH. Comparison of computer-assisted navigation and conventional instrumentation for bilateral total knee arthroplasty. J Arthroplasty. 2009;24:668–73. doi: 10.1016/j.arth.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Dutton AQ, Yeo SJ, Yang KY, Lo NN, Chia KU, Chong HC. Computer-assisted minimally invasive total knee arthroplasty compared with standard total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Am. 2008;90:2–9. doi: 10.2106/JBJS.F.01148. [DOI] [PubMed] [Google Scholar]
- 54.Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89:1153–60. doi: 10.2106/JBJS.F.00940. [DOI] [PubMed] [Google Scholar]
- 55.Karachalios T, Giotikas D, Roidis N, Poultsides L, Bargiotas K, Malizos KN. Total knee replacement performed with either a mini-midvastus or a standard approach: a prospective randomised clinical and radiological trial. J Bone Joint Surg Br. 2008;90:584–91. doi: 10.1302/0301-620X.90B5.20122. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001;83-A:1814–22. doi: 10.2106/00004623-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 58.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–95. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–7. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 60.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–86. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 61.Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10:645–54. doi: 10.1016/s0363-5023(85)80201-x. [DOI] [PubMed] [Google Scholar]
- 62.Tomaino MM, Pellegrini VD, Jr, Burton RI. Arthroplasty of the basal joint of the thumb. Long-term follow-up after ligament reconstruction with tendon interposition. J Bone Joint Surg Am. 1995;77:346–55. doi: 10.2106/00004623-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Hastings H, 2nd, Weiss AP, Quenzer D, Wiedeman GP, Hanington KR, Strickland JW. Arthrodesis of the wrist for post-traumatic disorders. J Bone Joint Surg Am. 1996;78:897–902. doi: 10.2106/00004623-199606000-00013. [DOI] [PubMed] [Google Scholar]
- 64.DiDonna ML, Kiefhaber TR, Stern PJ. Proximal row carpectomy: study with a minimum of ten years of follow-up. J Bone Joint Surg Am. 2004;86-A:2359–65. [PubMed] [Google Scholar]
- 65.Watson HK, Weinzweig J, Guidera PM, Zeppieri J, Ashmead D. One thousand intercarpal arthrodeses. J Hand Surg Br. 1999;24:307–15. doi: 10.1054/jhsb.1999.0066. [DOI] [PubMed] [Google Scholar]
- 66.Anderson MC, Adams BD. Total wrist arthroplasty. Hand Clin. 2005;21:621–30. doi: 10.1016/j.hcl.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Vicar AJ, Burton RI. Surgical management of the rheumatoid wrist--fusion or arthroplasty. J Hand Surg Am. 1986;11:790–7. doi: 10.1016/s0363-5023(86)80224-6. [DOI] [PubMed] [Google Scholar]
- 68.Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 468:147–57. doi: 10.1007/s11999-009-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–30. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 70.Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–64. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 72.Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37 (Suppl 1):10S–9S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 73.Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–46. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 74.Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 75.van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37:2134–9. doi: 10.1177/0363546509336725. [DOI] [PubMed] [Google Scholar]
- 76.Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694–706. doi: 10.1007/s00167-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 77.van Tienen TG, Hannink G, Buma P. Meniscus replacement using synthetic materials. Clin Sports Med. 2009;28:143–56. doi: 10.1016/j.csm.2008.08.003. [DOI] [PubMed] [Google Scholar]