Abstract
Cultured adherent bone marrow stromal cells (BMSCs) are capable of forming ectopic hematopoietic microenvironments (HMEs) in immunodeficient mice. However, the cell surface phenotype of the native bone marrow stem/progenitor cell that gives rise to BMSCs that support hematopoiesis remains poorly defined. We recently reported the derivation of human BMSC-like cells (CD133BMSCs) by magnetic cell sorting against Prominin-1 (CD133), an epitope expressed by embryonic, fetal, and adult stem cells. Here we demonstrate that CD133BMSCs are capable of forming ectopic HMEs. Cultured adherent CD133BMSCs derived from sorted CD133-positive cells lacked CD133 expression, but were uniformly positive for CD146, an epitope recently described to identify self-renewing osteoprogenitor cells that could transfer the HME. CD133BMSCs were genetically-tagged by lentivirus, expanded, and seeded into HA/TCP/fibrin constructs that were implanted subcutaneously. After 60 days, CD133BMSCs produced human osteocytes, osteoblasts, adipocytes, and reticular cells that supported murine hematopoiesis. CD133BMSCs that were not transduced with lentivirus also formed HMEs. Control constructs seeded with human dermal fibroblasts formed connective tissue, but failed to form HMEs. Our data indicate that CD133 expression identifies a native human bone marrow stem/progenitor cell that gives rise to BMSCs capable of forming the HME.
Keywords: CD133, Stem cell, Bone marrow, Niche, Hematopoiesis, MSC
Introduction
Reciprocal signaling between non-hematopoietic cells that comprise hematopoietic stem cell niches and hematopoietic stem cells (HSCs) is critical for the development and maintenance of the hematopoietic system. Non-hematopoietic multipotent bone marrow stromal cells (BMSCs) contribute to both the sinusoidal and endosteal niche environments by producing cells that regulate HSC proliferation, mobilization, quiescence, and differentiation through paracrine and direct cell-cell signaling [1–5]. Cultured BMSCs are well-known to generate stromal fibroblasts, adipocytes, chondrocytes, and osteoblasts [6]. Cultured BMSCs transplanted in vivo have been shown to produce pericytes, reticular cells, adipocytes, chondrocytes, osteoblasts and osteocytes [7–11], and in some cases to establish ectopic hematopoietic microenvironments (HME) that are similar in structure to the natural bone marrow environment [8; 11; 12].
HSCs can be enriched and purified from adult murine or human bone marrow mononuclear cells by sorting against various combinations of cell surface epitopes [3; 13]. In contrast, the cell surface phenotype of the native adult non-hematopoietic stem cell in vivo is poorly defined; this cell is likely to give rise to cultured adherent BMSCs. Sacchetti et al. (2007) described the CD146 epitope (Melanoma Cell Adhesion Molecule, MCAM) as an antigen associated with self-renewing human osteoprogenitors capable of HME formation when transplanted subcutaneously in hydroxyapatite/tri-calcium phosphate (HA/TCP)/fibrin constructs [11]. Generation of HMEs by BMSCs transplanted to ectopic sites suggests that such cells retain cell-autonomous information necessary for niche formation. Transplanted BMSCs that form HMEs also presumably secrete chemokines such as stromal-derived factor 1 alpha (SDF-1) that attract host-derived CD34-positive vascular and hematopoietic progenitors that then initiate hematopoiesis [14]. Several reports have demonstrated the prospective isolation of human BMSC-like cells by numerous cell surface epitopes such as STRO-1 [15], CD49b, CD73, CD90, CD105, CD130, CD146, CD200, integrin alphaV/beta5 [16], and also CD140b, CD340, and CD349 [17], but did not determine their ability to form HMEs in vivo.
CD133 (Prominin-1) is a pentaspanin glycoprotein localized to membrane protrusions of stem and progenitor cells of various origin [18–21] that display self-renewal and pluripotency [22] or multipotency for differentiation [23]. Here we show that adult bone marrow stem/progenitor cells prospectively isolated by sorting against CD133 generate BMSCs in culture that upon transplantation form ectopic HMEs in immunodeficient mice. Notably, our data indicate that the native adult human non-hematopoietic bone marrow stem cell expresses the CD133 antigen.
Materials and Methods
Isolation and preparation of CD133BMSCs
Isolation and preparation of human CD133BMSCs was performed as previously described [24]. CD133BMSCs were cultured in Nunclon Delta–coated 150 cm2 dishes (Nunc; Thermo Fisher Scientific, Rochester, NY) with complete culture medium (CCM) containing alpha minimum essential medium (α-MEM, Invitrogen, Carlsbad, CA) containing 20% fetal bovine serum (lot selected for rapid growth of hMSCs; Atlanta Biologicals, Lawrenceville GA), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/L L-glutamine (Mediatech Inc., Herndon, VA). For cell tracking in vivo, CD133BMSCs were transduced with lentivirus to express DsRed2 targeted to mitochondria as previously reported [25]. For proliferation assays cell number was determined by dye binding of nucleic acids (CyQuant, Invitrogen).
Differentiation of CD133BMSCs
Differentiation of CD133BMSCs was performed as previously described [24]. Confluent cultures of cells were prepared by plating CD133BMSCs at 1,000 cells/cm2 and incubating them in CCM for 5–7 days. The culture medium was then changed to either osteogenic or adipogenic medium. Osteogenic medium consisted of α-MEM containing 10% FCS, 1 nM dexamethasone, 0.2 mM ascorbic acid, and 10 mM β-glycerol phosphate (Sigma-Aldrich, St. Louis, MO). After incubation in osteogenic medium for 3 weeks with changes of medium every 3 or 4 days, the cells were washed with PBS (pH 7.4), fixed (10% neutral-buffered formalin, Fisher Scientific, Fair-lawn, NJ) for 20 min, and stained with 0.5% Alizarin Red S (pH 4.1; Sigma) for 20 min and washed three times for 5 min each with PBS. The adipogenic medium consisted of α-MEM containing 10% FCS, 0.5 μM hydrocortisone, 0.5 mM isobutylmethylxanthine, and 60 μM indomethacin (Sigma). After incubation in adipogenic medium for 3 weeks with media changes every 3 to 4 days, the cultures were washed with PBS, fixed with 10% formalin (Fisher Scientific), and stained with Oil Red O (Sigma) for 30 minutes and washed three times with PBS. The Oil Red O solution was prepared by diluting 3 parts of 0.5 % v/v stain in isopropanol with 2 parts water and clarified by filtration through a 0.2 μm PES filter (Nunc).
Phenotypic analysis by flow cytometry
Cell surface phenotyping of CD133BMSCs was performed as previously described [24]. Briefly, pellets of 1 × 105 to 5 × 105 cells were suspended in 0.5 ml PBS (pH 7.4) and were incubated for 30 minutes at 4 °C with fluorophore-conjugated monoclonal anti-human CD146 antibody that was pre-titered for flow cytometry (BD Biosciences, San Diego, CA). Non-specific antibody of the same isotype was used as negative control (BD Biosciences). After antibody labeling, cells were washed twice (PBS, pH 7.4) and analyzed by closed-stream flow cytometry (Epics XL; Beckman Coulter).
Hydroxyapatite tricalcium phosphate (HA/TCP) construct preparation
For each construct, an aliquot of 2 × 106 cells passage 5–6 in 100 μl of α-MEM was mixed with a previously autoclaved (sterile) aliquot of 40–50 mg of sintered hydroxyapatite/beta-tricalcium phosphate mixture (HA/TCP, ratio 20/80 by percent; 100–250 μm pore size; Himed Inc., Old Bethpage, NY). Fibrinogen (50 μl of 16mg/ml solution in α-MEM; final concentration of 3.2 μg/ml) and thrombin (100 μl of 50 units/ml in α-MEM with 2% CaCl2) were added to the cell/HA/TCP mixture in 24-well plates (Nunc) and mixed thoroughly. Fibrinogen and thrombin were from Sigma. The resultant viscous mixture was incubated for 90 minutes in a 37 °C tissue culture incubator (Model 3130; Thermo Electron Corporation, Houston, TX) and then implanted into prepared subcutaneous pockets in immunodeficient mice.
Implant surgery
All animal work was approved by the University of Vermont College of Medicines’ Office of Animal Care in accordance with American Association for Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. Female and male immunodeficient mice (NOD/SCID Interleukin-2-receptor gamma null, Strain name: NOD. Cg-Prkdcscid Il2rgtm1Wjl/SzJ, Jackson Laboratory, Bar Harbor ME) 9–12 weeks of age were anaesthetized with isoflurane (1–5%, to effect) and body temperature was maintained with a heated pad. The back/scapula area was shaved and sterilized by swabbing the area with topical ethanol followed by iodine three times (Sigma). A 15–20 mm longitudinal incision was made with fine scissors at the site of the scapula medial to the dorsal midline, and a 5–8 mm cavity between the dermis of the skin and the fascia underneath was made to accommodate the implant by spreading the fascia with forceps. Cellularized constructs were placed into the cavity with the use of sterile fine forceps. 1–4 sutures (6-0 monofilament nylon, Henry Schein, Melville, NY) and/or Vetbond (3M, St. Paul, MN) were used to seal the incision after each construct implantation. Sutures were removed 7 days after surgery. All mice received buprenorphine (0.05 mg/kg, s.c.) immediately and 24 hours after construct implantation.
Preparation of tissues for histological and immunohistochemical analysis
Cell-seeded HA/TCP constructs were removed from mice 60 days after implantation and fixed for two days (10% neutral buffered formalin, Fisher Scientific, Rochester NY) followed by decalcification (Decal®, Decal Chemical Corp. Tallman, NY) for 24 hours. Implants were mounted onto paraffin blocks and sectioned by microtome into 8 micron thick specimens on glass microscope slides (Superfrost Plus, Fisher Scientific, Rochester NY). After standard ethanol deparaffinization and hydration of sections, slides were stained with Hematoxylin and Eosin (H & E) as follows: 10 minutes in Harris Hematoxylin, rinse in tap water, 2 dips in acid alcohol (1% HCl in 70% ethanol), 1 minute in tap water, 2 dips in blue in ammonia (0.25% NaOH in distilled water), 5 minutes in tap water, 1.5 minutes in Eosin, and rinse in tap water. Slides were then dehydrated a standard gradient of ethanol solutions and 3 changes of xylenes. Sections were mounted with Permount (Fisher Scientific). Specimens on slides for IHC analysis were also deparaffinized and hydrated by through xylenes and a standard gradient of ethanol solutions. Two washes were performed with PBS (pH 7.4) for 5 minutes before and after each of the following treatments for specimens on Superfrost Plus microscope slides: 30 minutes in ice cold methanol (Sigma), antigen retrieval by 10 minutes of microwave treatment in 2X saline sodium citrate (SSC, Invitrogen), and 10 minutes protease digest in 50 μg/ml proteinase K (Invitrogen) in PBS at 37°C. Slides were blocked in 5% goat serum with 0.4% triton X-100 for 1 hr. The following antibodies were then diluted into blocking solution and incubated on the tissue sections: rabbit anti-human β-2-microglobulin (B2M, 1:1,000, Dako Corp., Carpinteria CA); rat anti-RFP (clone 5F8, 1:250, Chromotek, Planegg-Martinsried, Germany). Rabbit IgG was used as an isotype control for anti-B2M (1:1,000, 1 μg/ml). Rat IgG was used as an isotype control for anti-RFP (1:250, 4 μg/ml). After 3 × 5 min PBS washes, goat-anti-rabbit IgG Alexa-fluor 488 (1:500, 4 μg/ml) and goat-anti-rat IgG Alexa-fluor 594 (1:800, 2.5 μg/ml) (Molecular Probes, Invitrogen) were used as secondary antibodies. Following 3 additional washes in PBS, slides were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA).
Results
Cultured CD133BMSCs express CD146 and are multipotent
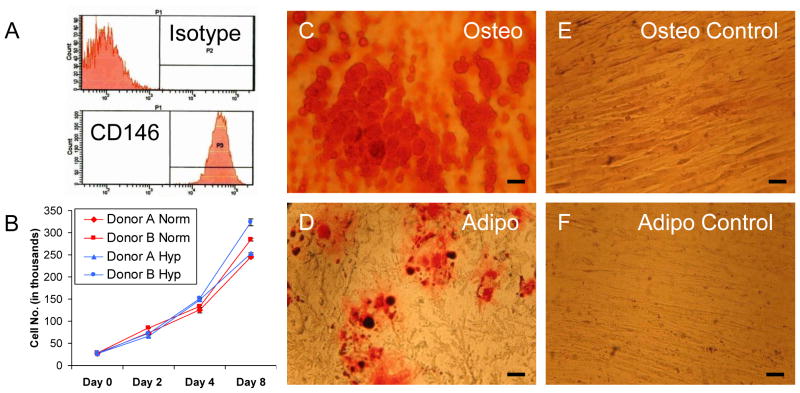
Cell surface phenotyping demonstrated that 100% of passage 3 CD133BMSCs were uniformly positive for the CD146 epitope (N=3 donors, Fig. 1A and data not shown). To assay growth potential, CD133BMSCs from 2 donors were subjected to a high density analysis of cell proliferation under both normoxic and hypoxic (1% oxygen) conditions. CD133BMSCs plated at 1,042 cells/cm2 in 6-well plates were grown in CCM for 10 days. Two days after plating (day 0), some cultures were exposed to hypoxic conditions whereas replica cultures were maintained under normoxic conditions (21% oxygen). Cell numbers were then determined over 8 days. CD133BMSCs from both donors expanded equally well under normoxic and hypoxic conditions (Fig. 1B). To assay differentiation potential, CD133BMSCs from 3 donors were plated at 1000 cells/cm2, expanded with CCM to 100% confluence, and then exposed to either osteogenic differentiation medium, adipogenic differentiation medium, or to fresh CCM (control) with medium changes every 3 days (Fig. 1C–F). After 3 weeks of culture in the various mediums, CD133BMSCs exposed to osteogenic medium displayed abundant calcium deposition that was detected by Alizarin Red S staining (Fig. 1C), while replica CD133BMSC cultures maintained in CCM showed no Alizarin Red S staining (Fig. 1E). CD133BMSCs cultured in adipogenic medium displayed adipocyte differentiation as detected by Oil-red-O staining for lipid deposition (Fig. 1D). Oil Red O staining was absent in replica control cultures grown in CCM (Fig. 1F).
Fig. 1. Characterization of CD133BMSCs.
A) Cell surface phenotyping for CD146 (MCAM). Note that isotype control is negative. B) High density growth of CD133BMSCs (N=2 donors) seeded at 1,042 cm2 in normoxic (21% oxygen; red lines) or hypoxic (1% oxygen; blue lines) conditions for 8 days. C–F) Phase contrast photomicrographs of cultured CD133BMSCs differentiated over 3 weeks into osteoblasts (Osteo) and adipocytes (Adipo). Calcium deposition was stained by Alizarin Red S (C) and lipid deposition was stained by Oil Red O (D). Alizarin Red S and Oil Red O staining was absent in CD133BMSCs treated with control growth medium (E, F). C–F, 40X magnification.
Production and implantation of cell-seeded constructs
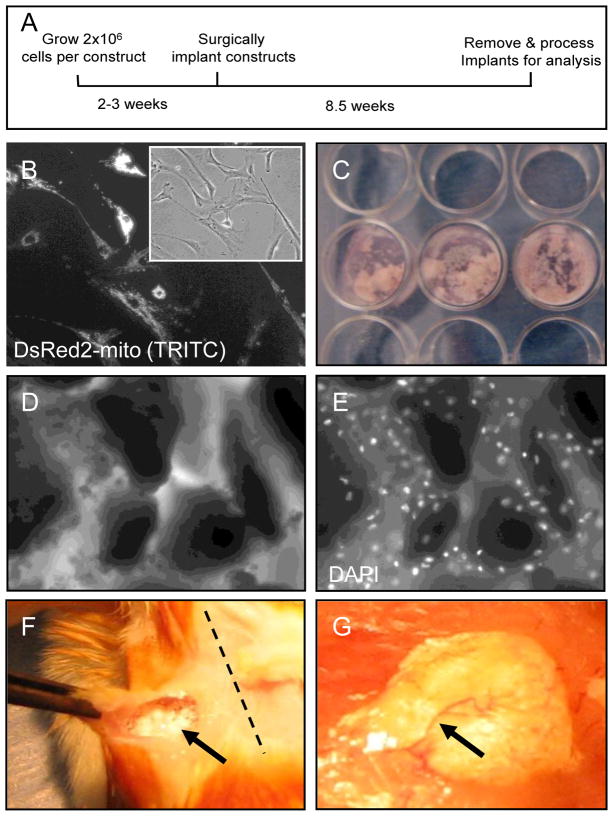
Based on previous reports describing HME transfer [11; 26; 27], we developed a protocol to produce human cell-seeded HA/TCP/fibrin constructs to assay ectopic HME formation in immunodeficient mice (Fig. 2). For cell tracking in vivo, CD133BMSCs from 1 donor were transduced with lentivirus to express a mitochondria-targeted DsRed2 reporter (Fig. 2B). To form constructs we combined aliquots of sterile HA/TCP sintered particles, 2 × 106 CD133BMSCs or fibroblasts (cell control), and clotting reagents (CaCl2, thrombin, and fibrinogen) (Fig. 2C–E). The resultant mixture was placed into a 37 ºC tissue culture incubator for 90 minutes to increase construct viscosity. Within 90 minutes cells attached to HA/TCP particles of various shapes and sizes within the constructs (Figs. 2D and E). After 90 minutes of incubation, cell-seeded constructs were implanted into subcutaneous pockets in immunodeficient mice (Fig. 2F). At 60 days after implantation, constructs became solidified and vascularized (Fig. 2G).
Fig. 2. Preparation and implantation of cell-seeded HA/TCP/fibrin constructs.
A) Schematic of HME transfer protocol. B) Epifluorescent imaging of CD133BMSCs expressing DsRed2-mito reporter (20X magnification). Inset: phase contrast photomicrograph of CD133dMSCs pictured in (B). C) Picture of constructs prepared in 24-well plates and incubated for 90 minutes at 37ºC in a cell culture incubator prior to implantation. D) Phase contrast photomicrograph of HA/TCP particles incubated with cells and clotting factors (10X magnification). E) Epifluorescent image of (D). Note that CD133BMSCs are evenly dispersed throughout the construct. White: DAPI nuclear stain. F) At the time of surgery, gelatinous construct material was implanted into subcutaneous pockets (arrow) in immunodeficient mice at the scapula proximal or medial to the dorsal midline (dotted line). G) At 60 days after implantation, solidified constructs were integrated into host tissue and were vascularized (arrow).
HME formation by CD133BMSCs
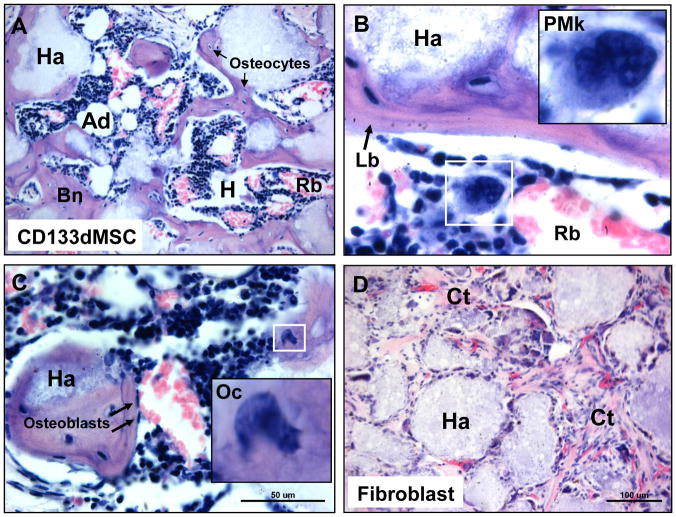
Sixty days after implantation, constructs were removed, sectioned, and stained with H & E. CD133BMSCs created characteristic HMEs with gross structural similarity to native bone marrow compartments (Fig. 3A–C). CD133BMSCs from 2 out of 2 donors tested formed HMEs. One donor was transduced with lentivirus for lineage tracing and the other was not transduced to control for lentiviral infection. CD133BMSC seeded constructs contained all of the cellular requisites for blood formation including: osteocytes, osteoblasts, reticular cells, adipocytes, and endothelial cells (Figure 3). Both endosteal-like and sinusoidal-like niches were observed in the implants containing CD133BMSCs. Hematopoietic progenitors and blood cells at various stages of maturation were observed throughout the niches: promegakaryocytes, erythroblasts, erythrocytes, neutrophils, and others (Fig. 3A–C). Human dermal fibroblasts seeded into control HA/TCP/fibrin constructs formed connective tissue, but did not form HMEs (Fig. 3D).
Fig. 3. Histology of cell-seeded constructs 60 days after implantation.
A) H & E stain of CD133BMSC-seeded construct showing HME formation with blood forming hematopoietic cells (Hp), enucleated red blood cells (Rb), and adipocytes (Ad), that are interspersed throughout bone (Bn) (10X magnification). Note that bone surrounds the HA/TCP carrier (Ha). Osteocytes (Os) were observed residing within lacunae (arrows). B) Concentrically-layered lamellar bone (Lb, arrow) had formed on HA/TCP surfaces (Ha) (40X magnification). Inset: promegakaryocyte (PMk). C) Erythropoiesis at various stages was observed throughout the HMEs. Inset: osteoclast (Oc). Arrows: osteoblasts. D) HA/TCP/fibrin construct seeded with 2 × 106 human fibroblasts. Fibroblasts adhered to the HA/TCP matrix (Ha) formed connective tissue (Ct) containing microvasculature throughout the implant, but did not support hematopoiesis.
Differentiation of CD133BMSCs into functional elements of the HME
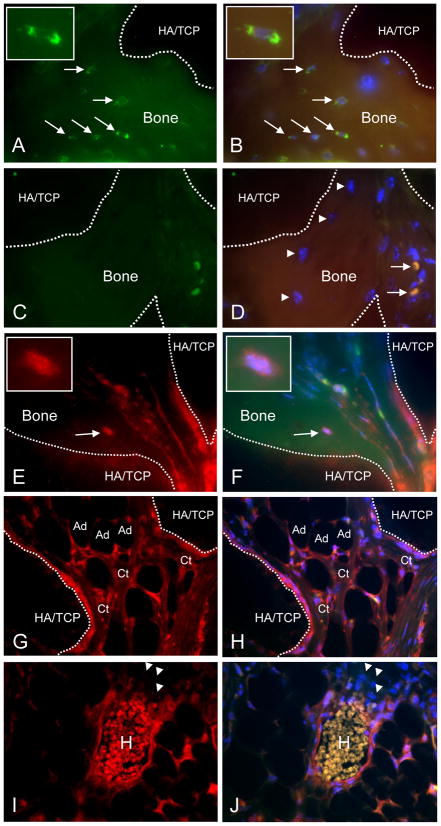
Immunohistochemical analysis of the niches formed by CD133BMSCs revealed donor and host origin of non-hematopoietic and hematopoietic cells, respectively. The presence of human CD133BMSCs within HMEs was confirmed by immunostaining for human-specific β2-microglobulin (β2M) and for DsRed2-mito (genetic tag). Human β2M-positive osteocytes were observed in lacunae within human bone that surrounded islands of HA/TCP (Fig. 4A and B). In contrast, human osteocytes were not stained on sections that were incubated with isotype control antisera (Fig. 4C and D). Osteocytes within bone also stained positive for DsRed, which was appropriately localized to mitochondria in the human cells (Fig. 4E and F). Isotype control staining for DsRed in HMEs was negative (data not shown). DsRed2-positive inter-sinusoidal and adventitial reticular cells (ARCs) within the HMEs surrounded areas of active hematopoiesis. Adipocytes and fibrous connective tissues were also DsRed2-positive (Fig. 4G–J). Notably, all hematopoietic cells were DsRed2-negative (Fig. 4I and J). Staining with antisera specific to human von Willebrand Factor did not identify human endothelial cells in the HMEs (data not shown).
Fig. 4. Immunohistochemical evaluation of CD133dMSC-seeded constructs.
A, B) Human-specific β2M staining (green, FITC channel) of osteocytes within bone formed from CD133BMSCs (arrows) (100X magnification). Dotted lines delineate bone (autofluorescent) and HA/TCP (dark areas). FITC/TRITC merge with DAPI (B). Insets: magnified view of an osteocyte. C, D) Isotype control staining (1 μg/ml IgG, 100X magnification). FITC/TRITC merge with DAPI (D). Arrowheads: osteocytes negative for staining by isotype. Arrows: strongly autofluorescent red blood cells. E, F) Staining for DsRed demonstrates characteristic punctate pattern of mitochondria within a bone-residing osteocyte (TRITC channel, E) and merge (F). Insets: DsRed-positive osteocyte. G, H) DsRed-positive inter-sinusoidal reticular cells, connective tissue (Ct), and adipocytes (Ad) in the implant. I, J) DsRed-positive peri-vascular reticular cells surrounding a sinus. H, Autofluorescent host-derived red blood cells. Arrowheads: Host-derived erythroblasts did not stain positive for DsRed. TRITC channel (E–I). TRITC/FITC merge with DAPI (F–J).
Discussion
Our data demonstrate that human CD133BMSCs seeded into HA/TCP/fibrin constructs can establish ectopic hematopoietic niches in immunodeficient mice. The presence of human cells in the HMEs was confirmed by staining for both human-specific β2M and a genetic tag (DsRed2-mito). We found that genetically-tagged CD133BMSCs differentiated into many non-hematopoietic cells known to contribute to HMEs including osteoblasts, osteocytes, adipocytes, and reticular cells. Notably, we found that CD133BMSCs contributed to both endosteal-like niches as well as sinusoidal-like niches within ectopic HMEs. In agreement with previous reports, we found that the endothelial cells and hematopoietic cells within ectopic HMEs were host-derived [11; 28].
Several different cell types present in bone marrow have been linked to the regulation of hematopoiesis. Osteoblasts that line endosteal HSC niches express the Notch receptor ligand Jagged 1 and regulate HSC activity [2], in part, through Notch-1 signaling [4]. In co-culture studies with HSCs, osteoblasts were reported to mediate long-term maintenance of HSCs (i.e. self-renewal) whereas short-term proliferation was influenced to a greater extent by sub-endosteal reticulocytes [29]. Sinusoidal endothelial cells also function in hematopoiesis [3; 30], and have been shown to regulate thrombopoiesis from megakaryocytes [30]. Sub-endothelial adventitial reticular cells (ARCs) also express Jagged 1 and contribute both structurally and functionally to sinusoidal HSC niches [11]. Human ARCs have been localized in vivo by immunohistochemistry against the p75 low affinity nerve growth factor receptor (p75LNGFR) [31]. and CD146 (MCAM) [11]. Notably, these cell surface proteins are expressed by early passage cultures of human BMSCs and antibodies against either can be used to directly isolate BMSCs from aspirated bone marrow mononuclear cells [11; 32].
Sacchetti et al. (2007) isolated self-renewing human osteoprogenitor cells on the basis of CD146 expression and demonstrated that clonal populations of the CD146-positive/CD133-negative cells were capable of forming ectopic HMEs [11]. CD146 is also expressed by endothelial cells, keratinocytes, smooth muscle cells, myofibroblasts, and lymphocytes in normal human tissues [33–35]. Previously, we demonstrated that magnetic-activated cell sorting against CD133 could be used to isolate a subpopulation of native human bone marrow stem/progenitor cells that gave rise to CD133-negative CD133BMSCs in culture [24]. Freshly-isolated adherent human CD133-positive cells were small, grew slowly, and had a distinct polyploid morphology with long, thin tenopodia and spoon-like lobopodia. In contrast, CD133BMSCs were larger, grew rapidly, and had the typical spindle-shaped morphology of cultured human BMSCs [24]. These observations were consistent with many reports showing that Prominin-1 (CD133) marks primitive stem cells (embryonic, fetal, and adult) and is downregulated during differentiation [36]. Whereas CD133 was lacking on CD133BMSCs, CD146 was uniformly and highly expressed through multiple passages. Pozzobon et al. (2009) reported that human bone marrow stem/progenitor cells isolated on the basis of CD133 expression were initially negative for CD146, but acquired its expression during expansion on cell culture plastic [37]. Taken together, the observations above indicate that a native CD133+/CD146− stem/progenitor cell from human bone can generate CD133−/CD146+ BMSCs in culture that are capable of forming ectopic HMEs. Thus, it is clearly of interest to determine whether such a lineage relationship exists in vivo for a CD133-positive stem/progenitor cell and downstream CD133-negative components of HSC niches such as osteoblasts and ARCs.
Conclusion
CD133 identifies a native human bone marrow stem/progenitor cell that gives rise to CD133BMSCs in culture. CD133BMSCs are non-hematopoietic progenitor cells that form ectopic HMEs when transplanted into immunodeficient mice.
Research highlights.
CD133-derived bone marrow stem cells can form ectopic hematopoietic niches in mice.
CD133 identifies bone marrow stem cells that lie upstream of CD146-positive cells.
CD133 is likely to identify the native human stem/progenitor cell in bone marrow.
Acknowledgments
This work was supported by grants from the National Institutes of Health: NHLBI HL085210 (JLS) and NIH/NCRR P20 RR016435 (Parsons R, COBRE PI, JLS, PI project 3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 3.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 5.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 6.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–5. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Krebsbach PH, Kuznetsov SA, Satomura K, et al. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 9.Miura Y, Gao Z, Miura M, et al. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells. 2006;24:2428–36. doi: 10.1634/stemcells.2006-0089. [DOI] [PubMed] [Google Scholar]
- 10.Muguruma Y, Yahata T, Miyatake H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–87. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 11.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Mankani MH, Kuznetsov SA, Robey PG. Formation of hematopoietic territories and bone by transplanted human bone marrow stromal cells requires a critical cell density. Exp Hematol. 2007;35:995–1004. doi: 10.1016/j.exphem.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 14.Slayton WB, Li XM, Butler J, et al. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–55. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Graves SE, Ohta S, et al. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–73. [PubMed] [Google Scholar]
- 16.Delorme B, Ringe J, Gallay N, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–5. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 17.Buhring HJ, Battula VL, Treml S, et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–71. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 18.Kuci S, Wessels JT, Buhring HJ, et al. Identification of a novel class of human adherent CD34- stem cells that give rise to SCID-repopulating cells. Blood. 2003;101:869–76. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- 19.Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–87. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 22.Sundberg M, Jansson L, Ketolainen J, et al. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009;2:113–24. doi: 10.1016/j.scr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–9. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakondi B, Shimada IS, Perry A, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–47. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spees JL, Olson SD, Whitney MJ, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mankani MH, Kuznetsov SA, Wolfe RM, et al. In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells. 2006;24:2140–9. doi: 10.1634/stemcells.2005-0567. [DOI] [PubMed] [Google Scholar]
- 27.Harris CT, Cooper LF. Comparison of bone graft matrices for human mesenchymal stem cell-directed osteogenesis. J Biomed Mater Res A. 2004;68:747–55. doi: 10.1002/jbm.a.20107. [DOI] [PubMed] [Google Scholar]
- 28.Chan CK, Chen CC, Luppen CA, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balduino A, Hurtado SP, Frazao P, et al. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319:255–66. doi: 10.1007/s00441-004-1006-3. [DOI] [PubMed] [Google Scholar]
- 30.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 31.Cattoretti G, Schiro R, Orazi A, et al. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–38. [PubMed] [Google Scholar]
- 32.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 33.Bardin N, Anfosso F, Masse JM, et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–84. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 34.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Weninger W, Rendl M, Mildner M, et al. Keratinocytes express the CD146 (Muc18/S-endo) antigen in tissue culture and during inflammatory skin diseases. J Invest Dermatol. 2000;115:219–24. doi: 10.1046/j.1523-1747.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 36.Kania G, Corbeil D, Fuchs J, et al. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells. 2005;23:791–804. doi: 10.1634/stemcells.2004-0232. [DOI] [PubMed] [Google Scholar]
- 37.Pozzobon M, Piccoli M, Ditadi A, et al. Mesenchymal stromal cells can be derived from bone marrow CD133+ cells: implications for therapy. Stem Cells Dev. 2009;18:497–510. doi: 10.1089/scd.2008.0003. [DOI] [PubMed] [Google Scholar]