Abstract
GABAB, μ-opioid, and adrenergic α2 receptors inhibit substance P release from primary afferent terminals in the dorsal horn. Studies in cell expression systems suggest that μ-opioid and GABAB receptors inhibit transmitter release from primary afferents by activating Src family kinases (SFKs), which then phosphorylate and inhibit voltage-gated calcium channels. This study investigated whether SFKs mediate the inhibition of substance P release by these three receptors. Substance P release was measured as neurokinin 1 receptor (NK1R) internalization in spinal cord slices and in vivo. In slices, NK1R internalization induced by high frequency dorsal root stimulation was inhibited by the μ-opioid agonist DAMGO and the GABAB agonist baclofen. This inhibition was reversed by the SFK inhibitor PP1. NK1R internalization induced by low frequency stimulation was also inhibited by DAMGO, but PP1 did not reverse this effect. In vivo, NK1R internalization induced by noxious mechanical stimulation of the hind paw was inhibited by intrathecal DAMGO and baclofen. This inhibition was reversed by intrathecal PP1, but not by the inactive PP1 analog PP3. PP1 produced no effect by itself. The α2 adrenergic agonists medetomidine and guanfacine produced a small but statistically significant inhibition of NK1R internalization induced by low frequency dorsal root stimulation. PP1 did not reverse the inhibition by guanfacine. These results show that SFKs mediate the inhibition of substance P release by μ-opioid and GABAB receptors, but not by α2 receptors, which is probably mediated by the binding of G protein βγ subunits to calcium channels.
Keywords: Calcium channel, dorsal horn, internalization, neurokinin 1 receptor, primary afferent
Primary afferent activity causes the release of glutamate and substance P into the spinal cord. Most dorsal horn neurons that express the neurokinin 1 receptor (NK1R) for substance P receive synapses from primary afferents and project directly to the brain (Todd et al., 2002; Todd et al., 2005). There is ample evidence that NK1Rs mediate hyperalgesia by increasing the excitability of these neurons (Traub, 1996; Mantyh et al., 1997; De Felipe et al., 1998; Henry et al., 1999; Laird et al., 2001). Substance P release from primary afferents is inhibited by several G protein-coupled receptors (GPCRs), including μ-opioid receptors (MORs) (Jessell & Iversen, 1977; Yaksh et al., 1980; Kondo et al., 2005), δ-opioid receptors (Kondo et al., 2005; Overland et al., 2009), GABAB receptors (Malcangio & Bowery, 1993; Riley et al., 1997; Marvizon et al., 1999a) and adrenergic α2 receptors (Kuraishi et al., 1985; Pang & Vasko, 1986; Ono et al., 1991a; Bourgoin et al., 1993; Takano et al., 1993). These receptors are present in substance P-containing terminals (Li et al., 1998; Stone et al., 1998; Ataka et al., 2000; Yang et al., 2001).
The conventional idea is that GPCRs inhibit neurotransmitter release through the inactivation of voltage-gated Ca2+ [Ca(V)] channels by the binding of G protein β γ subunits (Gβ γ), which is voltage-dependent (Dolphin, 2003; Evans & Zamponi, 2006; Dai et al., 2009). However, recent evidence suggests that some GPCRs may activate other signaling pathways. In primary afferents, Ca(V)2.1 (P/Q-type) and Ca(V)2.2 (N-type) channels control neurotransmitter release and are the ones inhibited by MORs (Rusin & Moises, 1995; Evans & Zamponi, 2006; Dai et al., 2009). Primary afferents contain a unique splice variant of Ca(V)2.2 channels having the 37a exon instead of the 37b exon (Bell et al., 2004; Castiglioni et al., 2006). The 37a exon contains a consensus site for tyrosine phosphorylation by Src family kinases (SFKs) that is absent in the 37b exon, and this makes these channels susceptible to a voltage-independent inhibition by MORs and GABAB receptors (Diverse-Pierluissi et al., 1997; Strock & Diverse-Pierluissi, 2004; Raingo et al., 2007). A similar voltage-independent inhibition by SFK phosphorylation may affect Ca(V)2.1 (type P/Q) channels (Weiss & Burgoyne, 2001). It has been proposed that, unlike MORs and GABAB receptors, α2 receptors do not inhibit Ca(V) channels through SFK phosphorylation, but only through Gβ γ binding (Strock & Diverse-Pierluissi, 2004).
SFKs are a group of ten enzymes that catalyze the phosphorylation of tyrosine residues and that form part of many key signaling pathways in mammalian cells (Thomas & Brugge, 1997). Five SFKs (Src, Fyn, Lck, Lyn and Yes) play important functions in the CNS, particularly in synaptic plasticity, by regulating the activity of Ca(V) channels and NMDA receptors and (Kalia et al., 2004; Xu et al., 2008; Zhang et al., 2008; Chen et al., 2010). Protein tyrosine phosphatases (PTPs) catalyze the dephosphorylation of the tyrosine residues phosphorylated by SFKs (Thomas & Brugge, 1997).
Here we investigate whether SFKs mediate the inhibition of substance P release by MORs, GABAB receptors and α2 receptors. We used NK1R internalization to measure substance P release, a method that is more sensitive than radioimmunoassay (Marvizon et al., 2003a), identifies the sites of release (Mantyh et al., 1995; Abbadie et al., 1997; Allen et al., 1997; Honore et al., 1999; Adelson et al., 2009) and reflects NK1R activation (Trafton et al., 1999; Trafton et al., 2001).
Materials and methods
Animals
Animals used in this study were male, Sprague-Dawley rats purchased from Harlan (Indianapolis, IND). A total of 66 rats were used in the study. Spinal cord slices were prepared from 17 juvenile rats (3–5 weeks old). Intrathecal catheters were implanted in 49 adult rats (2–4 months old). The anesthetic used and other procedural details are given below. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System, and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize the number of animals used and their suffering.
Chemicals and media
PP1 [1-(1,1-dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3, 4-d]pyrimidin-4-amine], PP2 [3-(4-chlorophenyl)-1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine], PP3 [1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine] and Tocrisolve-100 (20% soya oil emulsified in water with Pluronic F68) were from Tocris (Ellisville, MO). Dasatinib was from ChemieTek (Indianapolis, IN). Isoflurane was from Halocarbon Laboratories (River Edge, NJ). Prolong Gold was from Invitrogen (Eugene, OR). Baclofen, DAMGO and other chemicals were from Sigma.
Artificial cerebrospinal fluid (aCSF) contained (in mM) 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2 and 10 glucose; K+-aCSF contained 5 mM of KCl, and sucrose-aCSF contained 5 mM KCl and 215 mM sucrose instead of NaCl (iso-osmotic replacement). All these media were constantly bubbled with 95% O2/5% CO2.
Compounds were dissolved in water except for the following. For experiments in slices, PP1, PP2 and PP3 were dissolved at 10 mM in dimethyl-sulfoxide (DMSO) and then diluted to their desired concentrations in aCSF. For intrathecal injection of a 3 nmol dose of PP1, it was dissolved at 10 mM in DMSO and then diluted in saline, resulting in a final concentration of DMSO in the injectate of 3%. For intrathecal injection of a 10 nmol dose of PP1, it was dissolved at 100 mM in Tocrisolve-100 and then diluted in saline, resulting in a final concentration of Tocrisolve-100 in the injectate of 1%.
Spinal cord slices
Spinal cords were obtained from 3–5 weeks old male Sprague-Dawley rats by dorsal laminectomy. The rats were anesthetized with 3% isoflurane in an induction box and kept under isoflurane anesthesia during the extraction of the spinal cord, which took less than 2 min and included euthanasia by bilateral thoracotomy. Coronal slices (400 μm) with one dorsal root were cut with a vibratome (Integraslice 7550PSDS, Campden Instruments USA, Lafayette, IN) from a lumbar spinal cord segment (L2–L4), as described (Marvizon et al., 2003a; Lao & Marvizon, 2005; Adelson et al., 2009). The spinal cord segment was glued vertically to a block of agar on the stage of the vibratome and immersed in ice-cold sucrose-aCSF. Slices were cut using minimum forward speed and maximum vibration while observing them with a stereo microscope mounted over the vibratome. Fiber continuity between the dorsal root and the dorsal horn was assessed by examining the dorsal root and the dorsal surface of the slice with the stereo microscope. Slices were discarded if they did not meet the following criteria: 1) at least 80% of the dorsal funiculus had to be continuous with the dorsal root, and 2) the dorsal root had no cuts or compression damage. Slices were kept for one hour in K+-aCSF at 35 °C and then in regular aCSF at 35 °C, and were used within 3 hr of preparation.
Dorsal root stimulation of slices
The dorsal root attached to the slice was electrically stimulated using a custom-made chamber, as previously described (Marvizon et al., 2003b; Adelson et al., 2009). Slices were superfused at 3–6 ml/min with aCSF at 35 °C. The root was placed on a bipolar stimulation electrode (platinum wire of 0.5 mm diameter, 1 mm pole separation) in a compartment separated from the superfusion chamber by a grease bridge. The root and the electrodes were covered with mineral oil and any excess aCSF was suctioned away. This ensured that electrical current circulated through the root and that the stimulus was consistent between preparations. Electrical stimulation was generated by a Master-8 stimulator and an Iso-Flex stimulus isolating unit in constant voltage mode (A.M.P. Instruments, Jerusalem, Israel), and consisted of 1000 square pulses of 20 V and 0.4 ms (C-fiber intensity) delivered at 1 Hz or 100 Hz. Slices were superfused at 3–6 ml/min with aCSF at 35 °C. Drugs were present in the superfusate continuously starting 5 or 10 min before root stimulation. Ten minutes after the stimulus, slices were fixed by immersion in ice-cold fixative (4% paraformaldehyde, 0.18% picric acid in 0.1 M sodium phosphate buffer). A round hole was punched in the ventral horn of the slice ipsilateral to the stimulus in order to identify it in the histological sections after immunohistochemistry.
Intrathecal injections
To deliver drugs to the lumbar spinal cord, rats were surgically implanted with chronic intrathecal catheters inserted between the L5 and L6 lumbar vertebrae (Storkson et al., 1996; Chen & Marvizon, 2009). Rats (2–4 months old rats) were anesthetized with isoflurane (2–4% in oxygen) and kept under anesthesia on a metal platform kept at 35 °C by a feedback device. The skin and muscle were cut to expose vertebrae L5 and L6. A 20G needle was inserted between the L5 and L6 vertebrae to puncture the dura mater, which was inferred from a flick of the tail or paw and the backflow of spinal fluid. The needle was removed and the catheter (20 mm of PE-5 tube heat-fused to 150 mm of PE-10 tube) was inserted into the subdural space and pushed rostrally to terminate over L5–L6. The PE-10 catheter was then tunneled under the skin and externalized over the head. The skin was sutured, and the catheter was flushed with 10 μl saline and sealed. Rats were given an antibiotic (enrofloxacin) and an analgesic (carprofen) for 3 days after surgery. Rats were housed separately and used for the experiment 5–7 days after surgery. A criterion for immediate euthanasia of the rat was the presence of motor weakness or signs of paresis, but this did not occur in any of the rats in this study.
Intrathecal injection volume was 10 μl of injectate plus 10 μl saline flush (Zorman et al., 1982; Jensen & Yaksh, 1984; Aimone et al., 1987; Kondo et al., 2005). This volume leads to the distribution of the injectate over most of the spinal cord, but not into the brain (Yaksh & Rudy, 1976; Chen et al., 2007). Solutions are preloaded, in reverse order of administration, into a tube (PE-10), and delivered with a 50 μl Hamilton syringe within 1 min. The position of the catheter was examined postmortem. We established as exclusion criteria: 1) loss of the catheter, 2) termination of the catheter inside the spinal cord, and 3) occlusion of the catheter tip. Twelve of the 49 rats that were implanted with catheters were excluded from the study according with these criteria.
Noxious mechanical stimulation
A noxious mechanical stimulus was used to induce NK1R internalization in vivo, and was given 5–7 days after implanting the intrathecal catheters. Rats were anesthetized with isoflurane (2–3%) in an induction box and kept under isoflurane anesthesia until they were euthanized. After the intrathecal injections, one hind paw was clamped with a hemostat (closed to the first notch) for 30 sec (Le Bars et al., 1987). Ten minutes later, rats were euthanized with pentobarbital (100 mg/Kg). Rats were fixed immediately by aortic perfusion of 100 ml phosphate buffer (0.1 M sodium phosphate, pH 7.4) containing 0.01% heparin, followed by 400 ml of ice-cold fixative (4% paraformaldehyde, 0.18% picric acid in phosphate buffer).
Antibody characterization
The NK1R antibody was rabbit antiserum # 94168, made at CURE: Digestive Diseases Research Center, UCLA, under the sponsorship of Dr. Nigel Bunnett, UCSF. It was generated in rabbits using a peptide corresponding to the C-terminus of the rat NK1R (amino acids 393–407) coupled to KLH (Grady et al., 1996). It labeled by immunofluorescence cells transfected with rat NK1R, and it did not label nontransfected cells. Staining of the transfected cells was eliminated by preadsorption with its immunizing peptide. In Western blots from cells transfected with the NK1R, it produced a single band corresponding to a molecular weight of 100 kDa (Grady et al., 1996).
Immunohistochemistry
Spinal cord slices were fixed, cryoprotected, frozen and re-sectioned at 25 μm in a cryostat as described (Marvizon et al., 2003a; Adelson et al., 2009). Rats were fixed by aortic perfusion as described above, and the L4–L5 lumbar spinal cord segments were similarly processed and sectioned at 25 μm in the coronal plane (Chen et al., 2007; Lao et al., 2008). Sections were washed four times and then incubated overnight with the NK1R antiserum diluted 1:3000 in phosphate-buffered saline containing 0.3 % Triton X-100, 0.001 % thimerosal and 10 % normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, goat anti-rabbit IgG coupled to Alexa Fluor 488, Molecular Probes-Invitrogen, Eugene, OR) was applied at for 2 hours. Sections were washed four more times, mounted on glass slides, and coverslipped with Prolong Gold (Molecular Probes-Invitrogen). All incubations were done at room temperature.
Quantification of NK1R internalization
The amount of NK1R internalization was quantified using a standard method (Mantyh et al., 1995; Marvizon et al., 2003a; Adelson et al., 2009). NK1R neurons were visually counted while classifying them as with or without internalization, using a Zeiss Axio-Imager A1 microscope with a 63x oil immersion (numerical aperture [NA] 1.40) objective. The criterion for having internalization was the presence in the neuronal soma of ten or more NK1R endosomes, defined as a small region of bright staining separated from the cell surface (Fig. 1). The person counting the neurons was blinded to the treatment. All NK1R neurons in lamina I were counted in each histological section. In experiments in slices, at least three sections per slice were counted. In experiments in vivo, at least four sections were counted in the L4–L5 spinal segment for each rat.
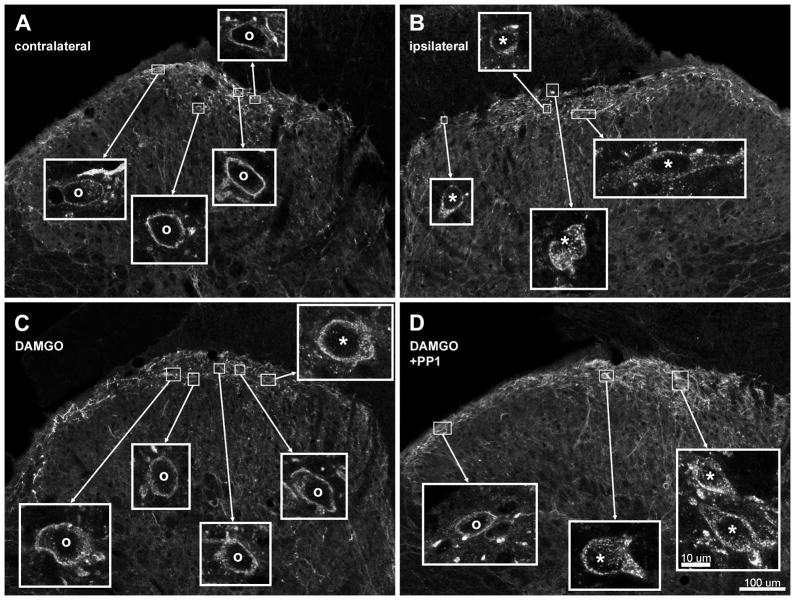
Fig. 1. Confocal images of NK1R neurons in lamina I after noxious stimulation of the hind paw in vivo.
Rats received a noxious stimulus (clamp for 30 sec) in the hind paw to induce substance P release and were fixed 10 min later for NK1R immunohistochemistry. Images were taken from sections of the L4–L5 spinal segments. A, B: Rats received an intrathecal injection of saline 10 min before the stimulus; A and B correspond to the contralateral and ipsilateral dorsal horns, respectively, of the same histological section. B: Rats received an intrathecal injection of DAMGO (2 nmol) 10 min before the stimulus; ipsilateral dorsal horn. C: Rats received intrathecal injections of DAMGO (2 nmol) and PP1 (10 nmol) 10 min before the stimulus and of PP1 (10 nmol) 70 min before the stimulus; ipsilateral dorsal horn. Main panels: images taken with a 10x objective, with a voxel size of 830 × 830 × 5983 nm and 2 confocal planes. Insets: images of lamina I neurons taken with a 63x objective, with a voxel size of 132 × 132 × 383 nm and 3 confocal planes. Neurons with NK1R internalization are indicated with “*” and neurons without internalization by “o”. Scale bars (in panel D) are 100 μm for the main panels and 10 μm for the insets.
Confocal microscopy
Images were acquired using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY), with 10x (NA 0.3) and 63x oil immersion (NA 1.4) objectives. Excitation light for the Alexa Fluor 488 fluorophore (emission peak 519 nm) was provided by the 488 nm line of an argon laser and the emission window was 500–580 nm. The pinhole was 1.0 Airy unit: 38.2 μm for the 10x objective and 51.5 μm for the 63x objective, as determined by the confocal microscope software. Images were acquired in grayscale as confocal stacks of sections of 1024×1024 pixels. Photomultiplier gain and offset was individually adjusted for each image to avoid pixel saturation and loss of background detail. Each section was averaged 2 or 4 times to reduce noise. The separation between confocal sections, optimized by the confocal microscope software using the Nyquist equation, was 5.98 μm for the 10x objective and 0.38 μm for the 63x objective.
Image processing
Images of the dorsal horn obtained with the 10x objective were used to show the location of the neurons imaged with the 63x objective (Fig. 1). The program Imaris 6.1.5 (x64, Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions. Images at 10x were cropped in x-y to 1024 × 768 pixels, and in the z dimension to the two brightest optical sections. Images at 63x were cropped in x-y to show the soma and proximal dendrites of the target neurons, and in the z dimension into three optical sections through the middle of the soma. Occasionally, several neurons were cropped from the same confocal stack. Image resolution was preserved in the cropping, so that pixels in Fig. 1 correspond to the pixels acquired by the confocal microscope. Voxel dimensions were 830 × 830 × 5983 nm with the 20x objective and 132 × 132 × 383 nm with the 100x objective. After cropping, a two-dimension projection picture was generated in Imaris and imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), which was used to make slight adjustments in the gamma of the images so that important details are clearly visible in Fig. 1. Adobe Photoshop was also used to compose the multi-panel figures and to add text and arrows.
Data analysis
Data were analyzed using Prism 5 (GraphPad Software, San Diego, CA). Statistical analyses consisted of two-way ANOVA and Bonferroni’s post-hoc test. Statistical significance was set at 0.05. The two variables were “drugs” (drug combinations) and “stimulus” (comparing the ipsilateral and contralateral sides). The Bonferroni’s post-hoc test was applied to the variable “drugs” to compare effects on the ipsilateral side.
Results
Effect of SFK inhibitors on MOR and GABAB receptor inhibition of substance P release in spinal cord slices
We hypothesized that the inhibition of substance P release produced by MORs and GABAB receptors is mediated by SKFs. If so, then SFK inhibitors should reverse the inhibition produced by MOR and GABAB receptor agonists. In a first experiment, substance P release was induced in rat spinal cord slices by electrical stimulation of the dorsal root at high frequency (100 Hz), using stimulation parameters previously found to be optimal to induce substance P release (Adelson et al., 2009). Substance P release was measured as NK1R internalization in lamina I neurons. The stimulus induced NK1R internalization in the ipsilateral dorsal horn, but not contralaterally. Superfusing the slices with the MOR agonist DAMGO (1 μM, Fig. 2A) or the GABAB receptor agonist baclofen (30 μM, Fig. 2B) significantly inhibited the evoked NK1R internalization. The concentration of DAMGO was chosen based on its concentration-response curve to induce MOR internalization in rat spinal cord slices (IC50 = 30 nM, 95% CI = 20–45 nM) (Marvizon et al., 1999b). Moreover, 1 μM DAMGO inhibited NK1R internalization induced by dorsal root stimulation in slices, and this inhibition was reversed by 10 μM naloxone (Kondo et al., 2005). The concentration of baclofen was chosen based on its concentration-response to inhibit NK1R internalization induced by dorsal root stimulation in slices (IC50 = 1.5 μM, 95% CI = 0.4–5.5 μM) (Lao & Marvizon, 2005). Therefore, the chosen concentrations of DAMGO and baclofen are near-saturation for their target receptors.
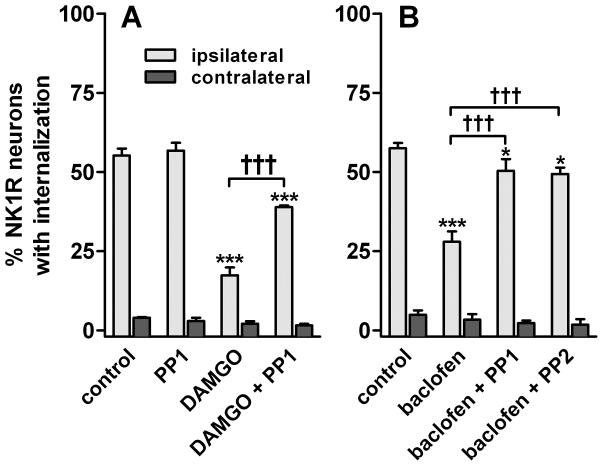
Fig. 2. NK1R internalization induced by 100 Hz dorsal root stimulation of spinal cord slices: SFK inhibitors reversed the inhibition by DAMGO and baclofen.
Spinal cord slices (n=3–4) were stimulated at the dorsal root at 100 Hz to induce substance P release. A. Slices were superfused with aCSF (control), 10 μM PP1, 1 μM DAMGO or DAMGO + PP1. B. Slices were superfused with aCSF, 30 μM baclofen, baclofen + 10 μM PP1 or baclofen + 10 μM PP2. Treatments with PP1 and PP2 were preceded by 1 hr preincubation with these drugs at 10 μM. Two-way ANOVA: p<0.0001 for stimulus and drugs. Bonferroni’s post-hoc test: *, p<0.05, ***, p<0.001 compared to control; †††, p<0.001 as indicated.
To determine whether the inhibition produced by MORs and GABAB receptors was mediated by SFKs, we used PP1 and PP2, which are selective inhibitor of SFKs, particularly of Lck and Fyn (Hanke et al., 1996; Liu et al., 1999). PP1 and PP2 inhibited human T-cell proliferation with IC50s ranging from 0.5 μM to 26 μM, depending of the stimulus used to induce proliferation (Hanke et al., 1996). Therefore, we chose a concentration of 10 μM for PP1 and PP2. As shown in Fig. 2, PP1 reversed the inhibition of the evoked NK1R internalization by DAMGO or baclofen, but produced no effect by itself. Inhibition by baclofen was also reversed by PP2. PP1 and PP2 were applied to the slices for 1 hr before the stimulus and then together with the agonist. This length of time appears to be necessary for inhibition of SFKs and/or de-phosphorylation of their substrates by PTPs in primary afferent neurons (McRoberts et al., 2007; Chen et al., 2010). Two-way ANOVA of the data with DAMGO (Fig. 2A) revealed significant effects of the variables ‘stimulus’ (F1=1346, p<0.0001), ‘drugs’ (F3=81, p<0.0001) and their interaction (F3=70, p<0.0001). Similarly, two-way ANOVA of the data with baclofen (Fig. 2B) revealed significant effects of ‘stimulus’ (F1=761, p<0.0001), ‘drugs’ (F3=16, p<0.0001) and their interaction (F3=14, p<0.0001).
Next, we investigated whether the inhibition of substance P release by DAMGO and its reversal by the SFK inhibitor PP1 also occurred when substance P release was evoked by low frequency (1 Hz) stimulation of the dorsal root (Fig. 3). Because this stimulus induces a relatively small amount of NK1R internalization (Marvizon et al., 1997; Marvizon et al., 2003b; Adelson et al., 2009), we increased it by using peptidase inhibitors (captopril and thiorphan, 10 μM) to reduce substance P degradation (Marvizon et al., 2003b; Adelson et al., 2009). Stimulation at 1 Hz with peptidase inhibitors induced a somewhat higher amount of NK1R internalization than 100 Hz stimulation without peptidase inhibitors (Fig. 2). DAMGO (10 μM) substantially inhibited the evoked NK1R internalization, but this time the inhibition was not reversed by 10 μM PP1. As before, PP1 was applied to the slices for 1 hr before the stimulus and then together with DAMGO. Two-way ANOVA of data in Fig. 3 revealed significant effects of the variables ‘stimulus’ (F1=98, p<0.0001), ‘drugs’ (F2=34, p<0.0001) and their interaction (F2=29, p<0.0001).
Fig. 3. NK1R internalization induced by 1 Hz dorsal root stimulation of spinal cord slices: a SFK inhibitor did not reverse the inhibition by DAMGO.
Spinal cord slices were stimulated at the dorsal root at 1 Hz to induce substance P release while being superfused with the peptidase inhibitors captopril (10 μM) and thiorphan (10 μM) alone (control), with 1 μM DAMGO or with 1 μM DAMGO + 10 μM PP1. Treatment with PP1 was preceded by 1 hr preincubation with 10 μM PP1. Two-way ANOVA: p<0.0001 for stimulus, drugs and interaction. Bonferroni’s post-hoc test: ***, p<0.001 compared to control. Number of slices (n) for each data set is given inside the columns.
SFK inhibitors reversed MOR and GABAB receptor inhibition of substance P release in vivo
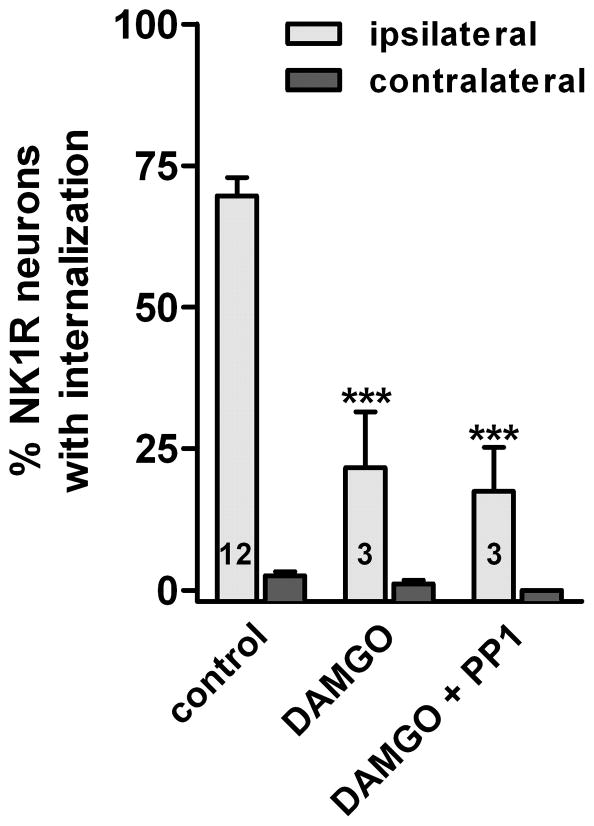
This experiment was performed in vivo by inducing substance P release with a noxious stimulus: clamping one hind paw with a hemostat for 30 sec, under anesthesia (Mantyh et al., 1995; Lao et al., 2008; Chen & Marvizon, 2009). This stimulus induced abundant NK1R internalization in lamina I of the ipsilateral L4–L5 segments (Fig. 1B), but none contralaterally (Fig. 1A). Quantitative results are shown in Fig. 4. The MOR agonist DAMGO (2 nmol, Fig. 1C) or the GABAB agonist baclofen (50 nmol) injected intrathecally 10 min before paw clamp significantly reduced the evoked NK1R internalization. This intrathecal dose of DAMGO was chosen because 1 nmol DAMGO strongly inhibited NK1R internalization and produced near-maximal analgesia in previous studies (Trafton et al., 1999; Kondo et al., 2005). Both effects were reversed by naloxone (Kondo et al., 2005). Likewise, the dose of baclofen was chosen based on its dose-responses to inhibit NK1R internalization induced by noxious stimulation (Riley et al., 2001) and to produce thermal antinociception (Malan et al., 2002). In both cases the effect of baclofen was near-maximal at 40 nmol.
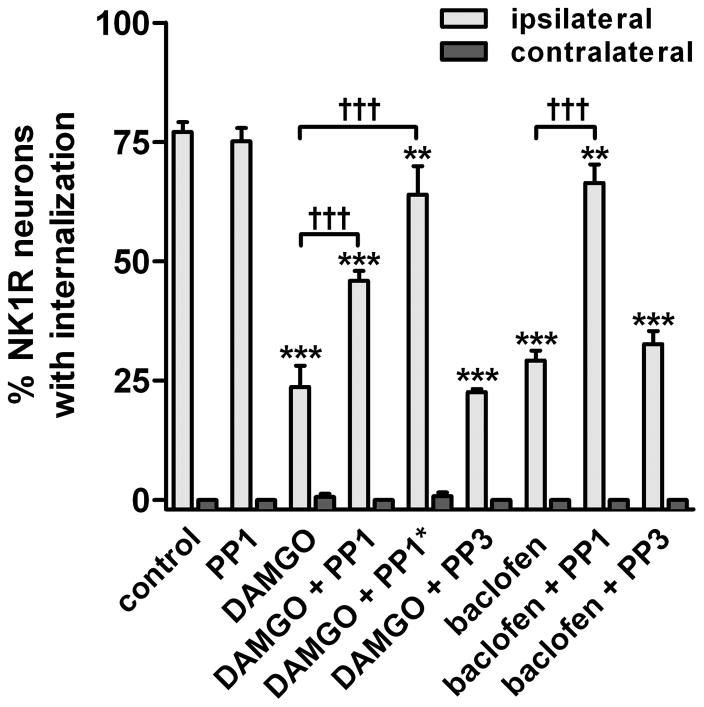
Fig. 4. SFK inhibitors reverse the inhibition by DAMGO and baclofen of NK1R internalization induced by noxious stimulation in vivo.
Rats (n=3) received an i.t. injection of 3 nmol PP1 (PP1*: 10 nmol), 3 nmol PP3, or no injection. One hour later they received an i.t. injection of DAMGO (2 nmol) or baclofen (50 nmol) with or without PP1 or PP3, as indicated. Noxious stimulation (hindpaw clamp for 30 sec) was delivered 10 min after the second injection, and rats were fixed 10 min later. NK1R internalization was measured in the L4–L5 spinal segments. Two-way ANOVA: p<0.0001 for ‘drugs’ and ‘stimulus’. Bonferroni’s post-hoc test: ***, p<0.001, **, p<0.01 compared to control; †††, p<0.001 as indicated.
To study the involvement of SFKs, the SKF inhibitor PP1 was injected intrathecally 1 hr before the injection of DAMGO or baclofen and then coinjected with these compounds. At a dose of 3 nmol, PP1 produced a near complete reversal of the inhibition by baclofen and a partial reversal of the inhibition by DAMGO (Fig. 4). Increasing the dose of PP1 to 10 nmol (PP1* in Fig. 4, see ‘Chemicals and media’ in Material and Methods) resulted in a near complete reversal of the inhibition by DAMGO, as can be observed in the example image in Fig. 1D. PP1 by itself (3 nmol intrathecally) did not affect the evoked NK1R internalization ipsilaterally or induced NK1R internalization contralaterally, showing that SFKs do not affect the internalization mechanism of NK1Rs. The inactive PP1 analog PP3 (3 nmol) or vehicle (1 % Tocrisolve-100) did not affect the inhibition produced by DAMGO or baclofen, indicating that the effects of PP1 were mediated by SFKs. Two-way ANOVA of the data in Fig. 4 revealed significant effects of the variables ‘stimulus’ (F1=1435, p<0.0001) and ‘drugs’ (F8=37, p<0.0001) and their interaction (F8=37, p<0.0001). These results indicate that inhibition of substance P release by MORs and GABAB receptors is mediated by SFKs.
A SFK inhibitor did not reverse the α2 adrenergic inhibition of substance P release
Substance P release from primary afferent terminals is also inhibited by α2 adrenergic receptors (Kuraishi et al., 1985; Pang & Vasko, 1986; Ono et al., 1991b; Bourgoin et al., 1993; Takano et al., 1993; Overland et al., 2009). Accordingly, we investigated whether α2 receptor agonists inhibit NK1R internalization evoked by stimulating spinal cord slices at the dorsal root at high and low frequencies, and whether this inhibition is mediated by SFKs.
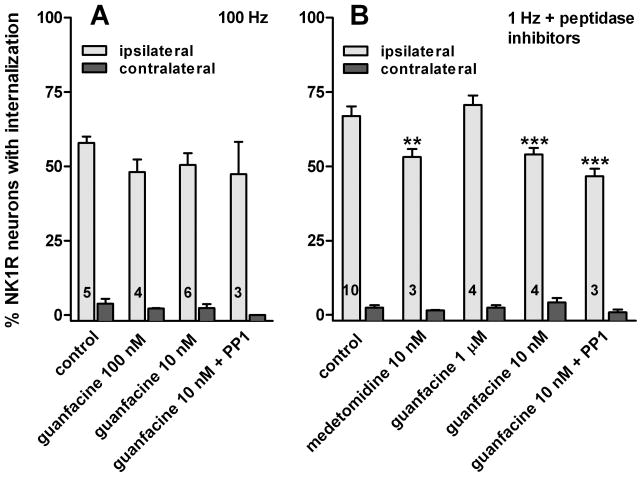
When NK1R internalization was evoked with high frequency (100 Hz) stimulation, the α2 agonist guanfacine (10 nM or 100 nM, Fig. 5A) produced a trend towards inhibition that was not significant in a two-way ANOVA (F1=358, p<0.0001 for ‘stimulus’; F3=1.46, p=0.25 for ‘guanfacine’, and F3=0.53, p=0.66 for their interaction). The SFK inhibitor PP1 (10 μM) produced no significant effects, either.
Fig. 5. Inhibition of NK1R internalization by α2 adrenergic agonists in spinal cord slices.
Spinal cord slices were stimulated at the dorsal root to evoke substance P release. The α2 agonists medetomidine and guanfacine were superfused to the slices at the concentrations indicated. PP1 (10 μM) was applied in a 1 hr preincubation and then added to guanfacine in the superfusate. Number of slices (n) for each data set is indicated by numbers inside the columns. A. 100 Hz stimulation; two-way ANOVA: p<0.0001 for ‘stimulus’, p=0.25 for ‘drugs’, p=0.66 for their interaction. B. 1 Hz stimulation in the presence of peptidase inhibitors (captopril and thiorphan, 10 μM); two-way ANOVA: p<0.0001 for ‘stimulus’, p=0.0002 for ‘drugs’, p=0.0002 for their interaction. Bonferroni’s post-hoc tests: **, p<0.01, ***, p<0.001 compared to control.
We then evoked NK1R internalization with low frequency (1 Hz) stimulation of the dorsal root. As in the experiment in Fig. 2, peptidase inhibitors (captopril and thiorphan, 10 μM) were used to reduce substance P degradation and hence boost NK1R internalization. In these conditions, the α2 agonists guanfacine and medetomidine at a concentration of 10 nM produced a small but statistically significant inhibition of the evoked NK1R internalization (Fig. 5B). Guanfacine produced no inhibition at a higher concentration (1 μM). Inhibition of substance P release by 10 nM guanfacine was not reversed by PP1 (10 μM, Fig. 5B). If anything, PP1 showed a trend (not significant in the Bonferroni’s post-hoc test) to increase the inhibition by guanfacine. Here again, PP1 was applied to the slices for 1 hr before the stimulus and then together with guanfacine. Two-way ANOVA of the data in Fig. 5B revealed significant effects of the variables ‘stimulus’ (F1=998, p<0.0001), ‘drugs’ (F4=7.2, p=0.0002) and their interaction (F4=6.18, p=0.0002). These results indicate that inhibition of substance P release by α2 receptors is not mediated by SFKs.
Discussion
Our results show that blockade of SFKs reverses the inhibition of substance P release produced by MORs and GABAB receptors, but not by α2 receptors.
SFKs mediate the inhibition of substance P release by MORs and GABAB receptors
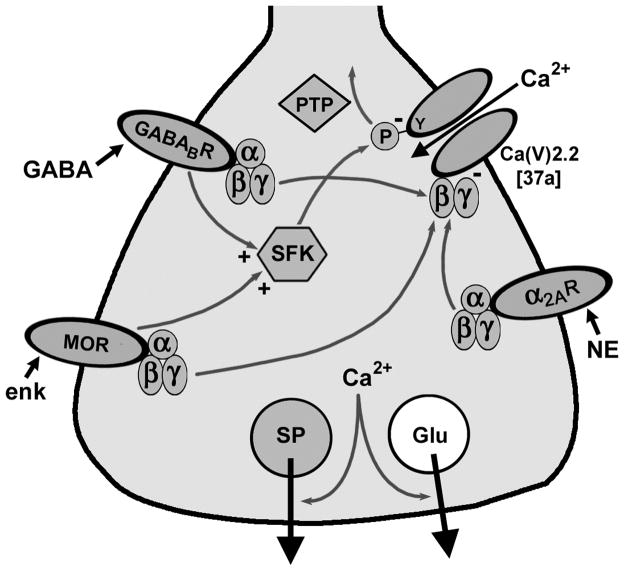
Our results, together with previous studies in cell expression systems (Raingo et al., 2007) and in cultures of embryonic chicken sensory neurons (Diverse-Pierluissi et al., 1997; Strock & Diverse-Pierluissi, 2004), support the two mechanisms for the inhibition of substance P release by GPCRs illustrated in Fig. 6. One of these mechanisms is the voltage-dependent blockade of Ca(V) channels by Gβ γ binding (Dolphin, 2003; Evans & Zamponi, 2006; Dai et al., 2009). The second mechanism involves the activation of SFKs, which then inhibit Ca(V) channels by tyrosine phosphorylation. Since the entry of Ca2+ in the terminal through Ca(V) channels triggers glutamate and substance P release, blockade of the channels results in inhibition of transmitter release.
Fig. 6. Mechanisms of inhibition of substance P release from primary afferent terminals.
Substance P (SP) and glutamate (Glu) are released when Ca2+ enters the terminal through Ca(V) channels. MORs, GABAB receptors (GABABRs) and α2A receptors inactivate (-) Ca(V)2.2 through Gβ γ. MORs and GABABRs also inactivate Ca(V)2.2 by Tyr (Y)-phosphorylation by SFKs. NE: norepinephrine; enk: enkephalin.
Ca(V) channels present in primary afferent terminals are mainly Ca(V)2.2 (N-type), although Ca(V)2.1 (P/Q-type) channels are also present (Rusin & Moises, 1995; 1998). In nodose and dorsal root ganglia, GABAB receptors inhibit exclusively Ca(V)2.2 channels, whereas MORs inhibit both Ca(V)2.2 and Ca(V)2.1 channels (Rusin & Moises, 1995; 1998). Ca(V)2.2 channels in primary afferent neurons have a unique splice variant of their α1 subunit containing the 37a exon instead of the 37b exon (Raingo et al., 2007), which is largely absent in other CNS neurons (Bell et al., 2004; Castiglioni et al., 2006). Importantly, the 37a exon, but not the 37b exon, contains a tyrosine residue susceptible to SFK phosphorylation (Raingo et al., 2007). In HEK293 cells expressing Ca(V)2.2e[37a] channels, MORs and GABAB receptors activate SFKs, which phosphorylate Ca(V)2.2 channels, inhibiting them. Similar findings were obtained in cultures of embryonic chicken sensory neurons (Diverse-Pierluissi et al., 1997; Strock & Diverse-Pierluissi, 2004), where Ca(V)2.2 channels were found to form a complex with Src kinase (Richman et al., 2004) and Src activation by GABAB receptors inhibited Ca(V)2.2 channels (Schiff et al., 2000). Although SFK inhibition of Ca(V)2.1 channels has not been studied in primary afferents, SFKs were found to phosphorylate and inhibit Ca(V)2.1 channels in adrenal chromaffin cells (Weiss & Burgoyne, 2001). Hence, this may contribute to the MOR inhibition of substance P release.
Our data show that SFK-mediated inhibition of Ca(V) channels does occur in mammalian primary afferents and plays a key role in controlling substance P release. When substance P release was evoked by high frequency stimulation, SFK inhibitors reversed most of the inhibition by GABAB receptors and MORs, indicating that SFKs is the primary inhibitory mechanism in these conditions. In contrast, when substance P release was evoked by low frequency stimulation, the SFK inhibitor PP1 did not reverse the inhibition by MORs. A possible explanation for this frequency dependence is that high frequency firing of primary afferents produces a large depolarization of their terminals, which hinders the inhibition of Ca(V) channels by Gβ γ binding (Raingo et al., 2007). In addition, our results suggest that the SFK mechanism is turned off during low frequency firing of primary afferents, and that Gβ γ binding mediates MOR inhibition in these conditions.
SFKs do not mediate the inhibition of substance P release by α2 adrenergic receptors
We show that the α2 agonists guanfacine and medetomidine inhibit NK1R internalization evoked by dorsal root stimulation in spinal cord slices, although their effect was small and detected only at low concentrations. Similar low concentrations (1–10 nM) of the α2 receptor agonist clonidine were found to inhibit the release of calcitonin gene-related peptide (CGRP) from rat spinal cord slices (Overland et al., 2009). Notably, inhibition of substance P release by guanfacine was only statistically significant when using low frequency stimulation. This is consistent with the idea that α2 receptors do not inhibit Ca(V) channels through SFK phosphorylation (Strock & Diverse-Pierluissi, 2004), but only through the voltage-dependent binding of Gβ γ (Fig. 6) (Dolphin, 2003; Evans & Zamponi, 2006; Dai et al., 2009). Thus, terminal depolarization produced by high frequency stimulation would prevent Gβ γ binding and therefore inhibition by the α2 receptors. Further support for this idea comes from the fact that the SFK inhibitor PP1 did not reverse the inhibition of substance P release by guanfacine.
A previous study in vivo by Nazarian et al. (2008) failed to detect inhibition of the evoked NK1R internalization by the α2 agonists ST-91 and dexmedetomidine (an enantiomer of medetomidine). This study used two different noxious stimuli to induce NK1R internalization: formalin injection and paw compression, and several doses of dexmedetomidine (0.3–10 μg or 1.3–43 nmol). We attribute the differences from our study to two possible causes. First, Nazarian et al. anesthetized the rats with pentobarbital, which may have blocked the effect of the α2 receptors. Second, these intense noxious stimuli probably induce high frequency firing of primary afferents, which we found hinders the inhibition by α2 receptors. Nazarian et al. pointed out that α2 receptor inhibition of substance P release was detected in studies using spinal cord slices (Pang & Vasko, 1986; Ono et al., 1991b; Takano et al., 1993) but not in vivo. However, the two studies in vivo (Lang et al., 1994; Zhao et al., 2004) that they cite were conducted in cats, suggesting that there may be species differences. Another study done in vivo in rabbits (Kuraishi et al., 1985) did report a noradrenergic inhibition of substance P release. In any case, the results by Nazarian et al. agree with our observation that the inhibition of substance P release by α2 receptors is much less robust than the inhibition produced by MORs and GABAB receptors. It is possible that α2 receptors in primary afferents work primarily in synergy with opioid receptors. Overland et al. (2009) recently reported a strong synergy between α2 and δ-opioid receptors to inhibit CGRP release from primary afferents and to produce analgesia. Another study (Tan et al., 2009) found that α2A receptors associate with MORs in primary afferents: they co-internalize and cross-desensitize.
Physiological relevance
An intriguing possibility is that the SFKs activated by MORs and GABAB receptors phosphorylate proteins other than the Ca(V) channels. Thus, we recently reported that NMDA receptors in primary afferent terminals require SFK phosphorylation to be activated (Chen et al., 2010). Although it is not clear whether these SFKs are the same that are activated by the MORs, this possibility is supported by another recent report (Zhou et al., 2010) showing that MORs present presynaptically in primary afferent terminals induce long-term potentiation of these synapses, and that this requires activation of NMDA receptors.
Pathological states like inflammation and neuropathic pain probably induce changes in MORs, GABAB receptors and α2 receptors, or in their signaling mechanisms, which may be result in hyperalgesia and altered responses to analgesics. The fact that NK1R antagonists reverse the hyperalgesia and allodynia produced by nerve injury (Cumberbatch et al., 1998; Cahill & Coderre, 2002) indicates that substance P does play a role in neuropathic pain. However, after nerve injury substance P synthesis decreases in C-fibers (Hokfelt et al., 1994) and occurs de novo in A-fibers (Marchand et al., 1994; Noguchi et al., 1994; Noguchi et al., 1995; Fukuoka et al., 1998). Therefore, it has been proposed that A-fibers are the source of substance P in neuropathic pain. Indeed, Malcangio et al. (2000) found that Aβ-fiber stimulation induced substance P release after spinal nerve lesion. However, other investigators studying nerve injury models could not detect substance P immunoreactivity in A-fiber terminals (Noguchi et al., 1995; Hughes et al., 2007) or substance P release induced by A-fiber stimulation (Allen et al., 1999; Hughes et al., 2007). Instead, Allen et al. (1999) found that nerve transection resulted in an increase in substance P release induced by C-fiber stimulation.
Morphine loses its effectiveness in neuropathic pain in humans (Arner & Meyerson, 1988; Portenoy et al., 1990) and when injected intrathecally in rodent nerve injury models (Mao et al., 1995; Ossipov et al., 1995b; a; Wegert et al., 1997). This seems to be largely caused by the downregulation of MORs in primary afferents (Zhang et al., 1998; Kohno et al., 2005). Ca(V)2.2 channels possessing the 37a exon and susceptible to SFK inhibition are also downregulated in neuropathic pain (Altier et al., 2007). However, the effect of this loss of the 37a exon is unclear, in view that GABAB receptors in primary afferents, whose effect is also mediated by SFKs, do not lose effectiveness in neuropathic pain. Unlike MORs, GABAB receptors in primary afferents are not downregulated by nerve injury (Engle et al., 2006). GABAB receptor agonists decrease neuropathic pain in rodents (Chen & Pan, 2003; Franek et al., 2004; Urban et al., 2005), and in humans are particularly effective to treat trigeminal neuralgia (Idanpaan-Heikkila & Guilbaud, 1999; Deseure et al., 2003) and in conjunction with spinal cord electrical stimulation (Lind et al., 2004; Lind et al., 2008).
After nerve injury, spinal application of the α2 adrenergic agonist clonidine produced antinociception in both rats (Luo et al., 1994; Duflo et al., 2002) and humans (Eisenach et al., 1995). However, it is not clear whether this effect is mediated by α2 receptors in primary afferents or in dorsal horn neurons. Primary afferents express α2A receptors, whereas dorsal horn neurons express α2C receptors (Stone et al., 1998). Since expression of α2A receptors decreases after nerve injury (Stone et al., 1999; Leiphart et al., 2003), it is possible that adrenergic effects on primary afferents decrease in neuropathic pain. Alternatively, α2 receptor analgesia may switch from α2A to α2B receptors after nerve injury (Duflo et al., 2002; Leiphart et al., 2004). In addition, changes in the signaling system used by the α2 receptors after nerve injury (Bantel et al., 2005) may be important in determining their analgesic effect.
In conclusion, knowledge of the signaling systems used by MORs, GABAB receptors and α2 adrenergic receptors in primary afferent terminals is important to understand the mechanisms of action of analgesic drugs and how these mechanisms change during chronic pain.
Acknowledgments
Supported by grant B4766I from the Rehabilitation Research & Development Service, Department of Veteran Affairs to J.C.M., who is also a recipient of grant R01 DA012609 from the National Institutes of Health.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- Ca(V)
voltage-gated calcium channel
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- DMSO
dimethyl-sulfoxide
- DAMGO
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- DRG
dorsal root ganglia
- Gβ γ
G protein β γ subunits
- GPCR
G protein-coupled receptor
- MOR
μ-opioid receptor
- NA
numerical aperture
- NK1R
neurokinin 1 receptor
- PKC
protein kinase C
- PP1
1-(1,1-Dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3, 4-d]pyrimidin-4-amine
- PP2
3-(4-chlorophenyl)-1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- PP3
1-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- PTP
protein tyrosine phosphatase
- SFK
Src family kinase
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience. 2009;161:538–553. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C- afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. doi: 10.1016/S0304-3959(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases alpha2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- Bourgoin S, Pohl M, Mauborgne A, Benoliel JJ, Collin E, Hamon M, Cesselin F. Monoaminergic control of the release of calcitonin gene-related peptide-like and substance P-like materials from rat spinal cord slices. Neuropharmacology. 1993;32:633–640. doi: 10.1016/0028-3908(93)90076-f. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Castiglioni AJ, Raingo J, Lipscombe D. Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J Physiol. 2006;576:119–134. doi: 10.1113/jphysiol.2006.115030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res. 2003;965:67–74. doi: 10.1016/s0006-8993(02)04123-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Marvizon JC. Acute inflammation induces segmental, bilateral, supraspinally mediated opioid release in the rat spinal cord, as measured by μ-opioid receptor internalization. Neuroscience. 2009;161:157–172. doi: 10.1016/j.neuroscience.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JCG. Comparing analgesia and μ-opioid receptor internalization produced by intrathecal enkephalin: Requirement for peptidase inhibition. Neuropharmacology. 2007;53:664–667. doi: 10.1016/j.neuropharm.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch MJ, Carlson E, Wyatt A, Boyce S, Hill RG, Rupniak NM. Reversal of behavioural and electrophysiological correlates of experimental peripheral neuropathy by the NK1 receptor antagonist GR205171 in rats. Neuropharmacology. 1998;37:1535–1543. doi: 10.1016/s0028-3908(98)00125-7. [DOI] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Deseure K, Koek W, Adriaensen H, Colpaert FC. Continuous administration of the 5-hydroxytryptamine1A agonist (3-Chloro-4-fluoro-phenyl)-[4-fluoro-4-[[(5-methyl-pyridin-2-ylmethyl) -amino]-methyl]piperidin-1-yl]-methadone (F 13640) attenuates allodynia-like behavior in a rat model of trigeminal neuropathic pain. J Pharmacol Exp Ther. 2003;306:505–514. doi: 10.1124/jpet.103.050286. [DOI] [PubMed] [Google Scholar]
- Diverse-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc Natl Acad Sci U S A. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Duflo F, Li X, Bantel C, Pancaro C, Vincler M, Eisenach JC. Peripheral nerve injury alters the alpha2 adrenoceptor subtype activated by clonidine for analgesia. Anesthesiology. 2002;97:636–641. doi: 10.1097/00000542-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Engle MP, Gassman M, Sykes KT, Bettler B, Hammond DL. Spinal nerve ligation does not alter the expression or function of GABA(B) receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience. 2006;138:1277–1287. doi: 10.1016/j.neuroscience.2005.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Franek M, Vaculin S, Rokyta R. GABA(B) receptor agonist baclofen has non-specific antinociceptive effect in the model of peripheral neuropathy in the rat. Physiol Res. 2004;53:351–355. [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, Ansel J, Portbury AL, Furness JB, McDonald DM, Bunnett NW. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Henry JL, Yashpal K, Pitcher GM, Chabot J, Coderre TJ. Evidence for tonic activation of NK-1 receptors during the second phase of the formalin test in the rat. J Neurosci. 1999;19:6588–6598. doi: 10.1523/JNEUROSCI.19-15-06588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;1:22–29. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Riddell JS, Todd AJ. Upregulation of substance P in low-threshold myelinated afferents is not required for tactile allodynia in the chronic constriction injury and spinal nerve ligation models. J Neurosci. 2007;27:2035–2044. doi: 10.1523/JNEUROSCI.5401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idanpaan-Heikkila JJ, Guilbaud G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviour. Pain. 1999;79:281–290. doi: 10.1016/s0304-3959(98)00172-9. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- Laird JM, Roza C, De Felipe C, Hunt SP, Cervero F. Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain. 2001;90:97–103. doi: 10.1016/s0304-3959(00)00394-8. [DOI] [PubMed] [Google Scholar]
- Lang C, Hope P, Grubb B, Duggan A. Lack of effect of microinjection of noradrenaline or medetomidine on stimulus-evoked release of substance P in the spinal cord of the cat: a study with antibody microprobes. Br J Pharmacol. 1994;112:951–957. doi: 10.1111/j.1476-5381.1994.tb13173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao L, Marvizon JCG. GABAA receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord. Neuroscience. 2005;130:1013–1027. doi: 10.1016/j.neuroscience.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Lao L, Song B, Chen W, Marvizon JC. Noxious mechanical stimulation evokes the segmental release of opioid peptides that induce μ-opioid receptor internalization in the presence of peptidase inhibitors. Brain Res. 2008;1197:85–93. doi: 10.1016/j.brainres.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Bourgoin S, Clot AM, Hamon M, Cesselin F. Noxious mechanical stimuli increase the release of Met-enkephalin-like material heterosegmentally in the rat spinal cord. Brain Res. 1987;402:188–192. doi: 10.1016/0006-8993(87)91066-3. [DOI] [PubMed] [Google Scholar]
- Leiphart JW, Dills CV, Levy RM. Decreased spinal alpha2a- and alpha2c-adrenergic receptor subtype mRNA in a rat model of neuropathic pain. Neurosci Lett. 2003;349:5–8. doi: 10.1016/s0304-3940(03)00610-4. [DOI] [PubMed] [Google Scholar]
- Leiphart JW, Dills CV, Levy RM. Alpha2-adrenergic receptor subtype specificity of intrathecally administered tizanidine used for analgesia for neuropathic pain. J Neurosurg. 2004;101:641–647. doi: 10.3171/jns.2004.101.4.0641. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Ding YQ, Li YQ, Li JS, Nomura S, Kaneko T, Mizuno N. Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res. 1998;794:347–352. doi: 10.1016/s0006-8993(98)00332-1. [DOI] [PubMed] [Google Scholar]
- Lind G, Meyerson BA, Winter J, Linderoth B. Intrathecal baclofen as adjuvant therapy to enhance the effect of spinal cord stimulation in neuropathic pain: a pilot study. Eur J Pain. 2004;8:377–383. doi: 10.1016/j.ejpain.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Lind G, Schechtmann G, Winter J, Meyerson BA, Linderoth B. Baclofen-enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: Long-term outcome of a pilot study. Eur J Pain. 2008;12:132–136. doi: 10.1016/j.ejpain.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bishop A, Witucki L, Kraybill B, Shimizu E, Tsien J, Ubersax J, Blethrow J, Morgan DO, Shokat KM. Structural basis for selective inhibition of Src family kinases by PP1. Chem Biol. 1999;6:671–678. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- Luo L, Puke MJ, Wiesenfeld-Hallin Z. The effects of intrathecal morphine and clonidine on the prevention and reversal of spinal cord hyperexcitability following sciatic nerve section in the rat. Pain. 1994;58:245–252. doi: 10.1016/0304-3959(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. GABAB, but not GABAA receptor activation, inhibits electrically evoked substance P-like immunoreactivity release from the rat spinal cord in vitro. J Pharmacol Exp Ther. 1993;266:1490–1496. [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–399. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- Marchand JE, Wurm WH, Kato T, Kream RM. Altered tachykinin expression by dorsal root ganglion neurons in a rat model of neuropathic pain. Pain. 1994;58:219–231. doi: 10.1016/0304-3959(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999a;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Waszak-McGee J, Mayer EA. Internalization of μ-opioid receptors in rat spinal cord slices. Neuroreport. 1999b;10:2329–2334. doi: 10.1097/00001756-199908020-00020. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003a;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Marvizon JCG, Wang X, Lao L, Song B. Effect of peptidases on the ability of exogenous and endogenous neurokinins to produce neurokinin 1 receptor internalization in the rat spinal cord. Br J Pharmacol. 2003b;140:1389–1398. doi: 10.1038/sj.bjp.0705578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Christianson CA, Hua XY, Yaksh TL. Dexmedetomidine and ST-91 analgesia in the formalin model is mediated by alpha2A-adrenoceptors: a mechanism of action distinct from morphine. Br J Pharmacol. 2008;155:1117–1126. doi: 10.1038/bjp.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;15:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Mishima A, Ono S, Fukuda H, Vasko MR. Inhibitory effects of clonidine and tizanidine on release of substance P from slices of rat spinal cord and antagonism by α-adrenergic receptor and antagonists. Neuropharmacology. 1991a;30:585–589. doi: 10.1016/0028-3908(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Ono H, Mishima A, Ono S, Fukuda H, Vasko MR. Inhibitory effects of clonidine and tizanidine on release of substance P from slices of rat spinal cord and antagonism by alpha-adrenergic receptor antagonists. Neuropharmacology. 1991b;30:585–589. doi: 10.1016/0028-3908(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett. 1995a;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995b;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. Protein kinase C mediates the synergistic interaction between agonists acting at α2-adrenergic and δ-opioid receptors in spinal cord. J Neurosci. 2009;29:13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Vasko MR. Morphine and norepinephrine but not 5-hydroxytriptamine and gamma-aminobutyric acid inhibit the potassium-stimulated release of substance P from rat spinal cord slices. Brain Res. 1986;376:268–279. doi: 10.1016/0006-8993(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990;43:273–286. doi: 10.1016/0304-3959(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci. 2007;10:285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman RW, Tombler E, Lau KK, Anantharam A, Rodriguez J, O’Bryan JP, Diverse-Pierluissi MA. N-type Ca2+ channels as scaffold proteins in the assembly of signaling molecules for GABAB receptor effects. J Biol Chem. 2004;279:24649–24658. doi: 10.1074/jbc.M312182200. [DOI] [PubMed] [Google Scholar]
- Riley RC, Loo C, Mantyh PW, Basbaum AI. GABA-B but not GABA-A receptors inhibit noxious stimulus-evoked internalization of the neurokinin-1 (NK-1) receptor in rat spinal cord. Society for Neuroscience Abstracts. 1997;23:175.117. [Google Scholar]
- Riley RC, Trafton JA, Chi SI, Basbaum AI. Presynaptic regulation of spinal cord tachykinin signaling via GABA(B) but not GABA(A) receptor activation. Neuroscience. 2001;103:725–737. doi: 10.1016/s0306-4522(00)00571-6. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Moises HC. μ-Opioid receptor activation reduces multiple components of high- threshold calcium current in rat sensory neurons. J Neurosci. 1995;15:4315–4327. doi: 10.1523/JNEUROSCI.15-06-04315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI, Moises HC. Mu-opioid and GABAB receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons. Neuroscience. 1998;85:939–956. doi: 10.1016/s0306-4522(97)00674-x. [DOI] [PubMed] [Google Scholar]
- Schiff ML, Siderovski DP, Jordan JD, Brothers G, Snow B, De Vries L, Ortiz DF, Diverse-Pierluissi M. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408:723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L, Riedl MS, Wang J, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on alpha-2A and alpha-2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience. 1999;93:1399–1407. doi: 10.1016/s0306-4522(99)00209-2. [DOI] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Strock J, Diverse-Pierluissi MA. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol Pharmacol. 2004;66:1071–1076. doi: 10.1124/mol.104.002261. [DOI] [PubMed] [Google Scholar]
- Takano M, Takano Y, Yaksh TL. Release of calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal polypeptide (VIP) from rat spinal cord: modulation by alpha 2 agonists. Peptides. 1993;14:371–378. doi: 10.1016/0196-9781(93)90055-l. [DOI] [PubMed] [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie CW. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous μ-opioid and alpha2A-adrenergic receptors in neurons. J Biol Chem. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Young S, Puskar Z. Fos induction in lamina I projection neurons in response to noxious thermal stimuli. Neuroscience. 2005;131:209–217. doi: 10.1016/j.neuroscience.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Basbaum AI. Differential contribution of substance P and neurokinin A to spinal cord neurokinin-1 receptor signaling in the rat. J Neurosci. 2001;21:3656–3664. doi: 10.1523/JNEUROSCI.21-10-03656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- Urban MO, Ren K, Park KT, Campbell B, Anker N, Stearns B, Aiyar J, Belley M, Cohen C, Bristow L. Comparison of the antinociceptive profiles of gabapentin and 3-methylgabapentin in rat models of acute and persistent pain: implications for mechanism of action. J Pharmacol Exp Ther. 2005;313:1209–1216. doi: 10.1124/jpet.104.081778. [DOI] [PubMed] [Google Scholar]
- Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Jr, Porreca F. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Burgoyne RD. Voltage-independent inhibition of P/Q-type Ca2+ channels in adrenal chromaffin cells via a neuronal Ca2+ sensor-1-dependent pathway involves Src family tyrosine kinase. J Biol Chem. 2001;276:44804–44811. doi: 10.1074/jbc.M103262200. [DOI] [PubMed] [Google Scholar]
- Xu J, Weerapura M, Ali MK, Jackson MF, Li H, Lei G, Xue S, Kwan CL, Manolson MF, Yang K, Macdonald JF, Yu XM. Control of Excitatory Synaptic Transmission by C-terminal Src Kinase. J Biol Chem. 2008;283:17503–17514. doi: 10.1074/jbc.M800917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang D, Li YQ. Distribution and depression of the GABA(B) receptor in the spinal dorsal horn of adult rat. Brain Res Bull. 2001;55:479–485. doi: 10.1016/s0361-9230(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Lacey G, Hendry IA, Morton CR. Substance P release in the cat spinal cord upon afferent C-fibre stimulation is not attenuated by clonidine at analgesic doses. Neurosci Lett. 2004;361:216–219. doi: 10.1016/j.neulet.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. Opioid-Induced Long-Term Potentiation in the Spinal Cord Is a Presynaptic Event. J Neurosci. 2010;30:4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorman G, Belcher G, Adams JE, Fields HL. Lumbar intrathecal naloxone blocks analgesia produced by microstimulation of the ventromedial medulla in the rat. Brain Res. 1982;236:77–84. doi: 10.1016/0006-8993(82)90035-x. [DOI] [PubMed] [Google Scholar]