Abstract
Pesticides are widely used in agricultural and other settings, resulting in continued human exposure. Pesticide toxicity has been clearly demonstrated to alter a variety of neurological functions. Particularly, there is strong evidence suggesting that pesticide exposure predisposes to neurodegenerative diseases. Epidemiological data has suggested a relationship between pesticide exposure and brain neurodegeneration. However, an increasing debate has aroused regarding this issue. Paraquat is a highly toxic quaternary nitrogen herbicide which has been largely studied as a model for Parkinson’s disease providing valuable insight into the possible mechanisms involved in the toxic effects of pesticides and their role in the progression of neurodegenerative diseases. In this work, we review the molecular mechanisms involved in the neurotoxic actions of pesticides, with a particular emphasis on the mechanisms associated with the induction neuronal cell death by paraquat as a model for Parkinsonian neurodegeneration.
Keywords: Pesticides, apoptosis, ROS, oxidative stress, redox, Parkinson, neurodegeneration, neurodegenerative, environmental, toxicants
1. INTRODUCTION
Much progress has been made in identifying genes involved in familial, or inherited, forms of different neurodegenerative diseases, including Parkinson’s disease (PD). However, the majority of disease cases are sporadic (not inherited) and their origin(s) still remain largely undetermined. The environment is a key contributor to human health and disease. Epidemiological evidence suggests that environmental factors play a role in the etiology of neurodegenerative diseases. For example, epidemiologic studies implicate the exposure to pesticides, metals, polychlorinated biphenyls, solvents and particulate matter as risk factors for PD [1–20]. Cells respond and adapt to the environment through multiple mechanisms that involve communication pathways or signal transduction processes [21]. Neurodegenerative diseases are most commonly associated with selective neuron loss by apoptosis. Environmental stressors are known to mediate a wide variety of toxic effects including cell death. However, the molecular mechanisms involved in the positive effects of environmental exposures in the progression of neurodegenerative diseases are still unknown.
2. PESTICIDES AND PARKINSONIAN NEURODEGENERATION
Pesticides are defined as any substance or mixture of substances intended for preventing, destroying, repelling or mitigating pests. Pesticides consist of multiple classes and subclasses and exhibit a vast array of chemically diverse structures. They are commonly referred to by the organisms designed to control (e.g., herbicides, insecticides, or fungicides) or by their chemical class (organophosphate, triazine) [22–24]. However, pesticides are not always selective for their intended target species, and adverse health effects can occur in non-target species including humans. Pesticide toxicity has been clearly demonstrated to alter a variety of physiological functions. In addition, evidence suggests that pesticide exposure increases the risk of cancer and neurodegenerative diseases. Recent evidence also demonstrates the ability of pesticides to act as endocrine disruptors, contributing to various adverse effects associated with reproductive and developmental toxicity [4, 25–30].
Parkinson’s disease (PD) is characterized by abnormalities of motor control such as resting tremors, bradykinesia (slowness of voluntary movement), rigidity, and a loss of postural reflexes. The majority of cases of PD are not inherited and thus it has been proposed that environmental factors are associated with this disease. A number of epidemiologic studies have found an association between PD and exposure to pesticides. Furthermore, increased levels of pesticides have been found in the brains of PD cases versus controls. Contradictory studies have also been published demonstrating no association between PD and pesticide exposure. [2, 3, 5, 8, 14, 31, 32]. It is clear now that PD is a multi-factorial disease with a complex etiology including genetic risk factors, environmental exposure and aging [33–35].
3. PARAQUAT AND PARKINSONS’S DISEASE
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) is a highly toxic quarternary nitrogen herbicide. Because of its low cost, rapid action, and environmental characteristics, paraquat is a widely used herbicide around the world. Many cases of acute paraquat poisoning and death have been reported over the past few decades. Paraquat is not readily absorbed from the gastrointestinal tract, and is even more slowly absorbed across the skin. Upon absorption, independent from the route of exposure, paraquat accumulates in the lung and the kidney where it exerts its major acute toxicological effects. It has been suggested that paraquat metabolites might be more readily absorbed than the parent compound, but their identities and toxicities are unknown. However, paraquat is very poorly metabolized and is excreted almost unchanged in the urine, although there is some controversy as to the possibility and extent of its metabolism by the gut microflora. Metabolism of paraquat has been reported to occur via demethylation (monomethyl dipyridone ion) or oxidation (paraquat pyridone ion and paraquat dipyridone ion) [36, 37].
On the other hand, chronic paraquat exposure has been suggested as an etiological factor for PD. Animal studies have demonstrated that paraquat can cause neurodegeneration of dopaminergic neurons [38, 39]. However, there is still a lot of controversy regarding whether the neurotoxicological actions of paraquat represent an accurate experimental model for studying the pathogenesis of PD [40–42]. Nevertheless, the study of paraquat’s neurotoxic properties has provided valuable information regarding the potential mechanisms involved in the progression of neurodegeneration associated with environmental toxicity. It is clear now that because PD is not found around the world, it is impossible for a single toxin such as paraquat, to be the sole environmental risk factor for PD. Thus, it is thought that a complex array of environmental exposures and gene interactions might exert synergistic effects toward the predisposition to sporadic PD. Paraquat is thought to be transported across the blood-brain barrier by the action of a neutral amino acid transporter carrier such as the system L carrier (LAT-1), which normally carries amino acids L-valine and L-phenylalanine, whose administration has been reported to prevent paraquat-induced neurotoxicity [39, 43]. However, a recent study found that paraquat is excluded from the rhesus macaque brain by the blood-brain barrier, arguing against the causative role of paraquat exposure in idiopathic Parkinson’s disease [44]. Paraquat has been largely demonstrated to induce cell death in a variety of cell types and tissues [45, 46]. We will focus next on the possible mechanisms involved in the regulation of cell death signaling in neural cells due to their relevance to PD.
4. MOLECULAR MECHANISMS OF PARAQUAT INDUCED NEUROTOXICITY
4.1 Environmental toxicity, oxidative stress and redox signaling
Environmental toxicants are known to induce oxidative stress by: 1) the induction of reactive oxygen species (ROS) as byproducts of detoxifying metabolism, 2) alterations in the mitochondrial metabolism or 3) by their own redox (reduction oxidation) cycling properties per se. Thus, redox signaling has been proposed as the central mechanism for the toxicological effects of environmental toxicants including pesticides. Toxicant-induced oxidative stress is well known to mediate a wide variety of toxic effects such as DNA damage or genotoxicity. However, it is known that many of the toxic effects induced by environmental toxicants are mediated by the regulation/induction of cell death, whose deregulation has been associated with the etiology of several environmental diseases [47–52]. Oxidative stress has been widely shown to regulate apoptosis [47, 50, 53]. However, the exact mechanisms are still unclear.
Oxidative stress is caused by an imbalance between the production of reactive oxygen and the ability to: 1) detoxify the reactive intermediates produced, or 2) repair the resulting damage. Ultimately, oxidative stress conveys the biomolecular alteration in cellular function caused by the reaction of reactive species with cellular constituents. Reactive oxygen species (ROS) include oxygen (O2)-derived free radicals (defined as molecules with one or more unpaired electrons in an outermost valence shell) such as superoxide anion (•O2−) and the hydroxyl radical (•OH), as well as nonradical derivatives of O2 such as hydrogen peroxide (H2O2). ROS production is the result of an aerobic environment. A significant amount of O2 consumed by mitochondria is converted to •O2−, although it can be produced through various enzymatic oxidation reactions catalyzed by cytochrome P450, other oxidoreductases and also by NADPH (nicotinamide adenine dinucleotide phosphate)-oxidase. •O2− reacts at diffusion-controlled rates with nitric oxide (•NO) produced by •NO-synthases (NOS) leading to the formation of a wide diversity of oxidizing and nitrosating/nitrating species such as peroxynitrite (ONOO−). •O2− is also dismutated nonenzymatically or enzymatically with the aid of superoxide dismutases (SODs) to hydrogen peroxide (H2O2). H2O2 can be also utilized by myeloperoxidases (MPO) to produce hypochlorous acid and other noxious chlorine derived oxidants. Furthermore, H2O2 can be reduced to •OH− through Fenton type reactions. Thus it is clear that the formation of a reactive species can ultimately lead to an amplification chain generating other more toxic reactive species.
Cells have intrinsic antioxidant mechanisms to detoxify ROS generated under both physiological and pathological conditions. Reduced glutathione (GSH) is the most important antioxidant molecule in the cell, and due to its high cytosolic concentration, it can directly scavenge ROS such as •O2−, •OH and •NO. H2O2 is reduced to H2O by GSH peroxidases (GPX) and catalase. The GSH reductase (GR) and thioredoxin (Trx)/thioredoxin reductase systems regenerate cellular GSH or reduced Trx, respectively, at the expense of NADPH. Other antioxidant molecules (such as ascorbate or vitamin E) and enzymes (such as peroxiredoxins) are important defenses against oxidative stress. Oxidative stress arises if detoxification systems and antioxidants are compromised or if ROS production is excessive, resulting in DNA, protein, and lipid oxidation [47, 50, 54].
The regulatory role of oxidative stress in signal transduction in physiological settings must exhibit substrate specificity. Until recently the study of the biomolecular alterations caused by the reaction of reactive species has been undertaken. Oxidative damage to DNA leads to the formation of lesions such as 8-oxo-deoxyguanosine, 8-oxo-deoxyadenosine, and deoxythymidine glycol which are selectively excised from DNA by DNA glycosylases. Lipid peroxidation refers to the oxidative degradation of lipids. Lipid peroxidation is initiated through a radical-mediated abstraction of a hydrogen atom from polyunsaturated fatty acids to make water and a fatty acid radical. Lipid peroxyl radicals (LOO•) are formed by the reaction of free fatty acid radicals with oxygen that subsequently reacts with another free fatty acid producing a different fatty acid radical and a lipid peroxide propagating the damage. Lipid peroxidation generates a number of lipid hydroperoxide products such as malondialdehyde, 4-hydroperoxy-2-nonenal, 4-oxo-2-nonenal and 4-hydroxy-2-nonenal (4HNE). These aldehyde products react with individual nucleotides and nucleophilic amino acids, thus inducing several signaling effects [50]. Oxidative protein modifications have been shown to regulate the activity of a wide variety of proteins such as kinases, phosphatases, proteases (caspases), molecular adaptors and chaperones, and transcription factors. Specific amino acid modifications in cysteine, methionine, tryptophan, and tyrosine residues are prone to oxidative modification. Oxidative protein modifications in general can be classified as reversible and irreversible modifications. Highly reactive oxidant species such as hypochlorous acid, ONOO−, and •OH are thought to oxidize biomolecules leading to the irreversible formation of, for example, 3-nitrotyrosine and protein carbonyls. Physiological oxidants such as •NO, •O2− and H2O2, have been implicated in reversible protein modifications at the cysteine level (nitrosylation, hydroxylation, glutathionylation, disulfide bond formation) that underlie homeostatic control and diverse biological responses. A wide variety of enzymes regulate these post-translational modifications including sulfiredoxins, thioredoxins, peroxiredoxins, glutaredoxins and methionine sulfoxide reductases [55, 56].
4.2 Paraquat-induced oxidative stress and its association with Parkinson’s disease
Parkinson’s disease (PD) is characterized by a selective degeneration of dopaminergic neurons in the substantia nigra (SN) pars compacta attributed to toxic accumulation and aggregation of proteins, mitochondrial dysfunction and oxidative stress. The occurrence of oxidative stress has been observed in the SN of PD brains as evidenced by increased lipid, protein, and DNA oxidation, increased total iron content, and significant decreases in GSH and GSH/glutathione disulfide (GSSG) ratio [57]. The main pathway of cell toxicity in PD involves misfolding and aggregation of α-synuclein [58]. Failure of α-synuclein clearance by the ubiquitin–proteasome system (UPS) leads to its accumulation over time and to the formation of fibrillar aggregates and Lewy bodies. α-Synuclein protofibrils can directly lead to oxidative stress that can further impair the UPS by reducing ATP levels, inhibiting the proteasome, and by the oxidation of parkin. Exposure to paraquat has been shown to induce proteasome dysfunction and α-synuclein aggregation [59–63]. Furthermore, paraquat has been shown to potentiate α-synuclein-induced toxicity [64, 65]. It has been hypothesized that mutated α-synuclein induces a reduction in vesicle number and the accumulation of cytoplasmic dopamine in association with enhanced ROS generation and initiation of the apoptotic cascade [66]. In the cytosol, dopamine is metabolized by monoamine oxidase which generates H2O2, or is auto-oxidized generating •O2−, H2O2, and dopamine-quinone species [67]. Autosomal recessive PD-associated genes, parkin, DJ-1, and PINK-1 (PTEN induced putative kinase 1) are involved in mitochondrial function, which suggests that mitochondrial dysfunction and the generation of ROS is a central event in the pathogenesis of PD. Interestingly, paraquat-induced toxicity and proteasome dysfunction is potentiated in DJ-1 deficiency [68–71]. Paraquat has also been demonstrated to induce parkin aggregation [72]. Mutations in leucine-rich repeat kinase 2 (LRRK2) cause familial Parkinson’s disease. LRRK2 overexpression has also been shown to protect against paraquat-induced toxicity [73]. These results support the hypothesis that environmental and genetic factors act cooperatively in PD neurodegeneration.
Pesticide-induced redox signaling has been demonstrated to mediate many of the toxicological effects of these chemicals. Exposure to a wide variety of pesticides induces oxidative stress reflected as the accumulation of ROS, lipid peroxidation and DNA damage [74]. In general, pesticides have been shown to alter cellular redox balance by different mechanisms including: 1) their enzymatic conversion to secondary reactive products and/or ROS; 2) depletion of antioxidant defenses; and 3) impairment of antioxidant enzyme function [49, 75]. Initially, structural similarity between paraquat and the toxic metabolite MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), raised the possibility that both toxicants could have similar mechanisms for the induction of neurotoxicity. MPTP crosses the blood-brain barrier and is biotransformed by glial monoamine oxidases and two-electron oxidation to MPP+ (1-methyl-4-phenylpyridinium). MPP+ is uptaken by neurons through dopamine transporters (DAT) and induces neuronal cell death by inhibition of mitochondrial complex I and induction of oxidative stress. Some studies have reported that inhibition of DAT protects against paraquat-induced toxicity suggesting a role for DAT in the cellular uptake of paraquat [76–79]. In fact, DAT genetic variability and pesticide exposure have been shown to interact, increasing PD risk [80]. However, recent studies report that paraquat is neither a substrate nor inhibitor of DAT [81].
Parkinson’s disease has been associated with impaired oxidative phosphorylation and decreased complex I activity, which induces reactive oxygen species (ROS) formation and oxidative stress. The role of mitochondria in paraquat toxicity is still unclear. In contrast to MPP+, paraquat has been reported to be a weak inhibitor of complex I of the electron transport chain (ETC) [81, 82]. Furthermore, paraquat induced toxicity has been reported to be independent from complex I inhibition [83]. Recently, paraquat was reported to act at the level of complex III to generate ROS [84, 85].
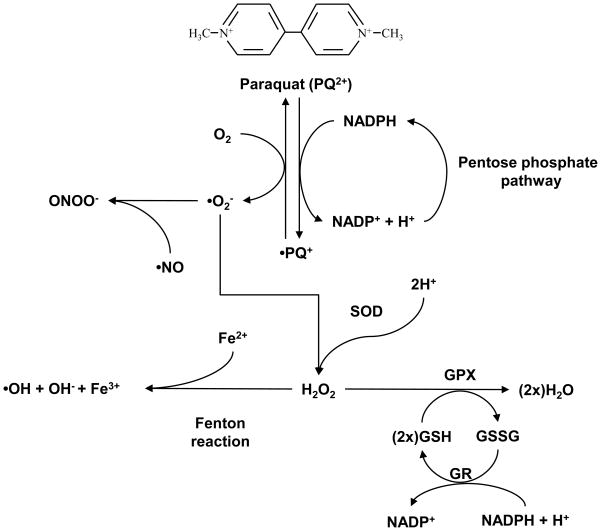
In other tissues including the lung, paraquat toxicity have been shown to be mediated by its cytosolic reduction by a NADPH-cytochrome P450 reductase to the mono-cation radical PQ+ (Figure 1). This radical reacts spontaneously with O2, leading to the generation of •O2−, regenerating the original paraquat dication, which can undergo the reduction-oxidation cycle again. Oxidation of NADPH by paraquat impairs GSSG recycling to GSH and thus the activity of several antioxidant systems. Although depletion of NADPH can stimulate the pentose phosphate and fatty acid synthesis pathways, restoration of NADPH cellular reducing equivalents promotes a continuous redox reaction involving paraquat and O2 and the formation of •O2− [86]. In the brain, specific cell types have been reported to express cytochrome P450 enzymes. The presence of cytochrome P450 enzymes in the brain should also be important in inducing bioactivation and cellular damage of pesticides. Many results support the possibility of a local metabolism of pesticides and other pollutants in the brain by cytochrome P450 enzymes into neurotoxic compounds, suggesting that brain metabolism could be a factor modulating the individual susceptibility to Parkinson’s disease during pesticide exposure. Although cytochrome P450 enzymes content in the brain is lower than in other tissues, local metabolism of pesticides by isoforms highly expressed in the brain might be an underestimated factor in pesticide-induced neurotoxicity [87, 88]. Other enzymes capable of initiating the redox cycling process of paraquat have been identified in microsomal, plasma membrane, and cytosolic components which include nitric-oxide synthase, NADPH-oxidase and thioredoxin reductase [89–91]. Uptake of paraquat into the mitochondria through a carrier-mediated membrane potential-dependent transport across the mitochondrial inner membrane and its reduction by complex I, have been reported to mediate its redox cycling and toxicity at the mitochondria [92]. More recently, paraquat has been shown to mediate oxidative stress by induction/activation of NADPH-oxidase [93–96]. Xantine oxidase system has also been reported to mediate the generation of ROS in neurons exposed to paraquat [97].
Figure 1. Redox cycling by paraquat.
Paraquat is a highly toxic quarternary nitrogen herbicide which has been shown capable to redox cycle in some cell types. Dication paraquat (PQ2+) is reduced to PQ+ by an NADPH-cytochrome P450 reductase. In the presence of O2, PQ2+ is oxidized with the concomitant production of superoxide anion (•O2−). SOD dismutates •O2− to H2O2 which is further metabolized by GPX. •O2− also reacts •NO leading to the formation of ONOO−. Overexpression of SOD and GPX protects against paraquat-induced toxicity [107]. However, PQ2+ can redox cycle again, leading to a continuous generation of ROS and oxidative stress, and depleting the intracellular pools of GSH and NADPH (required for GSSG recycling to GSH by GR). Accumulation of H2O2 is reduced further to •OH− through Fenton type reactions.
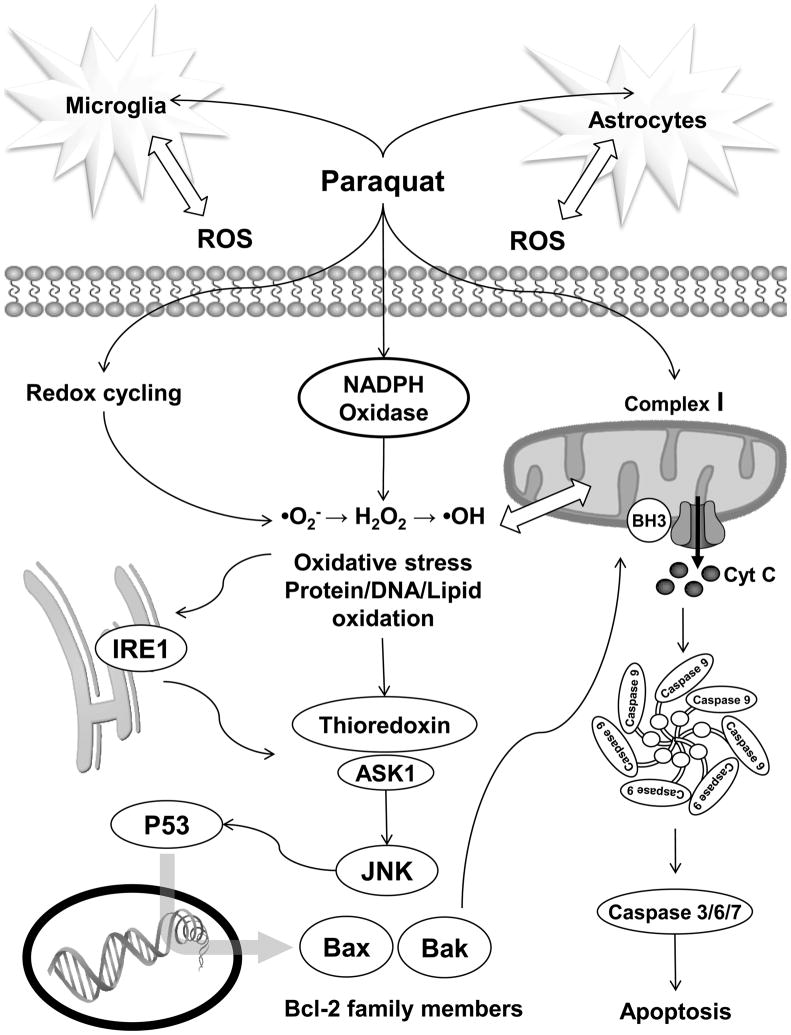
Although paraquat-induced ROS might arise from a number of cellular sources, it is clear that oxidative stress is a central player in the regulation of paraquat-induced neuronal cell death (Figure 2). The ability of paraquat to cause oxidative damage through a free radical mechanism may explain the selective vulnerability of dopaminergic neurons which are highly susceptible to oxidative damage due to the pro-oxidant properties of dopamine. In fact, a recent report suggests that paraquat toxicity is associated with an increase in the oxidative pathway of dopamine metabolism [98]. As mentioned above, redox cycling or mitochondrial function impairment by paraquat induces ROS formation, particularly •O2−. •O2− can promote the formation of other ROS, such as H2O2 and •OH [99]. SODs have been shown to protect against paraquat-induced neurotoxicity [100–102]. Accordingly, copper deficiency, which impairs Cu/Zn-SOD, was shown to potentiate paraquat induced cell death [103]. Paraquat has also been shown to induce GSH depletion in neurons [98, 104]. Accordingly, GSH/antioxidant supplementation has been clearly shown to prevent paraquat-induced toxicity in animal models [86, 97, 105, 106]. Overexpression of the antioxidant enzymes SOD and glutathione peroxidase has been shown to protect against paraquat/maneb neurotoxicity [107]. Finally, NF-E2-related factor-2 (Nrf2)-dependent regulation of antioxidant responsive element (ARE)-mediated gene expression has been shown to protect against paraquat by induction of heme-oxygenase 1, which is the rate-limiting enzyme involved in the oxidative degradation of free heme, preventing the heme-catalyzed production of •OH from H2O2 [108].
Figure 2. Molecular mechanisms involved in paraquat-induced neurotoxicity.
The mechanisms by which paraquat enters the cell are still unknown. However, it is clear that paraquat can promote the generation of ROS in the cytoplasm by three distinct mechanisms including: 1) redox cycling, 2) inhibition of mitochondrial electron transport chain, and/or 3) induction/activation of ROS generating enzymes such as NADPH-oxidases. Paraquat has also been shown to induce neuronal oxidative stress through the activation of glial cells. Accumulation of ROS leads to oxidative stress observed by the oxidative modification of lipids, proteins and nucleic acids which mediate the activation of cell death signaling cascades. Neuron loss in PD is mostly associated with apoptosis, and paraquat-induced apoptosis has been demonstrated to involve mainly intrinsic pathways. Oxidative stress can directly induce the activation of the mitochondrial pathway of apoptosis. However, paraquat-induced oxidative stress has also been suggested to trigger the activation of ASK1/JNK signaling cascade through the induction of endoplasmic reticulum stress and the activation of IRE1, and also by the direct oxidation of Trx. Activation of SAPKs such as JNK has been demonstrated to regulate the activation/induction of pro-apoptotic Bcl-2 family members, which can further trigger the mitochondrial pathway of apoptosis by inducing cytochrome C (Cyt C) release and the activation of caspases.
4.3 Paraquat-induced cell death
It is well known that environmental toxicants exert their toxicity, at least in part, by triggering cell death. Cell death is classified by biochemical and morphological criteria. According to the recommended classification of cell death [109], three distinct types of cell death pathways can be defined according to morphological criteria which are necrosis, apoptosis and autophagy, although there are numerous examples in which cell death displays mixed features. Necrotic cell death is characterized by a gain in cell volume, swelling of organelles, plasma membrane rupture and subsequent loss of intracellular contents. It is now recognized that execution of necrotic cell death may be regulated by a series of signal transduction pathways and catabolic processes [109]. Paraquat has been reported to induce necrosis when injected into different areas of the rat brain. However, this effect might be observed just at high doses [110].
Autophagy is a major catabolic pathway by which eukaryotic cells degrade and recycle macromolecules and organelles. It plays an essential role in differentiation and development, as well as in cellular response to stress. Autophagy can be activated during amino acid deprivation and has been associated with neurodegenerative diseases. Autophagy is initiated by the surrounding of cytoplasmic constituents by the crescent-shaped isolation membrane/phagophore, which forms a closed double-membrane structure, called autophagosome. The autophagosome subsequently fuses with a lysosome to become an autolysosome, and its content is degraded by acidic lysosomal hydrolases. Autophagic cell death is morphologically defined by massive autophagic vacuolization of the cytoplasm in the absence of chromatin condensation [109]. Neurons from patients with PD display characteristics of autophagy. Recent studies have demonstrated that low concentrations of paraquat induce autophagy which is followed by apoptosis. In this work, autophagy was shown to be modulated by DJ-1. Surprisingly, because inhibition of autophagy potentiated apoptosis induced by paraquat, it was proposed that autophagy might be acting as a protective mechanism against cell death progression [111–113].
Neurodegenerative diseases are most commonly associated with selective neuron loss by apoptosis. Paraquat-induced neuronal cell death has been demonstrated to involve primarily the activation of apoptosis. Apoptosis, or programmed cell death, is an evolutionary conserved process involved in a variety of biological processes. Under physiological conditions, apoptosis is important not only for the constant turnover of cells in all tissues but also during the normal development and senescence of the organism. Moreover, apoptosis deregulation has been widely observed to occur as either a cause or a consequence of distinct pathologies [114]. Apoptosis is a highly organized process characterized by the progressive activation of precise signaling pathways leading to specific biochemical and morphological alterations. Initial stages of apoptosis are characterized by cell shrinkage, loss of membrane lipid asymmetry and chromatin condensation, while later stages are associated with the activation of execution caspases (cysteine-dependent aspartate-directed proteases) and endonuclease, apoptotic body formation and cell fragmentation [115, 116]. Both extrinsic and intrinsic pathways have been described for the activation of apoptosis. Induction of apoptosis via the extrinsic pathway is triggered by the activation of death receptors leading to the formation of the death-inducing signaling complex (DISC) by the recruitment of the Fas-associated death domain (FADD) and initiator caspase 8. Death receptor-induced apoptosis is amplified by cleavage of the Bcl-2 (B-cell lymphoma 2)-family protein Bid by caspase 8, which triggers the mitochondrial pathway of apoptosis [117, 118]. Paraquat induced cytotoxicity has been recently suggested to be mediated via the activation of the Fas extrinsic pathway of apoptosis [119], but its relevance to the progression of PD remains unclear.
Apoptosis induced by paraquat has been demonstrated to involve mainly the intrinsic mitochondrial pathway (Figure 2). The intrinsic pathway of apoptosis, also referred to as the mitochondrial pathway, is activated by a wide variety of cytotoxic stimuli or environmental stressors. Although the mechanisms by which these stimuli trigger apoptosis differ between them, they convey the release of proapoptotic proteins from the mitochondria including cytochrome C. However, the exact mechanisms mediating cytochrome C release are still controversial [120, 121]. Distinct mitochondrial components and mitochondrial released proteins such as AIF (apoptosis inducing factor), EndoG (Endonuclease G), ANT (adenine nucleotide translocase), cyclophilin D, Bit1, p53AIP (p53-regulated Apoptosis Inducing Protein 1), GRIM-19 (gene associated with retinoic-interferon-induced mortality 19), DAP3 (death associated protein 3), Nur77/TR3/NGFB-1 (Nerve Growth factor IB), HtrA2 (HtrA serine peptidase 2)/Omi and Smac (second mitochondria-derived activator of caspases)/Diablo have been proposed to participate in the mitochondrial pathway to apoptosis [122]. The intrinsic pathway is also regulated by the Bcl-2 family of proteins. The BH3 (Bcl-2 homology domain 3)-only proteins Bad (Bcl-2-associated death promoter), Bid (Bcl-2 interacting domain), Bim, Bik (Bcl-2-interacting killer), NOXA, and PUMA (p53 upregulated modulator of apoptosis) regulate the anti-apoptotic Bcl-2 proteins Bcl-2 and Bcl-xl (B-cell lymphoma-extra large) to promote apoptosis. Bcl-2 and Bcl-xl inhibit Bax (Bcl-2–associated X protein) and Bak (Bcl-2 homologous antagonist/killer). BH3-only proteins de-repress Bax and Bak by direct binding and inhibition of Bcl-2 and other anti-apoptotic family members. Bax and Bak are known to mediate the release of cytochrome C. Released cytochrome C leads to the recruitment of APAF1 (apoptotic protease activating factor 1) into the apoptosome and activation of caspase-9 [121, 123, 124]. Once activated, initiator caspases converge in the cleavage/activation of execution caspases 3, 6 and 7 which further cleave different cellular substrate leading to the organized demise of the cell. A wide variety of enzymes such as protein kinases, phosphatases, calpains, transcription factors and several other adaptor or scaffolding proteins have been described to participate in several pathways of apoptosis in distinct ways [123, 125–128].
In PD, cell death by apoptosis has been proposed to result from mitochondrial dysfunction, leading to an increase in oxidative stress and a decline in ATP production. Paraquat induces cytochrome C release [59, 97] and caspase 9 activation, which are preceded by the induction/activation of pro-apoptotic Bax and Bak [59, 129]. Activation of Bak has been proposed to be associated to the induction of Bid, BNip3 (Bcl-2/adenovirus E1B 19 kDa protein-interacting protein 3) and NOXA [59]. Interestingly, exposure to maneb and paraquat enhances Bax-dependent cell death through an increased induction of Bax-activators Bik and Bim [107, 130]. Induction of pro-apoptotic Bax and apoptosis in response to paraquat were also reported to be dependent on p53 [103, 129]. Finally, absence of Bcl-2 enhances paraquat-induced oxidative stress by regulation of GSH-dependent antioxidant enzymes [131]. Paraquat neurotoxicity has also been reported to require the activation of stress activated protein kinases (SAPK) [132–138].
Other intrinsic pathways of apoptosis, such as endoplasmic reticulum (ER) stress and DNA damage, have been described, which can be dependent or independent from the mitochondrial pathway. The ER is highly sensitive to stresses that perturb cellular energy levels, the redox state and/or Ca2+ concentration. Such stresses result in the accumulation and aggregation of unfolded proteins which are toxic to cells. ER stress leads to the activation of the SAPK c-Jun N-terminal kinases (JNK) and induction of C/EBP homologous protein (CHOP), which by impairment of the anti-apoptotic function of Bcl-2, lead to the activation of Bim, Bax and Bak, transmission of the signal from the ER to the mitochondria and execution of death by activation of caspases [139, 140]. DNA damage is also known to trigger apoptosis. Blockage of DNA replication associated with DNA damage leads to the activation of p53 which induces the transcriptional activation of pro-apoptotic factors. However, non-transcriptional regulation of apoptosis by p53 and p53-independent pathways has also been described [141, 142]. Although the mitochondrial pathway of apoptosis has been largely linked to paraquat-induced cell death, other signaling pathways have also been implicated in paraquat toxicity. Recently, paraquat has been shown to induce DNA damage and ER stress. ER stress was associated with the activation of the inositol-requiring enzyme 1 (IRE1), apoptosis signal regulating kinase 1 (ASK1), and JNK [137, 138].
The molecular mechanisms linking paraquat-induced oxidative stress and apoptosis are still largely elusive. Recently, paraquat-induced oxidation of Trx has been reported as a possible mechanism for the activation of the ASK1/JNK signaling pathways [136–138]. Accordingly, Nrf2 dependent regulation of trx levels determines the sensitivity of paraquat toxicity by activation of the ASK1/JNK-p38 signaling [138]. Paraquat–induced tyrosine nitration and lipid peroxidation (4HNE) has been recently demonstrated [104, 143]. However, the molecular targets for these signaling events remain to be elucidated. It was recently demonstrated that oxidative stress induced by paraquat generates protein aggregation of the plasma membrane Ca2+-ATPase (PMCA) and its degradation by calpain [144].
Most of the studies regarding the molecular mechanisms of paraquat-induced apoptosis have been directed towards neuronal cell types. However, it is obvious that the complexity of brain tissue organization is given by the interaction of different neurons with glial cell types. Compared to neurons, astrocytes have been demonstrated to be more resistant to paraquat-induced oxidative stress by the responsive induction of a variety of antioxidant systems including catalase and SODs [104, 145, 146]. Initial studies have demonstrated that microglial cells react to pro-oxidant conditions suggesting that they can contribute to environmental stress-induced neurotoxicity [119]. Paraquat induces microglial activation which seems to precede PD neurodegeneration [93, 147, 148]. Low paraquat concentrations have been recently demonstrated to be toxic to neurons only in the presence of microglial cells. In this study it was observed that in neuron-microglia cultures exposed to paraquat, microglia was a source of paraquat-derived oxidative stress [95]. Interestingly, NADPH oxidase from glial cells has been proposed to mediate the generation of ROS [93, 95, 149], which seems to be regulated by the activation of protein kinase C (PKC) and ERK (extracellular signal-regulated kinase) signaling pathways [96, 149].
The extensive geographical overlap of paraquat use with other environmental agents has suggested the possibility of synergistic effects of other environmental toxicants with paraquat on the progression of PD [150–152]. Epidemiological studies have recently reported that exposure to paraquat/maneb increases the risk of developing PD [153]. Recent studies have undertaken the task to study this hypothesis. For example, simultaneous exposure to paraquat and maneb (manganese ethylene-bis-dithiocarbamate) induces additive/synergistic toxic effects in nigrostriatal dopamine systems in vivo [154, 155]. Paraquat/maneb-induced neuronal cell death was associated with the induction/activation of Bax but not Bak [130]. Furthermore, developmental exposure to paraquat/maneb has been reported to produce permanent and progressive damage to the nigrostriatal dopaminergic system enhancing adult susceptibility to these pesticides in the induction of neurodegeneration [156–158]. α-synuclein pathology has also been shown to be potentiated by paraquat/maneb treatment [65]. It has been demonstrated that iron increases paraquat/NADPH-cytochrome P450 reductase-dependent formation of •OH [159]. Interestingly, a recent study has also demonstrated that the combined environmental exposure to paraquat and iron accelerates the degeneration of nigrostriatal dopaminergic neurons [14]. These data support the notion that environmental exposures may act synergistically to produce neurodegeneration.
4.4 Neurotoxicity-induced by other pesticides
4.4.1 Diquat
Diquat (1,1′-ethylene-2,2′-dipyridylium dibromide) is used agriculturally for the same purposes as paraquat. Exposure to diquat has also been associated to PD [160]. Although structurally similar to paraquat, diquat toxicity has been demonstrated to be mediated by a somewhat different mechanism than that of paraquat. The ability of diquat to induce oxidative stress and ROS has been also suggested to be associated to its cycling properties [91, 161–163]. Like paraquat, diquat can be reduced to form a free radical and then reoxidized in the presence of oxygen, with the concomitant production of •O2−. Diquat has also been proposed to induce ROS by inhibition of complex I and III of the mitochondrial ETC [85]. Furthermore, diquat has also been shown to induce ROS formation from glial cells [161].
4.4.2 Maneb
Exposure to the fungicide maneb has been also associated with the induction of oxidative stress and PD [164–167]. Maneb has been shown to induce oxidative stress, protein carbonylation and α-synuclein aggregation due to proteasomal dysfunction, which were shown to be modulated by intracellular GSH [168, 169]. Both extracellular (microglial) and intracellular oxidases have been suggested to mediate ROS formation via redox cycling of maneb [170].
4.4.3 Rotenone
Rotenone (Rotenoid) is a broad-spectrum agricultural insecticide. Rotenone-induced apoptosis is considered to contribute to the etiology of Parkinson’s disease PD [171, 172]. It has been largely thought that rotenone induces ROS by inhibition the mitochondrial respiratory chain complex I [173]. Rotenone has been shown to inhibit the transfer of electrons from iron-sulfur centers in complex I to ubiquinone, which prevents NADH from being converted into ATP [171]. However, recent results have demonstrated that the pro-apoptotic effects of rotenone seem to be independent from complex I inhibition [83]. Rotenone enhances the amount of mitochondrial ROS production, which mediate cytochrome C release and caspase-dependent apoptotic cell death [174–180]. Accordingly, Bcl-2 overexpression protects against apoptosis induced by rotenone [181]. Antioxidant supplementation prevents while Nrf-2 deficiency augments rotenone-induced cell death [180, 182]. Rotenone has also been shown to induce caspase-independent cell death [183, 184]. Rotenone induces accumulation of α-synuclein [185, 186] and recent reports demonstrated that overexpression of α-synuclein, deletion of parkin, PINK1, and knock-down of DJ-1 enhance rotenone–induced toxicity [187–189]. In contrast, overexpression of LRKK2 protects against rotenone-induced mitochondrial dysfunction [73]. Rotenone also induces parkin aggregation [72] and oxidation of mitochondrial Trx [136]. Rotenone has been demonstrated to induce activation of GSK-3 (glycogen synthase kinase 3), JNK and p38 kinases, whose activity seems to be required for the progression of apoptosis [190–192]. Rotenone has also been reported to trigger ER stress and the activation of kinases IRE1 and PERK (PKR-like ER kinase) [193]. Recent reports demonstrated that ROS induced by rotenone are involved in dopamine redistribution to the cytosol, whose pro-oxidant conditions might potentiate rotenone-induced apoptosis of dopaminergic cells [182, 194, 195]. Similar to paraquat, rotenone activates microglial cells which might act as an important source for ROS [196]. Although rotenone has represented a useful experimental model of neurotoxicity, it lacks significant specificity for the central nervous system. Furthermore, there is no evidence of PD in association with rotenone exposure.
4.4.4 Other pesticides
Organochlorine insecticides (also called chlorinated hydrocarbons) have both neurotoxic and carcinogenic effects and have been banned from use in the United Sates. Initial studies demonstrated the presence of the organochlorine pesticides dieldrin in post-mortem brains of PD cases [197]. Dieldrin has been shown to induce apoptosis via GSH depletion and oxidative stress, triggering the intrinsic mitochondrial apoptotic pathway [198, 199]. Proteasomal inhibition was also recently shown to precede cell death after dieldrin treatment, which was potentiated by α-synuclein [200]. Dichlorodiphenyltrichloroethane (DDT) derivatives have been shown to induce neural cell death by apoptosis through the activation of MAPKs (mitogen-activated protein kinases) [201]. The organotins di-n-butyltin dichloride (DBTC) and tri-n-butyltin chloride (TBTC) have been demonstrated to induce ROS formation, SAPK activation and Ca2+ overload which precede cytochrome C release and apoptosis [202].
5. CONCLUSIONS AND PERSPECTIVES
Pesticide toxicity has been clearly demonstrated to alter neurological functions. Epidemiological studies have suggested a relationship between pesticide exposure and brain neurodegeneration. Particularly, paraquat exposure has been largely associated with Parkinson’s disease (PD). However, there are still a number of controversies regarding the relevance of these observations and the validity of paraquat neurotoxicity as a model for sporadic PD. Nevertheless, the study of paraquat-induced neurotoxicity has provided valuable insight into the mechanisms regulating neuronal cell death by environmental toxicants. To date, the molecular mechanisms involved in neuronal cell death by paraquat are still unclear. Research so far clearly demonstrates a role for oxidative stress and ROS in paraquat-induced neurotoxicity, which seems to be mediated by both mitochondrial and ER stress pathways. Several mechanisms including a) redox cycling, b) mitochondrial ETC inhibition, and c) activation of NADPH oxidases have been proposed as potential sources for paraquat-induced ROS formation, particularly for the accumulation of •O2−. However, the molecular targets being regulated by oxidative stress and ROS in response to paraquat remain elusive. Recent reports suggest that oxidation of signaling molecules such as thioredoxin might be a central event regulating the activation of specific signal transduction pathways such as ASK-1/SAPK and p53, which is translated into the regulation of pro-apoptotic protein expression. Because of the complexity of the effects of environmental exposures on human health, it has been proposed that the study of the exposure to the combination of several toxicants might be a more relevant area of research, which could uncover new mechanisms by which environmental exposures regulate neurodegenerative diseases. In this way, it has been demonstrated that exposure to paraquat together with other pesticides (maneb) or metals (iron) exert their toxicity by mechanisms involving synergistic processes or the activation of completely different signal transduction pathways. Indeed, this is an area of research whose results certainly have great potential to be translated in important advances in public health.
Acknowledgments
This work was supported by the National Institutes of Health Grant P20RR17675 Centers of Biomedical Research Excellence (COBRE) (R. Franco) and the School of Community Health Sciences and a Junior Faculty Research Award from the University of Nevada-Reno (MI Panayiotidis). Due to space limitations we would like to apologize in advance to all our colleagues whose research was not cited in this review but whose work has certainly advanced our understanding of this field of research.
Abbreviations
- APAF1
apoptotic protease activating factor
- ARE
antioxidant response element
- ASK1
apoptosis signal regulating kinase 1
- Bcl-2
B-cell lymphoma
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2–associated X protein
- BH3
Bcl-2 homology domain 3
- Bid
Bcl-2 interacting domain
- Bik
Bcl-2-interacting killer
- BNip3
Bcl-2/adenovirus E1B 19 kDa protein-interacting protein 3
- Caspases
cysteine-dependent aspartate-directed proteases
- CHOP
C/EBP homologous protein
- DAT
dopamine transporters
- Diquat
1,1′-ethylene-2,2′-dipyridylium dibromide
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- ETC
electron transport chain
- GPX
glutathione peroxidaxe
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- 4HNE
4-hydroxy-2-nonenal
- IRE1
inositol-requiring enzyme 1
- JNK
c-Jun N-terminal kinase
- Nrf-2
NF-E2-related factor-2
- LAT-1
large neutral amino acid transporter
- LOO•
lipid peroxyl radicals
- LRRK2
leucine-rich repeat kinase 2
- Maneb
manganese ethylene-bis-dithiocarbamate)
- MPO
myeloperoxidases
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NADPH
nicotinamide adenine dinucleotide phosphate
- •NO
nitric oxide
- NOS
nitric oxide synthase
- •O2−
superoxide anion
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- PERK
PKR-like ER kinase
- PD
Parkinson’s disease
- PINK-1
PTEN induced putative kinase 1
- PQ+
mono-cation radical
- Redox
reduction oxidation
- ROS
reactive oxygen species
- SAPK
stress activated protein kinases
- SN
substantia nigra
- SOD
superoxide dismutase
- Trx
thioredoxin
- UPS
ubiquitin-proteasome system
Footnotes
CONFLICT OF INTEREST
The author declares that there is no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lai BC, Marion SA, Teschke K, Tsui JK. Occupational and environmental risk factors for Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:297–309. doi: 10.1016/s1353-8020(01)00054-2. [DOI] [PubMed] [Google Scholar]
- 2.Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson’s disease. Cell Death Differ. 2010 doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- 4.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology. 1999;18:303–308. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- 7.Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Andersen JK. Paraquat and iron exposure as possible synergistic environmental risk factors in Parkinson’s disease. Neurotox Res. 2003;5:307–313. doi: 10.1007/BF03033150. [DOI] [PubMed] [Google Scholar]
- 9.Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, Gorell J. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect. 2006;114:1872–1876. doi: 10.1289/ehp.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner CE, Battistutta D, Dunne MP, Silburn PA, Mellick GD. Test-retest repeatability of self-reported environmental exposures in Parkinson’s disease cases and healthy controls. Parkinsonism Relat Disord. 2005;11:287–295. doi: 10.1016/j.parkreldis.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson’s disease. J Neurol Sci. 2004;217:169–174. doi: 10.1016/j.jns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell L, Maginnis C, Lewis S, Pickering N, Antoniak M, Hubbard R, Lawson I, Britton J. Occupational exposure to solvents and metals and Parkinson’s disease. Neurology. 2003;61:716–717. doi: 10.1212/wnl.61.5.716. [DOI] [PubMed] [Google Scholar]
- 14.Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J Neurosci. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, Jorgensen PJ, Budtz-Jorgensen E, Grandjean P. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 18.Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- 19.Rango M, Canesi M, Ghione I, Farabola M, Righini A, Bresolin N, Antonini A, Pezzoli G. Parkinson’s disease, chronic hydrocarbon exposure and striatal neuronal damage: a 1-H MRS study. Neurotoxicology. 2006;27:164–168. doi: 10.1016/j.neuro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J Neurol Sci. 2007;262:37–44. doi: 10.1016/j.jns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 22.Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annual review of public health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- 23.Costa LG, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–1249. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 24.Voccia I, Blakley B, Brousseau P, Fournier M. Immunotoxicity of pesticides: a review. Toxicology and industrial health. 1999;15:119–132. doi: 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- 25.Colborn T. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environ Health Perspect. 2006;114:10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson P, Talts U. Neonatal exposure to neurotoxic pesticides increases adult susceptibility: a review of current findings. Neurotoxicology. 2000;21:37–47. [PubMed] [Google Scholar]
- 27.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107(Suppl 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss B. Vulnerability to pesticide neurotoxicity is a lifetime issue. Neurotoxicology. 2000;21:67–73. [PubMed] [Google Scholar]
- 31.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 33.Ross CA, Smith WW. Gene-environment interactions in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S309–315. doi: 10.1016/S1353-8020(08)70022-1. [DOI] [PubMed] [Google Scholar]
- 34.Benmoyal-Segal L, Soreq H. Gene-environment interactions in sporadic Parkinson’s disease. J Neurochem. 2006;97:1740–1755. doi: 10.1111/j.1471-4159.2006.03937.x. [DOI] [PubMed] [Google Scholar]
- 35.Palomo T, Archer T, Beninger RJ, Kostrzewa RM. Gene-environment interplay in neurogenesis and neurodegeneration. Neurotox Res. 2004;6:415–434. doi: 10.1007/BF03033279. [DOI] [PubMed] [Google Scholar]
- 36.Shimada H, Furuno H, Hirai K, Koyama J, Ariyama J, Simamura E. Paraquat detoxicative system in the mouse liver postmitochondrial fraction. Arch Biochem Biophys. 2002;402:149–157. doi: 10.1016/S0003-9861(02)00059-0. [DOI] [PubMed] [Google Scholar]
- 37.Murray RE, Gibson JE. Paraquat disposition in rats, guinea pigs and monkeys. Toxicol Appl Pharmacol. 1974;27:283–291. doi: 10.1016/0041-008x(74)90199-9. [DOI] [PubMed] [Google Scholar]
- 38.Liou HH, Chen RC, Tsai YF, Chen WP, Chang YC, Tsai MC. Effects of paraquat on the substantia nigra of the wistar rats: neurochemical, histological, and behavioral studies. Toxicol Appl Pharmacol. 1996;137:34–41. doi: 10.1006/taap.1996.0054. [DOI] [PubMed] [Google Scholar]
- 39.Chanyachukul T, Yoovathaworn K, Thongsaard W, Chongthammakun S, Navasumrit P, Satayavivad J. Attenuation of paraquat-induced motor behavior and neurochemical disturbances by L-valine in vivo. Toxicol Lett. 2004;150:259–269. doi: 10.1016/j.toxlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 40.LoPachin RM, Gavin T. Response to “Paraquat: the red herring of Parkinson’s disease research”. Toxicol Sci. 2008;103:219–221. doi: 10.1093/toxsci/kfn028. author reply 222–213. [DOI] [PubMed] [Google Scholar]
- 41.Cory-Slechta DA, Thiruchelvam M, Di Monte DA. Letter regarding: “Paraquat: the red herring of Parkinson’s disease research”. Toxicol Sci. 2008;103:215–216. doi: 10.1093/toxsci/kfm309. author reply 217–218. [DOI] [PubMed] [Google Scholar]
- 42.Miller GW. Paraquat: the red herring of Parkinson’s disease research. Toxicol Sci. 2007;100:1–2. doi: 10.1093/toxsci/kfm223. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, Shiono H. Carrier-mediated processes in blood--brain barrier penetration and neural uptake of paraquat. Brain Res. 2001;906:135–142. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- 44.Bartlett RM, Holden JE, Nickles RJ, Murali D, Barbee DL, Barnhart TE, Christian BT, DeJesus OT. Paraquat is excluded by the blood brain barrier in rhesus macaque: An in vivo pet study. Brain Res. 2009;1259:74–79. doi: 10.1016/j.brainres.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 46.Chan BS, Lazzaro VA, Seale JP, Duggin GG. The renal excretory mechanisms and the role of organic cations in modulating the renal handling of paraquat. Pharmacol Ther. 1998;79:193–203. doi: 10.1016/s0163-7258(98)00015-1. [DOI] [PubMed] [Google Scholar]
- 47.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 48.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:RA141–147. [PubMed] [Google Scholar]
- 50.West JD, Marnett LJ. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxicol. 2006;19:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 51.Franco R, Panayiotidis MI. Environmental toxicity, oxidative stress, human disease and the “black box” of their synergism: how much have we revealed? Mutat Res. 2009;674:1–2. doi: 10.1016/j.mrgentox.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Franco R, Sanchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Filomeni G, Ciriolo MR. Redox control of apoptosis: an update. Antioxid Redox Signal. 2006;8:2187–2192. doi: 10.1089/ars.2006.8.2187. [DOI] [PubMed] [Google Scholar]
- 54.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 55.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- 58.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 59.Fei Q, McCormack AL, Di Monte DA, Ethell DW. Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J Biol Chem. 2008;283:3357–3364. doi: 10.1074/jbc.M708451200. [DOI] [PubMed] [Google Scholar]
- 60.Yang W, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. J Toxicol Environ Health A. 2007;70:1849–1857. doi: 10.1080/15287390701459262. [DOI] [PubMed] [Google Scholar]
- 61.Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 62.Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 63.Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 64.Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- 65.Norris EH, Uryu K, Leight S, Giasson BI, Trojanowski JQ, Lee VM. Pesticide exposure exacerbates alpha-synucleinopathy in an A53T transgenic mouse model. Am J Pathol. 2007;170:658–666. doi: 10.2353/ajpath.2007.060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson’s disease. Trends Mol Med. 2006;12:521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 68.Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16:2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- 69.Lavara-Culebras E, Paricio N. Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene. 2007;400:158–165. doi: 10.1016/j.gene.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 71.Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 72.Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, Troncoso J, Dawson VL, Dawson TM, Lim KL. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum Mol Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 73.Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–9218. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 75.Banerjee BD, Seth V, Ahmed RS. Pesticide-induced oxidative stress: perspectives and trends. Reviews on environmental health. 2001;16:1–40. doi: 10.1515/reveh.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- 76.Yang W, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: relevance to the dopaminergic pathogenesis. J Toxicol Environ Health A. 2005;68:1939–1961. doi: 10.1080/15287390500226987. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu K, Matsubara K, Ohtaki K, Shiono H. Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res. 2003;46:523–532. doi: 10.1016/s0168-0102(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 2003;976:243–252. doi: 10.1016/s0006-8993(03)02750-1. [DOI] [PubMed] [Google Scholar]
- 79.McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem. 2003;85:82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- 80.Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 82.Tawara T, Fukushima T, Hojo N, Isobe A, Shiwaku K, Setogawa T, Yamane Y. Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Arch Toxicol. 1996;70:585–589. doi: 10.1007/s002040050316. [DOI] [PubMed] [Google Scholar]
- 83.Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drechsel DA, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 87.Dutheil F, Beaune P, Loriot MA. Xenobiotic metabolizing enzymes in the central nervous system: Contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie. 2008;90:426–436. doi: 10.1016/j.biochi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Ghersi-Egea JF, Livertoux MH, Minn A, Perrin R, Siest G. Enzyme mediated superoxide radical formation initiated by exogenous molecules in rat brain preparations. Toxicol Appl Pharmacol. 1991;110:107–117. doi: 10.1016/0041-008x(91)90294-o. [DOI] [PubMed] [Google Scholar]
- 89.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gray JP, Heck DE, Mishin V, Smith PJ, Hong JY, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase: identification of the enzyme as thioredoxin reductase. J Biol Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- 91.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 92.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 93.Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cristovao AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 96.Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez-Polo RA, Rodriguez-Martin A, Moran JM, Niso M, Soler G, Fuentes JM. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Res. 2004;1011:170–176. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- 98.Kang MJ, Gil SJ, Koh HC. Paraquat induces alternation of the dopamine catabolic pathways and glutathione levels in the substantia nigra of mice. Toxicol Lett. 2009;188:148–152. doi: 10.1016/j.toxlet.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Ghersi-Egea JF, Maupoil V, Ray D, Rochette L. Electronic spin resonance detection of superoxide and hydroxyl radicals during the reductive metabolism of drugs by rat brain preparations and isolated cerebral microvessels. Free Radic Biol Med. 1998;24:1074–1081. doi: 10.1016/s0891-5849(97)00387-0. [DOI] [PubMed] [Google Scholar]
- 100.Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Rispoli V, Nistico R, Rotiroti D, Salvemini D. The role of oxidative stress in paraquat-induced neurotoxicity in rats: protection by non peptidyl superoxide dismutase mimetic. Neurosci Lett. 2003;335:163–166. doi: 10.1016/s0304-3940(02)01168-0. [DOI] [PubMed] [Google Scholar]
- 101.Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 102.Choi HS, An JJ, Kim SY, Lee SH, Kim DW, Yoo KY, Won MH, Kang TC, Kwon HJ, Kang JH, Cho SW, Kwon OS, Park J, Eum WS, Choi SY. PEP-1-SOD fusion protein efficiently protects against paraquat-induced dopaminergic neuron damage in a Parkinson disease mouse model. Free Radic Biol Med. 2006;41:1058–1068. doi: 10.1016/j.freeradbiomed.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Rossi L, Marchese E, Lombardo MF, Rotilio G, Ciriolo MR. Increased susceptibility of copper-deficient neuroblastoma cells to oxidative stress-mediated apoptosis. Free Radic Biol Med. 2001;30:1177–1187. doi: 10.1016/s0891-5849(01)00533-0. [DOI] [PubMed] [Google Scholar]
- 104.Schmuck G, Rohrdanz E, Tran-Thi QH, Kahl R, Schluter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res. 2002;4:1–13. doi: 10.1080/10298420290007574. [DOI] [PubMed] [Google Scholar]
- 105.Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47:904–915. doi: 10.1016/j.neuropharm.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 106.Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Neuroprotective effects of alpha-tocopherol on oxidative stress in rat striatal cultures. Eur J Pharmacol. 2003;465:15–22. doi: 10.1016/s0014-2999(03)01495-x. [DOI] [PubMed] [Google Scholar]
- 107.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 108.Minelli A, Conte C, Grottelli S, Bellezza I, Emiliani C, Bolanos JP. Cyclo (His-Pro) upregulates heme oxygenase 1 via activation of Nrf2-ARE signalling. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06376.x. [DOI] [PubMed] [Google Scholar]
- 109.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Calo M, Iannone M, Passafaro M, Nistico G. Selective vulnerability of hippocampal CA3 neurones after microinfusion of paraquat into the rat substantia nigra or into the ventral tegmental area. J Comp Pathol. 1990;103:73–78. doi: 10.1016/s0021-9975(08)80136-3. [DOI] [PubMed] [Google Scholar]
- 111.Gonzalez-Polo RA, Niso-Santano M, Ortiz-Ortiz MA, Gomez-Martin A, Moran JM, Garcia-Rubio L, Francisco-Morcillo J, Zaragoza C, Soler G, Fuentes JM. Relationship between autophagy and apoptotic cell death in human neuroblastoma cells treated with paraquat: could autophagy be a “brake” in paraquat-induced apoptotic death? Autophagy. 2007;3:366–367. doi: 10.4161/auto.4194. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Polo R, Niso-Santano M, Moran JM, Ortiz-Ortiz MA, Bravo-San Pedro JM, Soler G, Fuentes JM. Silencing DJ-1 reveals its contribution in paraquat-induced autophagy. J Neurochem. 2009;109:889–898. doi: 10.1111/j.1471-4159.2009.06020.x. [DOI] [PubMed] [Google Scholar]
- 113.Gonzalez-Polo RA, Niso-Santano M, Ortiz-Ortiz MA, Gomez-Martin A, Moran JM, Garcia-Rubio L, Francisco-Morcillo J, Zaragoza C, Soler G, Fuentes JM. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol Sci. 2007;97:448–458. doi: 10.1093/toxsci/kfm040. [DOI] [PubMed] [Google Scholar]
- 114.Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 115.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 116.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 117.Khosravi-Far R, Esposti MD. Death receptor signals to mitochondria. Cancer Biol Ther. 2004;3:1051–1057. doi: 10.4161/cbt.3.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 119.Vogt M, Bauer MK, Ferrari D, Schulze-Osthoff K. Oxidative stress and hypoxia/reoxygenation trigger CD95 (APO-1/Fas) ligand expression in microglial cells. FEBS Lett. 1998;429:67–72. doi: 10.1016/s0014-5793(98)00562-6. [DOI] [PubMed] [Google Scholar]
- 120.Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 121.Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- 122.Ekert PG, Vaux DL. The mitochondrial death squad: hardened killers or innocent bystanders? Curr Opin Cell Biol. 2005;17:626–630. doi: 10.1016/j.ceb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 124.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 125.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 127.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 128.Demarchi F, Schneider C. The calpain system as a modulator of stress/damage response. Cell Cycle. 2007;6:136–138. doi: 10.4161/cc.6.2.3759. [DOI] [PubMed] [Google Scholar]
- 129.Yang W, Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J Toxicol Environ Health A. 2008;71:289–299. doi: 10.1080/15287390701738467. [DOI] [PubMed] [Google Scholar]
- 130.Fei Q, Ethell DW. Maneb potentiates paraquat neurotoxicity by inducing key Bcl-2 family members. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05293.x. [DOI] [PubMed] [Google Scholar]
- 131.Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- 132.Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- 133.Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem. 2001;76:1010–1021. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 134.Niso-Santano M, Moran JM, Garcia-Rubio L, Gomez-Martin A, Gonzalez-Polo RA, Soler G, Fuentes JM. Low concentrations of paraquat induces early activation of extracellular signal-regulated kinase 1/2, protein kinase B, and c-Jun N-terminal kinase 1/2 pathways: role of c-Jun N-terminal kinase in paraquat-induced cell death. Toxicol Sci. 2006;92:507–515. doi: 10.1093/toxsci/kfl013. [DOI] [PubMed] [Google Scholar]
- 135.Klintworth H, Newhouse K, Li T, Choi WS, Faigle R, Xia Z. Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 2007;97:149–162. doi: 10.1093/toxsci/kfm029. [DOI] [PubMed] [Google Scholar]
- 136.Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- 137.Yang W, Tiffany-Castiglioni E, Koh HC, Son IH. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2009 doi: 10.1016/j.toxlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 138.Niso-Santano M, Gonzalez-Polo RA, Bravo-San Pedro JM, Gomez-Sanchez R, Lastres-Becker I, Ortiz-Ortiz MA, Soler G, Moran JM, Cuadrado A, Fuentes JM. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death. Modulation by the Nrf2/Trx axis. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 139.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 141.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 142.Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–2808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 143.McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- 144.Zaidi A, Fernandes D, Bean JL, Michaelis ML. Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase. Free Radic Biol Med. 2009;47:1507–1514. doi: 10.1016/j.freeradbiomed.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rohrdanz E, Schmuck G, Ohler S, Kahl R. The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res. 2001;900:128–136. doi: 10.1016/s0006-8993(01)02277-6. [DOI] [PubMed] [Google Scholar]
- 146.Olesen BT, Clausen J, Vang O. Characterization of the transcriptional profile in primary astrocytes after oxidative stress induced by Paraquat. Neurotoxicology. 2008;29:13–21. doi: 10.1016/j.neuro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 147.Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 148.Saint-Pierre M, Tremblay ME, Sik A, Gross RE, Cicchetti F. Temporal effects of paraquat/maneb on microglial activation and dopamine neuronal loss in older rats. J Neurochem. 2006;98:760–772. doi: 10.1111/j.1471-4159.2006.03923.x. [DOI] [PubMed] [Google Scholar]
- 149.Kim S, Hwang J, Lee WH, Hwang DY, Suk K. Role of protein kinase Cdelta in paraquat-induced glial cell death. J Neurosci Res. 2008;86:2062–2070. doi: 10.1002/jnr.21643. [DOI] [PubMed] [Google Scholar]
- 150.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 151.Cory-Slechta DA, Thiruchelvam M, Barlow BK, Richfield EK. Developmental pesticide models of the Parkinson disease phenotype. Environ Health Perspect. 2005;113:1263–1270. doi: 10.1289/ehp.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23:457–470. doi: 10.1016/j.reprotox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 153.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res. 2000;873:225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- 155.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology. 2002;23:621–633. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 157.Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 158.Barlow BK, Richfield EK, Cory-Slechta DA, Thiruchelvam M. A fetal risk factor for Parkinson’s disease. Dev Neurosci. 2004;26:11–23. doi: 10.1159/000080707. [DOI] [PubMed] [Google Scholar]
- 159.Clejan L, Cederbaum AI. Synergistic interactions between NADPH-cytochrome P-450 reductase, paraquat, and iron in the generation of active oxygen radicals. Biochem Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- 160.Sechi GP, Agnetti V, Piredda M, Canu M, Deserra F, Omar HA, Rosati G. Acute and persistent parkinsonism after use of diquat. Neurology. 1992;42:261–263. doi: 10.1212/wnl.42.1.261. [DOI] [PubMed] [Google Scholar]
- 161.Bayol-Denizot C, Daval JL, Netter P, Minn A. Xenobiotic-mediated production of superoxide by primary cultures of rat cerebral endothelial cells, astrocytes, and neurones. Biochim Biophys Acta. 2000;1497:115–126. doi: 10.1016/s0167-4889(00)00047-1. [DOI] [PubMed] [Google Scholar]
- 162.Bonneh-Barkay D, Langston WJ, Di Monte DA. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid Redox Signal. 2005;7:649–653. doi: 10.1089/ars.2005.7.649. [DOI] [PubMed] [Google Scholar]
- 163.Slaughter MR, Thakkar H, O’Brien PJ. Effect of diquat on the antioxidant system and cell growth in human neuroblastoma cells. Toxicol Appl Pharmacol. 2002;178:63–70. doi: 10.1006/taap.2001.9322. [DOI] [PubMed] [Google Scholar]
- 164.Ferraz HB, Bertolucci PH, Pereira JS, Lima JG, Andrade LA. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurology. 1988;38:550–553. doi: 10.1212/wnl.38.4.550. [DOI] [PubMed] [Google Scholar]
- 165.Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand J Work Environ Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- 166.Fitsanakis VA, Amarnath V, Moore JT, Montine KS, Zhang J, Montine TJ. Catalysis of catechol oxidation by metal-dithiocarbamate complexes in pesticides. Free Radic Biol Med. 2002;33:1714–1723. doi: 10.1016/s0891-5849(02)01169-3. [DOI] [PubMed] [Google Scholar]
- 167.Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, Montine TJ. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem. 2003;84:336–346. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Y, Shie FS, Piccardo P, Montine TJ, Zhang J. Proteasomal inhibition induced by manganese ethylene-bis-dithiocarbamate: relevance to Parkinson’s disease. Neuroscience. 2004;128:281–291. doi: 10.1016/j.neuroscience.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 169.Barlow BK, Lee DW, Cory-Slechta DA, Opanashuk LA. Modulation of antioxidant defense systems by the environmental pesticide maneb in dopaminergic cells. Neurotoxicology. 2005;26:63–75. doi: 10.1016/j.neuro.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 170.Domico LM, Cooper KR, Bernard LP, Zeevalk GD. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology. 2007;28:1079–1091. doi: 10.1016/j.neuro.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson’s disease. J Neurochem. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 172.Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seaton TA, Cooper JM, Schapira AH. Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res. 1997;777:110–118. doi: 10.1016/s0006-8993(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 174.Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem. 2003;87:914–921. doi: 10.1046/j.1471-4159.2003.02068.x. [DOI] [PubMed] [Google Scholar]
- 175.Pei W, Liou AK, Chen J. Two caspase-mediated apoptotic pathways induced by rotenone toxicity in cortical neuronal cells. FASEB J. 2003;17:520–522. doi: 10.1096/fj.02-0653fje. [DOI] [PubMed] [Google Scholar]
- 176.Clayton R, Clark JB, Sharpe M. Cytochrome c release from rat brain mitochondria is proportional to the mitochondrial functional deficit: implications for apoptosis and neurodegenerative disease. J Neurochem. 2005;92:840–849. doi: 10.1111/j.1471-4159.2004.02918.x. [DOI] [PubMed] [Google Scholar]
- 177.Radad K, Rausch WD, Gille G. Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int. 2006;49:379–386. doi: 10.1016/j.neuint.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 178.Lee J, Huang MS, Yang IC, Lai TC, Wang JL, Pang VF, Hsiao M, Kuo MY. Essential roles of caspases and their upstream regulators in rotenone-induced apoptosis. Biochem Biophys Res Commun. 2008;371:33–38. doi: 10.1016/j.bbrc.2008.03.149. [DOI] [PubMed] [Google Scholar]
- 179.Watabe M, Nakaki T. Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther. 2004;311:948–953. doi: 10.1124/jpet.104.071381. [DOI] [PubMed] [Google Scholar]
- 180.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 181.Tada-Oikawa S, Hiraku Y, Kawanishi M, Kawanishi S. Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 2003;73:3277–3288. doi: 10.1016/j.lfs.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 182.Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone-elicited dopamine redistribution from vesicles to cytosol in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther. 2007;323:499–507. doi: 10.1124/jpet.107.127597. [DOI] [PubMed] [Google Scholar]
- 183.Li J, Spletter ML, Johnson DA, Wright LS, Svendsen CN, Johnson JA. Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J Neurochem. 2005;92:462–476. doi: 10.1111/j.1471-4159.2004.02872.x. [DOI] [PubMed] [Google Scholar]
- 184.Lim ML, Mercer LD, Nagley P, Beart PM. Rotenone and MPP+ preferentially redistribute apoptosis-inducing factor in apoptotic dopamine neurons. Neuroreport. 2007;18:307–312. doi: 10.1097/WNR.0b013e32801b3ca6. [DOI] [PubMed] [Google Scholar]
- 185.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, Wakabayashi K, Itoyama Y. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem. 2008;283:23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- 187.Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- 189.Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem. 2006;97:934–946. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- 190.Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z. Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci. 2004;79:137–146. doi: 10.1093/toxsci/kfh089. [DOI] [PubMed] [Google Scholar]
- 191.King TD, Jope RS. Inhibition of glycogen synthase kinase-3 protects cells from intrinsic but not extrinsic oxidative stress. Neuroreport. 2005;16:597–601. doi: 10.1097/00001756-200504250-00016. [DOI] [PubMed] [Google Scholar]
- 192.Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem. 2009;284:3001–3011. doi: 10.1074/jbc.M808698200. [DOI] [PubMed] [Google Scholar]
- 193.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ahmadi FA, Grammatopoulos TN, Poczobutt AM, Jones SM, Snell LD, Das M, Zawada WM. Dopamine selectively sensitizes dopaminergic neurons to rotenone-induced apoptosis. Neurochem Res. 2008;33:886–901. doi: 10.1007/s11064-007-9532-5. [DOI] [PubMed] [Google Scholar]
- 195.Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol Pharmacol. 2008;74:933–940. doi: 10.1124/mol.108.048546. [DOI] [PubMed] [Google Scholar]
- 196.Shaikh SB, Nicholson LF. Effects of chronic low dose rotenone treatment on human microglial cells. Mol Neurodegener. 2009;4:55. doi: 10.1186/1750-1326-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 198.Kitazawa M, Anantharam V, Kanthasamy A, Kanthasamy AG. Dieldrin promotes proteolytic cleavage of poly(ADP-ribose) polymerase and apoptosis in dopaminergic cells: protective effect of mitochondrial anti-apoptotic protein Bcl-2. Neurotoxicology. 2004;25:589–598. doi: 10.1016/j.neuro.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 199.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 200.Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- 201.Shinomiya N, Shinomiya M. Dichlorodiphenyltrichloroethane suppresses neurite outgrowth and induces apoptosis in PC12 pheochromocytoma cells. Toxicol Lett. 2003;137:175–183. doi: 10.1016/s0378-4274(02)00401-0. [DOI] [PubMed] [Google Scholar]
- 202.Nakatsu Y, Kotake Y, Ohta S. Concentration dependence of the mechanisms of tributyltin-induced apoptosis. Toxicol Sci. 2007;97:438–447. doi: 10.1093/toxsci/kfm039. [DOI] [PubMed] [Google Scholar]