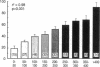Abstract
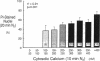
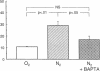
The role of cytosolic free Ca2+ ([Ca2+]i) in hypoxic injury was investigated in rat proximal tubules. [Ca2+]i was measured using fura-2 and cell injury was estimated with propidium iodide (PI) in individual tubules using video imaging fluorescence microscopy. [Ca2+]i increased from approximately 170 to approximately 390 nM during 5 min of hypoxia. This increase preceded detectable cell injury as assessed by PI and was reversible with reoxygenation. 1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA; 100 microM) reduced [Ca2+]i under basal conditions (approximately 80 nM) and during hypoxia (approximately 120 nM) and significantly attenuated hypoxic injury. When [Ca2+]i and hypoxic cell injury were studied concurrently in the same individual tubules, the 10 min [Ca2+]i rise correlated significantly with subsequent cell damage observed at 20 min. 2 mM glycine did not block the rise in [Ca2+]i, yet protected the tubules from hypoxic injury. These results indicate that in rat proximal tubules, hypoxia induces an increase of [Ca2+]i which occurs before cell damage. The protective effect of BAPTA supports a role for [Ca2+]i in the initiation of hypoxic proximal tubule injury. The glycine results, however, implicate calcium-independent mechanisms of injury and/or blockade of calcium-mediated processes of injury such as activation of phospholipases or proteases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida A. R., Bunnachak D., Burnier M., Wetzels J. F., Burke T. J., Schrier R. W. Time-dependent protective effects of calcium channel blockers on anoxia- and hypoxia-induced proximal tubule injury. J Pharmacol Exp Ther. 1992 Feb;260(2):526–532. [PubMed] [Google Scholar]
- Balaban R. S., Mandel L. J. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol. 1988 Mar;254(3 Pt 2):F407–F416. doi: 10.1152/ajprenal.1988.254.3.F407. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Gordon J. A., Bulger R. E., Dobyan D. C., Schrier R. W. Protective effect of intrarenal calcium membrane blockers before or after renal ischemia. Functional, morphological, and mitochondrial studies. J Clin Invest. 1984 Nov;74(5):1830–1841. doi: 10.1172/JCI111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger J. D., Robinette J. B., Schrier R. W. Smooth muscle calcium and endothelium-derived relaxing factor in the abnormal vascular responses of acute renal failure. J Clin Invest. 1988 Aug;82(2):532–537. doi: 10.1172/JCI113628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan K. A., Macdonald G. J., Charlesworth J. A., Pussell B. A. Verapamil prevents post-transplant oliguric renal failure. Clin Nephrol. 1985 Dec;24(6):289–291. [PubMed] [Google Scholar]
- Goldfarb D., Iaina A., Serban I., Gavendo S., Kapuler S., Eliahou H. E. Beneficial effect of verapamil in ischemic acute renal failure in the rat. Proc Soc Exp Biol Med. 1983 Mar;172(3):389–392. doi: 10.3181/00379727-172-41576. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jacobs W. R., Ferrari C. M., Brazy P. C., Mandel L. J. Cytosolic free calcium regulation in renal tubules from spontaneously hypertensive rats. Am J Physiol. 1990 Jan;258(1 Pt 2):F175–F182. doi: 10.1152/ajprenal.1990.258.1.F175. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Sgambati M., Gomez G., Vilaro P., Higdon M., Bell P. D., Mandel L. J. Role of cytosolic Ca in renal tubule damage induced by anoxia. Am J Physiol. 1991 Mar;260(3 Pt 1):C545–C554. doi: 10.1152/ajpcell.1991.260.3.C545. [DOI] [PubMed] [Google Scholar]
- Kohda C., Gemba M. Effect of verapamil on the calcium and magnesium transports of rat kidney cortex mitochondria. Jpn J Pharmacol. 1979 Oct;29(5):745–751. doi: 10.1254/jjp.29.745. [DOI] [PubMed] [Google Scholar]
- Kribben A., Wetzels J. F., Wieder E. D., Burke T. J., Schrier R. W. New technique to assess hypoxia-induced cell injury in individual isolated renal tubules. Kidney Int. 1993 Feb;43(2):464–469. doi: 10.1038/ki.1993.68. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayer H. H., Wagner K. Prevention of delayed graft function in cadaver kidney transplants by diltiazem: outcome of two prospective, randomized clinical trials. J Cardiovasc Pharmacol. 1987;10 (Suppl 10):S170–S177. [PubMed] [Google Scholar]
- Phelps P. C., Smith M. W., Trump B. F. Cytosolic ionized calcium and bleb formation after acute cell injury of cultured rabbit renal tubule cells. Lab Invest. 1989 May;60(5):630–642. [PubMed] [Google Scholar]
- Sasaki D. T., Dumas S. E., Engleman E. G. Discrimination of viable and non-viable cells using propidium iodide in two color immunofluorescence. Cytometry. 1987 Jul;8(4):413–420. doi: 10.1002/cyto.990080411. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Arnold P. E., Van Putten V. J., Burke T. J. Cellular calcium in ischemic acute renal failure: role of calcium entry blockers. Kidney Int. 1987 Sep;32(3):313–321. doi: 10.1038/ki.1987.211. [DOI] [PubMed] [Google Scholar]
- Schwertschlag U., Schrier R. W., Wilson P. Beneficial effects of calcium channel blockers and calmodulin binding drugs on in vitro renal cell anoxia. J Pharmacol Exp Ther. 1986 Jul;238(1):119–124. [PubMed] [Google Scholar]
- Smith M. W., Phelps P. C., Trump B. F. Injury-induced changes in cytosolic Ca2+ in individual rabbit proximal tubule cells. Am J Physiol. 1992 Apr;262(4 Pt 2):F647–F655. doi: 10.1152/ajprenal.1992.262.4.F647. [DOI] [PubMed] [Google Scholar]
- Takano T., Soltoff S. P., Murdaugh S., Mandel L. J. Intracellular respiratory dysfunction and cell injury in short-term anoxia of rabbit renal proximal tubules. J Clin Invest. 1985 Dec;76(6):2377–2384. doi: 10.1172/JCI112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojyo Y., Matsumoto Y. Inhibitory effects of loading with the calcium-chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) on amylase release and cellular ATP level in rat parotid cells. Biochem Pharmacol. 1990 Jun 1;39(11):1775–1779. doi: 10.1016/0006-2952(90)90124-4. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Wagner K., Schultze G., Molzahn M., Neumayer H. H. The influence of long-term infusion of the calcium antagonist diltiazem on postischemic acute renal failure in conscious dogs. Klin Wochenschr. 1986 Feb 3;64(3):135–140. doi: 10.1007/BF01732639. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Roeser N. F., Venkatachalam M. A. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest. 1991 Feb;87(2):581–590. doi: 10.1172/JCI115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Wetzels J. F., Yu L., Wang X., Kribben A., Burke T. J., Schrier R. W. Calcium modulation and cell injury in isolated rat proximal tubules. J Pharmacol Exp Ther. 1993 Oct;267(1):176–180. [PubMed] [Google Scholar]
- Wilson P. D., Schrier R. W. Nephron segment and calcium as determinants of anoxic cell death in renal cultures. Kidney Int. 1986 Jun;29(6):1172–1179. doi: 10.1038/ki.1986.124. [DOI] [PubMed] [Google Scholar]