Abstract
Objective
To determine if specific measures of heart rate variability (HRV) are associated with the total score on a seven-item inventory for Sudden Unexplained Death in Epilepsy (SUDEP).
Methods
Nineteen subjects with intractable partial seizures, at least three per month, were enrolled in a randomized clinical trial of omega-3 fatty acids in epilepsy. At study entry, subjects underwent a 1-hour ECG recording for the determination of HRV. To estimate the risk of SUDEP, we assembled a seven-item inventory (The SUDEP-7 Inventory) from risk factors prospectively validated by Walczak al. [6] The SUDEP-7 score was then correlated with measures of HRV using the Pearson Correlation and other parametric and non-parametric methods.
Results
Subjects were highly refractory, with a mean seizure frequency of 22.8 seizures per month. Scores on the SUDEP-7 inventory ranged from 1 – 7 of a maximum possible score of 12. RMSSD, a measure of high frequency HRV was inversely correlated with the SUDEP-7 score, r = −0.64, p =0.004. Subjects with higher SUDEP-7 scores had reduced levels of HRV (RMSSD). Other time-dependent measures of HRV (SDNN, SDANN) were not significantly correlated with SUDEP risk scores.
Conclusions
RMSSD, a measure of HRV, which reflects the integrity of vagus nerve-mediated autonomic control of the heart, is highly associated with the total score on a new 7-item SUDEP risk inventory. Lower RMSSD values were associated with higher risk scores on a new SUDEP risk inventory. This provides new evidence that HRV (specifically RMSSD) is a marker of SUDEP risk.
Keywords: Seizures, Epilepsy, Heart Rate Variability, Sudden Death in Epilepsy (SUDEP)
Introduction
Sudden unexplained death in epilepsy (SUDEP) is a major cause of death in people with epilepsy.[1] Risk factors for SUDEP have been identified; however, biomarkers have yet to be developed. Heart rate variability (HRV), a measure of autonomic regulation of the heart, is predictive of sudden death in heart disease, and is hypothesized to play a role in the mechanism of SUDEP. [2,3] However, in a small SUDEP series of seven matched subjects, HRV was not significantly lower in SUDEP victims, an unexpected finding confounded by small sample size.[4,5]
The objective of this study was to test the hypothesis that HRV is associated with SUDEP risk in subjects with severe epilepsy. We chose SUDEP risk as a surrogate marker for actual SUDEP, and assembled an inventory of seven validated SUDEP risk factors (the SUDEP-7) adapted from the prospective study of Walczak et al.[6] The use of an inventory of validated risk factors may be helpful in estimating SUDEP risk. We correlated the SUDEP-7 inventory with time-dependent measures of HRV recorded in subjects with intractable partial seizures.
Methods
Subjects were enrolled in a double-blind study of fish oil for intractable seizures. Inclusion criteria were a history of poorly controlled simple partial, complex partial, or tonic/clonic seizures; age 18–70; ≥ three partial-onset seizures/month; exposure to ≥ two antiepileptic drugs at therapeutic doses. At entry, subjects underwent a one-hour electrocardiogram while awake (Philips Digitrak-Plus 24). Sampling rate was 175 Hz, and bandpass was 0.5 to 60 Hz. Beat to beat intervals (R-R intervals) were calculated after correction for artifacts. The following standard time dependent measures of HRV were included for analysis, as defined by Stein et al: SDNN, SDANN, and RMSSD.[7] HRV measures are defined as follows: SDNN (the mean of the standard deviations for all R-R intervals); SDANN (standard deviation of all R-R intervals in successive five-minute epochs), and RMSSD (root-mean square differences of successive R-R intervals).[7]
The SUDEP-7 inventory was assembled from the large prospective cohort study of SUDEP reported by Walczak et al. [6] The core risk factors identified by Walczak et al were consolidated to a 7-item inventory. These items were adapted from table 3 of Walczak et al. (See Walczak et al 2001, page 522).[6] Risk factors with low odds ratios (0 to 2) were not included. The risk factor “any seizures, average per month” was consolidated into two core risk factors: any seizures in the last year, or > 50 seizures per month. Weighting for each risk factor was determined as the natural log of the odds ratio, rounded to the nearest integer. The weighted SUDEP-7 inventory was scored from 0 to 12 (See table 1).
Table 1.
The SUDEP-7 Risk Inventory, derived from the large prospective cohort study reported by Walczak et al. [6]
| SUDEP-7 RISK FACTOR INVENTORY | |||||
|---|---|---|---|---|---|
| SUDEP RISK FACTORS | ODDS RATIO/CONFIDENCE INTERVAL | WEIGHTING LOGE X O.R. | YES OR NO | SCORE | |
| 1 | More than 3 Tonic-Clonic Seizures In Last Year |
8.1 [2.2–30] Walczak et al 2001 |
0 or 2 | ||
| 2 | 1 or more Tonic-Clonic Seizures In Last Year |
2.4 [1.8–30.5] Walczak et al 2001 |
0 or 1 | ||
| 3 | One or more seizures of any type over the last 12 months |
2.2, 3.8, 4.6** [0.3–14.4, .8–19.2, .5–40.9] Walczak et al 2001 |
0 or 1 | ||
| 4 | > 50 seizures of any type per month over the last 12 months |
11.5 [1.3–99.3] Walczak et al 2001 |
0 or 2 | ||
| 5 | Duration of Epilepsy ≥ 30 years |
13.9 [3.4–57.1] Walczak et al 2001 |
0 or 3 | ||
| 6 | Current use of three or more anti-epileptic drugs |
4.0 [1.4–11.7] Walczak et al 2001 |
0 or 1 | ||
| 7 | Mental Retardation, I. Q. <70, or too impaired to test |
5.0 [1.3–19.3] Walczak et al 2001 |
0 or 2 | ||
| Total Weighted Score (0–12) | |||||
Multiple Odds ratios reflect the following average frequencies of any seizures in the last month over a 12-month period: ≤1, > 1 to ≤ 15, >15 to ≤50, from Walczak et al 2001.
SUDEP-7 inventory scores were correlated with the time dependent measures of HRV. The study neuropsychologist determined IQ estimates prospectively at study entry. Correlations were assessed using the Pearson product moment correlation and the Spearman non-parametric rank correlations. Group differences were determined using a two tail students t-test. Significance levels are reported at p < 0.05.
Results
Nineteen consecutive subjects with poorly controlled partial-onset seizures were consented; eighteen completed enrollment. Table 2 summarizes data for the eighteen completed subjects. The mean age for the subjects was 34.8, SD 9.4. The mean total seizure frequency for the group was 0.76 seizures per day SD 1.37 (mean 22.8 seizures per month).
Table 2.
Summary of Data.
| SUBJECT # | AGE | SDNN (msec) | SDANN (msec) | RMSSD (msec) | SUDEP-7 SCORE (WEIGHTED) |
|---|---|---|---|---|---|
| 01 | 32 | 70.5 | 31.6 | 30.4 | 5 |
| 02 | 28 | 40.0 | 8.9 | 20.9 | 5 |
| 03 | 56 | 37.0 | 29.3 | 15.1 | 5 |
| 04 | 44 | 91.1 | 61.1 | 32.0 | 5 |
| 05 | 37 | 46.7 | 13.7 | 21.3 | 4 |
| 06 | 32 | 54.5 | 29.5 | 23.8 | 4 |
| 07 | 23 | 74.8 | 25.2 | 31.3 | 1 |
| 08 | 45 | 58.1 | 44.2 | 14.9 | 7 |
| 09 | 28 | 29.1 | 9.9 | 11.8 | 6 |
| 10 | 19 | 61.2 | 29.6 | 26.5 | 4 |
| 11 | 23 | 74.1 | 45.0 | 47.5 | 1 |
| 12 | 37 | 54.2 | 30.3 | 31.5 | 1 |
| 14 | 29 | 89.3 | 72.8 | 33.6 | 5 |
| 15 | 40 | 63.7 | 54.8 | 20.9 | 4 |
| 16 | 32 | 67.1 | 43.0 | 23.3 | 2 |
| 17 | 34 | 44.6 | 27.9 | 23.5 | 5 |
| 18 | 42 | 45.5 | 27.5 | 26.4 | 4 |
| 19 | 45 | 74.7 | 43.9 | 21.4 | 5 |
| Mean | 34.8 | 59.8 | 34.9 | 25.3 | 4.1 |
| SD | 9.4 | 17.5 | 17.0 | 8.3 | 1.7 |
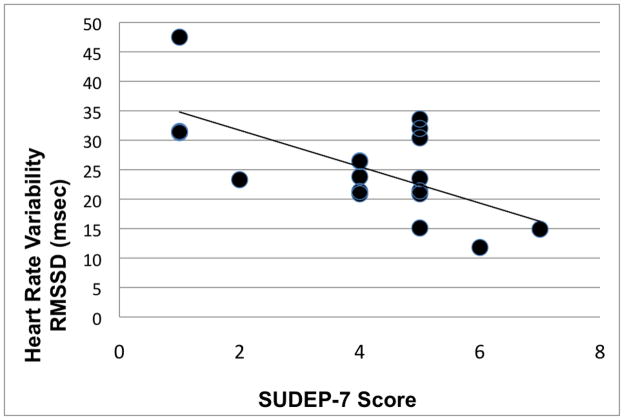
The total score on the SUDEP-7 inventory ranged from 1 to 7, mean 4.1. The mean RMSSD for subjects with a SUDEP score of 0 – 1 was 36.7 msec, versus a mean of 23.0 msec for subjects with SUDEP scores of 2 to 7, p = 0.005, student t-test. Consistent with this mean difference, the SUDEP-7 inventory score was inversely correlated with RMSSD, Pearson r = −0.64, p = 0.004 (Figure 1). Similar results were obtained using the Spearman rank correlation, r =−.512, p = 0.03. The two other HRV measures, SDNN, SDANN, had correlations that were not statistically significant. There was a borderline significant correlation with age (r = 0.40, p = 0.099).
Figure 1.
Scatter plot of RMSSD versus SUDEP-7 score. The inverse correlation was highly significant, r = −.64, p=0.004.
Discussion
The results indicate that a measure of vagus-mediated control of the heart, RMSSD, is highly associated with the SUDEP-7 risk inventory score. Subjects with low RMSSD values (poor vagus-mediated HRV) tended to have higher SUDEP-7 scores (higher risk for SUDEP). Although these findings will require validation in a larger cohort, the results provide preliminary evidence that a measure of HRV is associated with risk factors of SUDEP.
Reduced HRV is an accepted biomarker for mortality and sudden death in heart disease, and is hypothesized to be a biomarker for SUDEP.[2,3] The association between the SUDEP-7 score and RMSSD provides further evidence of a link between risk factors for SUDEP and HRV. Confounding are the results from the important study by Surges et al., who retrospectively evaluated HRV in seven victims of SUDEP.[4.5] Surges et al did not find significant differences in HRV between seven SUDEP victims and matched controls.[4] However, their study did find substantial yet non-significant differences in RMSSD and high frequency power (HF power), both measures of vagus-mediated autonomic control of the heart. [4,5] It is very possible that a larger sample size may indeed find statistical differences in vagus mediated measures of HRV in victims of SUDEP.[4,5]
The central nervous system exerts beat-to-beat control of the heart via the vagus nerve, which modulates heart rate in response to inspiration, expiration, wake and sleep state, and levels of activity.[7, 8] Heart rate variability is measured in time-dependent and frequency-dependent fashion.[7] Time dependent measures include the standard deviation of normal-to-normal intervals (SDNN). SDNN provides a measure of HRV in different states, such as sleep, wake, and activity.[7] RMSSD, the square root of the mean squared differences of successive R-R intervals, is another time-dependent measure of HRV.[7] RMSSD is associated with short-term, rapid changes in heart rate, and is correlated with vagus-mediated components of HRV.[7]
HRV, especially the high frequency components, is regulated by parasympathetic influences from the vagus nerve; only parasympathetic action can mediate the rapid changes accompanying such high frequency variation.[7,8] The vagus nerve exerts its influence by release of acetylcholine at postganglionic muscarinic receptors and the sinoatrial node, and by inhibition of presynaptic norepinephrine release.[8] The effect of the vagus nerve is protective; during myocardial ischemia, the vagus nerve acts to reduce heart rate, increase coronary artery perfusion, and stabilize the myocardium, preventing ventricular fibrillation. [9]
Evidence is accumulating that vagus-mediated autonomic control of the heart is defective in persons with severe epilepsy.[3,10,11] There is an increase in resting sympathetic tone, and decreased parasympathetic (vagus-mediated) tone in epilepsy.[3,10, 11] Diminished vagus influences could lead to inadequate protection from exaggerated sympathetic stress which occurs during seizures. Furthermore, hypoxia may occur in over 30% of seizures, with an average minimum oxygen saturation of 75% after tonic-clonic seizures.[12] Hypoxia can be sustained, with an average duration of desaturation and hypoxia lasting over 1 minute. [12] The combination of sustained and severe hypoxia, with decreased protection by the vagus nerve, may further increase the risk of myocardial injury and lethal arrhythmias, contributing to the risk of SUDEP.
This is an exploratory study, and is limited by the relatively small sample size, and the use of time-dependent, not frequency dependent HRV measures. We plan to validate the data in larger numbers using frequency-dependent measures, including low frequency power (LF), high frequency power (HF), LF/HF ratios, and approximate entropy (ApEn). Ultimately, this inventory should be applied to a cohort who died of SUDEP, compared to age-matched controls. We anticipate the SUDEP-7 inventory will require refinement in the future.
The data from this preliminary study do indicate that RMSSD may be a biomarker of SUDEP risk, and represents a first step toward the development of new tools to assess and estimate SUDEP risk. The SUDEP-7 inventory and measures of HRV may provide ways to identify people at risk, and could serve as outcome measures for new interventions to prevent SUDEP.
Acknowledgments
The study is supported by grants from the National Institutes of Health, NIH/NCCAM R21 AT003420-02 and M01-RR00865; Mr. and Mrs. James and Beverly Peters, the Salter Family Trust, Mr. and Mrs. Marc and Terry Jacoby, Mr. and Mrs. Robert and Linda Brill, the Lagermeier and Lester families.
Footnotes
Presented at the 2009 American Epilepsy Society Annual Meeting, Boston, Massachusetts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurology. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- 2.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. Journal of Cardiovascular Electrophysiology. 2005;16:13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee S, Tripathi M, Chandra P, Yadav R, et al. Cardiovascular autonomic functions in well-controlled and intractable partial epilepsies. Epilepsy Research. 2009;85:261–269. doi: 10.1016/j.eplepsyres.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Surges R, Henneberger C, Adjei P, Scott CA, et al. Do alterations in inter-ictal heart rate variability predict sudden unexpected death in epilepsy? Epilepsy Research. 2009;87:277–280. doi: 10.1016/j.eplepsyres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.DeGiorgio CM, DeGiorgio AC. SUDEP and Heart Rate Variability. Epilepsy Research. 2010 doi: 10.1016/j.eplepsyres.2010.03.013. [EPUB] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walczak TS, Leppik IE, D’Amelio M, Rarick J, et al. Incidence and risk factors in sudden unexpected death in epilepsy. A prospective cohort study. Neurology. 2001;56:519–525. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 7.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart Rate Variability: A measure of cardiac autonomic tone. American Heart Journal. 1993;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 8.Verrier RL, Antzelevitch C. Autonomic aspects of arrythmogenesis: the enduring and the new. Current Opinion in Cardiology. 2004;19:2–11. doi: 10.1097/00001573-200401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 10.Ansakorpi H, Korpelainen JT, Huikuri HV, Tolonen U, et al. Heart rate dynamics in refractory and well controlled temporal lobe epilepsy. J Neurology Neurosurgery Psychiatry. 2002;72:26–30. doi: 10.1136/jnnp.72.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotta H, Koizumi K, Stewart M. Cardiac sympathetic nerve activity during kainic acid-induced limbic cortical seizures in rats. Epilepsia. 2009;50:923–927. doi: 10.1111/j.1528-1167.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 12.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–45. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]