Abstract
Objectives
Efavirenz-based HIV therapy is associated with breast hypertrophy and gynecomastia. Here, we tested the hypothesis that efavirenz induces gynecomastia through direct binding and modulation of estrogen receptor (ER).
Methods
To determine the effect of efavirenz on growth, the estrogen-dependent, ER-positive breast cancer cell lines MCF-7, T47D and ZR-75-1 were treated with efavirenz under estrogen-free conditions in the presence or absence of the anti-estrogen ICI 182,780. Cells treated with 17β-estradiol in the absence or presence of ICI 182,780 served as positive and negative controls, respectively. Cellular growth was assayed using the crystal violet staining method and an in vitro receptor binding assay was used to measure efavirenz’s ER binding affinity.
Results
Efavirenz induced growth in MCF-7 cells with an estimated EC50 of 15.7µM. This growth was reversed by ICI 182,780. Further, efavirenz binds directly to ER (IC50 of ~52µM) at roughly 1000-fold higher concentration than observed with E2.
Conclusions
Our data suggest that efavirenz-induced gynecomastia may be due, at least in part, to drug-induced ER activation in breast tissues.
Keywords: efavirenz, HAART, gynecomastia, estrogens, estrogen receptor
Introduction
The introduction of highly active antiretroviral therapy (HAART) multi-drug combination regimens has considerably improved the prognosis of patients infected with human immunodeficiency virus (HIV) by reducing AIDS-related morbidity and mortality (1). However, chronic treatment with these regimens is associated with multiple adverse effects, non-adherence and eventually therapy failure (2). Treatment regimens containing the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz are preferred in treatment-naive patients and are widely used in other settings (3). While efavirenz is generally well tolerated, concentration-dependent side effects that impact drug adherence and promote resistance have been documented (4). Common adverse effects of efavirenz include central nervous system symptoms, occurring in up to 50% of patients (5), but other less common adverse effects have also been reported. An increasing number of reports suggest that the use of HAART, in particular efavirenz-based therapy, is associated with breast hypertrophy or gynecomastia (6–11). While mechanisms underlying efavirenz-induced gynecomastia are not well understood, a number of hypotheses exist, including a direct estrogenic effect, induction of immune response, or altered steroid hormone metabolism by cytochrome P450 enzymes. To our knowledge, none of these hypotheses have been tested directly. In this study, we tested whether efavirenz can induce breast cancer cell growth by binding and modulating estrogen receptor activity. We examined the ability of efavirenz to (a) induce the growth of the estrogen-dependent, ER-positive breast cancer cell lines MCF-7, T47D and ZR-75-1, in the presence or absence of the pure anti-estrogen ICI 182,780; and (b) directly bind ER using an in vitro fluorescence polarization-based receptor binding assay.
Results
Efavirenz induces breast cancer cell growth
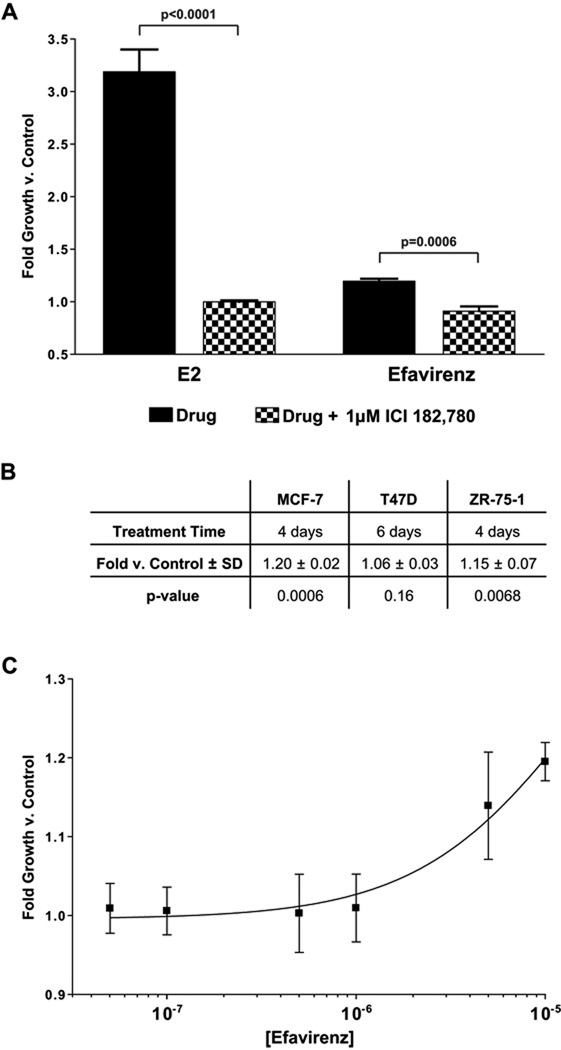
Efavirenz (10µM) stimulated the growth of MCF-7 cells ~1.2-fold greater than vehicle treatment (Figure 1A; right, solid bar). This effect was blocked by the anti-estrogen ICI 182,780 (Figure 1A; right, checkered bar). As expected, E2 (10nM) maximally stimulates growth (~3.2-fold) versus vehicle treatment (Figure 1A; left, solid bar). ICI 182,780 completely blocked E2-induced growth (Figure 1A; left, checkered bar). Efavirenz induced a similar amount of growth in ZR-75-1 cells following 4 days of treatment (Figure 1B), and this growth was blocked by ICI 182,780 (data not shown). However, efavirenz did not stimulate the growth of T47D cells following 6 days of treatment (Figure 1B).
Figure 1.
MCF-7 cells were grown in estrogen-free conditions as described in Materials and Methods. (A) E2 was added to a final concentration of 10nM, and efavirenz was added to a final concentration of 10µM. Cells were treated in the absence (solid bars) or presence (checkered bars) of ICI 182,780 at a final concentration of 1µM. Bars represent 4-day growth vs. vehicle-treated control ± SD of experiments in triplicate. (B) Growth induced by 10µM efavirenz in breast cancer cell lines. SD, Standard Deviation. P-values were determined for efavirenz-treated cells versus vehicle control. (C) Efavirenz was added at increasing concentrations from 50nM – 10µM. Points represent 4-day growth vs. vehicle-treated control ± SD of experiments in triplicate.
The concentration-effect curve for efavirenz-induced growth in MCF-7 cells is shown in Figure 1C. Efavirenz induced cellular growth was concentration-dependent up to 10µM. Growth induced at any concentration was completely blocked by 1µM ICI 182,780 (data not shown). Higher efavirenz concentrations (50 or 100µM) were growth inhibitory to MCF-7, T47D, and ZR-75-1 cells; this effect could not be blocked by ICI 182,780 (data not shown). Although this growth inhibition at high concentrations prevented full characterization of the concentration-effect relationship, we estimated an EC50 of approximately 15.7µM using the data obtained from lower concentrations (1–10µM).
Efavirenz directly binds estrogen receptor alpha
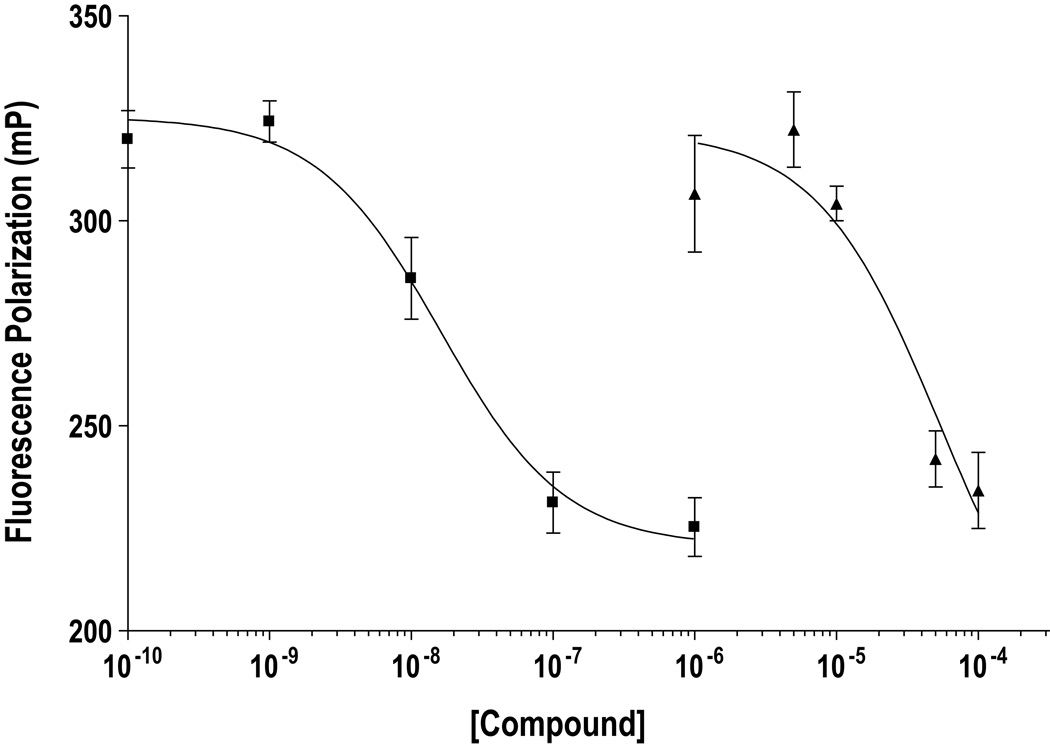
The relative affinity of efavirenz binding to the ER relative to that of E2 was determined using a competitive binding assay as described in the Materials and Methods section. Efavirenz bound ER-alpha at a >1000-fold higher concentration (IC50 of ~52µM) than E2 (IC50 of ~16nM) under these experimental conditions (Figure 2).
Figure 2.
Fluorescence polarization based ER-alpha binding assays were performed as described in Materials and Methods. Decreasing polarization (Y-axis) represents increased receptor occupancy by the test compound, 17β-estradiol (■) or efavirenz (▲). Points represent polarization ± SD of experiments in triplicate.
Discussion
Reports show that 1.8 – 8.4% of male patients develop gynecomastia with efavirenz treatment.(6–11) However, the precise mechanism of this adverse effect remains unknown. Our data suggest that efavirenz-induced gynecomastia may be due to direct estrogenic effects in breast tissues. We demonstrate that efavirenz induces the growth of the estrogen-dependent, ER-positive breast cancer cell lines MCF-7 and ZR-75-1 and that this effect is completely reversed by the anti-estrogen ICI 182,780. We have also provided evidence that efavirenz binds directly to ER-alpha. These data provide the first evidence that efavirenz-induced breast hypertrophy and gynecomastia may be due in part to the ability of the drug to directly activate ER.
Our data is the first to directly demonstrate that efavirenz indeed binds to ER-alpha and that it induces cell growth in an E2-dependent breast cancer model. While efavirenz induced growth at ~105-fold greater concentrations than E2, it bound ER-alpha in vitro at relatively much lower concentrations (only 103-fold greater concentration than E2), consistent with the hypothesis that efavirenz acts as a weak agonist of ER. Further, although efavirenz was much less potent than E2 in inducing growth (EC50’s of 15.7µM vs. 5pM (12)), our findings may be clinically important, because efavirenz concentrations that induce growth in our cell model are within the therapeutic plasma concentration range achieved after daily oral administration of 600mg daily (mean steady state Cmin and Cmax of 5.6µM and 12.9µM respectively, with inter-patient variability ranging from 0.4µM to 48µM)(4, 13). In addition, given the lipophilicity of efavirenz and thus very large volume of distribution, it is likely that the concentration in breast tissues is much higher than in plasma. Efavirenz steady state plasma concentrations in HIV patients exhibit wide intersubject variability due to the effects of genetic polymorphisms and drug interactions (4, 13). Given the concentration-dependent ER-alpha binding and MCF-7 growth induction observed in our study, and that patients with higher efavirenz exposure are at increased risk for adverse effects (4, 13), it is possible that patients achieving higher plasma concentrations of efavirenz are more likely to experience breast hypertrophy and gynecomastia.
The fact that efavirenz induces growth in MCF-7 and ZR-75-1 cells, but not T47D cells, suggests that the efavirenz-induced growth may be dependent on the expression of specific ER transcription co-factors. Unique nuclear receptor co-factor expression is known to play a role in the transcriptional activity of other clinically used agents, particularly the selective estrogen receptor modulator (SERM) tamoxifen, which has differing estrogenic and anti-estrogenic activity in varying target tissues(14).
We were unable to study the effect of efavirenz at high concentrations (>10µM), due to non-specific cytotoxicity or cytostatic effects. However, the fact that high-dose efavirenz-induced growth inhibition was not blocked by the ICI 182,780 suggests that this is unrelated to its estrogenic activity. Interestingly, we found that high concentrations of efavirenz (1–10µM) could antagonize growth induced by 5pM E2, providing additional evidence that efavirenz indeed acts as a weak or partial agonist of ER-alpha (data not shown). However, we could not confirm that this growth antagonism was specifically due to competition for binding to ER-alpha with E2.
Our data may have implications beyond efavirenz’s potential role in gynecomastia. Evidence exists for an increased incidence of AIDS-defining and certain non-AIDS-defining cancers, including breast cancer, in HIV-infected patients. Generally, HAART use has been shown to be protective for AIDS-defining cancers, although the extent of this protection for non-AIDS-defining cancers seems limited. A recent meta-analysis of the incidence of non-AIDS-defining cancers in HIV patients suggests that the incidence of breast cancer in these patients has significantly increased since the implementation of HAART as standard therapy (15). Further epidemiological studies comparing efavirenz-based and non-efavirenz-based therapies will be needed to rule out the possibility that the estrogenic activity of efavirenz may promote breast cancer. It also remains to be seen whether efavirenz interferes with endocrine treatment of breast cancer and contributes to drug resistance.
This study demonstrates that efavirenz directly binds and activates ER, providing a plausible mechanistic explanation for efavirenz-induced gynecomastia in HIV patients. Additional indirect support to this suggestion has been provided by Kegg and Lau, who reported a case of efavirenz-induced gynecomastia that was successfully reversed using 20mg daily tamoxifen (16). Tamoxifen has been widely used for the treatment and prophylaxis of anti-androgen induced gynecomastia in prostate cancer patients with high efficacy and low toxicity (17, 18) in addition to its widespread use as a front line therapy for the treatment and prevention of breast cancer. As multiple anti-retroviral drugs are currently available to treat HIV infection, switching from efavirenz to alternative anti-retroviral drugs may be one potential strategy to alleviate this adverse effect. However, multiple factors need to be considered before switching to an alternate therapy. Based on our in vitro data and evidence from the literature, tamoxifen and other anti-estrogens may be useful in the treatment of efavirenz-induced gynecomastia. Importantly, before considering the addition of an anti-estrogen to a patient’s treatment regimen, other potential causes of gynecomastia should be assessed. A randomized control trial would be necessary to fully evaluate the utility, and more importantly tolerability, of anti-estrogens as a treatment for efavirenz-induced gynecomastia.
Materials and Methods
Cell lines and culture conditions
17β-estradiol (E2) was purchased from Sigma-Aldrich Inc. (St. Louis, MO). Efavirenz and ICI 182,780 were purchased from Toronto Research Chemical (Toronto, Ontario, Canada). The estrogen receptor-positive, estrogen-dependent breast cancer cell lines MCF-7, T47D and ZR-75-1 were obtained from the Tissue Culture Shared Resource (TCSR) at the Lombardi Comprehensive Cancer Center at Georgetown University. These cell lines are widely and routinely used for examinations of the activity of estrogens and anti-estrogens (12, 19). Cells were routinely cultured in modified IMEM (Biosource International Inc., Camarillo, CA) with 10% fetal bovine serum (Valley Biomedical Inc., Winchester, VA), at 37°C in a humidified 5% CO2 atmosphere. For growth assays in estrogen-free conditions, cells were repeatedly washed and grown in steroid depleted media (phenol red-free IMEM supplemented with 5% charcoal stripped calf bovine serum) as previously described (20). Cells were plated in steroid-depleted media at 2 × 103 cells/well in 96-well plates (Falcon, Lincoln Park, NJ) and allowed to attach overnight before treatment with test drugs. Cells in estrogen-free conditions were treated with vehicle (0.1% ethanol), E2 or efavirenz in the presence or absence of the anti-estrogen ICI 182,780. Relative cell number after 4–6 days of growth was determined using crystal violet and WST assays as described previously (21).
Receptor binding assay
Fluorescence polarization based competitive binding assays were performed to measure the relative binding affinity of efavirenz for ER-alpha using a commercially available kit (P2698, Invitrogen, Carlsbad, CA) according to the manufacturer’s specifications. We have previously described the use of this assay to evaluate the relative affinity of ligands for ER-alpha (19). Reactions (100µL) were carried out in black-wall, low-volume 96-well plates (6006270, PerkinElmer, Waltham, MA). Following 2 hours of incubation at room temperature, fluorescence polarization values were obtained using a BMG PolarStar Omega plate reader (BMG Labtech, Durham, NC).
Statistical analyses and curve fitting
Student’s t tests were used to compare treatments to respective controls (SigmaStat Version 3.5, Systat Software Inc, San Jose, CA). Curve fitting and effect concentration for half-maximal growth (EC50) or binding (IC50) were determined using GraphPad Prism Version 4.03 (GraphPad Software, San Diego, CA).
Acknowledgments
This work was supported in part by the Breast Cancer Research Foundation grant N003173 and by U-01 GM61373, T-32 GM007767 and R-01 GM078501-02 from the National from the National Institute of General Medical Sciences, Bethesda, MD.
Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 4.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 5.Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf. 2006;29:865–874. doi: 10.2165/00002018-200629100-00004. [DOI] [PubMed] [Google Scholar]
- 6.Caso JA, Prieto Jde M, Casas E, Sanz J. Gynecomastia without lipodystrophy syndrome in HIV-infected men treated with efavirenz. AIDS. 2001;15:1447–1448. doi: 10.1097/00002030-200107270-00018. [DOI] [PubMed] [Google Scholar]
- 7.Mercie P, Viallard JF, Thiebaut R, et al. Efavirenz-associated breast hypertrophy in HIV-infection patients. AIDS. 2001;15:126–129. doi: 10.1097/00002030-200101050-00021. [DOI] [PubMed] [Google Scholar]
- 8.Rahim S, Ortiz O, Maslow M, Holzman R. A case-control study of gynecomastia in HIV-1-infected patients receiving HAART. AIDS Read. 2004;14:23–24. 9–32, 5–40. [PubMed] [Google Scholar]
- 9.Manfredi R, Calza L, Chiodo F. Efavirenz versus nevirapine in current clinical practice: a prospective, open-label observational study. J Acquir Immune Defic Syndr. 2004;35:492–502. doi: 10.1097/00126334-200404150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Jover F, Cuadrado JM, Roig P, Rodriguez M, Andreu L, Merino J. Efavirenz-associated gynecomastia: report of five cases and review of the literature. Breast J. 2004;10:244–246. doi: 10.1111/j.1075-122X.2004.21392.x. [DOI] [PubMed] [Google Scholar]
- 11.Mira JA, Lozano F, Santos J, et al. Gynaecomastia in HIV-infected men on highly active antiretroviral therapy: association with efavirenz and didanosine treatment. Antivir Ther. 2004;9:511–517. [PubMed] [Google Scholar]
- 12.Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5alpha-androstane-3beta,17beta-diol (3betaAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–270. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Dobrzycka KM, Townson SM, Jiang S, Oesterreich S. Estrogen receptor corepressors -- a role in human breast cancer? Endocr Relat Cancer. 2003;10:517–536. doi: 10.1677/erc.0.0100517. [DOI] [PubMed] [Google Scholar]
- 15.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kegg S, Lau R. Tamoxifen in antiretroviral-associated gynaecomastia. Int J STD AIDS. 2002;13:582–583. doi: 10.1258/095646202760159756. [DOI] [PubMed] [Google Scholar]
- 17.Perdona S, Autorino R, De Placido S, et al. Efficacy of tamoxifen and radiotherapy for prevention and treatment of gynaecomastia and breast pain caused by bicalutamide in prostate cancer: a randomised controlled trial. Lancet Oncol. 2005;6:295–300. doi: 10.1016/S1470-2045(05)70103-0. [DOI] [PubMed] [Google Scholar]
- 18.Fradet Y, Egerdie B, Andersen M, et al. Tamoxifen as prophylaxis for prevention of gynaecomastia and breast pain associated with bicalutamide 150 mg monotherapy in patients with prostate cancer: a randomised, placebo-controlled, dose-response study. Eur Urol. 2007;52:106–114. doi: 10.1016/j.eururo.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 20.Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–149. doi: 10.1007/s10549-005-1483-4. [DOI] [PubMed] [Google Scholar]
- 21.Rae JM, Lippman ME. Evaluation of novel epidermal growth factor receptor tyrosine kinase inhibitors. Breast Cancer Res Treat. 2004;83:99–107. doi: 10.1023/B:BREA.0000010702.10130.29. [DOI] [PubMed] [Google Scholar]