Abstract
What are the neuroplastic mechanisms that allow some stroke patients to regain high quality control of their paretic leg, while others do not? One theory implicates ipsilateral corticospinal pathways projecting from the non-lesioned hemisphere. We devised a new transcranial magnetic stimulation protocol to identify ipsilateral corticospinal tract conductivity from the non-lesioned hemisphere to the paretic limb in chronic stroke patients. We also assessed corticospinal tract degeneration using diffusion tensor imaging and used an ankle tracking task to assess lower limb motor control. We found greater tracking error during antiphase bilateral ankle movement for patients with strong conductivity from the non-lesioned hemisphere to paretic ankle than those with weak or no conductivity. These findings suggest that, instead of assisting motor control, contributions to lower limb motor control from the non-lesioned hemisphere of some stroke survivors may be maladaptive.
Keywords: lower extremity, neuroplasticity, TMS, DTI, stroke
Introduction
Recovering the ability to walk is a critical pre-requisite to functional independence after stroke. But how does our brain re-reorganize the multitude of neural circuits located within the cortex, brain stem and spinal cord to enable recovery? We examined this question by assessing the effects of cortical drive to paretic lower limb spinal motoneurons originating in the lesioned and the non-lesioned hemispheres. In particular we wanted to investigate the potentially adaptive role of the non-lesioned motor cortex following stroke (Strens et al., 2003; Schwerin et al., 2008). Some researchers consider ipsilateral corticospinal projections to paretic upper limb motoneurons to be unhelpful or possibly maladaptive (Fregni & Pascual-Leone, 2006; Eyre, 2007). This notion is primarily based on the observation that poor functional outcomes are associated with ipsilateral motor evoked responses to transcranial magnetic stimulation (TMS) recorded from paretic upper limb muscles, and an increase in functional magnetic resonance imaging blood oxygen level dependent activity (fMRI BOLD) in the non-lesioned sensorimotor cortex (Ward et al., 2006; Schwerin et al., 2008). However, if there are few surviving corticospinal tract (CST) fibers projecting from the lesioned primary motor cortex (M1), non-lesioned sensorimotor cortical drive conducted over the ipsilateral CST may be a critically important physiological resource, despite associated poor functional outcomes.
Previously it was not possible to measure excitability of ipsilateral motor projections to lower limb motoneurons. The inherent low spatial resolution of TMS and the close proximity of the two lower limb motor cortices either side of the mid-sagittal fissure prevents the selective stimulation of one lower limb cortex. Our new TMS technique circumvented this limitation by taking advantage of the between-hemisphere asymmetry of motor system excitability typically evident in stroke survivors (Madhavan & Stinear, 2010) and calculating an index of corticospinal excitability to infer ipsilateral conductivity. CST integrity was estimated from fractional anisotropy (FA) using MRI diffusion tensor imaging (DTI). We regarded low FA asymmetry as an indication of intact CST connectivity.
To the best of our knowledge the association between FA asymmetry and lower limb function has not previously been reported. Improving voluntary control of the paretic ankle following stroke has been shown to translate to better walking outcomes (Mirelman et al., 2009). This finding raises the intriguing notion that using non-invasive brain stimulation as a therapeutic adjuvant to enhance voluntary control of ankle, especially in subacute patients, may translate into better walking recovery than has previously been achieved. The present study focused on investigating potential links between ipsilateral conductivity, connectivity, and ankle control by developing a visuo-motor task with unilateral (UNI) and bilateral limb movement patterns. Joint tracking has been used to examine upper and lower limb motor performance in stroke patients and healthy subjects (Careyet al., 1994; Careyet al., 1998; Carey et al., 2004). In our study bilateral movement involved a more difficult antiphase (AP) task and an easier in-phase (IP) task (Kelso, 1984). The difference in performance between the two bilateral tasks was taken as a measure of control difficulty. The present study therefore examined the hypothesis that patients with fewer CST projections from the lesioned motor cortex have greater conductivity from the non-lesioned hemisphere to the paretic lower limb and more degradation of tracking accuracy during the AP than the IP pattern of cyclic ankle flexion-extension.
Materials and Methods
Subjects
Fifteen chronic stroke patients (9 males; 6 females), age range 42 – 80 years, were recruited for this study. A variety of lesion locations and size was represented. Patients with contraindications to MRI or TMS, including those with metallic implants, a history of seizures and medications known to alter central nervous system excitability, and those with no visible ankle dorsiflexion were excluded. A written and verbal description of the study was provided and written informed consent approved by the Northwestern University Institutional Review Board was obtained from each subject. All methods conformed to the Declaration of Helsinki. A blinded clinical assessor rated each participant using the lower limb section of the FM scale (Fugl-Meyer et al., 1975). Each tested movement is given a score of 0 (movement cannot be performed), 1 (reduced strength, speed, amplitude or precision) or 2 (normal). Gait velocity was assessed using Gaitmat II (E.Q. Inc, PA). Cognitive impairment was assessed using the Mini Mental State Examination (MMSE) (Rovner & Folstein, 1987). Any score greater than 25 (out of 30) is considered normal. All patients participated in three sessions: TMS to assess ipsilateral conductivity, DTI to assess CST degeneration, and tracking to assess ankle motor control.
TMS
Patients were seated comfortably in a chair with knees and ankles flexed to 90 degrees. EMG was collected bilaterally from the TA muscle. An estimate of maximum voluntary isometric contraction (MVIC) was obtained for each muscle with the subject sitting. During TMS measurements the subject was given real time feedback of EMG on an oscilloscope to match a target contraction corresponding to 10% MVIC for each muscle. The subject’s feet were constrained by flexible weights placed over the dorsum of each foot to ensure isometric activation. TMS was used to generate motor evoked potentials (MEPs) from two coil positions – contralateral and ipsilateral – to each muscle being tested (details below). Surface Ag/AgCl electrodes (ConMed SureTrace, Utica NY) were placed over the muscle bellies of the TA of both legs. Before affixing the electrodes, hair was removed and the skin cleaned with alcohol to ensure adequate contact. The reference electrode was placed over the right patella. All EMG data were sampled at 2,000 Hz, amplified (1000 X) and bandpass-filtered (10–500 Hz) using an AMT-8 amplifier (Bortec Biomedical, Canada, Calgary, AB). EMG data were recorded using Spike2 software (Cambridge Electronic Design, UK).
Singe pulse TMS was delivered using a Magstim 200 stimulator (Magstim, Dyfed, Wales, UK) via a double cone coil (diameter 110 mm). Spike2 software was used to trigger the stimulator at 0.25 Hz, and to record the trigger pulses. A linen cap was tied tightly on the subject’s head. The vertex was marked on the cap. Two positions, 1 cm posterior and 1.5 cm left of the vertex and 1 cm posterior and 1.5 cm right of the vertex were marked on the cap. In previous experiments we have found that locating the double cone coil 1 cm posterior to the coronal plane reliably evokes responses in the leg muscles of the majority of subjects. The double cone coil was placed on the cap at either of the laterally offset positions where the intersection of the two embedded coils was located over the marked positions (Fig. 1). The coil was oriented to induce a posterior–anterior current flow in cortex. The coil cable was supported by a coil holder stand and the coil position was maintained manually by an assistant. The position of the coil was checked constantly during data collection to ensure that the coil was in the same position throughout. The coil located offset over the non-lesioned M1 was used to generate contralateral responses from the non-paretic leg and ipsilateral responses from the paretic leg. The coil located offset over the lesioned M1 generated contralateral responses from the paretic leg and ipsilateral responses from the non-paretic leg. Even though we acknowledge all responses are likely a mix of descending volleys from both hemispheres, the term “contralateral responses” refers to motor evoked responses obtained from the leg muscle contralateral to the coil position and “ipsilateral responses” refers to motor evoked responses obtained from the leg muscle ipsilateral to the coil position.
Figure-1.
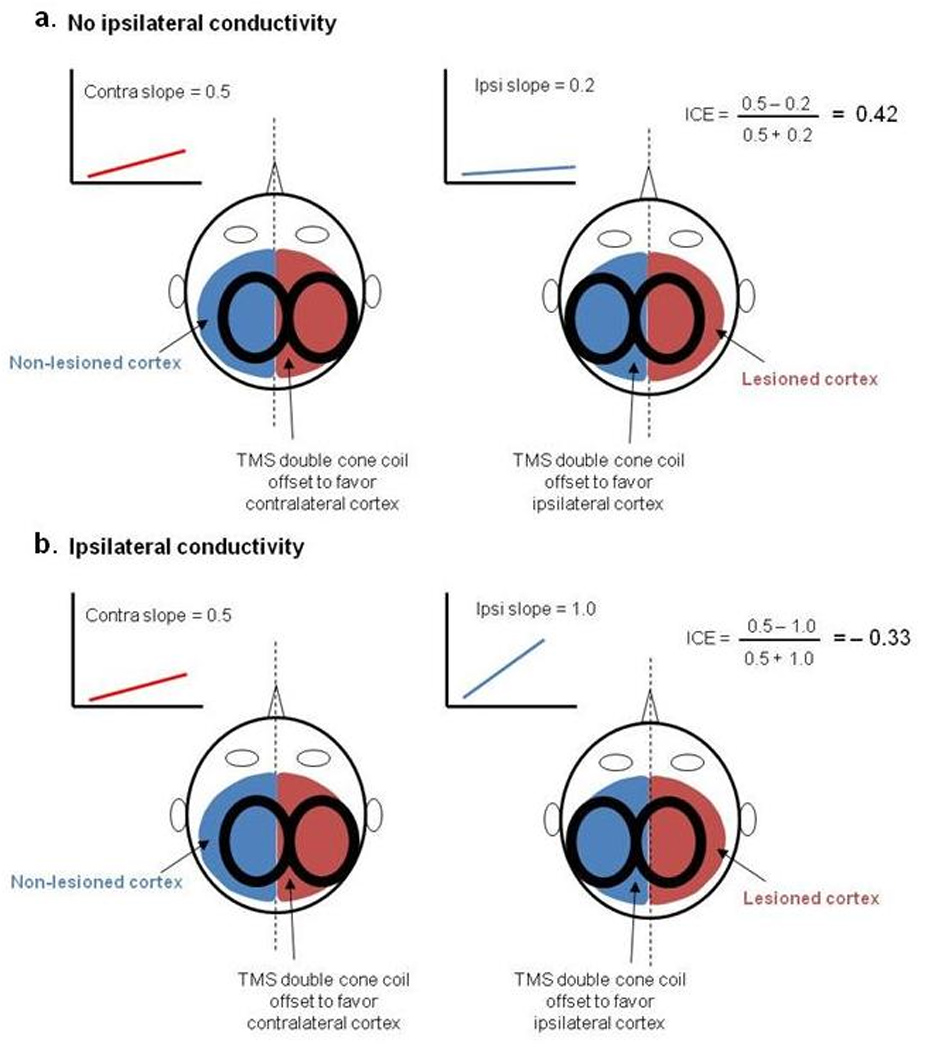
Schematic showing examples for the presence and absence of ipsilateral conductivity as determined from our TMS protocol. Panels a and b show recruitment curves when the TMS coil is positioned contralateral (blue) and ipsilateral (red) to the paretic muscle. Ipsilateral conductivity is assumed when the slope of the ipsilateral recruitment curve is steeper than the contralateral slope, thereby generating a negative value for the index of cortical excitability (ICE).
The coil was first placed offset over the non-lesioned M1. Responses were obtained at TMS intensities corresponding to 30%, 35%, 40%, 45%, 50%, 55%, 60% and 70% maximum stimulator output (MSO) for the non-paretic muscle contralateral to the coil to generate a recruitment curve (Fig. 1). This procedure was repeated with the coil in the same position and recruitment curve data were generated for the paretic muscle ipsilateral to the coil. The coil was then offset over the lesioned M1 and the same protocol was repeated to generate recruitment curve data for the paretic muscle contralateral to the coil and the non-paretic muscle ipsilateral to the coil. As we were interested in the linear component of the recruitment curve, we did not generate responses above 70% MSO. Intensities above 70% using a double cone coil are not well-tolerated. Post analysis showed that obtaining evoked responses between 30 – 70% MSO roughly corresponded to 80 – 140% active threshold, where active threshold was calculated as the stimulation intensity at which MEP amplitude (rectified integrated area) was at least 5% larger than the amplitude (rectified integrated area) of an equivalent window of pre-stimulus EMG. These thresholds were back calculated from recruitment curve data. Ten MEPs were obtained for each muscle at each coil position. Only one muscle was active at a time, thereby two sets of ten MEPs were collected from each coil position, at each intensity for each muscle.
MATLAB (The Mathworks, Inc, Natick, MA, USA) was used to analyze all data imported from Spike2. MEP area analysis was chosen as the key primary measure of corticomotor excitability. Our previous work has shown that the modulation of tonic motoneuron activity resulting from additional motoneuronal firing in response to weak TMS-induced volleys is best captured by calculating the rectified integrated area of EMG within a time window (Madhavan & Stinear, 2010). A MEP window was established for each muscle by finding the onset and offset latencies of large MEPs contralateral to the coil from the non-lesioned hemisphere. Because the onset latencies of upper limb MEPs ipsilateral to the coil and paretic MEPs contralateral to the coil are typically several milliseconds longer than MEPs from the non-lesioned hemisphere contralateral to the coil (Byrnes et al., 2001; Chen et al., 2003), we extended the window by 10 ms to capture increased motoneuron activity resulting from late arriving volleys. A window of identical width was set prior to the stimulus artifact to measure background activity. EMG area (mV.s) for the pre-stimulus window and MEP window was averaged for each muscle, intensity and coil position. The averaged MEP response as a percentage of background activity was plotted against the corresponding stimulus intensity and a linear function was used to fit this recruitment curve. Since we chose not to stimulate at intensities that elicit maximum MEPs, we used a conservative linear fit rather than a Boltzman fit, accepting the likelihood of not detecting a difference in slope. The slope of the steepest region was calculated to estimate the excitability (gain) of each hemisphere. The physiological index of corticospinal excitability (ICE) for each muscle was calculated using the equation:
Because of poor spatial resolution of the TMS coil, we expected our technique to induce responses that were a combination of ipsilateral and contralateral descending volleys. Hence, ipsilateral conductivity from the non-lesioned hemisphere was assumed only from negative values e.g., when steeper slopes were obtained in the paretic limb with the coil located preferentially over the non-lesioned cortex (ipsilateral to the paretic limb) than over the lesioned cortex (contralateral to the paretic limb).
MRI
DTI and anatomical scans were obtained using a Siemens 3T scanner located in the Center for Advanced Magnetic Resonance Imaging, Radiology Department, Northwestern University. T1 weighted anatomical images were obtained to identify lesion location using a magnetization prepared rapid acquisition gradient echo (MP-RAGE) protocol and the following parameters: TR/TE/TI 2300 ms/2.4 ms/900 ms, 9° flip angle, 1 mm3 voxel size.
An echo planar imaging based sequence was used to obtain DTI data using the following scanning parameters: TR/TE 4500ms/91ms; 90° flip angle, 2 mm3 voxel size; b=1000 s/mm2; 64 directions. Data were eddy current corrected and diffusion tensor fitting to each voxel was conducted using the Functional Magnetic Imaging of the Brain (FMRIB) Diffusion Toolbox (Oxford University, UK). FA images were transformed into Montreal Neurological Institute standard space using FMRIB's non-linear image registration tool (FNIRT). Eigenvalue decomposition of the diffusion tensors permits calculation of FA (Pierpaoli et al., 1996). FA is a unitless measure, where values approaching 1 indicate strongly isodirectional water molecule diffusion, which occurs in densely packed white matter tracts (Le Bihan et al., 2001). If the structural integrity of a tract is disrupted, FA decreases (Werring et al., 2000). FA was calculated for the posterior limb of the internal capsules (PLIC) bilaterally using a standard-space mask encompassing a region of interest extending from the posterior margin of the thalamus to the capsule's genu, bounded medially by the thalamus and laterally by the putamen, and from the plane of the anterior commissure to the inferior plane of the ventricles. FA was computed for the affected and unaffected PLICs, and used to calculate the asymmetry of PLIC integrity.
This yields a value between −1.0 and +1.0, where positive values indicate reduced FA in the affected PLIC, and a value of 0 indicates symmetrical FA in the PLICs.
Tracking
A custom built ankle tracking device was constructed for each limb, consisting of two adjustable plates and straps to support and secure the foot and shank. Voltages from a potentiometer affixed to the hinge aligned with the center of ankle joint rotation was used to measure ankle movement. The system was calibrated at the beginning of each testing session. The experimental task was to track a computer generated sinusoidal target with ankle dorsiflexion and plantarflexion. Spike2 software (Cambridge Electronic Design, UK) was used to generate the moving target. All data were sampled at 1000 Hz and recorded using Spike 2 software. The task was to follow the moving target (0.4 Hz) as accurately as possible. While one leg performed the tracking task, the other leg was under any of the following three conditions: stationary for the UNI task, moving in-phase where homologous muscles of both legs active simultaneously for the IP task, and are contracting alternately for the AP task.
Patients were given sufficient practice to confirm an understanding of the task (usually 60 seconds). At the beginning of the session, maximum active paretic and non-paretic ankle range of motion was obtained for each subject. The target sine wave was set to 70% of subject’s range of motion. Visual feedback of the sine wave and ankle response of the tracking leg only was provided, but movement data were collected from both ankles. Performance was monitored to ensure compliance with task requirements and verbal feedback was given when task requirements were not met. During unilateral movement, patients were prompted to avoid mirror movements or any movement with the non-target limb. The order of task and leg was randomized to counter learning effects. Errors in task performance were computed under these different movement conditions. The accuracy index (AI) for each of the three task periods was calculated according to the formula:
where E is the root-mean-square (rms) error (in degrees) between the target line and the response line, and P is the rms value (in degrees) between the sine wave and the midline separating the upper and lower phases of the sine wave. The maximum possible score is 100%. This measure has high intra-class correlation coefficient values in stroke patient data of > 0.88 (Carey et al., 1994). Tracking degradation induced by the AP task in relation to the IP task (AIAP-IP) was computed for the paretic and non-paretic ankle as a measure of control difficulty. Therefore, the IP task served as the control condition.
Statistical Analysis
STATA software (StataCorp LP, College Station TX, USA) was used to perform all statistical analyses. A two-way ANOVA was conducted for AI where the factors were ankle (paretic, non-paretic) and task (UNI, IP, AP); followed by post-hoc Tukey’s HSD comparisons. To examine the effect of ipsilateral conductivity on AI, patients were grouped into those with positive ICE values and those with negative ICE values. Two sample t-tests were conducted to examine the difference in mean AIAP-IP of each group. A linear regression analysis was conducted between FA and FM. The mean FA asymmetry was calculated for patients with positive ICE values and for those with negative ICE values. The difference between these means was examined using a two sample t-test. The adopted level of significance was 0.05.
Results
We recruited 15 chronic stroke patients with cortical and/or subcortical lesions from infarction. Patients with lower limb Fugl-Meyer (FM) assessment scores between 20 and 30 were recruited to ensure compliance with task requirements and a range of mild to moderate impairments. Out of the 15, four patients were excluded due to difficulties performing the tracking task and one patient was excluded due to damage to the cerebellum. Ten were included in the final analysis. Table 1 contains patients’ age, gender, lesion side and location, years since stroke, lower extremity FM score (maximum 34), gait velocity (m/s) and MMSE score.
Table 1.
Descriptive data of participants
| Patient | Lesioned hemisphere |
Lesion location | Gender | Age (yrs) |
Yrs since stroke |
LE FM score |
Gait vel. m/s |
MMSE |
|---|---|---|---|---|---|---|---|---|
| I | R | plic; pallidum | F | 58 | 2 | 28 | 0.63 | 30 |
| II | R | alic; putamen | F | 42 | 9 | 27 | 0.38 | 27 |
| III | L | plic; putamen; pallidum | F | 87 | 9 | 30 | 0.61 | 28 |
| IV | L | posterior border plic; inferior parietal | M | 60 | 1.4 | 26 | 0.90 | 29 |
| V | R | putamen; lateral border plic | M | 80 | 3.5 | 27 | 0.28 | 27 |
| VI | L | plic; thalamus; pallidum; putamen | F | 57 | 23 | 21 | 1.05 | 30 |
| VII | R | plic; pallidum; putamen | M | 60 | 16 | 25 | 0.71 | 27 |
| VIII | L | plic | M | 80 | 15 | 21 | 1.14 | 29 |
| IX | L | posterior border plic | M | 55 | 5.6 | 29 | 1.27 | 28 |
| X | R | plic; pallidum; putamen; inferior | F | 50 | 13 | 27 | 1.24 | 29 |
| Mean | 62.90 | 9.75 | 26.1 | 0.82 | 28.4 | |||
| SD | 14.5 | 6.99 | 3.03 | 0.35 | 1.17 | |||
| Min | 42 | 1.4 | 21 | 0.28 | 27 | |||
| Max | 87 | 23 | 30 | 1.27 | 30 |
plic, posterior limb of the internal capsule; alic, anterior limb of the internal capsule; LE FM, lower extremity Fugl Meyer (maximum score 32); MMSE, Mini Mental State Exam to assess cognitive impairment (maximum score 30)
Representative examples from two patients (VI and VII) showing diffusion weighted images, TMS recruitment curves for the paretic TA, and tracking data from the non-paretic ankle during the AP pattern are shown in Figure 2.
Figure-2.
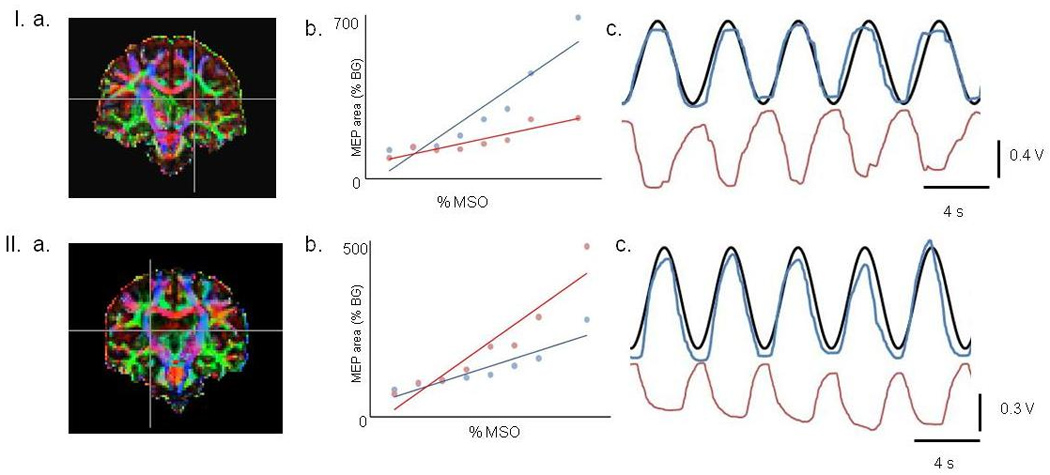
Representative examples from patients VI (top) and VII (bottom).
a. Coronal view of diffusion tensors. White matter direction is illustrated with anterior-posterior fibers in green, lateral fibers in red, and superior-inferior fibers in blue (e.g., the corticospinal tracts). Cross hairs are positioned to denote the degeneration of white matter in the posterior limb of internal capsule. Patient VI had a FA asymmetry of 0.17 and patient VII had a FA asymmetry of 0.05.
b. Recruitment curves from the paretic TA with the coil positioned contralateral (blue) and ipsilateral (red) to the muscle. The ICE values for patients VI and VII were 0.27 and −0.42 respectively. Note that the slope of the ipsilateral recruitment curve is higher than the contralateral curve on patient VII.
c. Non-paretic leg tracking during antiphase pattern. The black line is the target and the blue line is the response. The movement of the paretic ankle is shown in red. Note the degradation in coordination for patient VII.
The two-way ANOVA revealed a significant effect of task, F(1,9) = 19.80, p < 0.001, no effect of ankle (p > 0.7), and an interaction of ankle and task (p = 0.006) (Fig. 3). Tukey’s HSD comparisons revealed the interaction resulted from the AI of the non-paretic ankle being lower (more degraded) (mean: 23.2, SD ± 14.1) than the paretic ankle (34.8, ± 8.2) during the AP task, and the AI of the non-paretic ankle being higher (less degraded) (49.3, ± 7.3) than the paretic ankle during the UNI task (41.6, ± 8.1).
Figure-3.
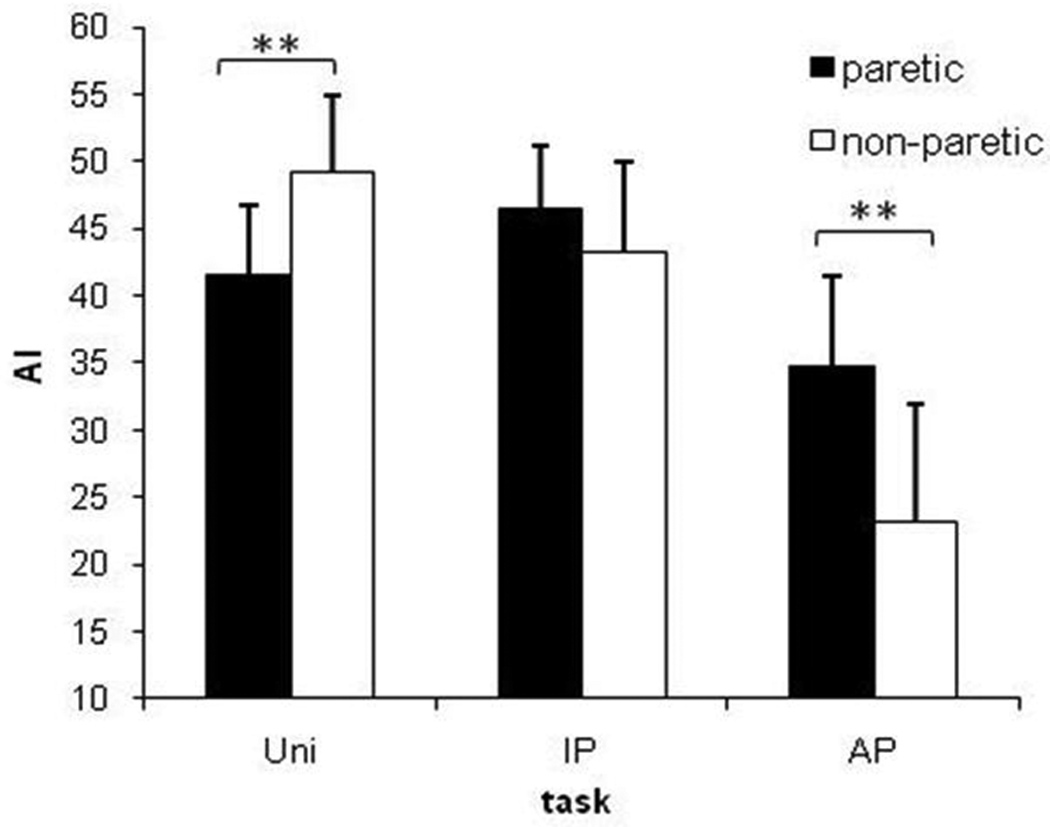
Tracking accuracy during different conditions for stroke patients. The y axis shows the accuracy index (AI) and the x axis shows the three different tasks – UNI, IP, and AP movement patterns. The black bars represent the paretic ankle during tracking and the white bars represent the non-paretic ankle during tracking. Data represent averages (± SEM) of 10 patients. A significant interaction was examined using t-tests (**, p < 0.01).
Negative ICE values were obtained for the paretic TA when a steeper recruitment curve slope was obtained with the coil offset from the mid-sagittal fissure over the non-lesioned M1 (ipsilateral to the paretic limb) than when the coil was offset over the lesioned M1 (contralateral to the paretic limb). More negative ICE values were taken to represent greater conductivity from the non-lesioned M1 to paretic limb motoneurons, and more positive ICE values were taken to indicate a weak or absent contribution to the paretic limb from the non-lesioned M1. The threshold to TMS in the paretic TA of one patient (X) was so high that reliable recruitment curves could not be obtained. Of the other nine patients, four had ICE values below zero, maximum − 0.43 and five had values above zero, maximum + 0.47. Ipsilateral conductivity was not detected in responses from the non-paretic TA i.e., all ICE values were positive (Fig. 4).
Figure-4.
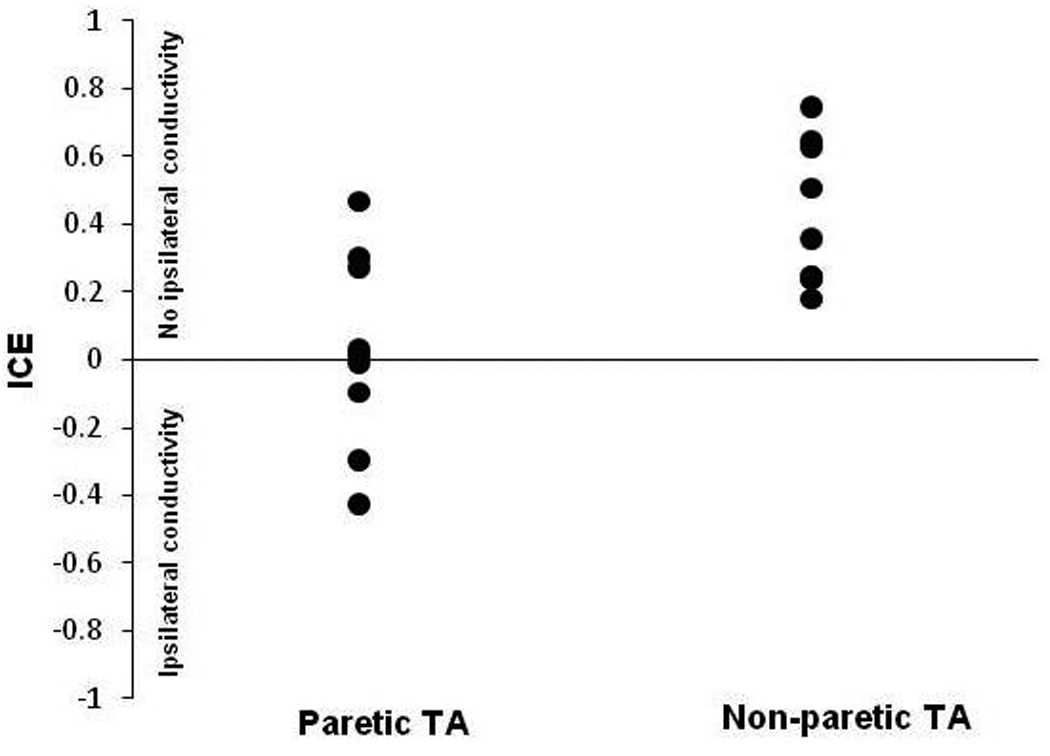
Index of corticospinal excitability (ICE). The y axis shows ICE values and x axis represents the paretic and non-paretic tibialis anterior (TA) muscle. Positive ICE values represent no ipsilateral conductivity and negative values represent ipsilateral conductivity. Note the absence of ipsilateral conductivity for the non-paretic TA.
The t-test of AIAP-IP means revealed that those with greater ipsilateral conductivity (negative ICE) had greater tracking degradation induced by the AP task in relation to the IP task (mean, s.e.m.) for the non-paretic ankle (−25, 8) compared with those who had no ipsilateral conductivity (positive ICE) (−8, 2), p = 0.01 (Figure 5). A smaller difference between AIAP-IP means for the paretic ankle was not significant (negative ICE group: −19, 12; positive ICE group: −7, 2). A Shapiro-Wilk test on STATA able to deal with a small sample size indicated data for each group were from a normal distribution. An F test indicated a between-group equality of variance.
Figure-5.
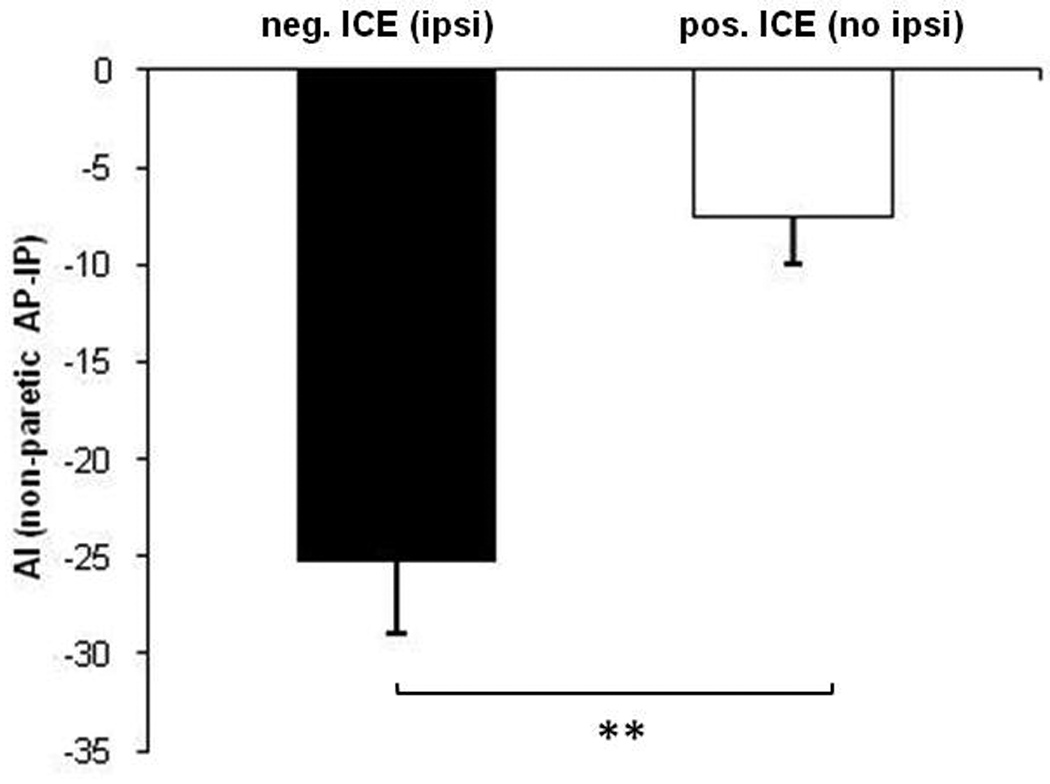
Relationship between ICE calculated from paretic limb recruitment curves when the coil was placed over the ipsilateral (non-lesioned) cortex, and the difference in AI between AP and IP calculated from the non-paretic ankle tracking data. The black bar represents the mean of the tracking error difference in patients (n = 4) who had negative values of ICE (evidence of ipsilateral conductivity to the paretic limb), and the white bar represents the mean in patients (n = 5) who had positive values of ICE (no evidence of ipsilateral conductivity to the paretic limb). More negative values represent greater degradation in AI during AP than IP tracking. Error bars represent 1 SEM. **, p < 0.01.
FA asymmetry ranged from 0.02 through 0.17. A linear regression analysis revealed a modest correlation with FM score, R2 = 0.55, F(1,9) = 9.87, p < 0.014. Greater FA asymmetry predicted lower FM scores. The mean (s.e.m) FA asymmetry for patients with positive ICE values was 0.05 (0.01) and for those with negative ICE values was 0.09 (0.06). But the difference between means failed to reach significance (p = 0.067).
Discussion
The novel finding from the present study is that the motor control of this carefully selected cohort of mild to moderately impaired stroke survivors (FM score 21 – 28) was degraded in a movement pattern where patients simultaneously activated an ankle dorsiflexor in one limb and a plantarflexor in the other limb (the AP task). An expected effect of ankle was not revealed by the ANOVA. This was because paretic ankle tracking accuracy was similar for all three tasks (UNI, IP, AP). However, the predictable inferior tracking accuracy of the paretic ankle compared with the non-paretic ankle was revealed by the post-hoc tests of means for the UNI task (Fig. 3). This finding supports the part of our hypothesis predicting that patients with greater ipsilateral conductivity from the non-lesioned hemisphere to the paretic limb would have greater degradation of tracking accuracy. Of particular interest is the finding that the AP control difficulty was revealed more strongly in the non-paretic ankle and was most evident in patients who had strong ipsilateral conductivity (Fig 5). Although our low FA asymmetry values correlated with high FM scores, as others have reported for upper limb studies following stroke (Stinear et al., 2007), the part of the hypothesis predicting that patients with greater CST degeneration would have stronger ipsilateral conductivity was not supported.
When strong ipsilateral conductivity from the non-lesioned cortex to the paretic limb was evident, tracking degradation of the non-paretic ankle was high. Conversely, when there was weak or no ipsilateral conductivity from the non-lesioned hemisphere, non-paretic ankle tracking was not degraded to the same extent. This key finding may be thought of as “motor conflict” within the non-lesioned hemisphere. The effect of this conflict is a paradox because, it is not intuitive to expect that activity in the non-lesioned hemisphere would result in such a robust degradation of non-paretic limb control. The conflict is not the conflict of movement selection described by cognitive neuroscientists (Rushworth et al., 2003) but can be thought of as an inappropriate transmission of mixed flexor–extensor signals from the non-lesioned motor cortex. For example, when patients with strong ipsilateral conductivity (from the non-lesioned cortex to the paretic limb) were flexing their paretic ankle during AP movement, degraded motor control could arise from paretic limb motoneurons receiving a mix of flexor drive from the contralateral lesioned cortex plus mixed flexor-extensor drive generated in the ipsilateral non-lesioned cortex. Due to high variance in the paretic ankle AIAP-IP measure for patients with ipsilateral conductivity (mean −19, s.e.m. 12) compared with patients with no ipsilateral conductivity (mean, −7, s.e.m. 2), statistically significant data are not available to support this notion. However, statistically significant data did support the explanation that when patients with strong ipsilateral conductivity were flexing their non-paretic ankle during AP movement, degraded motor control could arise from non-paretic limb motoneurons simultaneously receiving a mix of contralateral flexor and extensor drive from the non-lesioned cortex. Why is degradation of tracking accuracy more evident in the non-paretic than the paretic ankle? Based on the motor conflict idea, mixed flexor and extensor drive to the non-paretic ankle may have been stronger because it was transmitted via an intact crossed CST while the mixed drive to the paretic ankle was transmitted over relatively weaker ipsilateral pathways from the non-lesioned motor cortex. Why does the non-lesioned motor cortex generate mixed flexion–extension drive? If few lesioned corticospinal projections survive following stroke, pathways originating in the non-lesioned hemisphere are likely the only anatomical resource the motor system can use to regain lower limb motor control. However, Patients II and VII both of whom had low FA asymmetries of 0.04 therefore presumably had relatively intact crossed corticospinal projections to the paretic limb, also revealed a particularly large degradation of non-paretic AP ankle tracking. One possible explanation is an asymmetry in transcallosal inhibition. Although little is known about transcallosal circuitry relating to the lower limb motor cortices, weak transcallosal inhibition of homologous muscle representations in the non-lesioned lower limb cortex may allow the flexor representation to be active when the extensor representation alone should be active. It is well-established that AP patterns of bilateral human movement are less stable (more variable) than IP patterns especially at high cycle frequencies (Kelso, 1984; Stinear & Byblow, 2001). Patterns of bimanual movement involving asynchronous activation of homologous muscle representations in each cortex depend on an intact corpus callosum (Tuller & Kelso, 1989). Following stroke, an asymmetry develops in transcallosal inhibitory neuronal activity (Duque et al., 2005). Therefore, in the present study, a reduction in transcallosal inhibition from the lesioned cortex to the non-lesioned cortex may have contributed to the non-lesioned cortex delivering mixed flexor-extensor drive during AP movement.
We used our results to construct a clinical algorithm that could guide our development of non-invasive brain stimulation adjuvants to walking therapy following stroke. Data from the present study inform a simple algorithm. Our patients can be divided broadly into two groups: A. those with high ICE, and B. those with low ICE. The algorithm predicts that patients in group A would benefit from having the conflicted drive from their non-lesioned cortex suppressed using non-invasive brain stimulation during motor training to minimize ipsilateral drive to the paretic limb. While patients in group B would benefit from having their lesioned cortex facilitated during motor training to generally increase the drive to their paretic limb motoneurons. Constructing hypotheses to test this algorithm is expected to improve our understanding of post-stroke adaptive mechanisms, and inform the future application of stimulation-based therapeutic adjuvants. Importantly, the suppression of non-lesioned motor cortex excitability, which has been shown to improve upper limb function of stroke patients (Fregni & Pascual-Leone, 2006), may not be an optimal approach for all patients as an adjuvant for lower limb therapy. This concern is greater if the patient’s anatomical resources are limited to an extent that ipsilateral projections to paretic limb motoneurons are the predominant remaining anatomical resource.
The present study has several design, technical, and interpretive limitations. It is important to acknowledge that the functional task we used in the present study has no relationship to walking except that dorsiflexion of one ankle during the swing phase is accompanied by plantar flexion of the other ankle during the stance phase. Because spinal level cyclic modulation of neural circuits and brain stem contributions are associated with walking (Hultborn & Nielsen, 2007), we expected our seated single joint tracking task to be primarily a probe of lower limb cortical control not confounded by walking-related afferent modulation. Some of the dependent variables we used have restrictions, especially for stroke patients. For example, four patients initially recruited who met our inclusion criteria, were not included in the study because they could not generate any dorsiflexion against gravity. We also found that consistent tracking performance was dependent on eliminating mental distractions. Within a trial where reasonable tracking was generally evident, cycles were not included in the analysis when the trace stopped, or drifted in a random manner. The poor tracking performance of some patients may have been partly due to cognitive overload. However, this is unlikely because there was little difference between patients’ MMSE scores, and the two patients (II, VII) with particularly high tracking degradation had MMSE scores very close to the group mean. The behavioral variable that was the difference in tracking accuracy between two bipedal tasks likely imposeed similar cognitive loads. The index of corticospinal excitability is not valid when TMS fails to increase paretic muscle motoneuron firing above pre-TMS values (see Methods). For this reason ICE data from one patient (Patient X) were removed, although all other data from that patient were included. This limitation precludes the more disabled patient from participating in a study where ICE is an important variable. As ICE values become more positive they may not scale to the extent of weakening ipsilateral conductivity, and a positive value does not necessarily indicate a lack of any ipsilateral conductivity. In addition, because we selected a specific cohort of stroke survivors based on FM score, results from this small sample of stroke patients must not be generalized to the stroke population. Our ICE values may have been influenced by a pre-stroke between-hemisphere asymmetry in the conductivity of ipsilateral corticospinal pathways. Such an asymmetry is evident in the upper limb (MacKinnon et al., 2004). Because there is an inconsistent asymmetry unrelated to handedness (some subjects had strong left ipsilateral connections and others had strong right ipsilateral connections), and the asymmetry differed for different muscles, the influence of pre-stroke ipsilateral asymmetries on post-stroke ICE values is likely to remain a moot point. Finally, “ipsilateral conductivity” should not be interpreted as conduction over direct uncrossed monosynaptic pathways alone. There are a number of possible crossed and re-crossed pathways at the brain stem and spinal levels that could contribute to responses in the paretic limb elicited from TMS applied to the non-lesioned motor cortex. Notwithstanding these limitations, our novel approach is a break-through allowing data to be collected in the future to investigate the role of the non-lesioned hemisphere in the recovery of lower limb motor control following stroke.
This study provides a better understanding of neuroplastic mechanisms active in the lower limb corticomotor system following stroke. Of particular importance is the relationship between the extent of damage to the lesioned CST, the presence of ipsilateral conductivity from the non-lesioned hemisphere to the paretic limb, and the quality of voluntary ankle movement. It appears that strong ipsilateral drive from the non-lesioned cortex to paretic ankle motoneurons is maladaptive, even in some patients with a largely intact crossed CST. In the future, further characterization of this paradox is expected to provide a principled basis upon which noninvasive brain stimulation could be applied as a therapeutic adjuvant to enhance voluntary activation of paretic leg muscles.
Acknowledgements
We are especially grateful to Heidi Roth MSPT for patient recruitment and conducting the clinical evaluations and Nathanael Andrew for analyzing tracking data. The study was funded by NIH grants K01HD056216 (JWS), R21HD059287 (JWS), and the Olson Family. SM was supported by a grant from the Department of Education, NIDRR H133F090009.
References
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson KM, Kimberley TJ, Lewis SM, Auerbach EJ, Ugurbil K. fMRI analysis of ankle movement tracking training in subject with stroke. Exp. Brain Res. 2004;154:281–290. doi: 10.1007/s00221-003-1662-7. [DOI] [PubMed] [Google Scholar]
- Carey JR, Baxter TL, Di Fabio RP. Tracking control in the nonparetic hand of subjects with stroke. Arch. Phys. Med. Rehabil. 1998;79:435–441. doi: 10.1016/s0003-9993(98)90146-0. [DOI] [PubMed] [Google Scholar]
- Carey JR, Bogard CL, King BA, Suman VJ. Finger-movement tracking scores in healthy subjects. Percept. Mot. Skills. 1994;79:563–576. doi: 10.2466/pms.1994.79.1.563. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007;31:1136–1149. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Fregni FMDP, Pascual-Leone AMDP. Hand Motor Recovery After Stroke: Tuning the Orchestra to Improve Hand Motor Function. Cognitive and Behavioral Neurology. 2006;33:21. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. Spinal control of locomotion--from cat to man. Acta Physiol (Oxf) 2007;189:111–121. doi: 10.1111/j.1748-1716.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am. J. Physiol. 1984;246:R1000–R1004. doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Quartarone A, Rothwell JC. Inter-hemispheric asymmetry of ipsilateral corticofugal projections to proximal muscles in humans. Exp. Brain Res. 2004;157:225–233. doi: 10.1007/s00221-004-1836-y. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Stinear JW. Focal and bidirectional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimulation. 2010;3:42–50. doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40:169–174. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Folstein MF. Mini-mental state exam in clinical practice. Hosp. Pract. (Off. Ed) 1987;22 99, 103, 106, 110. [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20 Suppl 1:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp. Brain Res. 2008;185:509–519. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Phase transitions and postural deviations during bimanual kinesthetic tracking. Exp. Brain Res. 2001;137:467–477. doi: 10.1007/s002210000665. [DOI] [PubMed] [Google Scholar]
- Strens LH, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr. Biol. 2003;13:1201–1205. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- Tuller B, Kelso JA. Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Exp. Brain Res. 1989;75:306–316. doi: 10.1007/BF00247936. [DOI] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]


