Abstract
The cerebellum is classically considered to be a brain region involved in motor processing, but it has also been implicated innon-motor, and even cognitive, functions. Though previous research suggests that the cerebellum responds to noxious stimuli, its specific role during pain is unclear. Pain is a multidimensional experience that encompasses sensory discriminative, affective motivational, and cognitive evaluative components. Cerebellar involvement during the processing of pain could thus potentially reflect a number of different functional processes. This review will summarize the animal and human research to date that indicates that (1) primary afferents conduct nociceptive (noxious) input to the cerebellum, (2) electrical and pharmacological stimulation of the cerebellum can modulate nociceptive processing, and (3) cerebellar activity occurs during the presence of acute and chronic pain. Possible functional roles for the cerebellum relating to pain will be considered, including perspectives relating to emotion, cognition, and motor control in response to pain.
Keywords: cerebellum, pain, nociception, nociceptive, nocifensive, noxious
1. Introduction
The cerebellum is implicated in several neurological and psychiatric disorders (Gilman, 2000; Schmahmann, 2004). It is involved in a number of integrative functions, including: memory, associative learning, motor control (Ito, 2006; Schmahmann, 1991; Stoodley and Schmahmann, 2009), and more recently in somatosensory processing, including nociception (Saab and Willis, 2003). Nociception represents the neural circuitry that underlies the perception of pain, a multidimensional experience that encompasses sensory discriminative, affective motivational, and cognitive evaluative components (Melzack and Casey, 1968). Though most fMRI studies of pain show activation in the cerebellum (Table 1) (Apkarian et al., 2005; Borsook et al., 2008; Peyron et al., 2000), little is known about the specific role of the cerebellum in nociceptive processing. The purpose of this review is to summarize recent findings that suggest that the cerebellum may have a role in nociceptive processing and in pain. The review will cover a basic overview of the anatomy and connectivity of the cerebellum, the evidence that nociceptive afferents project to the cerebellum, the modulatory effects of cerebellar stimulation on nociceptive processing, how cerebellar activity has been linked to the perception of pain, and what pain-related activation in the cerebellum could functionally reflect.
Table 1.
Functional neuroimaging of cerebellar activation in experimental and pathological pain studies.
| A. Experimental pain | ||||||
|---|---|---|---|---|---|---|
| Paper | Method | Subjects | Stimulation | Site | Foci | |
| (Casey et al., 1996) | PET | 9 (3F) | Thermode | L arm | 1 (C) | |
| Cold water bath | L hand | 1 (C) | ||||
| (Hsieh et al., 1996) | PET | 4 (3F) | Ethanol injection | R arm | 2 (I) | |
| (Svensson et al., 1997) | PET | 11M | Laser | L arm | 1 (I) | |
| Electric | L arm | 1 (I) | ||||
| (Xu et al., 1997) | PET | 6M | Laser | L hand | 2 (B) | |
| (Derbyshire and Jones, 1998) | PET | 12M | Hot water bath | R hand | 1 (I) | |
| (Iadarola et al., 1998) | PET | 13 (5F) | Capsaicin injection | L arm | 5 (B) | |
| (May et al., 1998b) | PET | 7M | Capsaicin injection | R forehead | 3 (B) | |
| (Paulson et al., 1998) | PET | 10M | Thermode | L arm | 2 (I) | |
| 10F | Thermode | L arm | 3 (B) | |||
| (Svensson et al., 1998) | PET | 10 (4F) | Thermode (Tonic) | R arm | 1 (I) | |
| Thermode (Phasic) | R arm | 1 (I) | ||||
| (Becerra et al., 1999) | fMRI | 6M | Thermode (4x) | L arm | 1 (I) | |
| Thermode (3x) | L arm | 1 (I) | ||||
| (Coghill et al., 1999) | PET | 16 (7F) | Thermode | R arm | 2 (B) | |
| (Peyron et al., 1999) | PET | 12 (?F) | Thermode | R arm (L flipped) | 1 (B) | |
| (Becerra et al., 2001) | fMRI | 8M | Thermode | L hand | 3 (B) early | |
| 5 (B) late | ||||||
| (Casey et al., 2001) | PET | 14 (4F) | Thermode | L arm | 2 (B) | |
| (Coghill et al., 2001) | PET | 9 (5F) | Thermode | R arm | 17 (B) | |
| L arm | 15 (B) | |||||
| (Witting et al., 2001) | PET | 8 (2F) | Capsaicin injection | L arm | 1 (C) | |
| (Bingel et al., 2002) | fMRI | 14 (1F) | Laser | R/L hand | 2 (B) | |
| (Brooks et al., 2002) | fMRI | 18 (6F) | Thermode | R hand | 2 (B) | |
| L hand | 1 (I) | |||||
| (Derbyshire et al., 2002) | PET | 16 (11F) | Thermode | R hand | 1 (C) | |
| (Dimitrova et al., 2003) | PET | 16 (5F) | Electric | L tibial nerve | 13 (B) | |
| (Helmchen et al., 2003) | fMRI | 12 (3F) | Thermode | R hand | 16 (B) | |
| (Koyama et al., 2003) | fMRI | 9 (3F) | Thermode | R calf | 1 (I) | |
| (Nemoto et al., 2003) | PET | 12 (6F) | Laser | R arm | 2 (B) | |
| (Strigo et al., 2003) | fMRI | 7 (3F) | Balloon | Esophagus | 4 (B) | |
| Thermode | Upper chest | 5 (B) | ||||
| (Derbyshire et al., 2004) | fMRI | 8 (5F) | Thermode | R hand | 1 (I) | |
| (Giesecke et al., 2004) | fMRI | 11 (4F) | Pressure | L finger | 1 (I) | |
| (Helmchen et al., 2004) | fMRI | 16M | Thermode | R hand | 2 (I) | |
| (Ibinson et al., 2004) | fMRI | 6 (3F) | Electric | R median nerve | 1 (I) | |
| (Kupers et al., 2004) | PET | 10 (4F) | HT-saline injection | R face | 3 (B) | |
| (Wager et al., 2004) | fMRI | 24 (?F) | Electric | R wrist | 6 (I) | |
| 23 (?F) | Thermode | L arm | 6 (I) | |||
| (Botvinick et al., 2005) | fMRI | 12F | Thermode | L hand | 1 (I) | |
| (Koyama et al., 2005) | fMRI | 10 (2F) | Thermode | R leg | 3 (B) | |
| (Wiech et al., 2005) | fMRI | 15 (5F) | Thermode | L arm | 8 (C) | |
| (Albuquerque et al., 2006) | fMRI | 8F | Thermode | R face | 2 (B) | |
| (Carlsson et al., 2006) | fMRI | 10 (3F) | Electric | R wrist | 3 (B) | |
| (Choi et al., 2006) | fMRI | 18F | Hot water bath | L finger | 3 (B) | |
| (Farrell et al., 2006) | fMRI | 10M | Pressure | L finger | 1 (I) | |
| (Kong et al., 2006) | fMRI | 8M | Thermode | R arm | 2 (B) | |
| (Ruehle et al., 2006) | fMRI | 13 (9F) | Electric (ICS) | R foot | 3 (B) | |
| Electric (TCS) | R foot | 3 (B) | ||||
| (Oshiro et al., 2007) | fMRI | 12 (6F) | Thermode | L leg | 2 (B) | |
| (Seminowicz and Davis, 2007) | fMRI | 23 (12F) | Electric | L median nerve | 0 (C)** | |
| (Staud et al., 2007) | fMRI | 13F | Thermode | R foot | 1 (I) | |
| (Borsook et al., 2008) | fMRI | 12 (3F) | Thermode | R face | 4 (B)* | |
| (Helmchen et al., 2008) | fMRI | 14M | Thermode | L hand | 9 (B) | |
| Laser | L hand | 9 (B) | ||||
| (Tseng et al., 2009) | fMRI | 12 (6F) | Thermode | R foot | 2 (B) | |
| B. Pathological pain | ||||||
| Paper | Method | Subjects | Condition | Stimulation | Site | Foci |
| (Hsieh et al., 1995) | PET | 4 (1F) | Neuropathy (L) | Spontaneous pain | L | 1 (I) |
| 4 (3F) | Neuropathy (R) | Spontaneous pain | R varied | 1 (C) | ||
| 8 (4F) | Neuropathy (R+L) | Spontaneous pain | R+L varied (no flip) | 1 | ||
| (May et al., 1998a) | PET | 9M | Cluster headache | Triggered headache | L (R flipped) | 1 (C) |
| (Petrovic et al., 1999) | PET | 5 (3F) | Neuropathy | Brushallodynia (vs. rest) | R varied (L flipped) | 4 (B) |
| Brush allodynia (vs. touch) | R varied (L flipped) | 4 (B) | ||||
| (Derbyshire et al., 2002) | PET | 16 (12F) | Back pain | Thermode | R hand | 1 |
| (Giesecke et al., 2004) | fMRI | 11 (8F) | Back pain | Pressure | L finger | 1 (I) |
| 16 (12F) | Fibromyalgia | Pressure | L finger | 1 (I) | ||
| (Albuquerque et al., 2006) | fMRI | 8F | Burning mouth | Thermal hyperalgesia | R face | 1 (C) |
| (Becerra et al., 2006) | fMRI | 6 (5F) | Neuropathy | Cold allodynia | R face | 1 (I)* |
| Brush allodynia | R face | 2 (B)* | ||||
| Thermal hyperalgesia | R face | 2 (B)* | ||||
| (Ducreux et al., 2006) | fMRI | 6 (?F) | Syringomyelia | Cold allodynia | R hand (L flipped) | 1 (C) |
| (Schweinhardt et al., 2006) | fMRI | 8 (4F) | Neuropathy | Brush allodynia | R hand (L flipped) | 2 (B) |
| (Witting et al., 2006) | PET | 9 (3F) | Neuropathy | Brush allodynia | L varied (R flipped) | 1 (I) |
| (Geha et al., 2007) | fMRI | 11 (10F) | PHN | Spontaneous pain | L varied (R flipped) | 1 (I)* |
| (Borsook et al., 2008) | fMRI | 6 (5F) | Neuropathy (R) | Thermode | R face | 8 (B)* |
| Brush allodynia | R face | 19 (B)* | ||||
| (Geha et al., 2008) | fMRI | 11 (9F) | PHN | Brush allodynia | L varied (R flipped) | 1 (C)* |
Continuous pain ratings collected during imaging.
Did not report activation foci coordinates.
Abbreviations: B=bilateral, C=contralateral to stimuli, HT-saline=hypertonic saline, I=ipsilateral to stimuli, ICS=intracutaneous stimulation, TCS=transcutaneous stimulation
Continuous pain ratings collected during imaging.
Abbreviations: B=bilateral, C=contralateral to stimuli, HT-saline=hypertonic saline, I=ipsilateral to stimuli, ICS=intracutaneous stimulation, PHN=postherpetic neuralgia
2. Overview of Cerebellar Anatomy
The neural circuitry in the cerebellar cortex has a uniform structure featuring a three-layered sheet (Figure 1) (Eccles et al., 1967; Palay and Chan-Palay, 1974; Voogd and Glickstein, 1998). Purkinje cells are the largest neurons in the cerebellum in terms of cell body diameter. Their cell bodies are aligned in a single row to make up the Purkinje cell layer in the cerebellar cortex. Purkinje cells are inhibitory and provide the sole neural output from the cerebellar cortex. In terms of gross morphology, the human cerebellum is comprised of two hemispheres of complex folia joined across the midline by the vermis, with an anterior, a posterior, and a flocculonodular lobe. These three lobes are further divided into 10 lobules, designated I to X (Schmahmann et al., 2000).
Figure 1.
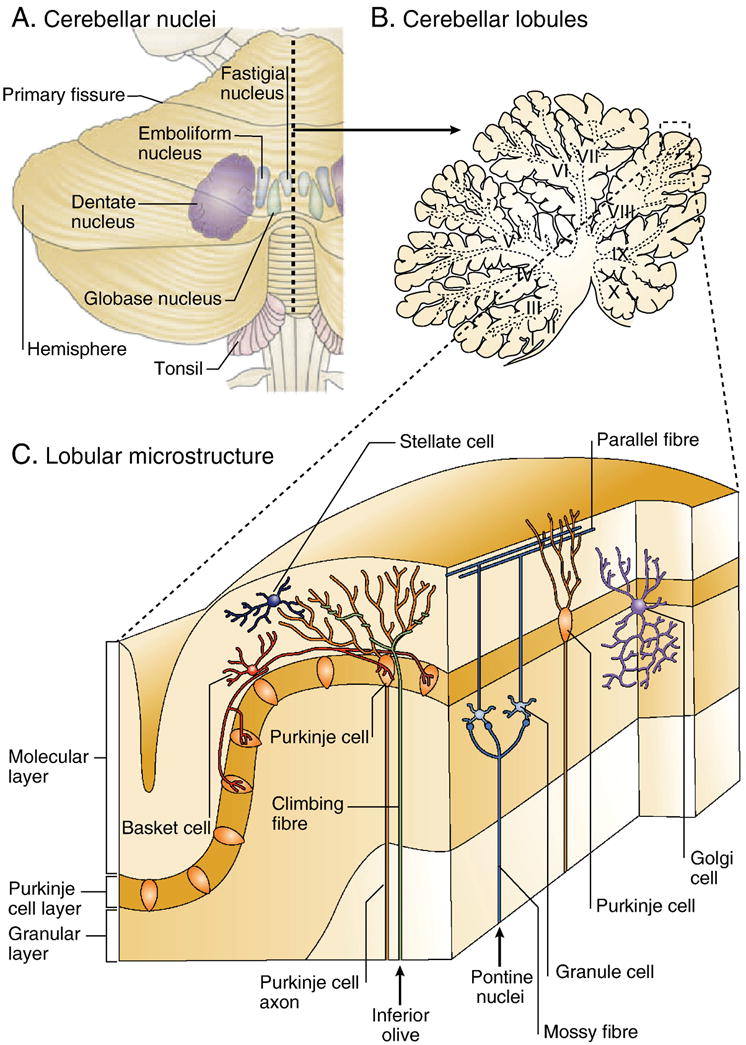
Cerebellar Anatomy. A) Location of the cerebellar nuclei beneath the cerebellar cortex, as viewed from the posterior aspect of the human cerebellum. B) Lobular organization of the human cerebellum. C) Magnified view of the microstructural organization of a representative lobule, highlighting the Purkinje cells and the main cerebellar inputs from the inferior olive and pontine nuclei. Figure modified with permission [pending] from (Ramnani, 2006).
The main ascending input routes to the cerebellum are through the mossy fibers, climbing fibers, and diffuse mono-aminergic and cholinergic afferents. Mossy fibers convey excitatory neural input from the pontine nuclei to granule cells, the axons of which ascend into the molecular layer and bifurcate to form parallel fibers that synapse on the distal dendrites of the Purkinje cells. Climbing fibers also provide excitatory cerebellar afferents, conveying input from the inferior olive to Purkinje cells directly. Both the mossy fibers and climbing fibers also send collateral projections to the deep cerebellar nuclei. The deep cerebellar nuclei project to brainstem nuclei and the thalamus, which relays these projections to different parts of the cerebral cortex.
3. Cortical and Sub-cortical Connectivity to and from the Cerebellum
The cerebellum receives massive cortical input through two major relays in the brainstem: the pontine nuclei and the inferior olive (Figure 2). With the identification of cortical connections to and from the cerebellum, distinctive cerebrocerebellar loops have been discovered that have been proposed to process motor control and cognitive functions (Jissendi et al., 2008; Kelly and Strick, 2003; Middleton and Strick, 2001; Schmahmann, 1996; Schmahmann and Pandya, 1997). Different parts of the cerebellum have anatomical and functional connectivity with specific cortical regions, indicating that the cerebellum has functional zones that may relate to the topography of its cortical connections (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2009; Sasaki et al., 1975; Schmahmann, 1996). A brief review of these connections will help define the possible pain-related functions that the cerebellum may process.
Figure 2.
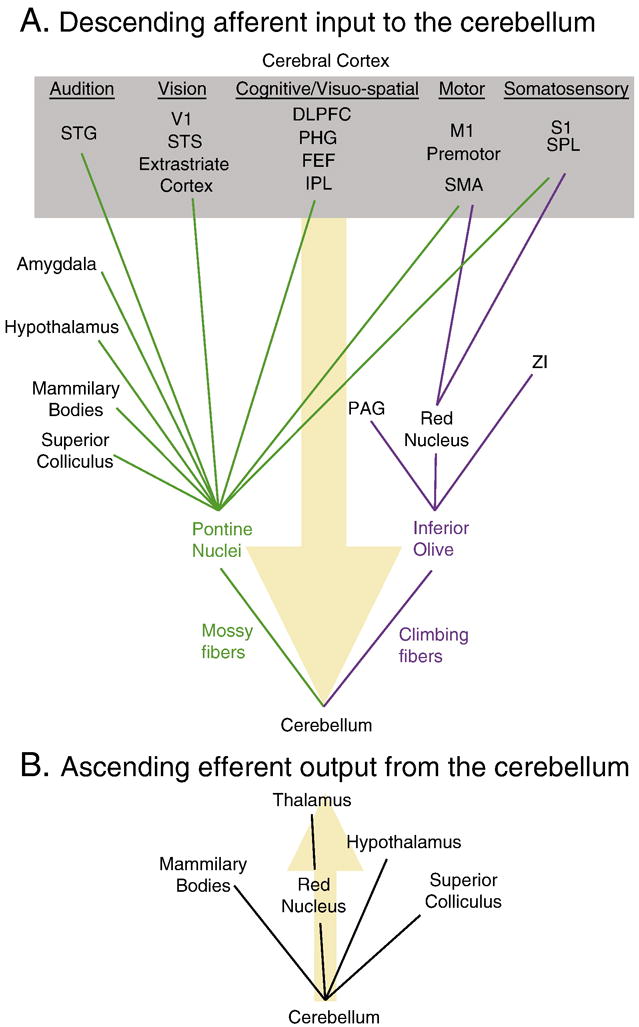
Cortical and sub-cortical connectivity to and from the cerebellum. A) Descending afferent input to the primate cerebellum (orange arrow). Cortical (gray box) and sub-cortical areas send neural inputs to the cerebellum through either the pontine nuclei (green) and inferior olive (purple). Descending connectivity based on (Cerminara et al., 2009; Schmahmann, 1996). B) Ascending efferent output from the primate cerebellum (orange arrow). Ascending connectivity based on (Haines and Dietrichs, 1984; Haines et al., 1997; May et al., 1990; Schmahmann, 1996). Abbreviations: dorsolateral prefrontal cortex (DLPFC), frontal eye fields (FEF), inferior parietal lobule (IPL), primary motor cortex (M1), periaqueductal gray (PAG), parahippocampal gyrus (PHG), primary somatosensory cortex (S1), supplementary motor area (SMA), superior parietal lobule (SPL), superior temporal gyrus (STG), superior temporal sulcus (STS), primary visual cortex (V1), zona incerta (ZI).
In the primate, the pontine nuclei receive extensive and diverse neural input from the cerebral cortex before sending mossy fiber input to the cerebellum through the middle cerebellar peduncle. Based on their areas of origin, cortical projections to the pons convey several different types of functional inputs that may pertain to pain, including motor, somatosensory, visuo-spatial, and cognitive information. Cerebral cortical efferents classically related to motor function arise from the primary motor cortex, premotor cortex, supplemental motor area, and frontal eye fields; while projections to the pons from traditionally somatosensory-related cortical areas arise from the primary somatosensory cortex and superior parietal lobule (Brodal, 1978; Glickstein et al., 1985; Jansen and Brodal, 1940; Nyby and Jansen, 1951; Schmahmann, 1996; Schmahmann and Pandya, 1997; Shook et al., 1990). The pons also receives cortical inputs from association areas relevant to cognition and/or visuo-spatial processing such as the inferior parietal lobule, parahippocampal gyrus, dorsolateral prefrontal cortex (DLPFC), and other regions within the prefrontal cortex (Brodal, 1978; Dum and Strick, 2003; Hartmann-von Monakow et al., 1981; May and Andersen, 1986; Middleton and Strick, 2001; Ramnani et al., 2006; Schmahmann and Pandya, 1997; Strick et al., 2009; Wiesendanger et al., 1979). The DLPFC efferents to the cerebellum are particularly interesting, given that it is a region implicated in cognitive control (Koechlin et al., 2003; MacDonald et al., 2000), as well as pain modulation (Apkarian et al., 2004; Lorenz et al., 2003; Wager et al., 2004; Zubieta et al., 2005). The DLPFC is part of a closed cerebrocerebellar loop (Kelly and Strick, 2003), which suggests that the cerebellum may have a feedback modulatory role in nociceptive processing.
The inferior olive, which sends climbing fiber input to the cerebellum through the inferior cerebellar peduncle, receives input from both cortical and subcortical sources. It receives most of its descending input from the parvicellular red nucleus, which in turn receives input from primary motor cortex, supplementary motor cortex, premotor cortex, primary somatosensory cortex, and the superior parietal lobe (Schmahmann, 1996). In addition to red nucleus input, the inferior olive also receives projections from zona incerta (Cintas et al., 1980; Saint-Cyr and Courville, 1980), which has a role in the expression of chronic pain (Masri et al., 2009). The periaqueductal gray (PAG), a brainstem structure related to descending pain modulation (Fields, 2000), sends projections to the inferior olive as well (Holstege, 1988; Rutherford et al., 1984; Van Bockstaele et al., 1991). Based on these connections, the red nucleus-inferior olive system seems to convey motor and some sensory efferents to the cerebellum, while the zona incerta and PAG inputs may have a role in the modulation of nociceptive processing.
4. Nociceptive Cerebellar Afferents
Though neuroanatomical tracers have shown that the cerebellum receives inputs from cutaneous primary afferents (Edgley and Gallimore, 1988; Randic et al., 1981; Snyder et al., 1978), direct evidence that it receives nociceptive afferents comes from electrophysiological studies. These studies suggest that afferent input from nociceptors reach the cerebellum, as stimulation of nociceptors evokes neural activity in the cerebellum (Figure 3) (Ekerot et al., 1987a; Ekerot et al., 1987b; Hayashi et al., 1984; VanGilder and Fitzmartin, 1973; VanGilder, 1975; Wu and Chen, 1990; Wu and Chen, 1992). In cats, stimulation of cutaneous A-delta and C fiber nociceptors activates climbing fibers that terminate on Purkinje cells in the cerebellar anterior lobe ipsilateral to stimulation (Ekerot et al., 1987a; Ekerot et al., 1987b). C fiber nociceptors convey neural input through the postsynaptic dorsal columns as part of a proposed spino-olivocerebellar pathway (Ekerot et al., 1991). In addition to climbing fiber input, C-fiber nociceptors may also act through mossy fibers to reach Purkinje cells in the cerebellum (Wu and Chen, 1992). In rats, noxious colorectal distention activates visceral nociceptive-specific neurons in the lateral medullary reticular formation, including several with direct projections to the cerebellar vermis (Ness et al., 1998). Likewise, nociceptive visceral stimulation can modulate Purkinje cell activity in the posterior cerebellar vermis (Saab and Willis, 2001). However, evidence of how nociceptive information is encoded once it reaches the cerebellum is lacking.
Figure 3.
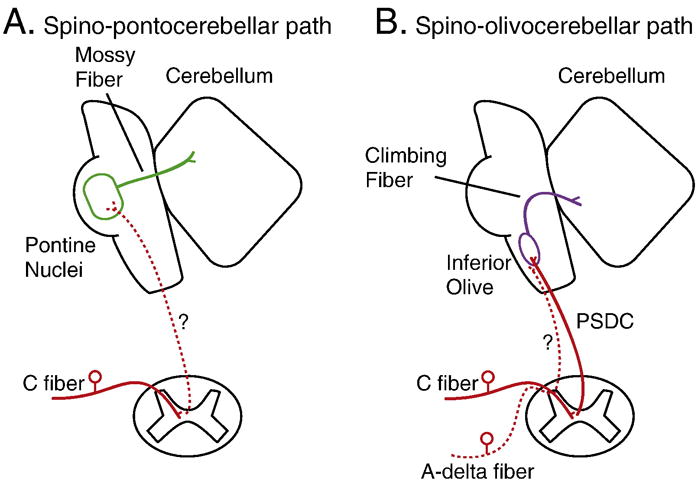
Cutaneous nociceptive afferents to the cerebellum. A) Spino-pontocerebellar path. Stimulation of C fiber nociceptors activates mossy fibers that project to the cerebellum through an unknown pathway. B) Spino-olivocerebellar path. C fiber nociceptors convey neural input through the post-synaptic dorsal column (PSDC) in the spinal cord to the inferior olive to activate climbing fibers that project to the cerebellum. A-delta fiber nociceptors stimulation activates climbing fibers through an unknown pathway.
5. Nociception is Modulated by Cerebellar Stimulation
Inferences from electrical and/or pharmacological stimulation of different parts of the cerebellum imply a modulatory role in nociceptive processing(Table 2). Electrical stimulation of the cerebellar lateral nucleus in rats modulates the encoding of noxious stimuli in intralaminar parafasicular neurons in the thalamus (Liu et al., 1993). In squirrel monkeys, electrical stimulation of the intermediate portion of the anterior cerebellar lobe can raise nociceptive thresholds to tail shock (Siegel and Wepsic, 1974). Microinjection of morphine into the anterior portion of the cerebellum of rats results in acute analgesia that is reversible by both systemic naloxone and electrical stimulation at the site of the microinjection (Dey and Ray, 1982). Stimulation of rat cerebellar cortex using electrical stimulation or chemical stimulation using D,L-homocysteic acid (DLH), a non-specific glutamate receptor agonist, increases neural responses to a noxious visceral stimulus in and around the termination sites of nociceptive afferents in the spinal cord (Saab and Willis, 2001). Chemical stimulation using DLH applied to the rat cerebellar cortex increases visceral nociceptive reflexes, whereas DLH applied to the cerebellar fastigial nucleus decreases these reflexes (Saab and Willis, 2002). This inhibitory effect led the authors to suggest that the cerebellum may engage the pain modulating circuitry in the brainstem (Fields, 2000), which includes the PAG and rostral ventromedial medulla. Perhaps related, a clinical study has demonstrated functional changes in primary somatosensory cortex following unilateral cerebellar lesions, though without accompanying perceptual changes (Restuccia et al., 2001). Though cerebellar activation through electrical and/or chemical means influences nociceptive processing, its role in the experience of pain is still not well defined.
Table 2.
Cerebellar stimulation modulates nociception.
| Stimulus | Cerebellar site | Effect | Nociception | Animal | Paper |
|---|---|---|---|---|---|
| Electric | Anterior lobe (intermediate part) | Increased tail shock thresholds | ↓ | Squirrel Monkeys | (Siegel and Wepsic, 1974) |
| Morphine | Anterior (culmen region) | Increased acute analgesia | ↓ | Rats | (Dey and Ray, 1982) |
| Electric | Lateral nucleus | Modulated parafasicular neurons, Intralaminar thalamus | ↑/↓ | Rats | (Liu et al., 1993) |
| Electric | Posterior vermis (lobule VI) | Increased midline neuron activity in lumbosacral spinal cord | ↑ | Rats | (Saab and Willis, 2001) |
| DLH | Posterior vermis (lobule VI) | Increased midline neuron activity in lumbosacral spinal cord | ↑ | Rats | (Saab and Willis, 2001) |
| DLH | Cortex (vermis, lobule VIII) | Increased nociceptive reflexes (abdominal) | ↑ | Rats | (Saab and Willis, 2002) |
| DLH | Fastigial nucleus | Decreased nociceptive reflexes (abdominal) | ↓ | Rats | (Saab and Willis, 2003) |
DLH (D, L homocysteic acid) = nonspecific glutamate receptor agonist
6. Cerebellar Activity and the Perception of Pain
Though pain neuroimaging studies often report cerebellar activation (Figure 4; Table 1), fMRI has only recently been applied to specifically addressing the nociceptive activity in the cerebellum and its relationship to pain. Helmchen and colleagues have identified differential patterns of cerebellar responses to innocuous and noxious thermal stimuli (Helmchen et al., 2003; Helmchen et al., 2004). Evidence suggests that nociceptive-specific activation is processed in the deep cerebellar nuclei, anterior vermis, and bilaterally in cerebellar hemispheric lobule VI (Helmchen et al., 2003). This dataset also indicated that in response to a 48.5oC stimulus, ipsilateral hemispheric lobules III-VI were significantly more active in subjects experiencing high pain vs. low pain. This was the first study to explicitly link nociceptive activity in the cerebellum with pain perception. In a follow-up study, cerebellar activity in lobule VI and the anterior vermis varied with pain ratings, but only when the stimuli were self-administered by the subjects being scanned (Helmchen et al., 2004). This contrasts with their earlier study, which found significant effects of pain intensity when stimuli were applied by the experimenters. This discrepancy between the two studies is not explained, and suggests that the cerebellum’s capacity for basic nociceptive encoding needs to be further explored.
Figure 4.
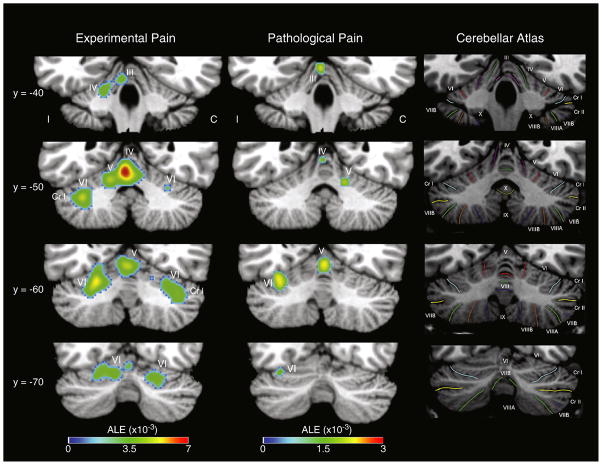
Activation estimate likelihood (ALE) meta-analysis of cerebellar activations reported by experimental (n=56 experiments, 195 foci) and pathological pain studies (n=20 experiments, 54 foci). ALE is a quantitative meta-analysis method that statistically evaluates the spatial distribution of activation foci across studies (Turkeltaub et al., 2002). Activation foci from experimental and pathological pain studies (Table 1) were entered as coordinates in Montreal Neurological Institute (MNI) space. Foci originally reported in Talairach coordinates were transformed into MNI space using the “Convert Foci” tool in GingerALE 2.0 (www.brainmap.org/ale/), which considers the analysis software used for spatial normalization in the reporting paper (i.e. FSL/SPM/Other). After MNI transformation, images were oriented such that the side of unilateral pain always occurred on the same side of the image (left). ALE maps were generated using GingerALE (Eickhoff et al., 2009; Laird et al., 2005), with a false discovery rate of p<0.001 and a minimum cluster volume of 150 mm3. ALE maps are displayed on the Colin27 in MNI space via Mango (ric.uthscsa.edu/mango/). The “Cerebellar Atlas” images in the right column show the corresponding slices from the MRI Atlas of the Human Cerebellum (Schmahmann et al., 2000). Experimental and pathological pain both activates vermal lobules IV/V, and bilateral hemispheric lobule VI. Abbreviations: contralateral to pain (C), Crus I (Cr I), ipsilateral to pain (I).
Data from our lab suggest that the pattern of cerebellar activation in patients who have neuropathic pain affecting the maxillary division of the trigeminal nerve (V2) is altered relative to healthy subjects (Borsook et al., 2008). In healthy subjects, noxious heat produced increased activation in areas thought to be involved in cognitive processing (lobules Crus II and VIIB), as well as sensory-motor integration (lobule VI) (Grodd et al., 2001; Schmahmann, 1996; Stoodley and Schmahmann, 2009; Timmann et al., 2009). Brush stimuli applied to the affected V2 region produced allodynia (pain to a normally innocuous stimulus) in these subjects and resulted in activation in areas involved in sensory-motor integration (lobules IV, V, and VI), secondary sensory processing (lobule VIIIB), cognition (lobules VIIB, Crus I, Crus II), and the dentate nucleus. Only painful stimuli triggered activation of cerebellar areas related to cognitive processing, while innocuous brushing did not. By contrast, painful heat and painful brushing in chronic neuropathic pain patients both activated cerebellar areas related to cognitive processing. This suggests that cognitive processing areas in the cerebellum may be related to the encoding of pain, possibly as a cognitive modulator.
7. Functional Aspects of the Cerebellum Non-specific to Pain
The cerebellum has been correlated with a wide variety of different cortical functional areas (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2009; Stoodley and Schmahmann, 2009; Strick et al., 2009). While the cerebellum appears to activate during the perception of pain, noxious stimuli may activate other processes related to pain, but not necessarily exclusive to it. Since pain itself is a multidimensional experience, other such functional processes that could be elicited include motor control, anticipation of pain, and negative emotions. Cerebellar activation could thus relate to aspects related to the pain experience separate from sensory discrimination. These may include nocifensive withdrawal responses, anticipation, emotion, and sensory-motor integration (Figure 5).
Figure 5.
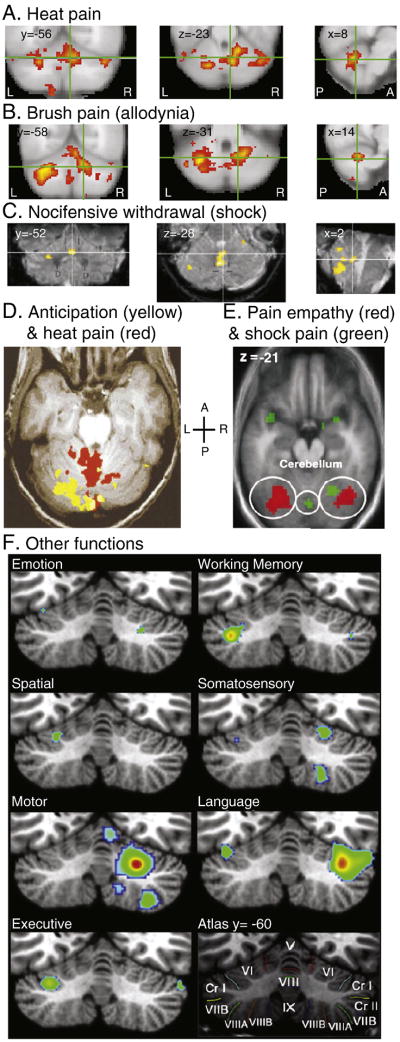
Cerebellar fMRI activation relating to pain, motor, and non-motor functions. A) Noxious thermal stimuli (pain threshold +1°C) applied to the right side of the face in neuropathic pain patients (Borsook et al., 2008) [with permission pending]. Crosshairs indicate approximate location of globose/fastigial deep cerebellar nuclei. B) Brushing of allodynic (painfully sensitive) skin on the right side of the face in neuropathic pain patients (Borsook et al., 2008) [with permission pending]. Crosshairs indicate approximate location of dentate deep cerebellar nucleus. C) Nociceptive leg withdrawal from noxious electrical stimuli applied to the left tibial nerve (Dimitrova et al., 2003) [with permission pending]. Crosshairs indicate approximate location of fastigial deep cerebellar nuclei. D) Anticipation of painful heat (yellow) and application of painful heat to the dorsum of the left hand (red) (Ploghaus et al., 1999) [with permission pending]. E) Observing one’s partner feeling pain (red) and experiencing pain oneself (green) from noxious electrical stimuli applied to the dorsum of the right hand (Singer et al., 2004) [with permission pending]. F) ALE meta-analysis of cerebellar activation elicited by a variety of tasks (Stoodley and Schmahmann, 2009) [with permission pending]. Abbreviations: anterior (A), left (L), posterior (P), right (R).
7.1. Withdrawal Responses
Lesions of the cerebellum can reduce the magnitude of a patient’s withdrawal from a noxious stimulus, though such patients do not show overt signs of altered sensation (Holmes, 1939). This observation led to the conclusion that the cerebellum is related more specifically to the generation of a motor response to pain than to pain itself. Indeed, imagining the execution of a motor task can activate the cerebellum (Boly et al., 2007; Lotze et al., 1999; Luft et al., 1998), but Holmes’ now classic finding does not rule out the possibility of a cerebellar role in nociceptive processing. More recently, the act of discriminating sensory information was found to significantly increase cerebellar activation (Gao et al., 1996). When a cutaneous discrimination task was added to a sensory experience, whether experimenter-applied or subject-initiated, cerebellar activation was significantly increased, suggesting a role for the cerebellum in sensory processing. Furthermore, a passive manipulation of limb position by an experimenter produces cerebellar activation similar to voluntary movement of the limb (Jueptner and Weiller, 1998). Together, these findings suggest that cerebellar activity observed during the execution of a motor task may be attributable to sensory processing. Thus far, functional imaging studies are often unable to differentiate cerebellar activation due to motor withdrawal vs. nociceptive sensory processing when using painful stimuli (Dimitrova et al., 2003; Maschke et al., 2002).
7.2. Inhibition of Nocifensive Behaviors/“Remaining Still”
Cerebellar responses to noxious stimuli in neuroimaging studies have often been ascribed to the inhibition of an escape response. Indeed, all human imaging studies include instructions to their subjects to remain still during scanning, which can be demanding during the application of painful stimuli. Stimulation of the PAG can lead to inhibition of withdrawal responses to nociceptive stimuli (Fields, 2000), which may involve the cerebellum. In a rodent model, chemical activation of the PAG by DLH has been shown to directly inhibit nociceptive input to the cerebellum, perhaps to help the animal disengage from an inescapable stressor (Cerminara et al., 2009). A pain study using human volunteers could theoretically engage a similar PAG-cerebellar process to reduce responsiveness to external stimuli. However, a reduction in nociceptive input to the cerebellum would seem to better explain a decrease in cerebellar cortical evoked potential amplitude rather than an increase, as was observed in the DLH study. During pain, increased cerebellar activation that would relate to inhibition of a motor response thus seems to require a mechanism separate from PAG-induced inhibition of cerebellar afferents.
The voluntary inhibition of a motor response to pain does not fully explain cerebellar activation during pain, since noxious heat can produce cerebellar activation even when subjects are under general anesthesia(Hofbauer et al., 2004). In this positron emission tomography study, noxious thermal stimuli were applied to the forearms of 15 healthy subjects while they were infused with different concentrations of propofol, a general anesthetic. When subjects were deeply sedated, noxious heat evoked significant cerebellar activation comparable in magnitude to when they were free of sedation. Interestingly, the cerebellum also showed activation with noxious heat when subjects were completely unconscious. Note that with deep sedation, almost half of the subjects showed involuntary movements of their limbs in response to noxious heat. Together, these results suggest that cerebellar activation to noxious heat: (1) can be involuntary; (2) is present in the absence of inhibition of movement; and (3) does not require the conscious perception of pain. Unfortunately, few if any other studies like this have been conducted to address the necessity of consciousness for cerebellar activation. Future research in this regard may help uncover the cerebellum’s role during nociceptive processing.
7.3. Anticipation of Pain
As well as being active during a painful event, the cerebellum shows activation during the anticipation of pain in humans with fMRI (Ploghaus et al., 1999). When subjects were shown a light cue to signal impending pain, the ipsilateral posterior cerebellum was active. During painful heat stimulation, a wholly different region of the cerebellum was active: bilateral anterior cerebellum. Yet in this study, the functional role of this cerebellar activation was ascribed to the motor response/preparation to painful stimuli. Another possibility is that the cerebellum is involved in pre-attentive detection of somatosensory input changes, as indicated by a recent mismatch negativity study (Restuccia et al., 2007). Considering the separate spatial locations of the anticipatory and pain activations, multiple parts of the cerebellum seem to respond to a painful event in different ways.
The cerebellum appears to have a more prominent role in processing unanticipated events than anticipated events, though it activates during the anticipation of an event before it occurs. In a recent imaging of spontaneous trigeminal neuralgia, also known as “tic doloureux” (Borsook et al., 2007b), no activation was observed in the cerebellum following self-triggered (expected) tics, but activation was observed following spontaneous (unexpected) tics. This information suggests that the cerebellum processes anticipated events differently than unanticipated events, akin to the relationship between efference copy and predicted sensory outcome (Sperry, 1950; Von Holst, 1954), and is perhaps involved in the preparation of impending pain. This indicates a role in processing anticipated sensory input with a high level of temporal accuracy, with optimization of temporal responses of anticipated events in the sensory and integrative systems (Blakemore et al., 1998; Ohyama et al., 2003; Tesche and Karhu, 2000). This putative comparative role of the cerebellum, in which the anticipation of pain is compared with the actual experience of pain, has not been thoroughly researched.
7.4. Emotional Aspects of Pain
In addition to responses to painful stimuli, cerebellar activation is also observed in studies of pain empathy (Jackson et al., 2005; Moriguchi et al., 2007; Singer et al., 2004), fear (Murphy et al., 2003), and in generalized emotional perception (Bermpohl et al., 2006; Konarski et al., 2005; Murphy et al., 2003). Cerebellar activation with pain empathy, regardless whether it is an affective or cognitive process, gives further indication that the cerebellum processes more than motor function. Thus far, cerebellar responses to pain have not been compared to other forms of aversive stimuli, such as aversive pictures that also activate the cerebellum (Bermpohl et al., 2006). Such a comparison would help determine whether the cerebellum responds to pain specifically, or encodes it as a generalized aversive stimulus.
7.5. Integrative Functions
The cerebellum may have an integrative function that combines and coordinates a variety of functional inputs for a supramodal process. A possible role of the cerebellum in pain processing may be considered in light of the “dysmetria of thought” hypothesis (Schmahmann, 1991; Schmahmann, 2004). This hypothesis posits that the cerebellum has a fundamental function to optimize performance by automatically modulating behavior according to context, and is disrupted when the cerebellum is damaged. This hypothesis is built upon the Universal Cerebellar Transform, which theorizes that the cerebellum has a singular generalizable function that is applied to a variety of different types of functional inputs (Schmahmann, 2004). The dysmetria of thought hypothesis may be applied to learned behaviors in pain (sensory) processing, as cerebellar processing may influence monitoring and adjusting sensory acquisition (Bower, 1997). The cerebellum may be involved in processing motor control error signals in state estimation (Miall and King, 2008; Wolpert and Miall, 1996), and may be a comparator for errors in somatosensory processing (Apps and Garwicz, 2005; Eccles et al., 1967; Ekerot et al., 1987a). Such error signals may play a role in wrongly executed movements. While this hypothesis was originally related to somatosensory inputs to the cerebellum, it may also apply to nociceptive inputs (Cerminara et al., 2009).
7.6. Cerebellum and Pain Co-morbidities
Given that chronic pain conditions are co-morbid with psychiatric disorders (Gureje et al., 2001; Haley et al., 1985; Wilson et al., 2002), the cerebellum is interestingly linked to depression as well as pain. Sensory and affective neural systems involved with processing pain can become altered with chronic pain, resulting in the development of psychiatric illnesses, such as depression (Borsook et al., 2007a). Abnormal cerebellar responses to the anticipation of noxious stimuli have been suggested to be a potential marker for depression (Smith et al., 2002). Furthermore, patients with depression have a tendency for increased resting activity in the anterior cerebellum, as well as abnormal responses to stimuli evoking positive and negative affect (Fitzgerald et al., 2008). This suggests that in addition to being co-morbid, pain and depression may share a common mechanism within the cerebellum.
8. Conclusions
An improved knowledge of the cerebellar function in relation to pain has potential implications for the discovery of new understanding for pain control. Currently, less than a handful of studies have even attempted to discern the function of the cerebellum in regards to pain. Pain does not necessarily have a singular impact on what may be a variety of cerebellar functions, given that pain is a multi-dimensional experience itself. Based on relatively scant evidence, we speculatively propose that the cerebellum is an integrator of multiple effector systems including affective processing, pain modulation, as well as sensorimotor processing. The cerebellum appears to play a cross-modal modulatory role in regards to pain, with noxious stimuli impacting the processing of generalized aversion as well as sensorimotor adaptations to pain and/or injury. Clearly, more research in this domain is required to explicitly define the significance of nociceptive processing in the human cerebellum. The role of the cerebellum as it pertains to motor control in response to nociceptive stimuli also needs further study. Such research may lead to an enhanced understanding of perturbations in chronic pain states which appear to have altered cerebellar functionality (Borsook et al., 2008).
Acknowledgments
This work has been supported by a K01 grant from NIDA to EA (DA25289), a K24 grant from NINDS to DB (NS064050), and the Birmingham foundation and the MINDLink foundation (JDS).
Footnotes
The authors declare no conflicts of interest regarding the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122:223–34. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–57. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–57. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage. 2006;30:588–600. doi: 10.1016/j.neuroimage.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–21. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998;1:635–40. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Boly M, Coleman MR, Davis MH, Hampshire A, Bor D, Moonen G, Maquet PA, Pickard JD, Laureys S, Owen AM. When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage. 2007;36:979–92. doi: 10.1016/j.neuroimage.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007a;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Pendse G, Morris S, Cole SH, Aiello-Lammens M, Scrivani S, Becerra LR. Comparison of evoked vs. spontaneous tics in a patient with trigeminal neuralgia (tic doloureux) Mol Pain. 2007b;3:34. doi: 10.1186/1744-8069-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum. 2008;7:252–72. doi: 10.1007/s12311-008-0011-6. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–9. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Bower JM. Control of sensory data acquisition. Int Rev Neurobiol. 1997;41:489–513. doi: 10.1016/s0074-7742(08)60367-0. [DOI] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101:251–83. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76:571–81. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951–9. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Koutsikou S, Lumb BM, Apps R. The periaqueductal grey modulates sensory input to the cerebellum: a role in coping behaviour? Eur J Neurosci. 2009;29:2197–206. doi: 10.1111/j.1460-9568.2009.06760.x. [DOI] [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, Kim JW, Kim SY, Lee SG, Lee MS. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- Cintas HM, Rutherford JG, Gwyn DG. Some midbrain and diencephalic projections to the inferior olive in the rat. In: Courville J, de Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. Raven Press; New York: 1980. pp. 73–96. [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–12. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76:127–35. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16:158–68. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Dey PK, Ray AK. Anterior cerebellum as a site for morphine analgesia and post-stimulation analgesia. Indian J Physiol Pharmacol. 1982;26:3–12. [PubMed] [Google Scholar]
- Dimitrova A, Kolb FP, Elles HG, Maschke M, Forsting M, Diener HC, Timmann D. Cerebellar responses evoked by nociceptive leg withdrawal reflex as revealed by event-related FMRI. J Neurophysiol. 2003;90:1877–86. doi: 10.1152/jn.00053.2003. [DOI] [PubMed] [Google Scholar]
- Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–76. doi: 10.1093/brain/awl016. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–9. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Eccles J, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Springer-Verlag; Berlin, Heidelberg, New York: 1967. [Google Scholar]
- Edgley SA, Gallimore CM. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988;397:99–111. doi: 10.1113/jphysiol.1988.sp016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Gustavsson P, Oscarsson O, Schouenborg J. Climbing fibres projecting to cat cerebellar anterior lobe activated by cutaneous A and C fibres. J Physiol. 1987a;386:529–38. doi: 10.1113/jphysiol.1987.sp016549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Oscarsson O, Schouenborg J. Stimulation of cat cutaneous nociceptive C fibres causing tonic and synchronous activity in climbing fibres. J Physiol. 1987b;386:539–46. doi: 10.1113/jphysiol.1987.sp016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Garwicz M, Schouenborg J. The postsynaptic dorsal column pathway mediates cutaneous nociceptive information to cerebellar climbing fibres in the cat. J Physiol. 1991;441:275–84. doi: 10.1113/jphysiol.1991.sp018751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Egan GF, Zamarripa F, Shade R, Blair-West J, Fox P, Denton DA. Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci U S A. 2006;103:2416–21. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–53. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–7. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;138:641–56. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–23. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Gilman S. The spinocerebellar ataxias. Clin Neuropharmacol. 2000;23:296–303. doi: 10.1097/00002826-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, 3rd, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235:343–59. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195–200. doi: 10.1016/s0304-3959(00)00483-8. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DE, Dietrichs E. An HRP study of hypothalamo-cerebellar and cerebello-hypothalamic connections in squirrel monkey (Saimiri sciureus) J Comp Neurol. 1984;229:559–75. doi: 10.1002/cne.902290409. [DOI] [PubMed] [Google Scholar]
- Haines DE, Dietrichs E, Mihailoff GA, McDonald EF. The cerebellar-hypothalamic axis: basic circuits and clinical observations. Int Rev Neurobiol. 1997;41:83–107. doi: 10.1016/s0074-7742(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Haley WE, Turner JA, Romano JM. Depression in chronic pain patients: relation to pain, activity, and sex differences. Pain. 1985;23:337–43. doi: 10.1016/0304-3959(85)90003-X. [DOI] [PubMed] [Google Scholar]
- Hartmann-von Monakow K, Akert K, Kunzle H. Projections of precentral, premotor, and prefrontal cortex to the basilar pontine grey and to nucleus reticularis tegmenti pontis in the monkey (Macaca fascicularis) Schweiz Arch Neurol Neurochir Psychiatry. 1981;129:189–208. [PubMed] [Google Scholar]
- Hayashi H, Sumino R, Sessle BJ. Functional organization of trigeminal subnucleus interpolaris: nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol. 1984;51:890–905. doi: 10.1152/jn.1984.51.5.890. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Petersen D, Nitschke MF. Differential cerebellar activation related to perceived pain intensity during noxious thermal stimulation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;335:202–6. doi: 10.1016/s0304-3940(02)01164-3. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Binkofski F. Cerebellar neural responses related to actively and passively applied noxious thermal stimulation in human subjects: a parametric fMRI study. Neurosci Lett. 2004;361:237–40. doi: 10.1016/j.neulet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Roehl M, Bingel U, Lorenz J, Buchel C. Common neural systems for contact heat and laser pain stimulation reveal higher-level pain processing. Hum Brain Mapp. 2008;29:1080–91. doi: 10.1002/hbm.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology. 2004;100:386–94. doi: 10.1097/00000542-200402000-00031. [DOI] [PubMed] [Google Scholar]
- Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- Holstege G. Direct and indirect pathways to lamina I in the medulla oblongata and spinal cord of the cat. Prog Brain Res. 1988;77:47–94. doi: 10.1016/s0079-6123(08)62778-8. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–36. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–14. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121 (Pt 5):931–47. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- Ibinson JW, Small RH, Algaze A, Roberts CJ, Clark DL, Schmalbrock P. Functional magnetic resonance imaging studies of pain: an investigation of signal decay during and across sessions. Anesthesiology. 2004;101:960–9. doi: 10.1097/00000542-200410000-00022. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jansen J, Brodal A. Experimental studies on the intrinsic fibers of the cerebellum. II. The corico-nuclear projection. J Comp Neurol. 1940;73:267–321. [PubMed] [Google Scholar]
- Jissendi P, Baudry S, Baleriaux D. Diffusion tensor imaging (DTI) and tractography of the cerebellar projections to prefrontal and posterior parietal cortices: a study at 3T. J Neuroradiol. 2008;35:42–50. doi: 10.1016/j.neurad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121(Pt 8):1437–49. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–21. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The single-epoch fMRI design: validation of a simplified paradigm for the collection of subjective ratings. Neuroimage. 2003;19:976–87. doi: 10.1016/s1053-8119(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–5. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–97. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers RC, Svensson P, Jensen TS. Central representation of muscle pain and mechanical hyperesthesia in the orofacial region: a positron emission tomography study. Pain. 2004;108:284–93. doi: 10.1016/j.pain.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALEmeta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FY, Qiao JT, Dafny N. Cerebellar stimulation modulates thalamic noxious-evoked responses. Brain Res Bull. 1993;30:529–34. doi: 10.1016/0361-9230(93)90079-q. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–91. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Stefanou A, Klose U, Voigt K. Comparing motion-and imagery-related activation in the human cerebellum: a functional MRI study. Hum Brain Mapp. 1998;6:105–13. doi: 10.1002/(SICI)1097-0193(1998)6:2<105::AID-HBM3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maschke M, Erichsen M, Drepper J, Jentzen W, Muller SP, Kolb FP, Diener HC, Timmann D. Limb flexion reflex-related areas in human cerebellum. Neuroreport. 2002;13:2325–30. doi: 10.1097/00001756-200212030-00031. [DOI] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol. 2009;102:181–91. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998a;352:275–8. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- May A, Kaube H, Buchel C, Eichten C, Rijntjes M, Juptner M, Weiller C, Diener HC. Experimental cranial pain elicited by capsaicin: a PET study. Pain. 1998b;74:61–6. doi: 10.1016/S0304-3959(97)00144-9. [DOI] [PubMed] [Google Scholar]
- May JG, Andersen RA. Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Exp Brain Res. 1986;63:265–78. doi: 10.1007/BF00236844. [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience. 1990;36:305–24. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- Melzack R, Casey KL. Sensory, motivational and central control determinants of chronic pain: a new conceptual model. In: Kenshalo DR, Thomas Charles C, editors. The Skin Senses. Springfield; 1968. pp. 423–443. [Google Scholar]
- Miall RC, King D. State estimation in the cerebellum. Cerebellum. 2008;7:572–6. doi: 10.1007/s12311-008-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Empathy and judging other’s pain: an fMRI study of alexithymia. Cereb Cortex. 2007;17:2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nemoto H, Toda H, Nakajima T, Hosokawa S, Okada Y, Yamamoto K, Horiuchi R, Endo K, Ida I, Mikuni M, Goto F. Fluvoxamine modulates pain sensation and affective processing of pain in human brain. Neuroreport. 2003;14:791–7. doi: 10.1097/00001756-200305060-00003. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Follett KA, Piper J, Dirks BA. Characterization of neurons in the area of the medullary lateral reticular nucleus responsive to noxious visceral and cutaneous stimuli. Brain Res. 1998;802:163–74. doi: 10.1016/s0006-8993(98)00608-8. [DOI] [PubMed] [Google Scholar]
- Nyby O, Jansen J. An experimental investigation of the corticopontine projection in macaca mulatta. Skrifterutgitt av Det Norske Videnskaps-Akademi: Oslo; 1. Mat Naturv Klasse. 1951;3:1–47. [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–7. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27:3388–94. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay S, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer-Verlag; Berlin, Heidelberg, New York: 1974. [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–9. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–70. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–80. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–81. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ, Gross CG, Robson MD, Kastner S, Matthews PM. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb Cortex. 2006;16:811–8. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Randic M, Miletic V, Loewy AD. A morphological study of cat dorsal spinocerebellar tract neurons after intracellular injection of horseradish peroxidase. J Comp Neurol. 1981;198:453–66. doi: 10.1002/cne.901980306. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Valeriani M, Barba C, Le Pera D, Capecci M, Filippini V, Molinari M. Functional changes of the primary somatosensory cortex in patients with unilateral cerebellar lesions. Brain. 2001;124:757–68. doi: 10.1093/brain/124.4.757. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Della Marca G, Valeriani M, Leggio MG, Molinari M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain. 2007;130:276–87. doi: 10.1093/brain/awl236. [DOI] [PubMed] [Google Scholar]
- Ruehle BS, Handwerker HO, Lennerz JK, Ringler R, Forster C. Brain activation during input from mechanoinsensitive versus polymodal C-nociceptors. J Neurosci. 2006;26:5492–9. doi: 10.1523/JNEUROSCI.2059-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford JG, Anderson WA, Gwyn DG. A reevaluation of midbrain and diencephalic projections to the inferior olive in rat with particular reference to the rubro-olivary pathway. J Comp Neurol. 1984;229:285–300. doi: 10.1002/cne.902290213. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD. Nociceptive visceral stimulation modulates the activity of cerebellar Purkinje cells. Exp Brain Res. 2001;140:122–6. doi: 10.1007/s002210100824. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD. Cerebellar stimulation modulates the intensity of a visceral nociceptive reflex in the rat. Exp Brain Res. 2002;146:117–21. doi: 10.1007/s00221-002-1107-8. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD. The cerebellum: organization, functions and its role in nociception. Brain Res Brain Res Rev. 2003;42:85–95. doi: 10.1016/s0165-0173(03)00151-6. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Courville J. Projections from the motor cortex, midbrain, and vestibular nuclei to the inferior olive in the cat: Anatomical and functional correlates. In: Courville J, de Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. Raven Press; New York: 1980. pp. 97–124. [Google Scholar]
- Sasaki K, Oka H, Matsuda Y, Shimono T, Mizuno N. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp Brain Res. 1975;23:91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–87. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32:256–65. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007;17:1412–22. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol. 1990;301:618–42. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- Siegel P, Wepsic JG. Alteration of nociception by stimulation of cerebellar structures in the monkey. Physiol Behav. 1974;13:189–94. doi: 10.1016/0031-9384(74)90033-x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith KA, Ploghaus A, Cowen PJ, McCleery JM, Goodwin GM, Smith S, Tracey I, Matthews PM. Cerebellar responses during anticipation of noxious stimuli in subjects recovered from depression. Functional magnetic resonance imaging study. Br J Psychiatry. 2002;181:411–5. doi: 10.1192/bjp.181.5.411. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Faull RL, Mehler WR. A comparative study of the neurons of origin of the spinocerebellar afferents in the rat, cat and squirrel monkey based on the retrograde transport of horseradish peroxidase. J Comp Neurol. 1978;181:833–52. doi: 10.1002/cne.901810409. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of spontaneous optokinetic responses produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003;89:3294–303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol. 1997;78:450–60. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- Svensson P, Johannsen P, Jensen TS, Arendt-Nielsen L, Nielsen J, Stodkilde-Jorgensen H, Gee AD, Baarsgaard Hansen S, Gjedde A. Cerebral blood-flow changes evoked by two levels of painful heat stimulation: a positron emission tomography study in humans. Eur J Pain. 1998;2:95–107. doi: 10.1016/s1090-3801(98)90001-5. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu JJ. Anticipatory cerebellar responses during somatosensory omission in man. Hum Brain Mapp. 2000;9:119–42. doi: 10.1002/(SICI)1097-0193(200003)9:3<119::AID-HBM2>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review Cortex. 2009 doi: 10.1016/j.cortex.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Tseng MT, Tseng WY, Chao CC, Lin HE, Hsieh ST. Distinct and shared cerebral activations in processing innocuous versus noxious contact heat revealed by functional magnetic resonance imaging. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–27. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- VanGilder JC, Fitzmartin JA. Evoked potentials from cat cerebellum following non-medullated C fiber stimulation of peripheral nerve. Brain Res. 1973;50:430–6. doi: 10.1016/0006-8993(73)90746-4. [DOI] [PubMed] [Google Scholar]
- VanGilder JC. Cerebellar evoked potentials from ‘C’ fibers. Brain Res. 1975;90:302–6. doi: 10.1016/0006-8993(75)90310-8. [DOI] [PubMed] [Google Scholar]
- Von Holst E. Relations between the central nervous system and the peripheral organs. Brit J Anim Behav. 1954;2:89–94. [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–5. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27:59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R, Wiesendanger M, Ruegg DG. An anatomical investigation of the corticopontine projection in the primate (Macaca fascicularis and Saimiri sciureus)--II. The projection from frontal and parental association areas. Neuroscience. 1979;4:747–65. doi: 10.1016/0306-4522(79)90004-6. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Witting N, Kupers RC, Svensson P, Arendt-Nielsen L, Gjedde A, Jensen TS. Experimental brush-evoked allodynia activates posterior parietal cortex. Neurology. 2001;57:1817–24. doi: 10.1212/wnl.57.10.1817. [DOI] [PubMed] [Google Scholar]
- Witting N, Kupers RC, Svensson P, Jensen TS. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. Pain. 2006;120:145–54. doi: 10.1016/j.pain.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen PX. Cerebellar evoked potential elicited by stimulation of C-fiber in saphenous nerve of cat. Brain Res. 1990;522:144–6. doi: 10.1016/0006-8993(90)91590-d. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen PX. Discharge response of cerebellar Purkinje cells to stimulation of C-fiber in cat saphenous nerve. Brain Res. 1992;581:269–72. doi: 10.1016/0006-8993(92)90717-n. [DOI] [PubMed] [Google Scholar]
- Xu X, Fukuyama H, Yazawa S, Mima T, Hanakawa T, Magata Y, Kanda M, Fujiwara N, Shindo K, Nagamine T, Shibasaki H. Functional localization of pain perception in the human brain studied by PET. Neuroreport. 1997;8:555–9. doi: 10.1097/00001756-199701200-00035. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]