Abstract
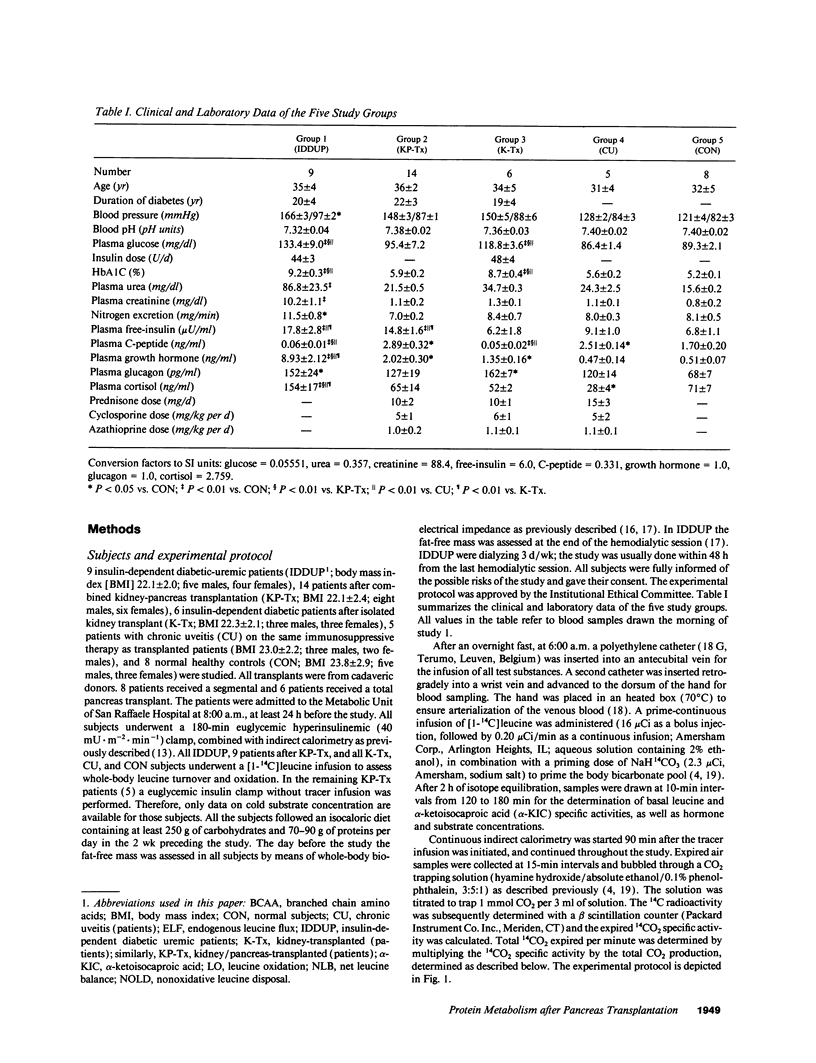
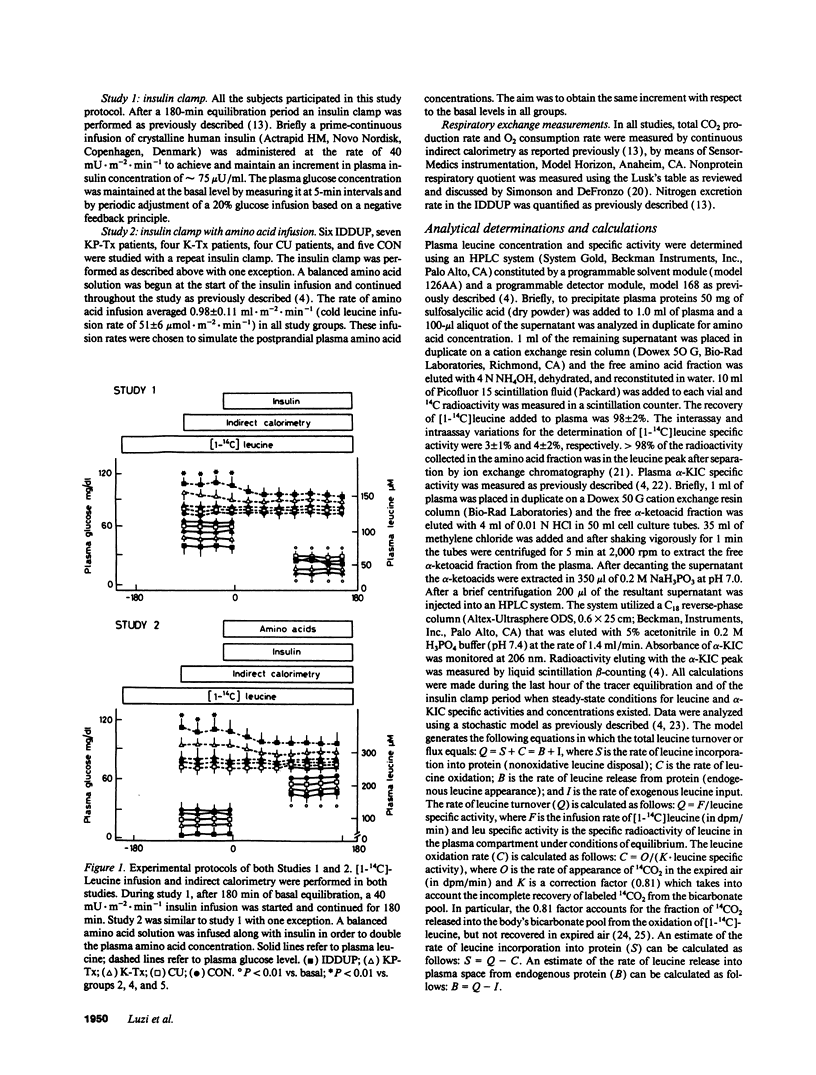
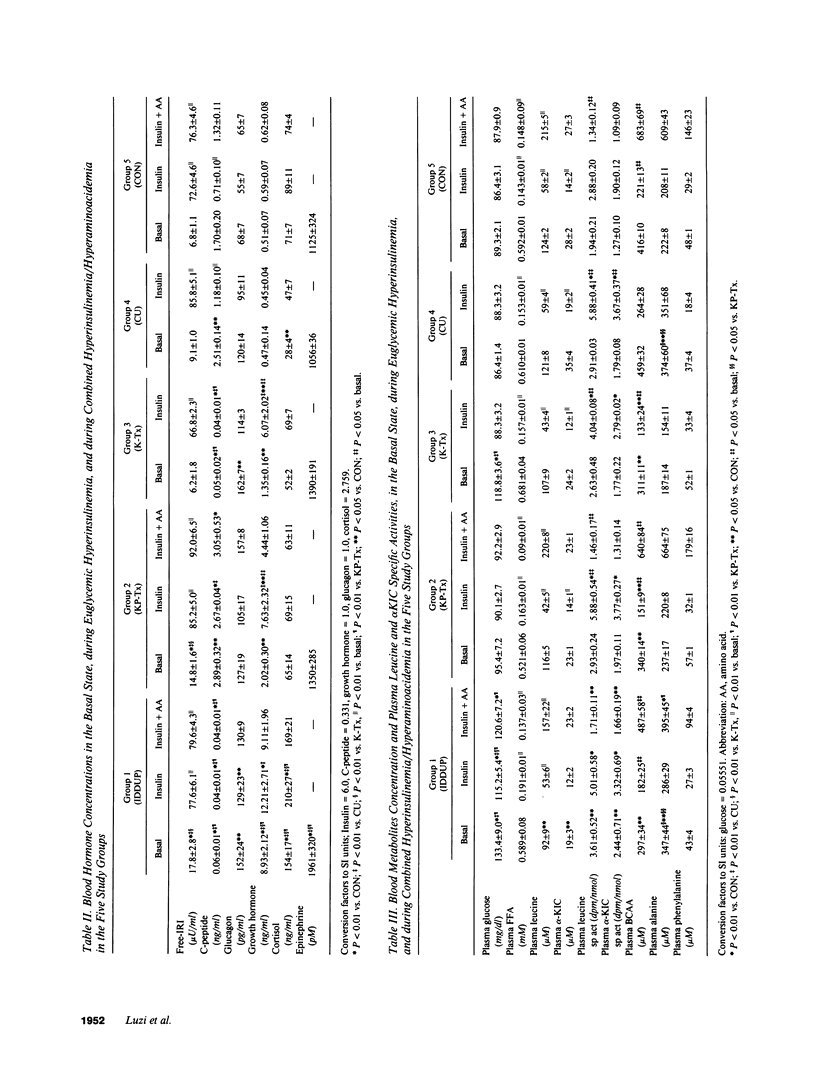
In order to assess the combined and separate effects of pancreas and kidney transplant on whole-body protein metabolism, 9 insulin-dependent diabetic-uremic patients (IDDUP), 14 patients after combined kidney-pancreas transplantation (KP-Tx), and 6 insulin-dependent diabetic patients with isolated kidney transplant (K-Tx), were studied in the basal postabsorptive state and during euglycemic hyperinsulinemia (study 1). [1-14C]Leucine infusion and indirect calorimetry were utilized to assess leucine metabolism. The subjects were studied again with a combined infusion of insulin and amino acids, given to mimic postprandial amino acid levels (study 2). In the basal state, IDDUP demonstrated with respect to normal subjects (CON): (a) higher free-insulin concentration (17.8 +/- 2.8 vs. 6.8 +/- 1.1 microU/ml, P < 0.01) (107 +/- 17 vs. 41 +/- 7 pM); (b) reduced plasma leucine (92 +/- 9 vs. 124 +/- 2 microM, P < 0.05), branched chain amino acids (BCAA) (297 +/- 34 vs. 416 +/- 10 microM, P < 0.05), endogenous leucine flux (ELF) (28.7 +/- 0.8 vs. 39.5 +/- 0.7 mumol.m-2.min-1, P < 0.01) and nonoxidative leucine disposal (NOLD) (20.7 +/- 0.2 vs. 32.0 +/- 0.7 mumol.m-2. min-1, P < 0.01); (c) similar leucine oxidation (LO) (8.0 +/- 0.1 vs. 7.5 +/- 0.1 mumol.m-2.min-1; P = NS). Both KP-Tx and K-Tx patients showed a complete normalization of plasma leucine (116 +/- 5 and 107 +/- 9 microM), ELF (38.1 +/- 0.1 and 38.5 +/- 0.9 mumol.m-2.min-1), and NOLD (28.3 +/- 0.6 and 31.0 +/- 1.3 mumol.m-2.min-1) (P = NS vs, CON). During hyperinsulinemia (study 1), IDDUP showed a defective decrease of leucine (42% vs. 53%; P < 0.05), BCAA (38% vs. 47%, P < 0.05), ELF (28% vs. 33%, P < 0.05), and LO (0% vs. 32%, P < 0.05) with respect to CON. Isolated kidney transplant reverted the defective inhibition of ELF (34%, P = NS vs. CON) of IDDUP, but not the inhibition of LO (18%, P < 0.05 vs. CON) by insulin. Combined kidney and pancreas transplanation normalized all kinetic parameters of insulin-mediated protein turnover. During combined hyperinsulinemia and hyperaminoacidemia (study 2), IDDUP showed a defective stimulation of NOLD (27.9 +/- 0.7 vs. 36.1 +/- 0.8 mumol.m-2.min-1, P < 0.01 compared to CON), which was normalized by transplantation (44.3 +/- 0.8 mumol.m-2.min-1).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. Tracer priming the bicarbonate pool. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Dunn F. L. Effects of insulin treatment on lipoprotein composition and function in patients with IDDM. Diabetes. 1992 Oct;41 (Suppl 2):107–110. doi: 10.2337/diab.41.2.s107. [DOI] [PubMed] [Google Scholar]
- Berkelhammer C. H., Baker J. P., Leiter L. A., Uldall P. R., Whittall R., Slater A., Wolman S. L. Whole-body protein turnover in adult hemodialysis patients as measured by 13C-leucine. Am J Clin Nutr. 1987 Nov;46(5):778–783. doi: 10.1093/ajcn/46.5.778. [DOI] [PubMed] [Google Scholar]
- Blackman J. D., Polonsky K. S., Jaspan J. B., Sturis J., Van Cauter E., Thistlethwaite J. R. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes. 1992 Oct;41(10):1346–1354. doi: 10.2337/diab.41.10.1346. [DOI] [PubMed] [Google Scholar]
- Brayman K. L., Sutherland D. E. Factors leading to improved outcome following pancreas transplantation--the influence of immunosuppression and HLA matching. Transplant Proc. 1992 Aug;24(4 Suppl 2):91–95. [PubMed] [Google Scholar]
- Brodsky I. G., Robbins D. C., Hiser E., Fuller S. P., Fillyaw M., Devlin J. T. Effects of low-protein diets on protein metabolism in insulin-dependent diabetes mellitus patients with early nephropathy. J Clin Endocrinol Metab. 1992 Aug;75(2):351–357. doi: 10.1210/jcem.75.2.1639934. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino P., Luzi L., Del Prato S., DeFronzo R. A. Dissociation of the effects of epinephrine and insulin on glucose and protein metabolism. Am J Physiol. 1990 Jan;258(1 Pt 1):E117–E125. doi: 10.1152/ajpendo.1990.258.1.E117. [DOI] [PubMed] [Google Scholar]
- Castellino P., Luzi L., Simonson D. C., Haymond M., DeFronzo R. A. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest. 1987 Dec;80(6):1784–1793. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C., Saccomani M. P., Tessari P., Biolo G., Luzi L., Matthews D. E. Compartmental model of leucine kinetics in humans. Am J Physiol. 1991 Oct;261(4 Pt 1):E539–E550. doi: 10.1152/ajpendo.1991.261.4.E539. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Zehr P. Quality of life in diabetic recipients of kidney transplants is better with the addition of the pancreas. Clin Transplant. 1990 Aug;4(4):238–241. [PubMed] [Google Scholar]
- Dahlmann B., Rutschmann M., Reinauer H. Effect of starvation or treatment with corticosterone on the amount of easily releasable myofilaments in rat skeletal muscles. Biochem J. 1986 Mar 15;234(3):659–664. doi: 10.1042/bj2340659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem P., Abid M., Redmon J. B., Sutherland D. E., Robertson R. P. Systemic venous drainage of pancreas allografts as independent cause of hyperinsulinemia in type I diabetic recipients. Diabetes. 1990 May;39(5):534–540. doi: 10.2337/diab.39.5.534. [DOI] [PubMed] [Google Scholar]
- Diem P., Redmon J. B., Abid M., Moran A., Sutherland D. E., Halter J. B., Robertson R. P. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest. 1990 Dec;86(6):2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenger R. B., Blifeld C., Prince H., Gradus D. B., Cho S., Sekiya N., Salusky I. B., Fine R. N. The pediatric nephrologist's dilemma: growth after renal transplantation and its interaction with age as a possible immunologic variable. J Pediatr. 1987 Dec;111(6 Pt 2):1022–1025. doi: 10.1016/s0022-3476(87)80049-5. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Sherwin R., Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med. 1977 Apr;137(4):507–513. [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., Shank M., Petrides A. S., DeFronzo R. A. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991 Jan;87(1):83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth C. G., Collste H., Lundgren G., Wilczek H., Klintmalm G., Ringdén O., Gunnarsson R., Ostman J. Successful outcome of segmental human pancreatic transplantation with enteric exocrine diversion after modifications in technique. Lancet. 1982 Sep 4;2(8297):522–524. doi: 10.1016/s0140-6736(82)90601-8. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P. Role of glucose transporters in glucocorticoid-induced insulin resistance. GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes. 1992 Jun;41(6):728–735. doi: 10.2337/diab.41.6.728. [DOI] [PubMed] [Google Scholar]
- Hindmarsh P. C., Matthews D. R., Di Silvio L., Kurtz A. B., Brook C. G. Relation between height velocity and fasting insulin concentrations. Arch Dis Child. 1988 Jun;63(6):665–666. doi: 10.1136/adc.63.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan M., Nicholson C. P., Katz H., Perkins J., Haymond M., Jensen M., Butler P., Rizza R. Effects of chronic systemic insulin delivery on insulin action in dogs. Diabetologia. 1991 Oct;34(10):702–708. doi: 10.1007/BF00401514. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Wong W. W., Shulman R. J., Smith E. O., Klein P. D. [13C]bicarbonate kinetics in humans: intra- vs. interindividual variations. Am J Physiol. 1983 Aug;245(2):R190–R202. doi: 10.1152/ajpregu.1983.245.2.R190. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Wong W. W., Wong W. M., Boutton T. W., Shulman R. J., Lifschitz C. L., Malphus E. W., Helge H., Klein P. D. Rapid determination of whole-body bicarbonate kinetics by use of a digital infusion. Am J Physiol. 1984 Oct;247(4 Pt 2):R709–R716. doi: 10.1152/ajpregu.1984.247.4.R709. [DOI] [PubMed] [Google Scholar]
- Katz H., Homan M., Velosa J., Robertson P., Rizza R. Effects of pancreas transplantation on postprandial glucose metabolism. N Engl J Med. 1991 Oct 31;325(18):1278–1283. doi: 10.1056/NEJM199110313251804. [DOI] [PubMed] [Google Scholar]
- Kopple J. D. Abnormal amino acid and protein metabolism in uremia. Kidney Int. 1978 Oct;14(4):340–348. doi: 10.1038/ki.1978.134. [DOI] [PubMed] [Google Scholar]
- Krolewski A. S., Warram J. H., Christlieb A. R., Busick E. J., Kahn C. R. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985 May;78(5):785–794. doi: 10.1016/0002-9343(85)90284-0. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Kathpalia S. C., Henriquez C. Endocrine abnormalities associated with chronic renal failure. Med Clin North Am. 1978 Nov;62(6):1341–1361. doi: 10.1016/s0025-7125(16)31740-0. [DOI] [PubMed] [Google Scholar]
- Lindholm A., Albrechtsen D., Tufveson G., Karlberg I., Persson N. H., Groth C. G. A randomized trial of cyclosporine and prednisolone versus cyclosporine, azathioprine, and prednisolone in primary cadaveric renal transplantation. Transplantation. 1992 Oct;54(4):624–631. doi: 10.1097/00007890-199210000-00011. [DOI] [PubMed] [Google Scholar]
- Luzi L., Battezzati A., Perseghin G., Bianchi E., Vergani S., Secchi A., La Rocca E., Staudacher C., Spotti D., Ferrari G. Lack of feedback inhibition of insulin secretion in denervated human pancreas. Diabetes. 1992 Dec;41(12):1632–1639. doi: 10.2337/diab.41.12.1632. [DOI] [PubMed] [Google Scholar]
- Luzi L., Castellino P., Simonson D. C., Petrides A. S., DeFronzo R. A. Leucine metabolism in IDDM. Role of insulin and substrate availability. Diabetes. 1990 Jan;39(1):38–48. doi: 10.2337/diacare.39.1.38. [DOI] [PubMed] [Google Scholar]
- Luzi L., Secchi A., Facchini F., Battezzati A., Staudacher C., Spotti D., Castoldi R., Ferrari G., Di Carlo V., Pozza G. Reduction of insulin resistance by combined kidney-pancreas transplantation in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1990 Sep;33(9):549–556. doi: 10.1007/BF00404143. [DOI] [PubMed] [Google Scholar]
- May R. C., Clark A. S., Goheer M. A., Mitch W. E. Specific defects in insulin-mediated muscle metabolism in acute uremia. Kidney Int. 1985 Sep;28(3):490–497. doi: 10.1038/ki.1985.155. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Halliday D., Matthews D. E., Welle S. L. Hyperglucagonemia during insulin deficiency accelerates protein catabolism. Am J Physiol. 1987 Aug;253(2 Pt 1):E208–E213. doi: 10.1152/ajpendo.1987.253.2.E208. [DOI] [PubMed] [Google Scholar]
- Nashel D. J. Mechanisms of action and clinical applications of cytotoxic drugs in rheumatic disorders. Med Clin North Am. 1985 Jul;69(4):817–840. doi: 10.1016/s0025-7125(16)31021-5. [DOI] [PubMed] [Google Scholar]
- Nissen S. L., Van Huysen C., Haymond M. W. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr. 1982 Oct 8;232(1):170–175. doi: 10.1016/s0378-4347(00)86021-1. [DOI] [PubMed] [Google Scholar]
- Osei K., Henry M. L., O'Dorisio T. M., Tesi R. J., Sommer B. G., Ferguson R. M. Physiological and pharmacological stimulation of pancreatic islet hormone secretion in type I diabetic pancreas allograft recipients. Diabetes. 1990 Oct;39(10):1235–1242. doi: 10.2337/diab.39.10.1235. [DOI] [PubMed] [Google Scholar]
- Ostman J., Bolinder J., Gunnarsson R., Brattström C., Tydén G., Wahren J., Groth C. G. Effects of pancreas transplantation on metabolic and hormonal profiles in IDDM patients. Diabetes. 1989 Jan;38 (Suppl 1):88–93. doi: 10.2337/diab.38.1.s88. [DOI] [PubMed] [Google Scholar]
- Ripamonti M., Mosca A., Rovida E., Luzzana M., Luzi L., Ceriotti F., Cottini F., Rossi-Bernardi L. Urea, creatinine, and glucose determined in plasma and whole blood by a differential pH technique. Clin Chem. 1984 Apr;30(4):556–559. [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Genest J., Baker B. A., Gerich J. E. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985 Feb;28(2):70–75. doi: 10.1007/BF00279918. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Scherrer U., Vissing S. F., Morgan B. J., Rollins J. A., Tindall R. S., Ring S., Hanson P., Mohanty P. K., Victor R. G. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990 Sep 13;323(11):693–699. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]
- Secchi A., Dubernard J. M., La Rocca E., LeFrancois N., Melandri M., Martin X., Touraine J. L., Traeger J., Pozza G. Endocrinometabolic effects of whole versus segmental pancreas allotransplantation in diabetic patients--a two-year follow-up. Transplantation. 1991 Mar;51(3):625–629. doi: 10.1097/00007890-199103000-00016. [DOI] [PubMed] [Google Scholar]
- Simonson D. C., DeFronzo R. A. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990 Mar;258(3 Pt 1):E399–E412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- Spotti D., Librenti M. C., Melandri M., Slaviero G., Quartagno R., Vedani P., Tagliabue V., Pozza G. Bioelectrical impedance in the evaluation of the nutritional status of hemodialyzed diabetic patients. Clin Nephrol. 1993 Mar;39(3):172–174. [PubMed] [Google Scholar]
- Sutherland D. E., Najarian J. S., Greenberg B. Z., Senske B. J., Anderson G. E., Francis R. S., Goetz F. C. Hormonal and metabolic effects of a pancreatic endocrine graft. Vascularized segmental transplantation in insulin-dependent diabetic patients. Ann Intern Med. 1981 Nov;95(5):537–541. doi: 10.7326/0003-4819-95-5-537. [DOI] [PubMed] [Google Scholar]
- Tessari P., Nosadini R., Trevisan R., De Kreutzenberg S. V., Inchiostro S., Duner E., Biolo G., Marescotti M. C., Tiengo A., Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidation in type 1, insulin-dependent diabetes mellitus. Evidence for insulin resistance involving glucose and amino acid metabolism. J Clin Invest. 1986 Jun;77(6):1797–1804. doi: 10.1172/JCI112504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberti G. C., Bilous R. W., Mackintosh D., Keen H. Monitoring glomerular function in diabetic nephropathy. A prospective study. Am J Med. 1983 Feb;74(2):256–264. doi: 10.1016/0002-9343(83)90624-1. [DOI] [PubMed] [Google Scholar]
- Zeller K., Whittaker E., Sullivan L., Raskin P., Jacobson H. R. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991 Jan 10;324(2):78–84. doi: 10.1056/NEJM199101103240202. [DOI] [PubMed] [Google Scholar]



