Abstract
Background
Severe traumatic injury elicits a neuroendocrine response that activates the sympathetic nervous system. Our previous work suggests that norepinephrine (NE) influences the bone marrow (BM) erythropoietic response. However, the dose-response relationship between NE and erythropoiesis remains unclear.
Materials and Methods
Two days following chemical sympathectomy with 6-hydroxydopamine (6-OHDA) or injection with saline vehicle (SHAM), male Sprague-Dawley rats were infused continuously with either saline (NS) or increasing doses of NE for 5 days via osmotic pumps. Erythropoiesis was assessed by growth of erythroid progenitor colonies (BFU-E and CFU-E for early and late progenitors, respectively).
Results
Following chemical sympathectomy with 6-OHDA, both BFU-E and CFU-E growth is inhibited (42%* and 43%* vs. 100% SHAM, *P < 0.05). SHAM rats with continuous infusion of exogenous NE show a clear dose-response inhibition of both BFU-E and CFU-E colony growth. In the 6-OHDA rats, continuous infusion of NE restored BFU-E and CFU-E growth at 10−8g/hr and 10−9g/hr, respectively.
Conclusions
Erythroid precursor colony growth is inhibited in sympathectomized rats. In addition, supraphysiologic doses of exogenous NE inhibit normal erythropoiesis in a dose-dependent fashion. Following chemical sympathectomy with 6-OHDA, exogenous NE restores erythropoiesis in a narrow window. Therefore, NE has a complex interaction within the BM and the elevation of NE following traumatic injury impacts BM erythropoietic function.
Keywords: BFU-E, CFU-E, bone marrow, 6-hydroxydopamine, catecholamine, chemical sympathectomy
INTRODUCTION
Bone marrow (BM) dysfunction has been shown to occur both clinically and experimentally following hemorrhagic shock, hypoxia, soft tissue injury, and thermal injury (1–5). Following severe injury BM dysfunction is commonly observed in the erythroid compartment and is manifest as a persistent anemia. The etiology of injury-associated anemia is multifactorial and thought to be due to acute blood loss, repeated phlebotomies, transfusion of stored blood, inadequate production of red blood cells, and nutritional deficiencies (6, 7). In addition, the prolonged inflammatory state caused by critical illness is yet another mechanism that has led to the terms ‘anemia of inflammation’ or ‘anemia of critical illness’ (7). In fact, circulating inflammatory cytokines have also been shown to impair the growth of erythroid progenitors within the BM (7, 8).
The initial stress response to injury is neuroendocrine, metabolic, hematologic and immunologic in nature. This stress state involving catecholamines is well described, and acts to support vital organ perfusion and mobilize fuel substrates (9–12). A poorly understood aspect of the host response to traumatic injury is the interaction of sympathetic nerves with the hematologic and immune systems (13, 14). It is known that after severe injury there is evidence of destruction of noradrenergic neurons innervating the injured tissues resulting in a massive release of norepinephrine (NE) into the peripheral circulation (14–17). Catecholamine levels have been shown to be elevated two-ten fold following severe injury (9, 11, 14, 18, 19).
There are noradrenergic and cholinergic innervations within the BM that provide a basis for the neural modulation of hematopoiesis (20). Our previous work demonstrated that adrenergic agonists at physiologic levels in vitro have a clear stimulatory effect on the growth of normal primitive erythroid colony precursors (21). In addition, when the levels of epinephrine (EPI) and NE were increased to supraphysiologic stress levels, the growth of erythroid progenitors was inhibited (9). This suggests that the effect of catecholamines on erythroid progenitor colony growth is concentration dependent.
To further characterize the role of NE in normal BM erythropoiesis in vivo, erythroid function in sympathectomized animals is required. Chemical sympathectomy using the neurotoxic agent, 6-hydroxydopamine (6-OHDA), has been shown to reversibly impair the sympathetic nerve terminal (22, 23). When 6-OHDA is injected intravenously or intraperitoneally, it accumulates in peripheral sympathetic nerve terminals, resulting in selective destruction. This results in a 80–95 % decrease in the amount of circulating NE (22, 23). Using this NE depletion model, the critical need for NE for normal erythropoiesis in vivo was assessed by examining its impact on the erythropoietic maturation process.
MATERIALS AND METHODS
Reagents
Bovine serum albumin (BSA), glutamine, RPMI-1640, 2-mercaptoethanol (2-ME), 6-OHDA, and methylcellulose were purchase from Sigma (St. Louis, MO). Fetal calf serum (FCS) was obtained from Atlanta Biologicals (Norcross, GA). Recombinant human erythropoietin (rhEpo) and IL-3 were purchased from R&D Systems (Minneapolis, MN). NE Bitartrate was purchased from Hospira, Inc. (Lake Forest, IL).
Animals
Male Sprague-Dawley rats (300–350g) were obtained from Charles River Laboratories and housed in barrier-sustained conditions in an animal room maintained at 22 ± 2°C on a 12hr light:12 hr dark cycle in accordance with the Guide for the Care and Use of Laboratory Animals. Standard rat chow and water were available ad libitum.
6-OHDA Treated Rats
6-OHDA solutions were prepared at a concentration per animal weight of 100mg/kg suspended in 1mL normal saline. 10 microliters of 0.5M ascorbic acid was added as an anti-oxidant. Rats in the 6-OHDA group received two intraperitoneal injections of the drug 24 hours apart. Control rats received injections of saline vehicle only (SHAM). All rats were sacrificed 24 hours after the second injection of 6-OHDA or saline and BM mononuclear cells were harvested for cell culture. Treatment with 6-OHDA did not result in any animal mortality.
Osmotic Pump Implantation
NE bitartrate was delivered at different rates (0.001 ng/hr, 0.01 ng/hr, 0.1 ng/hr, and 1 ng/hr) continuously using mini-osmotic pumps (ALZET model 1007D; Durect Corp., Cupertino, CA) implanted into the peritoneal cavity. Control rats were implanted with osmotic pumps that were filled with saline vehicle (NS) only. Rats were sacrificed 5 days after pump implantation for BM harvest and cell culture.
Similarly, NE bitartrate was delivered to 6-OHDA treated rats at continuous rates (0.1 ng/hr, 1 ng/hr, 10 ng/hr, and 100 ng/hr) using mini osmotic pumps. SHAM rats were implanted with osmotic pumps that were filled with NS only. All pumps were implanted 24 hours after the second injection of 6-OHDA or NS. Rats were sacrificed 5 days after pump implantation for BM harvest and culture.
BFU-E and CFU-E Cell Cultures
Bilateral femurs were aseptically extracted from the rats and placed in 70% ethanol for approximately 10 minutes. The femurs were then washed once with a phosphate buffered solution. The BM was harvested and resuspended in RPMI 1640 (Sigma) containing 10% FCS. BM cells were plated (2 × 106 cells/plate) in duplicate in rat spleen-enriched Iscove’s media containing 30% FCS, 2% BSA, 1% methylcellulose, 2 × 10−4 mol/L 2-ME, and glutamine with 6 U/mL rhIL-3, and 1.3 U/mL rhEpo. Cultures were incubated at 37°C in 5% CO2. BFU-E colonies were enumerated on day 15 and CFU-E colonies were enumerated on day 10 based on the number of cells and their morphology by an observer who was blinded to the origin of the samples.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using Student’s t test or analysis of variance and the Tukey-Kramer multiple comparisons test where appropriate. Results were considered significant if P < 0.05.
RESULTS
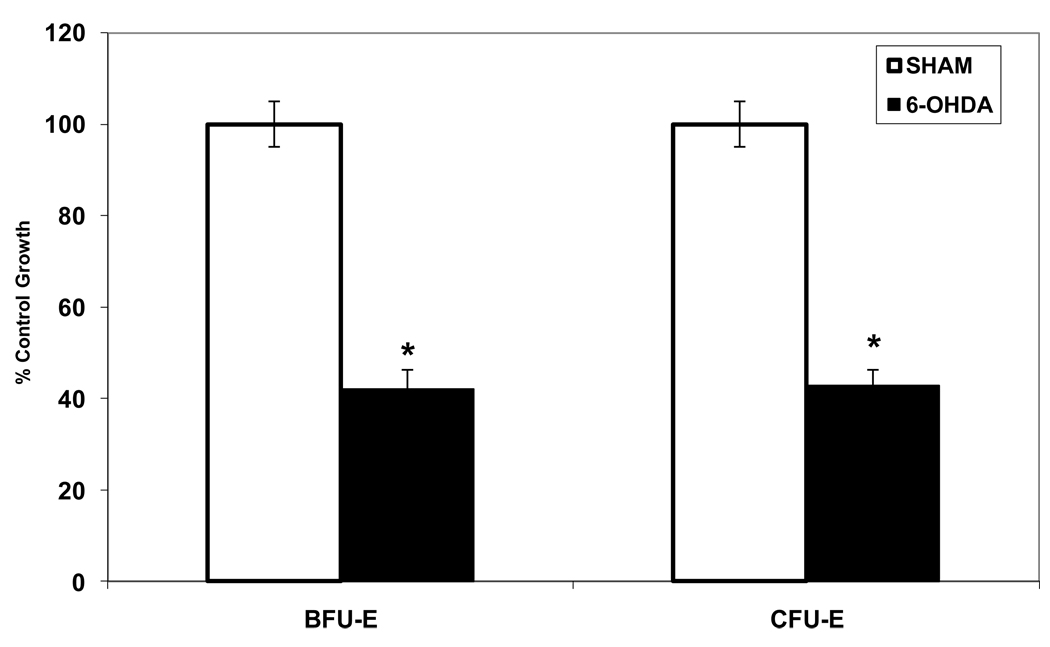
Erythroid precursor colony growth was decreased in NE depleted rats (6-OHDA) as compared to SHAM rats (Figure 1). NE depletion with 6-OHDA significantly inhibited BFU-E colony growth as compared to SHAM animals (42%* vs. 100%; *P < 0.05). Similarly, a more differentiated erythroid progenitor, CFU-E, was also significantly suppressed in the 6-OHDA group as compared to SHAM rats (Figure 1).
Figure 1.
Suppression of BFU-E and CFU-E colony growth following depletion of NE by chemical sympathectomy with 6-OHDA. *P < 0.05 (NE=norepinephrine, SHAM=normal saline injection, 6-OHDA=6-hydroxydopamine) N = 6–10 rats/group
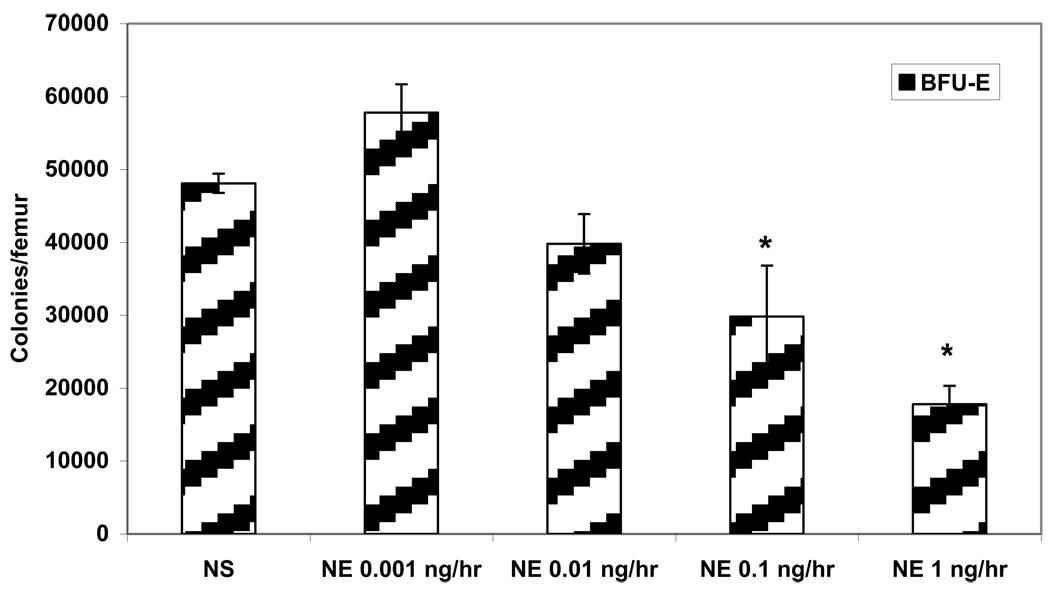
To determine the effects of exogenous NE, osmotic pumps were implanted in normal rats with varying concentrations of NE and erythroid progenitor colony growth was evaluated. As the concentration of NE increased, erythroid progenitor growth was suppressed in a dose-dependent fashion (Figure 2). Exogenous NE inhibited growth of BFU-E colonies beginning at a dose of 0.01 ng/hr, but reached a statistically significant inhibition of BFU-E growth at 0.1 ng/hr and 1 ng/hr as compared to SHAM implanted with NS. Growth of BFU-E colonies decreased 48% when NE was delivered at 0.1 ng/hr and growth was further decreased 63% with NE at 1 ng/hr (Figure 2). Low dose exogenous NE (0.001 ng/hr) had a mild stimulatory affect on BFU-E colony growth compared to SHAM that was not statistically significant (Figure 2).
Figure 2.
Dose-dependent suppression of BFU-E growth with exogenous NE delivered via an osmotic pump in normal rats. *P < 0.05 (NE=norepinephrine) N = 4–6 rats/group
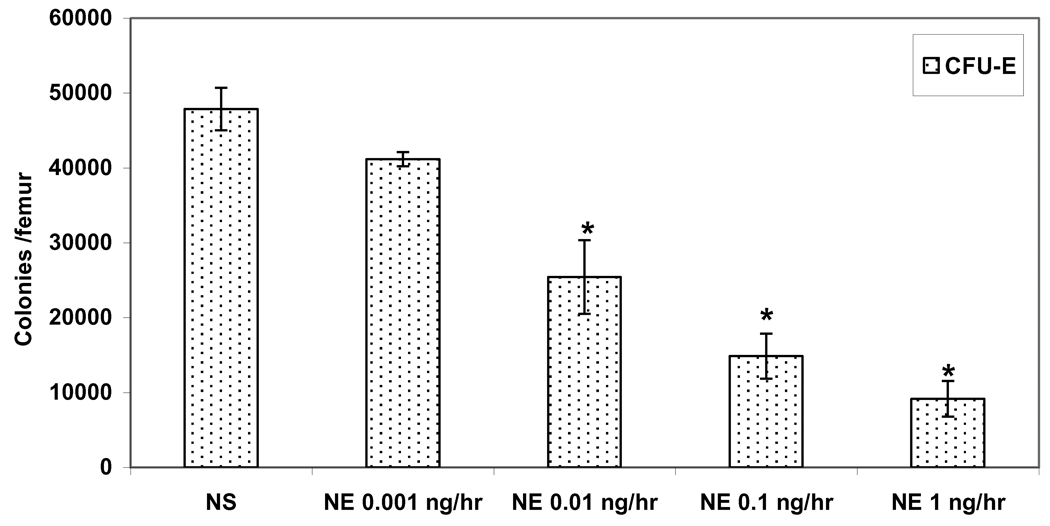
Similarly, the growth of CFU-E, was also inhibited by exogenous NE in a dose-dependent fashion (Figure 3). The suppression of CFU-E due to exogenous NE was more profound than that seen with BFU-E. The suppression of CFU-E colonies ranged from 15% when NE was delivered at 0.001 ng/hr to 81% with NE delivered at 1 ng/hr as compared to SHAM animals implanted with NS (Figure 3).
Figure 3.
Dose-dependent suppression of CFU-E growth with exogenous NE delivered via an osmotic pump in normal rats. *P < 0.05 (NE=norepinephrine) N = 4–6 rats/group
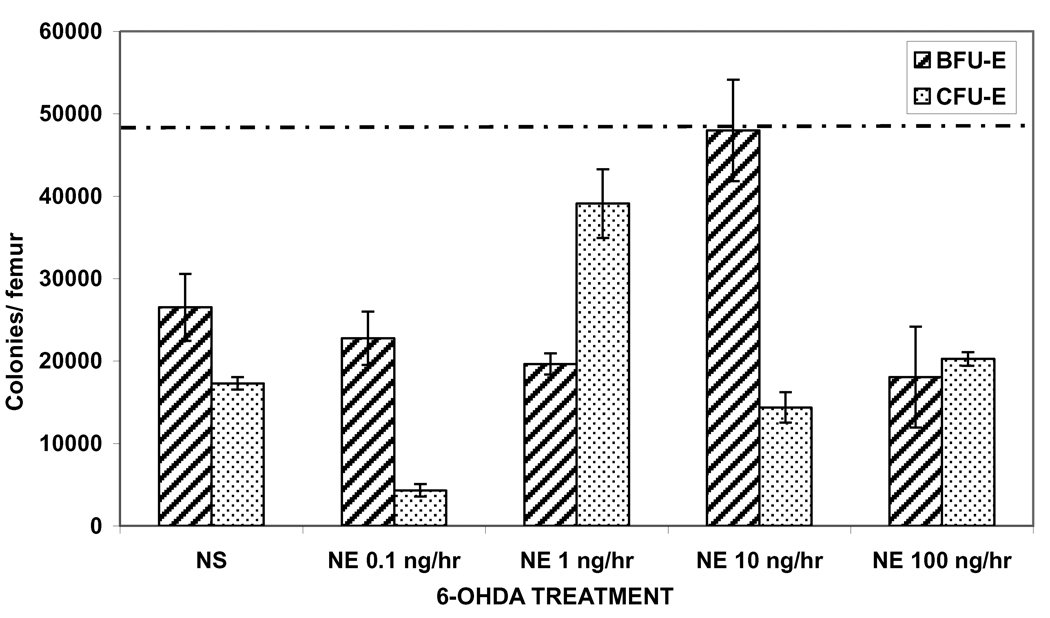
To determine if NE was essential and would restore erythroid progenitor growth, in 6-OHDA treated rats, osmotic pumps were implanted with increasing doses of NE. First, NS was added to osmotic pumps as a control to ensure that pump implantation did not act as a significant stress for 6-OHDA rats. As expected, growth of BFU-E and CFU-E colonies was not restored in 6-OHDA rats implanted with NS pumps (Figure 4). Full recovery of BFU-E colony growth was seen in 6-OHDA rats infused with NE at 10 ng/hr (Figure 4). Whereas, partial recovery of CFU-E colony growth was seen in rats when NE was delivered at 1 ng/hr (Figure 4).
Figure 4.
Restoration of erythroid progenitor growth following chemical sympathectomy in a narrow window with NE given via osmotic pumps. Experimental groups are compared to SHAM+NS which is represented by a dashed line. (SHAM=normal saline injection, NS=normal saline, NE=norepinephrine) N = 4–6 rats/group
DISCUSSION
Following severe injury, persistent anemia is one significant manifestation of hematopoietic failure (9, 14, 24–26). This erythropoietic dysfunction can last for the duration of the patient’s hospital stay regardless of the number of blood transfusions (25). Similarly, the adrenergic response to severe injury results in an elevation of catecholamine levels two to ten times normal (9, 11, 14, 18, 19). In our previous work, urinary NE levels in trauma patients were significantly increased and remained elevated for up to a week following severe injury (9). Catecholamines have already been shown to impact the pathophysiology of sepsis by attenuating proinflammatory cytokines and augmenting anti-inflammatory cytokines released by macrophages (27). Our previous work, in fact, demonstrated a direct role for catecholamines in BM dysfunction (9, 21). NE had both stimulatory and inhibitory effects on erythropoiesis in vitro at both extremes depending on the concentration (9, 21). Fonseca et al. (9) established that increasing levels of NE in vitro led to a dose-dependent reduction in erythroid progenitor colony growth. Similarly, Brown et al. (28) showed a similar dose response trend with the use of isoproterenol and epinephrine. The present study sought to further delineate the role of NE in vivo in the modulation of erythroid progenitor growth.
To further examine the role of NE in erythropoiesis in vivo, a chemical sympathectomy model was utilized to determine the effect of NE depletion. Chemical sympathectomy with 6-OHDA is a well established model of NE depletion. It has been shown in multiple studies to result in an 80–95% decrease in plasma NE levels (15, 22, 23, 29). In rodent BM progenitor cultures, we have demonstrated that NE depletion with 6-OHDA significantly inhibited both BFU-E and CFU-E colony growth in a similar fashion, with greater than 50% inhibition as compared to control. This inhibition of erythroid progenitor growth is supported by Obayashi et al. (22) who determined that 6-OHDA treatment does induce a reversible anemia. Obayashi and colleagues (22) found that in peripheral blood, treatment with 6-OHDA led to decreases in hemoglobin, hematocrit and red blood cell levels that do not return to baseline until thirty days after treatment (22). These findings indicate that NE plays a significant role in normal erythropoiesis and that NE is needed for normal erythroid function.
To determine the effect of supraphysiologic levels of NE, similar to what is seen following severe injury, osmotic pumps were implanted and erythropoiesis was assessed. At NE dose of 0.1 ng/hr, growth of BFU-E was inhibited nearly 40% and when NE was delivered at a dose of 1 ng/hr, growth of BFU-E colonies was further suppressed 63% (Figure 2). When studying a more differentiated erythroid progenitor, CFU-E, exogenous NE exhibited an even more profound suppression of colony growth (Figure 3). In our previous studies, we found BFU-E colonies to be more responsive to adrenergic agonists than CFU-E colonies. Therefore, it appeared that the adrenergic stimulus had its greatest effect at the earliest stage of committed erythroid cell maturation. However, in contrast, these studies show a more profound inhibition of CFU-E growth when compared to BFU-E growth. One possible explanation for these findings is due to the in vivo exposure of NE over a prolonged period, as opposed to the in vitro shorter exposure to adrenergic agents in previous experiments. These findings demonstrate that the NE concentration and duration of exposure affects lineage specific erythroid proliferation. This may also have clinical relevance with regard to the persistence of anemia seen after seen severe injury as it relates to the suppression of multiple erythroid progenitor cells.
To determine if erythropoietic suppression with 6-OHDA could be reversed, NE was then delivered via osmotic pumps. Our analysis demonstrated that chemical sympathectomy resulted in a reduction of erythropoiesis that is fully restored by exogenous NE for BFU-E colonies, but only in a narrow window (Figure 4). There was only a partial restoration of CFU-E colony growth with exogenous NE in the 6-OHDA rats. It is possible that the effect of NE at different concentrations is due to the variability of interaction with its adrenergic receptors. Muthu et al. (30) demonstrated that hematopoietic progenitor cells, inclusive of pluripotent stem cells, express alpha-1, alpha-2 and beta-2 adrenergic receptors. However, as progenitor cells differentiate the activity of adrenergic receptors may change (30). Previously, beta receptors have been shown to be more important in erythropoiesis than alpha receptors (31). Study of wound macrophage function in vitro by Gosain et al. (15) demonstrated that NE works through the beta adrenergic receptors at lower doses and predominantly through the alpha receptors at higher doses. NE has also been shown to compete for beta-adrenoreceptor binding at low potency (32). In addition, high doses of catecholamines have been shown to lead to the internalization of the beta-adrenoreceptor (33). It is also possible that since following chemical sympathectomy a higher concentration of NE is needed to restore erythroid progenitor growth there is a direct effect of 6-OHDA pretreatment on the adrenergic receptors. This finding is supported by Obayashi et al. (22) who showed that 6-OHDA treatment down regulates beta adrenergic receptors in rats (22). So the dose-dependent effects of NE could be explained by possible interaction of different adrenergic receptors at different concentrations, down regulation of beta receptors at higher doses, or internalization of beta receptors leading to suppression of erythroid progenitor growth.
Only one study has demonstrated the presence of endogenous catecholamines, including NE, EPI and dopamine, within the BM by high performance liquid chromatography (34). After harvesting bilateral femurs in mice the concentration of NE in the BM harvested is 0.003 ng/mg tissue, which is not surprisingly lower than that circulating in normal rat plasma (1.6 ng/mL) (34). The inability to routinely measure actual NE levels within the BM is a limitation. However, this study reveals that minimal changes in the circulating concentration of NE and the temporal relation of NE exposure significantly impacts erythropoietic function. Therefore, it is possible that in severely injured trauma patients both the duration and level of elevation of catecholamine exposure is involved in the persistence of anemia. It is unknown if there is a true persistent elevation or a daily surge of NE in these severely injured patients and the relation of this elevated circulating catecholamine to its concentration in the BM has not been investigated. Additionally, catecholamines are not the only agents that have dose-dependent effects on erythroid progenitor growth. Osterode et al. (35) demonstrated dose-dependent effects of lead on BFU-E growth as one of the causes involved in the multifactorial development of lead-induced anemia. It is suggested that the dose dependent reduction of BFU-E cells during exposure to lead is due to apoptosis (35). Recently, Jacobs-Helber and colleagues (36) observed that TNF-alpha inhibits the proliferation of maturing erythroid cells and decreases the formation of BFU-E in human and murine cells. Further studies looking at the adrenergic effects of BM erythroid progenitors and apoptosis may elucidate the mechanisms involved in the erythropoietic dysfunction seen following severe injury.
In summary, our results indicate that the presence of NE is critical for normal erythropoiesis. However, as the dose of NE increases, normal erythroid precursor cell growth is inhibited suggesting tight regulation of normal erythropoiesis. In sympathectomized rats treated with 6-OHDA, erythropoiesis was suppressed and with exogenous administration of NE erythropoiesis is restored, albeit in a narrow window. These data suggest that following severe traumatic injury there may be a direct correlation between the magnitude and duration of the stress response and the resultant erythropoietic dysfunction. Furthermore, NE has a complex interaction within the BM and an attenuation of the stress response following severe injury may aid in decreasing the morbidity of injury-associated anemia.
ACKNOWLEDGEMENTS
This research was supported in part by the NIH K08GM078304-01 and the Clowes ACS/AAST Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gamelli R, He L, Liu H. Marrow granulocyte-macrophage progenitor cell response to burn injury as modified by endotoxin and indomethacin. J Trauma. 1994;37:339–346. doi: 10.1097/00005373-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Livingston DH, Gentile PS, Malangoni MA. Bone marrow failure after hemorrhagic shock. Circ Shock. 1990;30:255–263. [PubMed] [Google Scholar]
- 3.Raff G, Livingston D, Wang M, et al. Hemorrhagic shock abolishes the myelopoietic response to turpentine-induced soft tissue injury. J Surg Res. 1995;59:75–79. doi: 10.1006/jsre.1995.1134. [DOI] [PubMed] [Google Scholar]
- 4.Fontes B, Moore FA, Moore EE, et al. Gut ischemia induces bone marrow failure and increases risk of infection. J Surg Res. 1994;57:505–509. doi: 10.1006/jsre.1994.1176. [DOI] [PubMed] [Google Scholar]
- 5.Mohr AM, Upperman JS, Taneja R, et al. The differential effects of acute hypoxia and endotoxin on the secretion and expression of bone marrow interleukin-1 and interleukin-6. Shock. 1997;7:324–331. doi: 10.1097/00024382-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Livingston DH, Anjaria D, Wu J, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sihler KC, Napolitano LM. [Accessed 8/11/2007];Anemia in critical care. 2007 February; [Society Critical Care Medicine Web site], Available at: http://www.sccm.org/SCCM/Publications/Critical+Connections/Archives. [Google Scholar]
- 8.Wu J, Livingston DH, Hauser CJ, et al. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF β1 by bone marrow stroma. Ann Surg. 2001;234:224–232. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseca RB, Mohr AM, Wang L, et al. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 10.Epstein J, Breslow MJ. The stress response of critical illness. Crit Care Clin. 1999;15:17–33. doi: 10.1016/s0749-0704(05)70037-3. [DOI] [PubMed] [Google Scholar]
- 11.Udelsman R, Holbrook NJ. Endocrine and molecular responses to surgical stress. Curr Prob Surg. 1994;31:653–720. [PubMed] [Google Scholar]
- 12.Woiciechowsky C, Schoning B, Cobanov J, et al. Early IL-6 concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52:339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Muthu K, Gamelli R, et al. Adrenergic modulation of splenic macrophage cytokine release in polymicrobrial sepsis. Am J Physiol Cell Physiol. 2004;287:C730–C736. doi: 10.1152/ajpcell.00562.2003. [DOI] [PubMed] [Google Scholar]
- 14.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 15.Gosain A, Muthu K, Gamelli RL, DiPietro LA. Norepinephrine suppresses wound macrophage phagocytic efficiency through alpha and beta adrenoreceptor dependent pathways. Surgery. 2007;142:170–179. doi: 10.1016/j.surg.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spengler RN, Allen RM, Remick DG, et al. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immuno. 1990;145:1430–1434. [PubMed] [Google Scholar]
- 17.Zetterstrom BE, Palmerio C, Fine J. Protection of functional and vascular integrity of the spleen in traumatic shock by denervation. Proc Soc Exp Biol Med. 1964;117:373–376. doi: 10.3181/00379727-117-29584. [DOI] [PubMed] [Google Scholar]
- 18.Breslow MJ, Ligier B. Hyperadrenergic states. Crit Care Med. 1991;19:1566–1579. doi: 10.1097/00003246-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Benedict CR, Grahame-Smith DG. Plasma noradrenaline and adrenaline concentrations and dopamine-beta-hydroxylase activity in patients with shock due to septicemia, trauma and haemorrhage. Q J Med. 1978;47:1–20. [PubMed] [Google Scholar]
- 20.Artico M, Bosco S, Cavallotti C, et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med. 2002;10:77–80. [PubMed] [Google Scholar]
- 21.Fonseca RB, Mohr AM, Wang L. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. J Surg Inf. 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 22.Obayashi K, Ando Y, Terazaki H, et al. Mechanism of anemia associated with autonomic dysfunction in rats. Autonom Neuroscience. 2000;82:123–129. doi: 10.1016/S0165-1838(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 23.Thoenen H, Tranzer JP. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-hydroxydopamine. Arch Pharmacol. 1968;261:271–288. doi: 10.1007/BF00536990. [DOI] [PubMed] [Google Scholar]
- 24.Heckbert SR, Vedder NB, Hoffman W, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Corwin HL, Surgenor SD, Gettinger A. Transfusion practice in the critically ill. Crit Care Med. 2003;31:S668–S671. doi: 10.1097/01.CCM.0000099348.99451.84. [DOI] [PubMed] [Google Scholar]
- 26.Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995;162:134–138. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Shankar R, Gamelli R, Jones S. Dynamic alterations in bone marrow: evidence of functional innervation. J Neuroimm. 1999;96:182–189. doi: 10.1016/s0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 28.Brown JE, Adamson JW. Modulation of in vitro erythropoiesis: The influence of beta-adrenergic agonists on erythroid colony formation. J Clin Invest. 1977;60:70–77. doi: 10.1172/JCI108771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali RA, Qureshi MA, McCorkle FM. Profile of chicken macrophage functions after exposure to catecholamines in vitro. Immunopharmacol Immunotoxicol. 1994;16:611–625. doi: 10.3109/08923979409019742. [DOI] [PubMed] [Google Scholar]
- 30.Muthu K, Iyer S, He LK, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimm. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mladenovic J, Adamson JW. Adrenergic modulation of erythropoiesis: in vitro studies of colony forming cells in normal and polycythemic man. Br J Hematol. 1984;56:323–332. doi: 10.1111/j.1365-2141.1984.tb03959.x. [DOI] [PubMed] [Google Scholar]
- 32.Abrass CK, O’Connor SW, Scarpace PJ, Abrass IB. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338–1341. [PubMed] [Google Scholar]
- 33.Elenkov IJ, Hasko G, Kovacs KJ, Vizi ES. Modulation of lipopoysaccaride-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J Neuroimmunol. 1995;61:123–131. doi: 10.1016/0165-5728(95)00080-l. [DOI] [PubMed] [Google Scholar]
- 34.Marino F, Consentino M, Bombelli R, et al. Measurement of catecholamines in mouse bone marrow by means of HPLC with electrochemical detection. Haematologica. 1997;82:392–394. [PubMed] [Google Scholar]
- 35.Osterode W, Barnas U, Geissler K. Dose dependent reduction of erythroid progenitor cells and inappropriate erythropoietin response in exposure to lead : new aspects of anemia induced by lead. Occup Environ Med. 1999;56:106–109. doi: 10.1136/oem.56.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs-Helber SM, Roh KH, Bailey D, et al. Tumor necrosis factor alpha expressed constitutively in erythroid cells or induced by erythropoietin has negative and stimulatory roles in normal erythropoiesis and erythroleukemia. Blood. 2003;101:524–531. doi: 10.1182/blood-2001-11-0084. [DOI] [PubMed] [Google Scholar]