Abstract
Statins are widely used to lower cholesterol levels by inhibiting cholesterol biosynthesis. Some evidence has indicated that statins might have therapeutic and preventive benefits for Alzheimer’s disease (AD). We and others also have shown the beneficial effect of statin treatment in reversing learning and memory deficits in animal models of AD. However, data from clinical trials are inconclusive. We previously documented that the adenovirus vector encoding 11 tandem repeats of Aβ1-6 fused to the receptor-binding domain (Ia) of Pseudomonas exotoxin A, AdPEDI-(Aβ1-6)11, is effective in inducing an immune response against amyloid-β protein (Aβ) and reducing brain Aβ load in Alzheimer’s mouse models. In the present study, we examined whether the administration of simvastatin can modulate immune and behavioral responses of C57BL/6 mice to vaccination. Simvastatin was given to the animals as a diet admixture for four weeks, followed by nasal vaccination with AdPEDI-(Aβ1-6)11 once per week for four weeks. The cholesterol-lowering action of simvastatin was monitored by measuring the cholesterol levels in plasma. Simvastatin significantly increased the number of the mice responding to vaccination compared with the mice receiving only AdPEDI-(Aβ1-6)11. Immunoglobulin isotyping revealed that the vaccination predominantly induced Th2 immune responses. Simvastatin treatment prevented Aβ-induced production of IFN-γ in splenocytes. The adenovirus vaccination altered mouse behavior in T- and elevated plus-maze tests and simvastatin counteracted such behavioral changes. Our results indicate that simvastatin clearly enhances the immune responses of C57BL/6 mice to the nasal vaccination with AdPEDI-(Aβ1-6)11. Simvastatin may be effective in preventing behavioral changes associated with vaccination.
Keywords: adenovirus, statins, sickness behavior, anxiety, Alzheimer’s disease, amyloid
1. Introduction
Alzheimer’s disease (AD) is a slowly progressive neurodegenerative disease and the most common type of dementia in the elderly. The characteristic pathological hallmarks of AD include the significant loss of neurons in the cerebral cortex and certain subcortical regions, the presence of intracellular neurofibrillary tangles and the formation of amyloid plaques outside neurons and in cerebral blood vessels. The etiology of AD is still not clear, but increasing lines of evidence support the hypothesis that accumulation of amyloid β-protein (Aβ), the major component in amyloid plaques, is the causative factor in the development of AD (Hardy and Selkoe, 2002; Price and Sisodia, 1994; Selkoe, 1994). The genetic abnormalities that account for the buildup of Aβ deposits have been identified in some autosomal dominant cases of AD, such as mutations in genes encoding presenilin-1 (PS1), presenelin-2 (PS2) and beta-amyloid precursor protein (APP) (Goate et al., 1991; Kowalska et al., 2004; Rogaev et al., 1995; Sherrington et al., 1995). Overexpression of the mutant forms of these genes in transgenic mice led to the high levels of Aβ accumulation in the brain as well as AD-like pathologic and behavioral alterations (Citron et al., 1997; Holcomb et al., 1998; Games et al., 1995; Hsiao et al., 1996).
To date, no satisfactory treatment is available for AD. A number of immunotherapeutic studies have shown that immunization with synthetic Aβ peptide prevented or reduced Aβ deposits (Schenk et al., 1999) and attenuated the memory and learning deficits in animal models of AD (Morgan et al., 2000; Janus et al., 2000). The clinical trials of Aβ1-42 vaccination (AN-1792) were halted because a subset of vaccinated patients (6%) developed brain inflammation presumably caused by T-cell mediated immune responses (Gilman et al., 2005; Nicoll et al., 2003; Orgogozo et al., 2003; Schenk and Yednock, 2002; Weiner and Selkoe, 2002). Other problems associated with Aβ immunotherapy are that only 20% of AD patients developed anti-Aβ antibodies due to poor immunogenicity of Aβ and aging (Gilman et al., 2005) and that one AD patient and aged AD mouse models developed cerebral microhemorrhages (Ferrer et al., 2004; Pfeifer et al., 2002; Racke et al., 2005). The subsequent reports of the vaccination clinical trials indicate that Aβ immunotherapy is effective in clearing Aβ deposits and improving cognitive deficits in a subset of AD patients (Gilman et al., 2005; Nicoll et al., 2003; Hock et al., 2003). Therefore, it is crucial to find a safe, efficacious immunotherapy. The synthetic Aβ1-42 peptide used in clinical trials contains both B- and T- cell epitopes, with Aβ1-15 identified as a B cell epitope (Cribbs et al., 2003; McLaurin et al., 2002; Town et al., 2001) and Aβ6-28 as a T cell epitope (Cribbs et al., 2003). To avoid the T-cell mediated side effects, we previously constructed an adenovirus vector encoding 11 tandem repeats of Aβ1-6 fused to the receptor-binding domain (Ia) of Pseudomonas exotoxin A, AdPEDI-(Aβ1-6)11 (Kim et al., 2005). The immunization study revealed that AdPEDI-(Aβ1-6)11 predominantly induced IgG1 isotype anti-Aβ antibodies (Kim et al., 2005) and upregulated IL-10 expression in AD mouse models (Kim et al., 2007b). The above results indicate that vaccination with AdPEDI-(Aβ1-6)11 effectively induces the Th2-type immune responses against Aβ.
Statins, the 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors, are widely used in clinical practice to lower cholesterol levels by inhibiting cholesterol biosynthesis. In addition to the cholesterol lowering effect, statins have many pleiotropic effects such as reducing Aβ production, suppressing inflammatory responses, stabilizing the blood-brain barrier integrity, protecting neurons from excitotoxins, apoptosis and oxidative stresses, and promoting synaptogenesis (Liao and Laufs, 2005; McFarlane et al., 2002; Wang et al., 2008). While several retrospective studies reported beneficial effects of statins on preventing AD (Jick et al., 2000; Li et al., 2007; Wolozin et al., 2000; Zamrini et al., 2004), others did not, or even on the cognitive function of the elderly (Li et al., 2004; Shepherd et al., 2002). Potentially beneficial effects of statins on AD have been demonstrated by a number of investigators using cell culture and animal models. For example, simvastatin and lovastatin were shown to reduce Aβ42 and Aβ40 levels in primary cultures of hippocampal and neocortical neurons and in the cerebrospinal fluid and brain homogenates in guinea pigs (Fassbender et al., 2001). Atorvastatin remarkably attenuated Aβ deposition in the brain of an AD mouse model (Petanceska et al., 2002). We also have shown that simvastatin was effective in reversing learning and memory deficits of an aged AD mouse model (Li et al., 2006). These results suggest that statins may have a beneficial role in preventing and/or treating AD. The other effects of statins, such as suppressing inflammation and stabilizing the blood-brain barrier integrity, may be beneficial to AD immunotherapy, particularly in avoiding its side effects, as well as sickness behaviors that may be evoked through the circulation by increases in proinflammatory cytokines associated with vaccinations. In the current study, we tested the feasibility of the combined treatment of an adenovirus vaccine, AdPEDI-(Aβ1-6)11, with simvastatin in young C57BL/6 mice by determining the effects of vaccination and/or statin treatment on their immune responses and behavioral functions such as exploratory activity, anxiety, and motor coordination.
2. Results
2.1. Simvastatin treatment
Two groups of 10 mice were treated daily with 50 mg/kg of simvastatin for 13 weeks. The total cholesterol levels in plasma were measured at 4 weeks (week 4) after the initiation of simvastatin treatment. Simvastatin treatment significantly reduced the total plasma cholesterol levels by 15% compared to the mice consuming regular diet without simvastatin (Table 1). There was no difference in the total cholesterol levels between simvastatin only and simvastatin plus AdPEDI-(Aβ1-6)11 groups. No difference in the total cholesterol levels was found between PBS only and AdPEDI-(Aβ1-6)11 only groups, also. During the treatment period, there were no differences in physical appearance, body weight, food consumption, or mortality among the groups (data not shown). Long-term treatment with 50 mg/kg of simvastatin did not cause any generalized toxicity.
Table 1.
| Treatment Groups | ||||
|---|---|---|---|---|
| Plasma | PBS | Adv | simvastatin | simvastatin + Adv |
| Total Cholesterol (mg/dl) | 112.25±3.4 | 114.1±3.2 | 93.4±3.3a | 100.0±4.1a |
Plasma cholesterol levels after simvastatin administration. (N=10 for each treatment group,
P < 0.05).
2.2. Anti-Aβ antibody titers and IgG isotyping
Two groups of 10 mice were subjected to nasal AdPEDI-(Aβ1-6)11 inoculations 5 times at weeks 4, 5, 6, 7 and 10 with and without simvastatin treatment (Fig. 1; Table 1). Anti-Aβ antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) using sera at weeks 0, 4, 7, 10 and 13. The data on immune responses, anti-Aβ antibody titers and isotyping are summarized in Table 2. At week 7, 9 out of 10 mice treated with simvastatin together with AdPEDI-(Aβ1-6)11 developed anti-Aβ titers (seropositive) while AdPEDI-(Aβ1-6)11 vaccination without simvastatin elicited anti-Aβ titers in 5 out of 10 mice. When only the seropositive mice were compared at week 7, the mean serum titer (1.9 ± 0.7 µg/ml) of mice subjected to the combination treatment of AdPEDI-(Aβ1-6)11 and simvastatin was similar to that (1.8 ± 1.2 µg/ml) of mice treated with only AdPEDI-(Aβ1-6)11. At weeks 10 and 13, the seropositive rates and the average anti-Aβ titers of seropositive mice receiving AdPEDI-(Aβ1-6)11 only stayed at almost the same levels. Although the number of seropositive mice subjected to the combination treatment gradually decreased from 9 to 7 and 6 at weeks 10 and 13, respectively, the mean anti-Aβ titer (8.8 ± 2.4 µg/ml) of seropositive mice receiving the combination treatment at week 13 increased approximately 4-fold from weeks 7 and 10 (P < 0.05) and was significantly higher than that (2.5 ± 0.8 µg/ml) of seropositive mice treated with AdPEDI-(Aβ1-6)11 alone (P = 0.03). Thus, simvastatin treatment appears to increase seropositive rates in its early stages as well as antibody titers in its later stages in susceptible animals. As expected, anti-Aβ IgG in mice receiving phosphate buffered saline (PBS) or simvastatin only were undetectable by ELISA.
Fig. 1.
Simvastatin treatment and immunization schedule.
Table 2.
| No. of anti-Aβ seropositive mice |
Antibody titers (µg/ml) mean±S.E.M. |
Ig isotypes of Aβ42 antibodies |
|
|---|---|---|---|
| Week 7 | |||
| AdPEDI-(Aβ1-6)11 only | 5/10 | 1.8±1.2 | IgG1 >> IgG2aa |
| AdPEDI-(Aβ1-6)11 + simvastatin | 9/10 | 1.9±0.7 | IgG1 >> IgG2aa |
| Week 10 | |||
| AdPEDI-(Aβ1-6)11 only | 5/10 | 1.9±1.0 | IgG1 >> IgG2aa |
| AdPEDI-(Aβ1-6)11 + simvastatin | 7/10 | 2.3±0.9 | IgG1 >> IgG2aa |
| Week 13 | |||
| AdPEDI-(Aβ1-6)11 only | 6/10 | 2.5±0.8 | IgG1 >> IgG2aa |
| AdPEDI-(Aβ1-6)11 + simvastatin | 6/10 | 8.8±2.4b | IgG1 >> IgG2aa |
Characterization of anti-Aβ antibodies induced by AdPEDI-(Aβ1-6)11 vaccination with or without simvastatin.
Levels of anti-Aβ IgG2a were less than the minimum detectable level by the isotype-specific ELISA.
P = 0.03.
Immunoglobulin isotype-specific anti-Aβ titers were quantified by ELISA. The IgG isotyping revealed that the anti-Aβ antibodies induced by nasal vaccination with AdPEDI-(Aβ1-6)11 were predominantly of the IgG1 isotype in both groups regardless of the simvastatin treatment (Table 2). The measurement of anti-Aβ IgG2a in both groups is below the detectable level by ELISA.
2.3. ELISPOT assay for IFN-γ
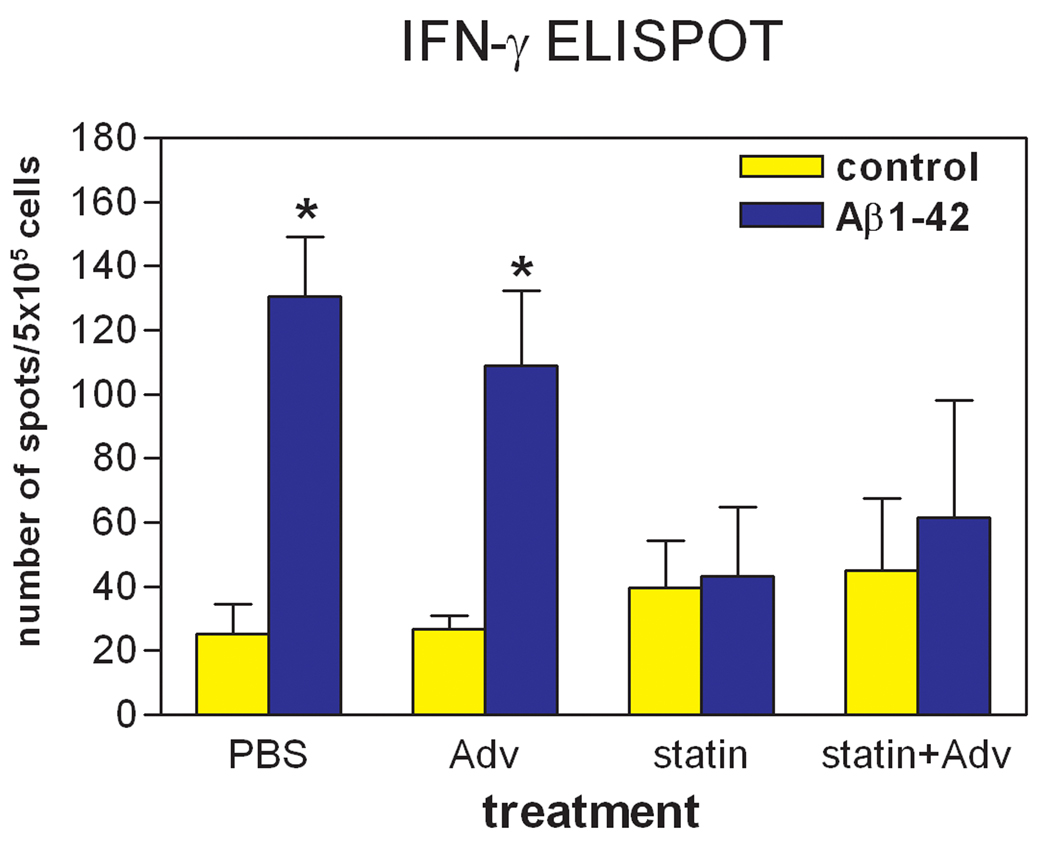
In addition to IgG antibody isotyping, to examine whether simvastatin can prevent Th1-type immune responses, enzyme-linked immunospot (ELISPOT) assay was carried out for determining the numbers of IFN-γ-producing cells in splenocytes from each mouse after the last AdPEDI-(Aβ1-6)11 immunization (week 13). The results are shown in Figure 2; in both PBS only and AdPEDI-(Aβ1-6)11 only treatment groups, the stimulation with Aβ1-42 peptide significantly increased the numbers of IFN-γ-producing splenocytes more than 4-fold compared to the non-stimulus conditions (P < 0.05). However, in the groups consuming simvastatin food, regardless of AdPEDI-(Aβ1-6)11 vaccination, the presence of Aβ1-42 peptide did not increase the number of IFN-γ-producing splenocytes. Thus, simvastatin treatment successfully prevented Aβ-induced production of IFN-γ in splenocytes.
Fig. 2.
ELISPOT assay to detect the immune responses against Aβ in splenocytes. Splenocytes were isolated from experimental animals and cultured in the presence or absence of 10 µg/ml of Aβ1-42 for 24 h. IFN-γ-producing splenocytes were determined by ELISPOT assay. For splenocytes isolated from the PBS- and AdPEDI-(Aβ1-6)11-treated mice, the numbers of IFN-γ-producing cells increased in response to Aβ stimulation (*P < 0.05). For mice treated with simvastatin regardless of AdPEDI-(Aβ1-6)11 vaccination, Aβ stimulation did not increase IFN-γ-producing splenocytes.
2.4. Exploratory activity, anxiety and motor coordination
To investigate possible effects of treatment on behavioral functions, mice were subjected to tests starting at week 8 after the completion of 4 vaccinations (Fig. 1). The exploratory tendencies were examined in T-maze spontaneous alternation, open-field, and elevated plus-maze tests, while motor coordination was measured in stationary beam, coat-hanger, and rotorod tests.
In T-maze spontaneous alternation test, the tendency of mice to switch arm choices on successive trials was evaluated. Thus, T-maze is a test dependent on working memory, anxiety levels, and cerebral activation. The mean spontaneous alternation rate was above the 50% chance level in all groups (P < 0.05, Mann-Whitney U test) (Table 3). There was a significant interaction in alternation rate between vaccine and simvastatin (F(1,35)=6.19, P < 0.05), due to the higher rate of mice treated with AdPEDI-(Aβ1-6)11 alone. Thus, simvastatin counteracted the effect of adenovirus vaccination. There was no difference in choice trial latencies.
Table 3.
| Test | PBS | simvastatin | Adv | simvastatin + Adv |
|---|---|---|---|---|
| T-maze | ||||
| Alternations (%) | 63±4 | 69±4 | 77±3a | 64±3 |
| Choice latencies/trial (s) | 8±1 | 10±1 | 10±1 | 8±1 |
| Elevated plus-maze | ||||
| Open arm entries | 13±1 | 10±1b | 10±0.7b | 14±1 |
| Enclosed arm entries | 16±1 | 16±2 | 14±1 | 15±1 |
| Open/total entries (%) | 46±2 | 37±4a | 433 | 47±3 |
| Open arm duration (s) | 67±8 | 60±10 | 69±7 | 79±6 |
| Enclosed arm duration (s) | 163±9 | 181±10 | 163±7 | 148±5 |
| Open/total duration (%) | 22±3 | 20±3 | 23±2 | 26±2 |
Effects of vaccine and simvastatin on exploratory activity in C57BL/6J mice. Values are means ± S.E.M.,
P < 0.05, and
P < 0.01 vs other groups.
In the elevated plus-maze test (Table 3), mice chose to explore either safe (enclosed) or anxiogenic (open) arms. On the initial testing day, there was a significant interaction in open arm entries (F(1,35)=10.64, P < 0.01), lower in the group receiving either AdPEDI-(Aβ1-6)11 or simvastatin alone relative to the others. Thus, combination treatment cancelled the anxiogenic effects of each treatment. No intergroup difference was evident for open arm duration, enclosed arm entries and duration, or for any measure on the following day (P > 0.05).
In the coat-hanger test (Table 4), mice treated with simvastatin regardless of adenovirus vaccination took a longer time before reaching the extremity of the horizontal wire (MT-2, F(1,35)=4.33, P < 0.05), with no difference found for any other measure.
Table 4.
| Tests | PBS | simvastatin | Adv | simvastatin + Adv |
|---|---|---|---|---|
| Coat-hanger | ||||
| MT-1 (s) | 51±12 | 64±18 | 28±9 | 64±18 |
| MT-2 (s) | 102±17 | 124±22a | 67±12 | 120±20a |
| 2-paw (s) | 151±17 | 163±21 | 122±14 | 169±16 |
| 3-paw (s) | 183±14 | 202±12a | 187±14 | 220±7a |
| 4-paw (s) | 230±5 | 214±8a | 197±14 | 230±5a |
| Midway (s) | 205±12 | 227±4 | 220±9 | 231±5 |
| Top (s) | 214±9 | 231±3 | 232±5 | 233±5 |
| Fall latencies | 214±9 | 232±3 | 232±5 | 233±5 |
| Rotorod | ||||
| Trial 1 (s) | 118±31 | 162±31 | 60±27 | 116±38 |
| Trials 2 (s) | 181±30 | 184±37 | 143±35 | 176±40 |
| Trial 3 (s) | 227±29 | 250±28a | 172±32 | 270±22a |
| Trial 4 (s) | 229±32 | 261±27 | 196±31 | 264±21 |
Effects of vaccine and simvastatin on motor coordination in C57BL/6J mice. Values are means ± S.E.M., and
P < 0.05 vs other groups.
In the rotorod test (Table 4), mice treated with simvastatin regardless of adenovirus vaccination stayed on the beam longer on the third trial block (F(1,35)=4.59, P < 0.05), indicating an improvement in motor coordination.
There was no intergroup difference for any measure in open-field and stationary beam tests (P > 0.05, data not shown).
It is possible that seronegative mice after nasal vaccination with AdPEDI-(Aβ1-6)11 may behave differently from seropositive mice. Therefore, we omitted seronegative mice from the two groups subjected to AdPEDI-(Aβ1-6)11 vaccination and, then, performed statistical analyses for intergroup differences in all the behavioral tests. The same intergroup differences were found in elevated plus-maze, coat-hanger, and rotorod tests even after deleting seronegative mice (P < 0.05, data not shown). No intergroup difference, however, was found for any measure in T maze. Thus, the improved alteration rate in the entire set of the AdPEDI-(Aβ1-6)11 only group in T maze is not due to the seropositivity (P > 0.05, data not shown). There was no intergroup difference for any measure in open-field and stationary beam tests, also (P > 0.05, data not shown).
3. Discussion
Immunomodulatory effects of statins on vaccination have been shown previously. In agreement with our observations, Lee et al. (2006) reported that a 10-day treatment with atorvastatin (40 mg) increased by a factor of 3 the humoral response of normal healthy volunteers receiving a tetanus toxoid followed by a booster on the fifth day. They suggested the use of statins to enhance humoral response to vaccination. On the contrary, Packard et al. (2007) found no differences in serum titers between an atorvastatin and placebo group of young healthy subjects after hepatitis A vaccination. In the latter study, atorvastatin treatment (40 mg) began on the same day as vaccination. The hepatitis A vaccine is considered to be a stronger antigen than tetanus toxoid, with a seroconversion rate higher than 98%. Therefore, the seroconversion rates cannot be compared between the two groups. Packard et al. (2007) also stated that atorvastatin treatment did not influence antibody responses in BALB/c mice subjected to vaccination of either a combined diphtheria-tetanus-pertussis-polio-Hib-hepatitis B vaccine or diphtheria-hepatitis vaccine. We, however, found that simvastatin treatment increased the seroconversion rate on average at the early treatment stage and the antibody titers at the later stage in C57BL/6 mice immunized with AdPEDI-(Aβ1-6)11. As we reported previously (Kim et al., 2005), when BALB/c and C57BL/6 mice were immunized with the same adenovirus vaccine only, the seroconversion rates were 100% and 57%, respectively. Therefore, statins may be more effective in enhancing immunoresponses to weak antigens and/or in immunocompromised subjects like the elderly.
While 50–60% of mice receiving only AdPEDI-(Aβ1-6)11 developed sizable anti-Aβ antibodies [this study and our previous study (Kim et al., 2005)], simvastatin treatment increased the number of the seropositive mice to 90% after 4 vaccinations (week 7). This finding is highly significant because only 20% of AD patients developed anti-Aβ antibodies in a phase II clinical trial of AN-1792 vaccine presumably due to low immunogenicity of Aβ and/or reduced immune responses in the elderly (Monsonego et al., 2001; Monsonego, 2005). The seroconversion rate of the combination treatment group dropped to 60% after the fifth vaccination (week 13) although the mean serum titer of the seropositive mice in the same group significantly increased. The reasons for the decrease in the seropositive rate at the late treatment stage are not clear. Because lovastatin treatment increases regulatory T (Treg) cells at the inflammation site in C57BL/6 mice (Mira et al., 2008), such an increase in Treg cells may induce immunological tolerance to antigens in susceptible animals (Hasselberg et al., 2009; Sun et al., 2010). In this regard, fine-tuning of the statin dosage as well as its duration is required to optimize beneficial effects of statins for AD immunotherapy: maximizing Th2 responses and minimizing adverse effects as well as immunological tolerance.
Due to meningoencephalitis presumably induced by Th1 responses associated with Aβ vaccination (Check, 2002; Nicoll et al., 2003; Orgogozo et al., 2003), the development of successful therapeutic vaccines against AD is thought to depend on identification of immunization strategies that can induce potent Aβ-specific anti-inflammatory Th2 responses while minimizing Th1 responses. We previously documented that nasal immunization with AdPEDI-(Aβ1-6)11 strongly polarized anti-inflammatory Th2 type response in mice (Kim et al., 2005; Kim et al., 2007a; Kim et al., 2007b). In the current study, regardless of the simvastatin treatment, IgG1 predominated in anti-Aβ antibodies induced by nasal vaccination of AdPEDI-(Aβ1-6)11 and anti-Aβ IgG2a was undetectable. In mice, the production of IgG1 is primarily induced by Th2-type cytokines, while IgG2a is produced through Th1-type cytokines. Thus, our observations are consistent with regard to nasal vaccination of AdPEDI-(Aβ1-6)11. Statins appear to have dual effects on two qualitatively different types of adaptive immune responses: enhancing anti-inflammatory Th2 and inhibiting pro-inflammatory Th1 responses. The mechanism by which statins promote a shift from a Th1-type response toward a Th2-type response was described in the previous studies (Arora et al., 2006; Ho and Glimcher, 2002; Murphy and Reiner, 2002; Robinson and O'Garra, 2002). Statin-treated dendritic cells promote Th2-cell differentiation by inducing expression of GATA-binding protein 3 (GATA3) and inhibit Th1 differentiation by downregulating activation of nuclear factor-κB (NF-κB) and T-bet (a T-box transcription factor) in CD4+ T cells. Furthermore, we have shown that simvastatin suppressed Aβ-induced proliferation of IFN-γ-producing splenocytes. This finding is consistent with the previous reports showing that statins suppress expression of Th1 type cytokines including IFN-γ (Arora et al., 2006; Hakamada-Taguchi et al., 2003). Naïve CD4 T-cells activated in the presence of IL-2 and IFN-γ tend to develop into Th1-cells and IFN-γ inhibits the proliferation of Th2-cells. The inhibition of IFN-γ production and Th1 differentiation by statins might have a significant role in providing a neuro-protective effect during Aβ vaccination because Aβ-induced meningoencephalitis is IFN-γ dependent and is associated with infiltration of Aβ-specific T cells in an animal model of AD (Monsonego et al., 2006). In response to Aβ stimulation, splenocites from mice in both PBS only and AdPEDI-(Aβ1-6)11 only treatment groups produced IFN-γ (Fig. 2). Because stimulation with Aβ is reported to increase production of IFN-γ in endothelial cells (Suo et al., 1998) and co-cultures of microglia and astrocytes (Yamamoto et al., 2007), this induction of IFN-γ in the PBS group does not represent antigen-specific T-cell responses. Nevertheless, our results indicate that statins are effective in inhibition of IFN-γ production by Aβ stimulation regardless of vaccination.
A broad spectrum of adverse effects of vaccines has been reported (Siegrist, 2007). Enhancing immunogenicity of vaccines can increase such adverse reactions (Jacobson et al., 2001). It is essential for successful vaccination to evoke innate immune responses prior to activation of adaptive immune responses. Activated innate and adaptive cells, however, produce pro-inflammatory cytokines acting on the brain through the circulation, altering brain functions and resulting in sickness, including affective, cognitive, and physical symptoms such as anxiety, depression, decreased motivation, impaired memory, and decreases in physical activities (Dantzer et al., 2008). Indeed, Salmonella typhi vaccine was used to model sickness behavior (Brydon et al., 2009; Wright et al., 2005). Therefore, we investigated possible adverse effects of our vaccination modalities on several behavioral tests. The only adverse effect of adenovirus vaccination was an increase in anxiety in the elevated plus-maze, an effect counteracted by simvastatin. In contrast, adenovirus vaccination had no effect on motor coordination and even improved the alternation rate of mice in the T-maze, and this effect was also counteracted by simvastatin. Thus, simvastatin counteracted both adverse and positive actions of vaccine treatment. Simvastatin alone improved motor coordination on the rotorod while slowing down motor speed on the horizontal bar of the coat-hanger. Overall, simvastatin appears to maximize the ability to coordinate movements on a moving beam, though at the expense of retarding movements on a stationary bar. The latter results are consistent with our previous observation that simvastatin treatment normalized hyperactivity of Tg2576 mice, an animal model of AD, in the open field test (Li et al., 2006). Thus, statins may have a calming effect on animals, which needs to be validated in the future.
The molecular mechanisms by which simvastatin offsets these alterations induced by adenovirus vaccination should be further explored. Because statins can reduce expression, production and circulating levels of proinflammatory cytokines such as tumor necrosis factor-α, IL-6 and IL-1β (Rosenson, 2001; Morgan et al., 2009; Youssef et al., 2002; Aprahamian et al., 2006; Aktas et al., 2003; Shimada et al., 2006), statins may ameliorate the negative impact of vaccines.
4. Methods and Materials
4.1. Preparation of AdPEDI-Aβ(1-6)11
AdPEDI-Aβ(1-6)11, was prepared as previously described (Kim et al., 2005).
4.2. Animals and treatments
C57BL/6 mice (6–8 weeks old, female) were obtained from Jackson Laboratories. Mice were randomly assigned to 4 treatment groups in such a manner as there was no significant intergroup difference in body weight. The 4 groups (n=10) were subjected to treatment with PBS, simvastatin, AdPEDI-(Aβ1-6)11 or simvastatin plus AdPEDI-(Aβ1-6)11. The simvastatin treatment groups were fed with a diet admixture containing 0.03% of simvastatin so that each mouse consumed a daily dose of approximately 50 mg simvastatin/kg body weight. Four weeks after the initiation of the simvastatin treatment, mice were nasally vaccinated with AdPEDI-Aβ(1-6)11 once every week for 4 weeks followed by a final booster shot on week 10 (Fig 1). Nasal vaccination was carried out by pipetting 1 × 108 plaque forming unites (PFU) of AdPEDI-(Aβ1-6)11 in 20 µl PBS into one of the nostrils of an anesthetized mouse. Control mice received the same amount of PBS. After the fourth vaccination, mice were subjected to behavioral assessment. Three weeks after the final vaccination, mice were sacrificed and their spleens were collected for isolation of splenocytes. A blood sample was taken through the tail vein at 0, 4, 7, 10 and 13 weeks after the initial vaccination to determine the levels of total cholesterol, anti-Aβ antibodies and the immunoglobulin isotypes. All animal protocols used for this study were prospectively reviewed and approved by the Institutional Animal Care and Use Committees of the University of Illinois College of Medicine at Peoria and the University of Alabama at Birmingham (UAB).
4.3. Determination of total cholesterol level in plasma
Plasma was prepared by centrifuging blood samples at 1500 × g for 10 min at room temperature. The total cholesterol levels in plasma were determined colorimetrically with commercial reagents (Infinity™ cholesterol reagent; Thermo Electron Corporation, Melbourne, Australia).
4.4. Determination of anti-Aβ antibodies in sera
The blood samples were incubated at room temperature for 1 h then transferred to 4°C. After overnight incubation, sera were separated by centrifugation at 10,000 × g for 10 min. Sera were stored at −80°C and thawed at the time of assay. ELISA was carried out to determine the titer of anti-Aβ antibodies and the immunoglobulin isotypes as previously described (Kim et al., 2005). To be brief, 96-well plate was coated with 500 ng synthetic Aβ1-42 peptide per well at 4°C overnight, followed by incubation with blocking buffer (1x PBS containing 0.5% BSA, 0.05% Tween-20 and 5% goat serum) at room temperature for 1 h. Then, diluted serum samples were added to microtiter wells and incubated at 4°C overnight. The next day, microtiter wells were washed 5 times using washing buffer (1x PBS containing 0.05% Tween-20), and then incubated with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h. In the following step, microtiter wells were washed with the washing buffer 5 times and then incubated with 100 µl of 3,3’,5,5’-tetramethylbenzidine (TMB) for 15 min. The reaction was stopped by adding 100 µl of 1 N H2SO4. The optic densities were determined at 450 nm using Microplate Reader. Serial dilutions of 6E10 (monoclonal anti-Aβ antibody) were used as the standard to determine the titer of anti-Aβ antibodies in the sera. Therefore, the concentrations (µg/ml) of the serum titers presented here reflect the concentrations of 6E10 antibody, which produce the same ELISA readings, and may not accurately represent the absolute amounts. Comparison of treatment groups was performed by one-way analysis of variance (ANOVA) and two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
4.5. Detection of IFN-γ-producing cells by ELISPOT
Spleens were individually isolated from mice. Single cell suspension of splenocytes was prepared by homogenizing a spleen tissue in 10 ml of RPMI 1640 medium and forcing cells through a cell strainer with 70 µm pores. Splenocytes were centrifuged at 380 g for 6 min and resuspended with 0.8 ml ACK lysing buffer (UAB Comprehensive Cancer Center) to lyse red blood cells. Cell suspension was centrifuged again at 380 g for 6 min and final cell pellets were suspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 20 µM 2-mercaptoethanol. The number of IFN-γ-producing splenocytes was determined by ELISPOT assay kit (eBioscience). To be brief, a 96-well PVDF membrane ELISPOT plate (Millipore) was coated with anti-mouse IFN-γ capture antibody at 4°C overnight and then treated with the blocking buffer for 2 h at room temperature. After washing with the washing buffer 3 times, 5 × 105 splenocytes were seeded in each well and stimulated by adding 100 µl of the culture medium containing Aβ1-42 at 10 µg/ml. After incubation at 37°C for 24 h, the medium was aspirated and the plate was washed with washing buffer (1x PBS containing 0.05% Tween-20). The detection antibody was then added to the 96-well plate and incubated at room temperature. Two hours later, the plate was washed 3 times and incubated with Streptavidin-HRP for 1 h. The 3-Amino-9-Ethylcarbazole (AEC) substrate solution (Sigma) was added to each well, and allowed to stand for development of the spots. The plate was air-dried in a hood and the spots were scanned by Immunospot Plate Scanning Services (CTL analyzers LLC, Cleveland OH).
4.6. Assessment of behavioral functions
The behavioral alterations potentially associated with vaccination and statin treatment were tested in the methods previously described (Lalonde et al., 2005). For each test day, spontaneous alternation was evaluated first, followed by open-field (days 1 to 3), elevated plus-maze (days 4 and 5), stationary beam (day 6), coat-hanger (day 6), and rotorod (days 7 to 9) tests.
Spontaneous alternation was tested with a T-maze. The maze was made of white acrylic and consisted of a central stem flanked on each side by 2 arms. The maze width was 9 cm, the wall height was 20 cm, and each arm was 30 cm in length. On the initial trial, the mice were placed in the stem with the right arm blocked by a plastic barrier (forced choice). After entering the available arm, the mice were kept in it for 1 min by closing the barrier behind them. The mice were then retrieved and after removing the barrier placed back in the stem for a free-choice trial, either into the same arm or the opposite one (4-paw criterion). On the following 9 days, the same 2-trial procedure was repeated, except that the blocked arm was switched from right on odd days to left on even days. The number of alternations and latencies before responding during the choice trial was measured.
Motor activity was measured in the open-field made of white acrylic with a 50 cm × 50 cm surface area. A mouse was placed in a corner of the open field. The activity in central zone (25 cm × 25 cm surface) and peripheral zone was recorded in a 5-min session for 3 consecutive days and analyzed by video tracking software (SD Instruments, San Diego, CA). The distance travelled and the time spent resting (<2 cm/s), moving slow (2- cm/s), moving fast (>5 cm/s) in each zone were measured, as well as the time spent in the periphery and center of the apparatus.
The elevated plus-maze consisted of 4 arms in a cross-shaped form with a 10 cm × 10 cm central region. Two of the arms were enclosed on 3 sides by walls (10 cm in height) which faced each other while the other 2 were open, except for a minimal border (0.5 cm in height) used to minimize falls. A mouse was placed in the central region and then the number of entries and the time spent in enclosed and open arms were measured in a 5-min session for 2 continuous days. The open/total arm entries and duration ratios were calculated.
The stationary beam (diameter: 2.5 cm; length: 110 cm) was made of plastic covered by white masking tape to facilitate a firm grip. The beam was divided into 11 segments along its length and placed at a 40 cm height from a cushioned floor to prevent injury. A cardboard wall was inserted at each end to prevent escape. A trial began by placing the mice on the middle segment. The number of segments crossed (4-paw criterion), the latencies before falling, and the number of falls were measured in a single 4-trial session, with a 1 min cut-off period and a 15 min intertrial interval.
Motor speed was measured in the coat-hanger test. The triangular-shaped coat-hanger consisted of a horizontal steel wire (diameter: 2 mm, length: 41 cm) flanked at each end by 2 side-bars (length: 19 cm; inclination: 35° from the horizontal axis). The horizontal bar was placed at a 40 cm height from a cushioned floor. The mice were placed upside-down in the middle of the horizontal wire and released only after gripping with all 4 paws. Seven types of movement time (MT) were compiled, namely latencies before reaching (snout criterion) the first 10 cm segment (MT-1) or the extremity (MT-2) of the horizontal wire, latencies before reaching either side-bar with 2, 3 or 4 paws, and latencies before reaching (snout criterion) either the midway or the top of the side-bar. The latencies before falling and the number of falls were also measured. A trial ended when the mice either fell or reached the top of the apparatus. In the latter case, a maximal score of 60 s was given for latencies. This test was performed in a single session of 4 trials. For each trial, there was a 1 min cut-off period and a 15 min intertrial interval.
The accelerating rotorod (Model 7650, Stoelting, Wood Dale, IL, USA) consisted of a beam (diameter: 3 cm) made of ribbed plastic, elevated at a 13.5 cm height, and separated into 5 sections (width: 5.5 cm) by a plastic barrier. Facing away from the experimenter's view, the mice were placed on top of the already revolving rod (4 rpm) in the orientation opposite to its movement, so that falls could be avoided by forward locomotion. The rotorod accelerated gradually and smoothly from 4 to 40 rpm during the 5-min trial. Latencies before falling were measured in 4-trial sessions for 3 days, with a 15 min intertrial interval. Whenever a mouse clung to the rod without moving (passive rotation) for 2 complete revolutions in succession, it was retrieved and a fall registered.
4.7. Statistical Analysis
Student’s t test was used to determine the significant difference in the total plasma cholesterol level and the anti-Aβ antibody titers between the treatment groups. The measurement of IFNγ was analyzed by one-way ANOVA followed by Turkey’s post hoc test. The rotorod test was analyzed by a two-way ANOVA with repeated measurements on the trial factor. For the other tests, intergroup differences were evaluated by unpaired t-tests. In the spontaneous alternation test, groups were each compared with the Mann-Whitney U test to a theoretical group performing at 50% chance. In all cases, P < 0.05 was considered to be statistically significant.
5. Conclusion
The phase II clinical trial of AN-1792 vaccine revealed serious problems associated with this modality: (1) meningoencephalitis presumably caused by T-cell mediated autoimmune responses (Check, 2002; Nicoll et al., 2003; Orgogozo et al., 2003), (2) low immunogenicity of Aβ and reduced immune responses in the elderly (Monsonego et al., 2001; Monsonego, 2005) and (3) cerebral hemorrhages (Ferrer et al., 2004; Pfeifer et al., 2002; Racke et al., 2005; Wilcock et al., 2003). Therefore, safe and effective modalities for AD immunotherapy are yet to be identified. We propose to use statins to circumvent the problems. To test the feasibility in this study, C57BL/6 mice were subjected to combined treatment of AdPEDI-(Aβ1-6)11 vaccine and simvastatin and the results were compared to those from single-agent treatment. Simvastatin treatment appeared to increase the seropositive rate of vaccinated mice in its early stage and boosted the antibody titers by 350% in the late stage. Simvastatin treatment inhibited Aβ-induced proliferation of IFN-γ-producing splenocytes. Furthermore, simvastatin treatment counteracted some behavioral alterations that were associated with AdPEDI-(Aβ1-6)11 vaccination. These results suggest that combined treatment of Aβ-immunotherapy and statin is a viable option to a safer and effective AD immunotherapy.
Acknowledgments
We thank Jamaal A. Rehman for his excellent technical support and Linda Walter for assistance in preparation of this manuscript. This research was supported in part by grants from the National Institutes of Health (AG031846, AG031979, AG029818 and EY018478) and the Alzheimer’s Association (IIRG-07-59494 and NIRG-06-27725).
Abbreviations used
- AD
Alzheimer’s disease
- Aβ
amyloid-β protein
- AEC
3-Amino-9-Ethylcarbazole
- ANOVA
analysis of variance
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immunospot
- GATA3
GATA-binding protein 3
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-CoA
- HRP
horseradish peroxidase
- MT
movement time
- NF-κB
nuclear factor-κB
- PBS
phosphate buffered saline
- PFU
plaque forming unites
- TMB
3,3’,5,5’-tetramethylbenzidine
- Treg
regulatory T
- UAB
University of Alabama at Birmingham
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, Sallach S, Endres M, Brocke S, Nitsch R, Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, Walsh K. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177:3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, Ray P. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, Tsuda A, Steptoe A. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–224. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check E. Nerve inflammation halts trial for Alzheimer's drug. Nature. 2002;415:462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George HP, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyo-oka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hasselberg A, Schon K, Tarkowski A, Lycke N. Role of CTA1R7K-COL-DD as a novel therapeutic mucosal tolerance-inducing vector for treatment of collagen-induced arthritis. Arthritis Rheum. 2009;60:1672–1682. doi: 10.1002/art.24566. [DOI] [PubMed] [Google Scholar]
- Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109(Suppl):S109–S120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jacobson RM, Swan A, Adegbenro A, Ludington SL, Wollan PC, Poland GA. Making vaccines more acceptable--methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine. 2001;19:2418–2427. doi: 10.1016/s0264-410x(00)00466-7. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Kim HD, Jin JJ, Maxwell JA, Fukuchi K. Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer's disease. Immunol Lett. 2007a;112:30–38. doi: 10.1016/j.imlet.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Maxwell JA, Kong FK, Tang DC, Fukuchi K. Induction of anti-inflammatory immune response by an adenovirus vector encoding 11 tandem repeats of Abeta1-6: toward safer and effective vaccines against Alzheimer's disease. Biochem Biophys Res Commun. 2005;336:84–92. doi: 10.1016/j.bbrc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Kim HD, Tahara K, Maxwell JA, Lalonde R, Fukuiwa T, Fujihashi K, Van Kampen KR, Kong FK, Tang DC, Fukuchi KI. Nasal inoculation of an adenovirus vector encoding 11 tandem repeats of Abeta1-6 upregulates IL-10 expression and reduces amyloid load in a Mo/Hu APPswe PS1dE9 mouse model of Alzheimer's disease. J Gene Med. 2007b;9:88–98. doi: 10.1002/jgm.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska A, Pruchnik-Wolinska D, Florczak J, Modestowicz R, Szczech J, Kozubski W, Rossa G, Wender M. Genetic study of familial cases of Alzheimer's disease. Acta Biochim Pol. 2004;51:245–252. [PubMed] [Google Scholar]
- Lalonde R, Kim HD, Maxwell JA, Fukuchi K. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS1/DeltaE9 mice with amyloid plaques. Neurosci Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Lee PY, Scumpia PO, Byars JA, Kelly KM, Zhuang H, Shuster JS, Theriaque DW, Segal MS, Reeves WH, Brantly ML. Short-term atorvastatin treatment enhances specific antibody production following tetanus toxoid vaccination in healthy volunteers. Vaccine. 2006;24:4035–4040. doi: 10.1016/j.vaccine.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Li G, Higdon R, Kukull WA, Peskind E, Van Valen MK, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87:1451–1458. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Mira E, Leon B, Barber DF, Jimenez-Baranda S, Goya I, Almonacid L, Marquez G, Zaballos A, Martinez A, Stein JV, Ardavin C, Manes S. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol. 2008;181:3524–3534. doi: 10.4049/jimmunol.181.5.3524. [DOI] [PubMed] [Google Scholar]
- Monsonego A. Immunogenic aspects of amyloid B-peptide: implications for pathogenesis and treatment of Alzheimer's disease. Proceedings VIII Conference on Alzheimer's Disease and Related Disorders. 2005;423 [Google Scholar]
- Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, Fisher Y, Owens T, Weiner HL. Abeta-induced meningoencephalitis is IFN-gamma-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Zappitelli M, Gill P. Statin prophylaxis and inflammatory mediators following cardiopulmonary bypass: a systematic review. Crit Care. 2009;13:R165. doi: 10.1186/cc8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Packard RR, Schlegel S, Senouf D, Burger F, Sigaud P, Perneger T, Siegrist CA, Mach F. Atorvastatin treatment and vaccination efficacy. J Clin Pharmacol. 2007;47:1022–1027. doi: 10.1177/0091270007302169. [DOI] [PubMed] [Google Scholar]
- Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T, Duff K, Pappolla M, Refolo LM. Statin therapy for Alzheimer's disease: will it work? J Mol Neurosci. 2002;19:155–161. doi: 10.1007/s12031-002-0026-2. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS, O'Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–758. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Pluripotential mechanisms of cardioprotection with HMG-CoA reductase inhibitor therapy. Am J Cardiovasc Drugs. 2001;1:411–420. doi: 10.2165/00129784-200101060-00001. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schenk DB, Yednock T. The role of microglia in Alzheimer's disease: friend or foe? Neurobiol Aging. 2002;23:677–679. doi: 10.1016/s0197-4580(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shimada K, Park JK, Daida H. T helper 1/T helper 2 balance and HMG-CoA reductase inhibitors in acute coronary syndrome: statins as immunomodulatory agents? Eur Heart J. 2006;27:2916–2918. doi: 10.1093/eurheartj/ehl376. [DOI] [PubMed] [Google Scholar]
- Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007;137 Suppl 1:S46–S50. doi: 10.1016/j.jcpa.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sun JB, Czerkinsky C, Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand J Immunol. 2010;71:1–11. doi: 10.1111/j.1365-3083.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M. Alzheimer's beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- Town T, Tan J, Sansone N, Obregon D, Klein T, Mullan M. Characterization of murine immunoglobulin G antibodies against human amyloid-beta1-42. Neurosci Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420:879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Zamrini E, McGwin G, Roseman JM. Association between statin use and Alzheimer's disease. Neuroepidemiology. 2004;23:94–98. doi: 10.1159/000073981. [DOI] [PubMed] [Google Scholar]