Abstract
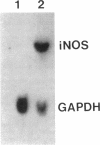
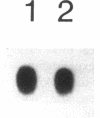
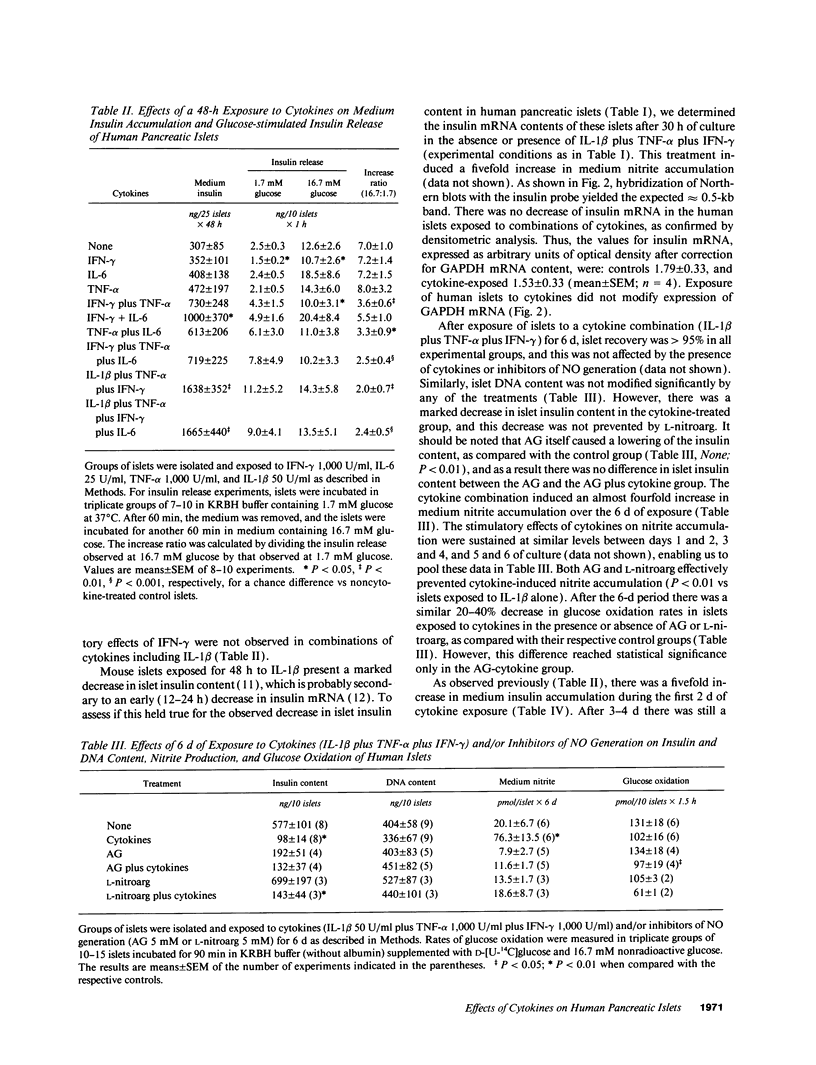
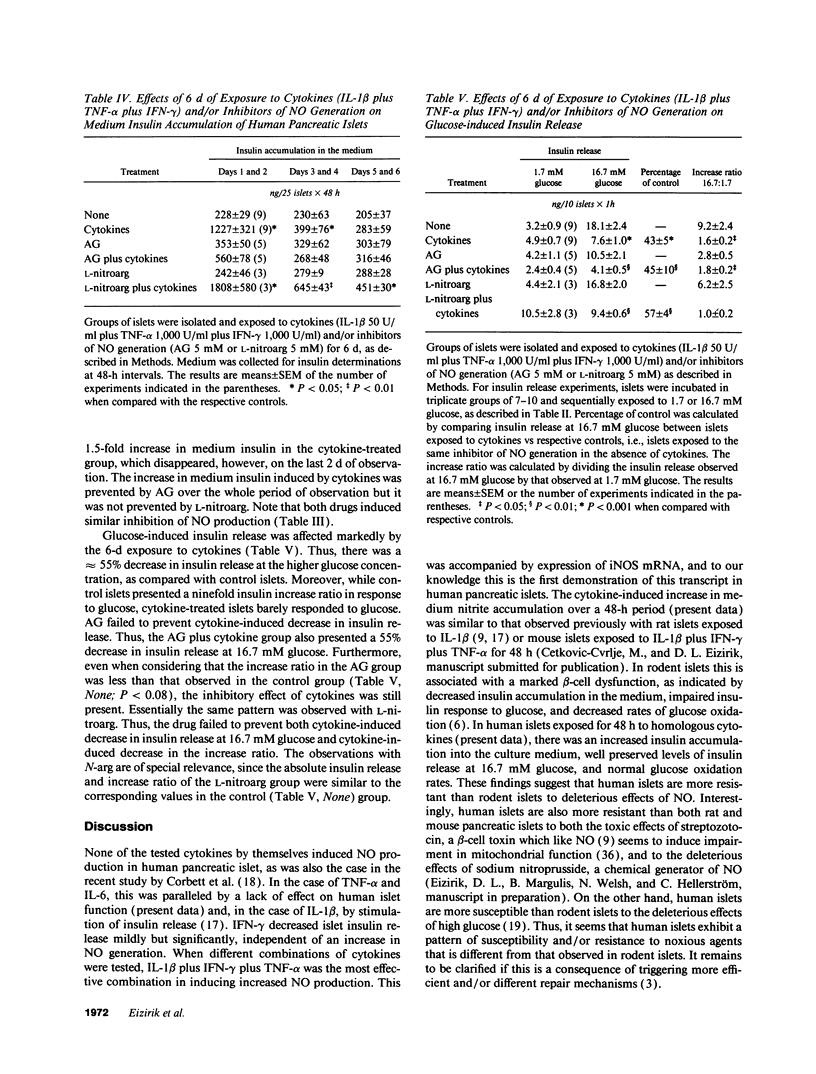
Cytokines have been proposed as inducers of beta-cell damage in human insulin-dependent diabetes mellitus via the generation of nitric oxide (NO). This concept is mostly based on data obtained in rodent pancreatic islets using heterologous cytokine preparations. The present study examined whether exposure of human pancreatic islets to different cytokines induces NO and impairs beta-cell function. Islets from 30 human pancreata were exposed for 6-144 h to the following human recombinant cytokines, alone or in combination: IFN-gamma (1,000 U/ml), TNF-alpha (1,000 U/ml), IL-6 (25 U/ml), and IL-1 beta (50 U/ml). After 48 h, none of the cytokines alone increased islet nitrite production, but IFN-gamma induced a 20% decrease in glucose-induced insulin release. Combinations of cytokines, notably IL-1 beta plus IFN-gamma plus TNF-alpha, induced increased expression of inducible NO synthase mRNA after 6 h and resulted in a fivefold increase in medium nitrite accumulation after 48 h. These cytokines did not impair glucose metabolism or insulin release in response to 16.7 mM glucose, but there was an 80% decrease in islet insulin content. An exposure of 144 h to IL-1 beta plus IFN-gamma plus TNF-alpha increased NO production and decreased both glucose-induced insulin release and insulin content. Inhibitors of NO generation, aminoguanidine or NG-nitro-L-arginine, blocked this cytokine-induced NO generation, but did not prevent the suppressive effect of IL-1 beta plus IFN-gamma plus TNF-alpha on insulin release and content. In conclusion, isolated human islets are more resistant to the suppressive effects of cytokines and NO than isolated rodent islets. Moreover, the present study suggests that NO is not the major mediator of cytokine effects on human islets.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Andersson A., Sandler S. Viability tests of cryopreserved endocrine pancreatic cells. Cryobiology. 1983 Apr;20(2):161–168. doi: 10.1016/0011-2240(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Bendtzen K. Immune hormones (cytokines); pathogenic role in autoimmune rheumatic and endocrine diseases. Autoimmunity. 1989;2(2):177–189. doi: 10.3109/08916938909019954. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Morling N., Fomsgaard A., Svenson M., Jakobsen B., Odum N., Svejgaard A. Association between HLA-DR2 and production of tumour necrosis factor alpha and interleukin 1 by mononuclear cells activated by lipopolysaccharide. Scand J Immunol. 1988 Nov;28(5):599–606. doi: 10.1111/j.1365-3083.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Borg L. A., Eizirik D. L. Short-term exposure of rat pancreatic islets to human interleukin-1 beta increases cellular uptake of calcium. Immunol Lett. 1990 Dec;26(3):253–258. doi: 10.1016/0165-2478(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992 Aug;41(8):897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Björklund A., Cagliero E. Genotoxic agents increase expression of growth arrest and DNA damage--inducible genes gadd 153 and gadd 45 in rat pancreatic islets. Diabetes. 1993 May;42(5):738–745. doi: 10.2337/diab.42.5.738. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Björklund A., Welsh N. Interleukin-1-induced expression of nitric oxide synthase in insulin-producing cells is preceded by c-fos induction and depends on gene transcription and protein synthesis. FEBS Lett. 1993 Feb 8;317(1-2):62–66. doi: 10.1016/0014-5793(93)81492-i. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L. Interleukin-1 beta induces an early decrease in insulin release, (pro)insulin biosynthesis and insulin mRNA in mouse pancreatic islets by a mechanism dependent on gene transcription and protein synthesis. Autoimmunity. 1991;10(2):107–113. doi: 10.3109/08916939109004814. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Korbutt G. S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992 Oct;90(4):1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Palmer J. P. Repair of pancreatic beta-cells. A relevant phenomenon in early IDDM? Diabetes. 1993 Oct;42(10):1383–1391. doi: 10.2337/diab.42.10.1383. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Sener A., Malaisse W. J. Defective catabolism of D-glucose and L-glutamine in mouse pancreatic islets maintained in culture after streptozotocin exposure. Endocrinology. 1988 Aug;123(2):1001–1007. doi: 10.1210/endo-123-2-1001. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Welsh M., Strandell E., Welsh N., Sandler S. Interleukin-1 beta depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology. 1990 Nov;127(5):2290–2297. doi: 10.1210/endo-127-5-2290. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Welsh N., Hellerström C. Predominance of stimulatory effects of interleukin-1 beta on isolated human pancreatic islets. J Clin Endocrinol Metab. 1993 Feb;76(2):399–403. doi: 10.1210/jcem.76.2.8432782. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Worsaae H., Bendtzen K. Detection of tumour necrosis factor from lipopolysaccharide-stimulated human mononuclear cells by enzyme-linked immunosorbent assay and cytotoxicity bioassay. Scand J Immunol. 1988 Feb;27(2):143–147. doi: 10.1111/j.1365-3083.1988.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Kawahara D. J., Kenney J. S. Species differences in human and rat islet sensitivity to human cytokines. Monoclonal anti-interleukin-1 (IL-1) influences on direct and indirect IL-1-mediated islet effects. Cytokine. 1991 Mar;3(2):117–124. doi: 10.1016/1043-4666(91)90031-8. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Type 1 (insulin-dependent) diabetes mellitus and nitric oxide. Diabetologia. 1992 Aug;35(8):796–797. doi: 10.1007/BF00429103. [DOI] [PubMed] [Google Scholar]
- Ling Z., In't Veld P. A., Pipeleers D. G. Interaction of interleukin-1 with islet beta-cells. Distinction between indirect, aspecific cytotoxicity and direct, specific functional suppression. Diabetes. 1993 Jan;42(1):56–65. doi: 10.2337/diab.42.1.56. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Helqvist S., Wogensen L. D., Mølvig J., Pociot F., Johannesen J., Nerup J. Cytokine and free radicals as effector molecules in the destruction of pancreatic beta cells. Curr Top Microbiol Immunol. 1990;164:169–193. doi: 10.1007/978-3-642-75741-9_9. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Suarez W. L., Thomas P. D., Strynadka K., Simpson I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992 May;35(5):409–413. doi: 10.1007/BF02342435. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Sumoski W., Rajotte R. V., Warnock G. L. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990 Jul;71(1):152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- Sandler S., Bendtzen K., Eizirik D. L., Welsh M. Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro. Endocrinology. 1990 Feb;126(2):1288–1294. doi: 10.1210/endo-126-2-1288. [DOI] [PubMed] [Google Scholar]
- Sandler S., Eizirik D. L., Svensson C., Strandell E., Welsh M., Welsh N. Biochemical and molecular actions of interleukin-1 on pancreatic beta-cells. Autoimmunity. 1991;10(3):241–253. doi: 10.3109/08916939109001895. [DOI] [PubMed] [Google Scholar]
- Soldevila G., Buscema M., Doshi M., James R. F., Bottazzo G. F., Pujol-Borrell R. Cytotoxic effect of IFN-gamma plus TNF-alpha on human islet cells. J Autoimmun. 1991 Apr;4(2):291–306. doi: 10.1016/0896-8411(91)90025-8. [DOI] [PubMed] [Google Scholar]
- Southern C., Schulster D., Green I. C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990 Dec 10;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- Sumoski W., Baquerizo H., Rabinovitch A. Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia. 1989 Nov;32(11):792–796. doi: 10.1007/BF00264909. [DOI] [PubMed] [Google Scholar]
- Svenson M., Bendtzen K. Inhibitor of interleukin 1 in normal human urine. Different effects on mouse thymocytes and on a murine T-cell line. Scand J Immunol. 1988 May;27(5):593–599. doi: 10.1111/j.1365-3083.1988.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Welsh N., Eizirik D. L., Bendtzen K., Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991 Dec;129(6):3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]
- Welsh N., Sandler S. Interleukin-1 beta induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochem Biophys Res Commun. 1992 Jan 15;182(1):333–340. doi: 10.1016/s0006-291x(05)80149-4. [DOI] [PubMed] [Google Scholar]