Abstract
Background
We prospectively assessed the incidence, risk factors, and costs associated with wound complications and lymphedema in melanoma patients undergoing inguinal lymph node dissection (ILND).
Materials and Methods
A total of 53 melanoma patients were accrued to 2 trials (June 2005 to July 2008) that included prospective evaluations of postoperative complications; 30-day wound complications included infection, seroma, and/or dehiscence. There were 20 patients who underwent limb volume measurement and completed a 19-item lymphedema symptom assessment questionnaire preoperatively and 3 months postoperatively. A multivariate analysis was performed to evaluate potential risk factors for complications. A microcosting analysis was also performed to evaluate the direct costs associated with wound complications.
Results
The 30-day wound complications were noted in 77.4% of patients. A BMI ≥ 30 (n = 28) increased the risk for wound complications (odds ratio [OR] = 11.4, 95% confidence interval [95%CI] 1.6–78.5, P = .01), while advanced nodal disease approached significance (OR = 9.0, 95%CI: 0.79–103.1, P = .08). Other risk factors, including diabetes, smoking, and the addition of a deep pelvic (iliac/obturator) dissection to ILND, were not significant. Of 20 patients, 9 (45%) developed limb volume change (LVC) ≥5% at 3 months, with associated mean symptom scores of 6.1 versus 4.6 for those without LVC. Costs for patients with wound complications were significantly higher than for those without wound complications.
Conclusions
Postoperative wound complications and early onset lymphedema occur frequently following ILND for melanoma. Obesity is an adverse risk factor for 30-day wound complications that can significantly increase postoperative costs, as is likely the case for advanced disease. Risk reduction practices and novel treatment approaches are needed to reduce postoperative morbidity.
Therapeutic lymphadenectomy is the standard treatment for patients with node-positive melanoma and has been shown to improve outcomes in some patients.1–4 However, inguinal lymph node dissection (ILND) has been associated with significant postoperative morbidity including infections, skin flap complications, and lower extremity lymphedema and often leads to extended length of hospitalization, reduced quality of life, and delayed return to normal activities.5–9 The need for additional surgical interventions in subsets of patients involving reconstruction or grafting to treat wound dehiscence and skin necrosis following ILND has also been described.10–13 Some studies have noted the incidence of short-term (30-day) and long-term (beyond 30-day) morbidity to be as high as 75%.8,9,14,15
Previous studies on ILND have primarily been retrospective in design and have reported various risk factors for postoperative complications, including medical comorbidities, pre-existing surgical incisions, obesity, and locally advanced disease.13,16–21 Bouchot et al. found that medical comorbidity defined by the American Society of Anesthesiologists (ASA) as grade III or greater was a predictive factor for wound complications.19 Shaw et al. found that previous surgical incisions from open lymph node biopsies prior to ILND were associated with 2 to 3 times the incidence of wound infections (33% vs 13%) and lymphedema (24% vs 13%) when compared with preoperative lymph node fine-needle aspiration (FNA).18 Sabel et al. found that obesity was a significant risk factor for wound complications (odds ratio [OR] = 1.11, 95% confidence interval [95% CI] 1.05–1.17, P = .0004), and that patients undergoing ILND for clinically palpable disease had more complications than those undergoing ILND for a positive sentinel node (OR = 2.28, 95% CI 1.07–4.88, P = 0.03).20 Likewise, Serpell et al. found that advanced disease was a significant risk factor, as the median size of largest involved node was associated with wound complications with an OR of 1.17 (P = .05), and when Rouzier et al. compared the incidence of postoperative cellulitis following ILND in obese and nonobese patients, the difference was significant (32.1% vs. 21.1%, P = .02).13,17
In this study, we prospectively examined the incidence and risk factors associated with postoperative morbidity following ILND. Specifically, the incidence of 30-day complications defined as infection requiring antibiotics, clinically apparent seroma, and/or poor wound healing (dehiscence) were examined along with the 90-day incidence of lymphedema and associated symptoms. A microcosting analysis was also performed to assess the direct costs associated with these complications.
METHODS
A total of 53 melanoma patients undergoing ILND were accrued to 2 clinical trials approved by the institutional review board at a single, tertiary cancer center. The first trial, completed in 2007, was designed to assess the effects of fibrin glue administration on the amount and duration of postoperative drain output following ILND.22 The primary objective of the second trial, which is ongoing, is to examine limb volume change (lymphedema) and quality of life after sentinel lymph node biopsy or lymph node dissection in patients with melanoma. Both trials included a prospective assessment of postoperative wound complications as well as demographic, clinical, treatment, and follow-up data. All patients underwent ILND, while some underwent a deep dissection that includes the iliac and obturator nodes. In concordance with institutional standards, all patients received preoperative prophylactic antibiotics. Jackson-Pratt drains were used in the wound cavity and remained in place until drain output was <30 ml per day for 48 hours averaging 3–4 weeks.
Wound Complications
Wound complications were defined in this study as wound infections, clinically relevant seromas, or wound dehiscences occurring within the 30 days of ILND. Each complication was further characterized as major or minor according to the extent of wound-related therapy required. Minor infections, as determined by the primary surgeon, were treated with oral antibiotics on an outpatient basis. Major infections required hospitalization and the administration of intravenous antibiotics or an intervention such as incision and drainage. Any seroma that was apparent on physical examination following routine postoperative drain removal was noted. Minor seromas were defined as those observed or managed by aspiration in the clinic setting. Major seromas were defined as those treated with either percutaneous drain placement or surgical incision and drainage. Wound dehiscence was defined as poor wound healing with a measured defect of at least 1 cm. Minor wound dehiscences were defined as those treated with local wound care consisting of packing or a hydrating dermal wound dressing gel. Major wound dehiscences were defined as those treated with a vacuum-assisted closure device. All wound dehiscences in this series healed secondarily, and none required skin grafting.
Lymphedema
Lymphedema was assessed qualitatively in 47 patients during routine follow-up clinic visits at approximately 3 months following surgery. Lymphedema was classified as none, mild, moderate, or severe according to criteria proposed by the NCI Common Toxicity Criteria (CTC) version 2.0.23 Postoperative qualitative assessments were unavailable for 6 patients who had postoperative follow-up outside of our institution. Quantitative measures of limb volume were performed in 20 patients using a perometer, which is an optoelectronic volumetric device (Juzo 1000M, Cuyahoga Falls, OH). For these patients, limb volume change for the affected limb was calculated as: volume percent (%) change = (postoperative limb volume – pre-operative limb volume)/preoperative limb volume. In patients who underwent bilateral ILND (n = 3), the limb with the greatest volume change was reported. In addition, a 19-item lymphedema symptom assessment questionnaire that was modified from a validated Lymphedema Breast Cancer Questionnaire (LBCQ) was administered preoperatively and at the time of limb volume assessment.24,25
Costs
A microcosting analysis was performed for all of the patients in the study (n = 53) using institutional financial reports. The analysis included 30-day postoperative direct costs; professional and nonprofessional postoperative costs, excluding costs for the initial surgical procedure (i.e., operating room, pathology, and the postanesthesia care unit), were calculated. Inpatient costs included room/board, pharmacy, and other services (e.g., laboratory, imaging, diagnostic studies, physical therapy, occupational therapy, respiratory therapy, equipment, and supplies). Outpatient costs included clinic visits, emergency room visits, materials, and procedures for wound-related complications. Outpatient costs incurred for clinic visits, imaging, and procedures not related to ILND or wound complications were excluded (e.g., routine staging imaging and laboratory costs).
Analysis
Patients were stratified into 3 groups based on complications (major, minor, and no complications), and outcomes were compared. A univariate risk analysis was carried out for all clinical, treatment, and pathologic factors for each of these groups to identify factors to be included in the multivariate model. Logistic regression analysis was performed to determine the significant risk factors associated with postoperative wound complications. Median costs were calculated for patients with and without wound complications, and the Wilcoxon rank sum test was used to assess cost differences between the 2 groups. All statistical analyses were performed using STATA/SE 9.2 for Windows (StataCorp LP, College Station, TX).
RESULTS
The clinical and pathologic characteristics of the cohort are summarized in Table 1. The majority of patients (n = 28, 52.8%) had a BMI ≥30 kg/m2. Regarding surgical treatment, 30 (56.6%) patients had a deep iliac/obturator lymph node dissection in addition to an ILND (Table 2). Most patients (n = 38, 71.7%) underwent a sartorius flap transposition, and the saphenous vein was spared in 16 patients (30.2%). The median number of lymph nodes removed was 17; the median number of positive lymph nodes was 1; 22.6% of patients had ≥4 positive lymph nodes. There were 29 patients (54.7%) who were determined to have pathologically confirmed extra-capsular nodal extension upon pathologic examination of their nodal specimens.
TABLE 1.
Clinical and pathologic characteristics of study cohort (n = 53)
| n = 53 | % | |
|---|---|---|
| Median age, years (range) | 55.6 (18–91) | |
| Sex | ||
| Male | 31 | 58.5 |
| Female | 22 | 41.5 |
| Median BMI, kg/m2 (range) | 30.2 (19.8–48.6) | |
| BMI, kg/m2 | ||
| ≥30 | 28 | 52.8 |
| <30 | 25 | 47.2 |
| Comorbiditiesa | ||
| Current tobacco use | 8 | 15.1 |
| Diabetes | 6 | 11.3 |
| Primary tumor (T) categories | ||
| T1 | 4 | 7.5 |
| T2 | 9 | 17.0 |
| T3 | 15 | 28.3 |
| T4 | 12 | 22.7 |
| Primary uncharacterized/unknown | 13 | 24.5 |
| AJCC stage | ||
| III A | 9 | 17.0 |
| III B | 13 | 24.5 |
| III C | 17 | 32.1 |
| Unknown primary | 13 | 24.5 |
| IV | 1 | 1.9 |
BMI body mass index, AJCC American Joint Committee on Cancer
Patients may belong to more than 1 category
TABLE 2.
Surgical treatment and postoperative complications
| n = 53 | % | |
|---|---|---|
| Extent of surgery | ||
| ILND alone | 23 | 43.4 |
| ILND and deep iliac/obturator node dissection | 30 | 56.6 |
| Surgical techniquea | ||
| Sartorius flap transposition | 38 | 71.7 |
| Saphenous vein spared | 16 | 30.2 |
| Lymph nodes harvested | ||
| Median number of lymph nodes removed (range) | 17 (5–56) | |
| Median number of positive nodes (range) | 1 (1–21) | |
| Patients with ≥4 positive lymph nodes | 12 | 22.6 |
| Patients with extra-nodal tumor extension | 29 | 54.7 |
| Patients with complications | 41 | 77.4 |
| Complications treated in the operating room | 2 | 3.8 |
| Complications treated in interventional radiology | 2 | 3.8 |
| Classification of complicationa | ||
| Wound infection | 29 | 54.7 |
| Major | 18 | 34.0 |
| Minor | 11 | 20.8 |
| Wound dehiscence | 28 | 52.8 |
| Major | 7 | 13.2 |
| Minor | 21 | 39.6 |
| Seroma | 15 | 28.3 |
| Major | 6 | 11.3 |
| Minor | 9 | 17.0 |
ILND inguinal lymph node dissection
Patients can belong to more than 1ne category; percentage standardized by sample size
The overall incidence of 30-day wound complications (major and minor) was 77.4%. Of these, wound infection was the most common complication (n = 29, 54.7%) with nearly two-thirds of infections requiring intravenous antibiotics or less commonly an invasive intervention. Two patients required a second operation for the management of their wound infection. Wound dehiscence was the second most common complication (n = 28, 52.8%), but most of these were minor and required only dressing changes. Seromas occurred in 28.3% of the cohort, with over a third of seromas leading to infections requiring either open incision and drainage or percutaneous drain placement. A univariate analysis assessing the odds of developing a complication was performed for age, comorbidity (e.g., tobacco use, diabetes), surgical procedure, number of positive nodes, and body mass index (BMI), and of these, BMI was the only statistically significant factor (OR = 4.7, 95% CI = 1.1–20.0, P = .04). For the multivariate analysis, the most significant prognostic factor was BMI (OR = 11.35, 95% CI: 1.6–78.5, P = .01), while disease stage approached significance (OR = 9.0, 95% CI: 0.8–103.1, P = .08) (Table 3).
TABLE 3.
Multivariate analysis of risk factors associated with postoperative wound complications
| ORa | 95% CI | P | ||
|---|---|---|---|---|
| BMI (kg/m2) | ≥30 vs <30 | 11.4 | 1.6–78.5 | .01 |
| Disease stage | IIIB, IIIC, MUP, IV, vs IIIA | 9.0 | 0.8–103.1 | .08 |
| Addition of iliac/obturator LND | Yes vs no | 0.5 | 0.1–2.8 | NS |
BMI body mass index, CI confidence interval, P probability value, MUP melanoma of unknown primary, LND lymph node dissection
Model adjusted for comorbidities (e.g., current tobacco use, diabetes)
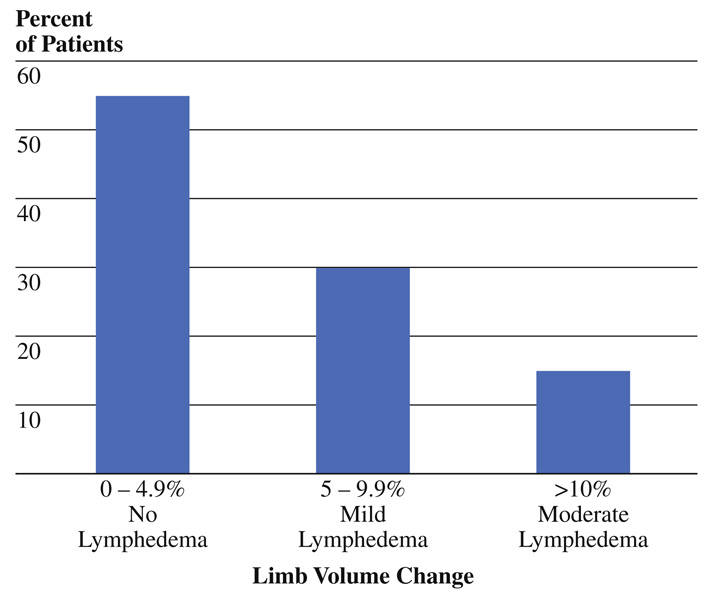
Qualitative clinical assessment of lymphedema identified swelling in 40 of 47 patients (85%) assessable at 3 months after surgery. The majority of patients (53%) with observable limb volume change were classified as having mild swelling, while 26% had moderate swelling, and 6% had severe swelling. Quantitative assessment of limb volume using the perometer was performed preoperatively and at the 3-month follow-up visit in a total of 20 patients. Limb volume change was noted in 9 patients (45%) with 6 suggestive of mild lymphedema (5–9.9% volume change) and 3 suggestive of moderate lymphedema (≥10% volume change) (Fig. 1).
FIG. 1.
Postoperative quantitative limb volume change at 3 months measured by perometer (n = 20)
Symptom Assessment
Of the 20 patients with both quantitative and qualitative measures available, 14 had symptom assessment measures at the 3-month follow-up visit. When stratifying by limb volume change assessed quantitatively (i.e., perometry), patients with <5% limb volume change reported a mean symptom score of 4.6, while those with limb volume change greater than or equal to 5% reported a mean symptom score of 6.1. When stratifying patients by limb volume change assessed qualitatively, a few more patients reported mild to severe limb swelling. Four patients with no significant limb volume change reported a mean symptom score of 3.75, while the 10 patients with mild to severe swelling reported a mean symptom score of 6.00. A significantly greater proportion of symptoms were reported for patients with self-reported limb swelling (P = .047).
Cost Analysis
The unadjusted postoperative costs for patients with wound complications were compared with those without wound complications by cost categories to ascertain the types of services that contributed most to the observed difference(s). Table 4 shows the difference in total medical costs within a 30-day follow-up for patients with complications compared with those without complications. The median total cost for patients with complications was $8025, with a wide range of $3976 to $56,708. The median total cost for patients without complications was $5439, with a range of $4338 to $14,648. The difference in median direct costs for patients with complications and those without complications was $2586 (P =.02). Differences were primarily related to nonprofessional costs ($7969 vs $5400, P =.03), with hospital costs (room/board) as the largest contributor ($5955 vs $ 4207, P =.04). Pharmacy costs were also significantly higher (median cost per patient with complications $543 vs $320 per patient without complications, P = .03). Finally, nonprofessional outpatient costs were higher in the group with wound complications (median cost per patient $357 vs $244, P = .01).
TABLE 4.
Comparison of direct postoperative costs for patients with complications (n = 41) versus those without complications (n = 12)
| Complications (n = 41) | Without complications (n = 12) | P value | |||
|---|---|---|---|---|---|
| Median costs (US dollars, $) per patient |
Range | Median costs (US dollars, $) per patient |
Range | ||
| Nonprofessional expenses | 7,969 | 3,976–54,519 | 5,400 | 4,339–14,447 | .03 |
| Inpatient | 7,610 | 3,809–53,866 | 5,218 | 4,060–14,162 | .05 |
| Room and board | 5,955 | 3,315–34,670 | 4,207 | 3,315–10,124 | .04 |
| Pharmacy | 543 | 65–4,952 | 320 | 35–1,534 | .03 |
| Other | 1,346 | 55–23,496 | 743 | 239–2,503 | NS |
| Outpatient | 357 | 0–5,556 | 244 | 0–470 | .01 |
| Clinic visits | 279 | 0–1,396 | 233 | 0–279 | .03 |
| Clinical procedures | 0 | 0–2,981 | – | – | – |
| Other | 85 | 0–4,666 | 0 | 0–237 | .01 |
| Professional expenses | 11 | 0–2,832 | 0 | 0–200 | .08 |
| Inpatient | 0 | 0–2,832 | 0 | 0–200 | NS |
| Outpatient | 0 | 0–736 | – | – | – |
| All expenses | 8,025 | 3,976–56,708 | 5,439 | 4,338–14,648 | .02 |
DISCUSSION
In this prospective study of melanoma patients undergoing ILND, 77% of patients had postoperative complications; 55% developed infections (major or minor), and 53% had some degree of wound dehiscence. Obesity (BMI ≥ 30 kg/m2) increased the risk of wound complications by more than 11-fold, and patients with advanced nodal disease were also at increased risk of developing postoperative complications, although this did not reach statistical significance. Of note is that the addition of a deep iliac/obturator lymph node dissection to ILND did not result in increased wound complications. Early posttreatment lymphedema was common, with 85% of patients identified with mild to moderate limb swelling using qualitative assessment by 3 months after surgery. Quantitative assessment of limb volume change using a perometer in a subset of patients (n = 20) revealed that 45% of patients had a greater than 5% change in limb volume compared with preoperative baseline measures and reported 35% more symptoms on average. Finally, 30-day resource utilization as measured by direct costs associated with the care of patients with wound complications ranged from $3976 to $56,708 and on average were 1.5 times greater than the costs for patients with an uncomplicated postoperative course.
The overall incidence of postoperative complications following ILND in this study is higher than that reported in the literature (1990–2008) for patients with melanoma, genitourinary malignancies, or gynecologic malignancies (Table 5). This discrepancy can be attributed to several factors. Most importantly, the majority of published reports identified postoperative complications retrospectively. The two studies that examined complications prospectively reported an incidence of wound complication similar to those in this report.17,21 Coit et al. reported complications in 64% of patients following inguinal lymph node dissection, and the authors acknowledged that the overall incidence of complications may have been even higher, since postdischarge wound complications were not included in their analysis.21 Serpell et al. also prospectively examined wound complications and reported an incidence of 71% following ILND.17 The discrepancies in the incidence of reported complications in the majority of the remaining literature likely reflect the limitations of capturing complication data retrospectively.
TABLE 5.
Published literature (1990–2009) reporting postoperative complications following ILND
| Author, Year | No. patients |
Study design | Malignancy | Infection (%) |
Seroma (%) |
Dehiscence/ necrosis (%) |
Lymphedema (%) |
|---|---|---|---|---|---|---|---|
| Shaw, 199018 | 58 | Retrospective | Melanoma | 25 | 12 | 10 | 23 |
| Coit, 199121 | 42 | Prospective | Melanoma | 19 (minor) 45 (major) | - | - | - |
| Beitsch, 19925 | 168 | Retrospective | Melanoma | 29 | 14 | 26 | 44 |
| Lin, 199257 | 76 | Retrospective | Vulvar carcinoma | 24 | 11 | - | 14 |
| Ravi, 199338 | 375 | Retrospective | Penile carcinoma | 18 | 5 | 61 | 25 |
| Karakousis, 199439 | 205 | Retrospective | Melanoma | 16 | 5 | 8 | 40 |
| Kulkarni, 199458 | 27 | Retrospective | Penile carcinoma | - | - | 26 | 77 |
| Bell, 200059 | 60 | Retrospective | Vulvar carcinoma | 8 | 12 | 10 | 14 |
| Hughes, 20003 | 132 | Retrospective | Melanoma | 15 | 2 | 7 | 36 |
| Gould, 200143 | 67 | Retrospective | Vulvar carcinoma | 35 | 13 | 19 | 5 |
| Bevan-Thomas, 200216 | 53 | Retrospective | Penile carcinoma | 10 | - | 8 | 23 |
| Gaarenstroom, 200326 | 101 | Retrospective | Vulvar carcinoma | 39 | 40 | 17 | 28 |
| Rouzier, 200313 | 206 | Retrospective | Vulvar carcinoma | 30 | - | 39 | 47 |
| Serpell, 200317 | 28 | Prospective | Melanoma | 25 | 46 | 25 | 29 |
| Tonouchi, 20039 | 20 | Retrospective | Mixed malignancies | 24 | 32 | 52 | 40 |
| Bouchot, 200419 | 58 | Retrospective | Penile carcinoma | 7 | 14 | 9 | 22 |
| deVries, 20068 | 14 | Retrospective | Melanoma | 29 | 7 | 7 | 64 |
| Sabel, 200720 | 212 | Retrospective | Melanoma | 19 | - | - | 27 |
| Guggenheim, 200832 | 43 | Retrospective | Melanoma | 33 | 42 | 7 | - |
| Current study, 2009 | 53 | Prospective | Melanoma | 55 | 28 | 53 | 45 |
The inclusive definitions for complications used in this prospective study clearly contribute to the high reported incidence. For example, patients with wound erythema are often treated with antibiotics and in this study were categorized as having had a wound infection, regardless of wound cultures or systemic signs of infection. Similarly, patients presenting with wound breakdown of ≥1 cm were included as having wound dehiscence; in contrast, other studies have used less-inclusive criteria, defining dehiscence as breakdown of ≥25–30% of the length of the wound.13,26 A third explanation for the high incidence of complications observed in our study is that the majority of our patients were obese. It is well established that obesity significantly increases the risk of wound complications.5,13,19–21,27 Most of the retrospective studies examining complications following ILND did not specify the number of obese patients. Given the modern epidemic of obesity in the general population, it is possible that contemporary patients are more obese than those who underwent ILND in the 1970s and 1980s.28,29 Also, studies conducted in Japan or Europe likely comprise patients with lower BMI than American patients; for example, the mean BMI for Japanese patients included in the study by Tonouchi et al. was 22.5 kg/m2, in contrast to the median BMI of 30.8 kg/m2 in the current study.9
Indications for ILND have changed over time, with possible implications resulting from an increased number of surgical interventions performed and also for the burden of disease. Historically, ILND would have been the first operation performed in the inguinal region, either for patients with a high-risk primary melanoma meeting the indications for an elective lymph node dissection (ELND), or for patients with clinically palpable disease undergoing a therapeutic lymph node dissection (TLND).30 Current practice standards recommend sentinel lymph node biopsy for patients with intermediate risk primary melanomas or needle/excisional biopsy for patients with clinically detectable nodal disease followed by selective ILND; for patients with positive nodes, this results in 2 operations.31 Multiple surgical interventions in the same anatomic region over a period of weeks, particularly with complicated healing of the initial wound, potentially contribute to an increased risk of subsequent wound complications.8,18
The incidence of postoperative complications following ILND is higher than for nodal dissections in other anatomic regions and is also higher than that expected for a typical “clean” operation (1–5%).17,18,32,33 Explanations that have been proposed for the increased incidence of wound complications associated with ILND include greater lymphatic flow in the inguinal nodes compared with axillary or cervical nodes, greater surface area associated with the dissection, relatively poor vascular supply to the skin and subcutaneous tissues in the region, surgical technique related to the relatively thin skin flaps routinely employed in ILND, density and pathogenicity of the flora of the inguinal region, and difficulty maintaining hygiene in this region, particularly in obese patients.17,18,21,34–37
Concerns have been raised about the potential for increased morbidity in patients undergoing an associated deep (iliac/obturator) lymph node dissection.1,3 In this analysis, the addition of iliac/obturator lymph node dissection to ILND did not significantly increase the risk for postoperative wound complications. These findings are in concordance with those reported by several groups comparing complication rates from ILND when stratifying by the extent of surgery (i.e., superficial ILND vs combined ILND and iliac/obturator lymph node dissection), as they did not find a significant difference between these groups.3,38–40 Lymphedema was more common after ILND alone in some studies, but 1 study specifically examining the incidence of lymphedema found no difference between the two procedures.3,5,20,41 There continues to be a lack of consensus about the complications of deep LND, and we suggest that when clinically indicated, concern about increased morbidity should not be a reason to avoid an iliac/obturator lymph node dissection.42
Secondary lymphedema is a chronic, progressive condition that can occur following lymph node dissection for melanoma or other malignancies.20,38,39 The relationship between early wound complications and the increased risk for the subsequent development of lymphedema has been noted.9,26,43 In our study, we noted that 45% of patients developed limb volume changes, and up to 85% had subjective swelling at approximately 3 months after surgery. The apparent increased sensitivity of the qualitative assessment potentially reflects the important prognostic role of patient symptom reporting in predicting subsequent quantitatively observable limb volume change.24 Given that the median onset of lymphedema has been reported to be at 18–24 months following surgery, our report likely underestimates the true incidence following ILND.44 Lymphedema is one of the primary endpoints of an ongoing longitudinal study at our institution. We anticipate that at the conclusion of that study we will have a better understanding of the natural history of lymphedema in our melanoma population.
There are several aspects of this study that set it apart from previous reports. The prospective nature of the study enables us to capture more accurate clinical data that may have been missed in retrospective evaluations. In addition to reporting the 30-day incidence of wound complications, our study estimated the costs of postoperative complications by comparing direct medical costs between those with complications and those without. Increased length of hospital stay, more frequent readmissions, and increased overall costs associated with postoperative wound infections have been well documented in other areas.45–48 Tracking postdischarge complications has increasingly been recognized as critical to assessing overall resource utilization, particularly in an era of shorter hospital stays.49 There are also several limitations associated with the study presented, including the relatively small sample size. By limiting our analysis to patients accrued prospectively to clinical trials rather than retrospectively examining all patients who had undergone ILND, our statistical power is limited. It is possible that with a larger cohort, risk factors associated with comorbidities such as diabetes and current tobacco use may have been significant. Our primary analysis was also limited to 30-day complications, which may not capture all postoperative complications. Finally, several patients were lost to follow-up because they chose to continue their postoperative care at facilities closer to their homes, affecting our ability to capture all costs. Despite these limitations, our study provides important information.
Postoperative complications occur in the majority of melanoma patients following ILND. The frequency of lymphedema after ILND suggests that objective assessment, education in risk reduction, and early referral for lymphedema treatment in patients with symptoms of heaviness and/or mild swelling should be a part of routine postoperative care. The increased costs associated with postoperative complications, although likely underestimated, still serve as an indicator of the overall cost to society and emphasize the importance of reducing wound complications. Some clinicians have suggested that altering surgical techniques may help reduce morbidity, such as using a straight rather than an S-shaped incision and sparing the saphenous vein.9,50–52 A more recent development has been the introduction of video endoscopic ILND, which is a minimally invasive technique reported to result in less postoperative morbidity in the first published reports.53–56 Such approaches must first demonstrate equivalent oncologic outcomes with respect to tumor recurrence. Following this, whether or not these approaches will prove to decrease the incidence of postoperative wound complications following ILND in larger studies is unclear, and further investigation is warranted.
REFERENCES
- 1.Hughes TM, Thomas JM. Combined inguinal and pelvic lymph node dissection for stage III melanoma. Br J Surg. 1999;86:1493–1498. doi: 10.1046/j.1365-2168.1999.01316.x. [DOI] [PubMed] [Google Scholar]
- 2.Essner R, Scheri R, Kavanagh M, Torisu-Itakura H, Wanek LA, Morton DL. Surgical management of the groin lymph nodes in melanoma in the era of sentinel lymph node dissection. Arch Surg. 2006;141:877–882. doi: 10.1001/archsurg.141.9.877. discussion 882–4. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TM, A’Hern RP, Thomas JM. Prognosis and surgical management of patients with palpable inguinal lymph node metastases from melanoma. Br J Surg. 2000;87:892–901. doi: 10.1046/j.1365-2168.2000.01439.x. [DOI] [PubMed] [Google Scholar]
- 4.Nathansohn N, Schachter J, Gutman H. Patterns of recurrence in patients with melanoma after radical lymph node dissection. Arch Surg. 2005;140:1172–1177. doi: 10.1001/archsurg.140.12.1172. [DOI] [PubMed] [Google Scholar]
- 5.Beitsch P, Balch C. Operative morbidity and risk factor assessment in melanoma patients undergoing inguinal lymph node dissection. Am J Surg. 1992;164:462–465. doi: 10.1016/s0002-9610(05)81181-x. discussion 465–6. [DOI] [PubMed] [Google Scholar]
- 6.Bland KI, Klamer TW, Polk HC, Jr, Knutson CO. Isolated regional lymph node dissection: morbidity, mortality and economic considerations. Ann Surg. 1981;193:372–376. doi: 10.1097/00000658-198103000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson CN, Shulver H, Lowe DC. The surgery of ‘inguinofemoral’ lymph nodes: is it adequate or excessive? Int J Gynecol Cancer. 2004;14:841–845. doi: 10.1111/j.1048-891X.2004.14518.x. [DOI] [PubMed] [Google Scholar]
- 8.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32:785–789. doi: 10.1016/j.ejso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Tonouchi H, Ohmori Y, Kobayashi M, Konishi N, Tanaka K, Mohri Y, et al. Operative morbidity associated with groin dissections. Surg Today. 2004;34:413–418. doi: 10.1007/s00595-003-2738-5. [DOI] [PubMed] [Google Scholar]
- 10.Abraham V, Ravi R, Shrivastava BR. Primary reconstruction to avoid wound breakdown following groin block dissection. Br J Plast Surg. 1992;45:211–213. doi: 10.1016/0007-1226(92)90079-d. [DOI] [PubMed] [Google Scholar]
- 11.Chester DL, Waters R. Adverse alteration of wound flora with topical negative-pressure therapy: a case report. Br J Plast Surg. 2002;55:510–511. doi: 10.1054/bjps.2002.3890. [DOI] [PubMed] [Google Scholar]
- 12.Han LY, Schimp V, Oh JC, Ramirez PT. A gelatin matrix-thrombin tissue sealant (FloSeal) application in the management of groin breakdown after inguinal lymphadenectomy for vulvar cancer. Int J Gynecol Cancer. 2004;14:621–624. doi: 10.1111/j.1048-891X.2004.14411.x. [DOI] [PubMed] [Google Scholar]
- 13.Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg. 2003;196:442–450. doi: 10.1016/S1072-7515(02)01895-1. [DOI] [PubMed] [Google Scholar]
- 14.Ingvar C, Erichsen C, Jonsson PE. Morbidity following prophylactic and therapeutic lymph node dissection for melanoma—a comparison. Tumori. 1984;70:529–533. doi: 10.1177/030089168407000610. [DOI] [PubMed] [Google Scholar]
- 15.Baas PC, Schraffordt Koops H, Hoekstra HJ, van Bruggen JJ, van der Weele LT, Oldhoff J, et al. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch Surg. 1992;127:281–286. doi: 10.1001/archsurg.1992.01420030043008. [DOI] [PubMed] [Google Scholar]
- 16.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. J Urol. 2002;167:1638–1642. [PubMed] [Google Scholar]
- 17.Serpell JW, Carne PW, Bailey M. Radical lymph node dissection for melanoma. ANZ J Surg. 2003;73:294–299. doi: 10.1046/j.1445-2197.2003.t01-1-02622.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JH, Rumball EM. Complications and local recurrence following lymphadenectomy. Br J Surg. 1990;77:760–764. doi: 10.1002/bjs.1800770715. [DOI] [PubMed] [Google Scholar]
- 19.Bouchot O, Rigaud J, Maillet F, Hetet JF, Karam G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol. 2004;45:761–765. doi: 10.1016/j.eururo.2003.12.003. discussion 765–6. [DOI] [PubMed] [Google Scholar]
- 20.Sabel MS, Griffith KA, Arora A, Shargorodsky J, Blazer DG, 3rd, Rees R, et al. Inguinal node dissection for melanoma in the era of sentinel lymph node biopsy. Surgery. 2007;141:728–735. doi: 10.1016/j.surg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Coit DG, Peters M, Brennan MF. A prospective randomized trial of perioperative cefazolin treatment in axillary and groin dissection. Arch Surg. 1991;126:1366–1371. doi: 10.1001/archsurg.1991.01410350056009. discussion 1371–2. [DOI] [PubMed] [Google Scholar]
- 22.Mortenson MM, Xing Y, Weaver S, Lee JE, Gershenwald JE, Lucci A, et al. Fibrin sealant does not decrease seroma output or time to drain removal following inguino-femoral lymph node dissection in melanoma patients: a randomized controlled trial ( NCT00506311) World J Surg Oncol. 2008;6:63. doi: 10.1186/1477-7819-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCI. Common Toxicity Criteria, Version 2.0. In Edition National Cancer Institute Cancer Therapy Evaluation Program 1998 [Google Scholar]
- 24.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Armer JM, Whitman M. The problem of lymphedema following breast cancer treatment: prevalence, symptoms, and self-management. Lymphology. 2002;35:153–159. [Google Scholar]
- 26.Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AA, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer. 2003;13:522–527. doi: 10.1046/j.1525-1438.2003.13304.x. [DOI] [PubMed] [Google Scholar]
- 27.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 30.Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988;6:163–172. doi: 10.1200/JCO.1988.6.1.163. [DOI] [PubMed] [Google Scholar]
- 31.NCCN. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology—Melanoma, Version 2. In Edition National Comprehensive Cancer Network 2009 [Google Scholar]
- 32.Guggenheim MM, Hug U, Jung FJ, Rousson V, Aust MC, Calcagni M, et al. Morbidity and recurrence after completion lymph node dissection following sentinel lymph node biopsy in cutaneous malignant melanoma. Ann Surg. 2008;247:687–693. doi: 10.1097/SLA.0b013e318161312a. [DOI] [PubMed] [Google Scholar]
- 33.Karakousis CP. Surgical procedures and lymphedema of the upper and lower extremity. J Surg Oncol. 2006;93:87–91. doi: 10.1002/jso.20349. [DOI] [PubMed] [Google Scholar]
- 34.Smith G. Primary postoperative wound infection due to staphylococcus pyogenes. Curr Probl Surg. 1979;16:1–56. doi: 10.1016/s0011-3840(79)80002-7. [DOI] [PubMed] [Google Scholar]
- 35.Bozzetti F, Terno G, Camerini E, Baticci F, Scarpa D, Pupa A. Pathogenesis and predictability of central venous catheter sepsis. Surgery. 1982;91:383–389. [PubMed] [Google Scholar]
- 36.Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17:426–435. doi: 10.1097/00129334-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher S. The challenges of obesity and skin integrity. Nurs Clin North Am. 2005;40:325–335. doi: 10.1016/j.cnur.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Ravi R. Morbidity following groin dissection for penile carcinoma. Br J Urol. 1993;72:941–945. doi: 10.1111/j.1464-410x.1993.tb16304.x. [DOI] [PubMed] [Google Scholar]
- 39.Karakousis CP, Driscoll DL. Groin dissection in malignant melanoma. Br J Surg. 1994;81:1771–1774. doi: 10.1002/bjs.1800811221. [DOI] [PubMed] [Google Scholar]
- 40.Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol. 2009;27:205–212. doi: 10.1007/s00345-008-0324-6. [DOI] [PubMed] [Google Scholar]
- 41.Karakousis CP, Heiser MA, Moore RH. Lymphedema after groin dissection. Am J Surg. 1983;145:205–208. doi: 10.1016/0002-9610(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 42.Badgwell B, Xing Y, Gershenwald JE, Lee JE, Mansfield PF, Ross MI, et al. Pelvic lymph node dissection is beneficial in subsets of patients with node-positive melanoma. Ann Surg Oncol. 2007;14:2867–2875. doi: 10.1245/s10434-007-9512-7. [DOI] [PubMed] [Google Scholar]
- 43.Gould N, Kamelle S, Tillmanns T, Scribner D, Gold M, Walker J, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol. 2001;82:329–332. doi: 10.1006/gyno.2001.6266. [DOI] [PubMed] [Google Scholar]
- 44.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Herwaldt LA, Cullen JJ, Scholz D, French P, Zimmerman MB, Pfaller MA, et al. A prospective study of outcomes, healthcare resource utilization, and costs associated with postoperative nosocomial infections. Infect Control Hosp Epidemiol. 2006;27:1291–1298. doi: 10.1086/509827. [DOI] [PubMed] [Google Scholar]
- 46.Green JW, Wenzel RP. Postoperative wound infection: a controlled study of the increased duration of hospital stay and direct cost of hospitalization. Ann Surg. 1977;185:264–268. doi: 10.1097/00000658-197703000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 48.Reilly J, Twaddle S, McIntosh J, Kean L. An economic analysis of surgical wound infection. J Hosp Infect. 2001;49:245–249. doi: 10.1053/jhin.2001.1086. [DOI] [PubMed] [Google Scholar]
- 49.Gastmeier P. Postdischarge surveillance for surgical site infection: the continuing challenge. Infect Control Hosp Epidemiol. 2006;27:1287–1290. doi: 10.1086/509000. [DOI] [PubMed] [Google Scholar]
- 50.Zhang SH, Sood AK, Sorosky JI, Anderson B, Buller RE. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer. 2000;89:1520–1525. [PubMed] [Google Scholar]
- 51.Dardarian TS, Gray HJ, Morgan MA, Rubin SC, Randall TC. Saphenous vein sparing during inguinal lymphadenectomy to reduce morbidity in patients with vulvar carcinoma. Gynecol Oncol. 2006;101:140–142. doi: 10.1016/j.ygyno.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Sheng X, Niu J, Li H, Li D, Tang L, et al. Sparing of saphenous vein during inguinal lymphadenectomy for vulval malignancies. Gynecol Oncol. 2007;105:722–726. doi: 10.1016/j.ygyno.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Tobias-Machado M, Tavares A, Molina WR, Jr., Forseto PH, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): initial case report and comparison with open radical procedure. Arch Esp Urol. 2006;59:849–852. doi: 10.4321/s0004-06142006000800020. [DOI] [PubMed] [Google Scholar]
- 54.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–957. doi: 10.1016/j.juro.2006.10.075. discussion 958. [DOI] [PubMed] [Google Scholar]
- 55.Tobias-Machado M, Tavares A, Silva MN, Molina WR, Jr, Forseto PH, Juliano RV, et al. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol. 2008;22:1687–1691. doi: 10.1089/end.2007.0386. [DOI] [PubMed] [Google Scholar]
- 56.Josephson DY, Jacobsohn KM, Link BA, Wilson TG. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology. 2009;73:167–170. doi: 10.1016/j.urology.2008.05.060. discussion 170–1. [DOI] [PubMed] [Google Scholar]
- 57.Lin JY, DuBeshter B, Angel C, Dvoretsky PM. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol. 1992;47:80–86. doi: 10.1016/0090-8258(92)90081-s. [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni JN, Kamat MR. Prophylactic bilateral groin node dissection versus prophylactic radiotherapy and surveillance in patients with N0 and N1-2A carcinoma of the penis. Eur Urol. 1994;26:123–128. doi: 10.1159/000475360. [DOI] [PubMed] [Google Scholar]
- 59.Bell JG, Lea JS, Reid GC. Complete groin lymphadenectomy with preservation of the fascia lata in the treatment of vulvar carcinoma. Gynecol Oncol. 2000;77:314–318. doi: 10.1006/gyno.2000.5790. [DOI] [PubMed] [Google Scholar]