Abstract
Autistic children show elevated serum levels of autoantibodies to several proteins essential for the function of normal brains. The voltage-dependent anion channel, VDAC, and hexokinase-I, a VDAC protective ligand, were identified as targets of this autoimmunity in autistic children. These autoantibodies were purified using immunoaffinity chromatographic techniques. Both antibodies induce apoptosis of cultured human neuroblastoma cells. Because VDAC and hexokinase-I are essential for brain protection from ischemic damage, the presence of these autoantibodies suggests a possible causal role in the neurologic pathogenesis of autism.
Keywords: autism, autoimmunity, voltage-dependent anion channel, hexokinase-I, apoptosis
1. Introduction
Autism is a neurodevelopmental syndrome of unknown etiology, characterized by distinct symptoms including marked social deficits, deviant language, and a restricted range of repetitive behaviors that appear within the first three years of life (Volkmar and Pauls, 2003). The syndrome is associated with abnormal brain development and manifests in several specific regions of the brain, especially the cerebellum, amygdala, and hippocampus (Volkmar and Pauls, 2003; Raymond et al., 2003; Belmonte et al., 2004). A number of findings strongly suggest immune system dysregulation or dysfunction in autism (Ashwood et al., 2006). These patients show decreased peripheral lymphocyte numbers, decreased responses to T cell mitogens, dysregulated apoptotic mechanisms, imbalance of serum immunoglobulin levels, and the presence of serum autoantibodies directed against components of the central nervous system (Ashwood et al., 2006). A possible link between autism and autoimmunity to regions in the brain was first reported in 1971 (Money et al., 1971). Although the pathophysiological significance of autoantibodies reported in children with autism remains uncertain, various anti-brain antibodies occur, including autoantibodies to myelin basic protein (Comi et al., 1999), neuron-axon filament protein (Singh et al., 1993), and glial fibrillary acidic protein (Singh et al., 1997). Elevated levels of antibodies against the neurologic antigens myelin basic protein, myelin-associated glycoprotein, ganglioside GM1, α,β-crystallin, sulfatide, chondroitin sulfate, and tubulin in the serum of autistic children have also been reported (Vojdani et al., 2002).
The voltage-dependent anion channel (VDAC) is a small, 30–35 kDa protein, originally discovered in the outer membrane of mitochondria where it functions as the major pore-forming protein (Colombini, 1979). VDAC is also found in the plasma membrane of a large number of tissues (De Pinto et al., 2003). In the brain, cell-surface VDAC performs a dual function: (1) maintenance of redox homeostasis in normal cells and (2) promotion of anion efflux in apoptotic cells (Elinder et al., 2005). A key protein in these mechanisms is hexokinase-I (HK-I), which acts as a gatekeeper maintaining a delicate balance between the opening and closing of VDAC (Oudard et al., 2004).
Studies in rats show that both VDAC and HK-I are densely localized in regions of the brain, including the caudate nucleus, hippocampus, hypothalamus and cerebellum (McEnery et al., 1993). Several studies have reported abnormalities in both the caudate nucleus and the cerebellum of autistic children (Hashimoto et al., 1995; Kern, 2002; Langen et al., 2007), but the associated molecular mechanisms have not been identified. For this reason, we investigated the possible reactivity of autoantibodies isolated from the serum of autistic children with VDAC in lysates from different areas of normal human brain. We found reactivity with a protein in the 35 kDa size range in caudate nucleus and cerebellum tissue lysates. This protein was isolated by electrophoresis and identified by MALDI-TOF analysis as VDAC. We also found autoantibodies to HK-I in the serum of autistic children. Both of these antibodies impair growth and induce apoptosis in human neuroblastoma cells in culture, suggesting a possible causal role in the neurologic damage observed in autistic children.
2. Materials and Methods
2.1. Patients
Thirty-four children with autistic disorder, 2 girls and 32 boys, were included in the study. All patients were recruited through family members of the Chilean Association of Parents with Autistic Children. These patients were diagnosed following procedures outlined by the American Psychiatric Association in the Diagnostic and Statistical Manual of Mental Disorders, DSM-IV (1994). The screening and diagnosis of autism was performed using the Childhood Autism Rating Scale (CARS) (Schopler et al., 1980). Autistic disorders were confirmed independently by two of the investigators (R.F. and S.C.), both of whom specialize in autistic disorders. Clinical assessment included medical and psychiatric history, demographic data, physical examination, and routine laboratory evaluations (Trottier et al., 1999). All 34 participants underwent the Autism Diagnostic Interview (ADI-R) (LeCouteur et al. 1989; Lord et al. 1994) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000) administered by a trained clinician (RF). The scores of these patients met cut-off values for the category designated as ‘Broad Autistic Spectrum Disorder (ASD),’ according to criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (CPEA) (Lainhart et al. 2006). Clinical assessment included medical and psychiatric history, demographic data, physical examination, and routine laboratory evaluations. Other disorders thought to be associated with autism (fragile X syndrome, Asperger’s syndrome, cerebral palsy, tuberous sclerosis, and neurofibromatosis) were excluded. Patients who had schizophrenia and other psychotic disorders were also excluded.
Autistic patients were investigated for immune disorders such as rheumatoid arthritis, juvenile rheumatoid arthritis, rheumatic fever, psoriatic arthritis, ankylosing spondylitis, systemic lupus erythematosus, dermatomyositis, polymyositis, psoriasis, vitiligo, myasthenia gravis, multiple sclerosis, amyothrophic lateral sclerosis, ulcerative colitis, Crohn’s disease, hyperthyroidism, hypothyroidism, idiopatic thrombocytopenic purpura, scleroderma, uveitis, polyarteritis nodosa, Wegener’s granulomatosis, Takayasu’s arteritis, vasculitis (other), type 1 diabetes, Addison’s disease, Sjogren syndrome, pemphigus, and Guillain Barré syndrome. Clinical assessment procedures included anthropomorphic measurements, dysmorphic examination, and neurologic examination. Baseline hematologic parameters, including complete blood count, ESR, creatinine, TSH, T3, T4, and cortisol, and urinalysis were also determined. Magnetic resonance imaging (MRI) was performed in this group of patients. Selected patients did not have significant abnormal findings by MRI. Peripheral blood samples were also obtained from age-matched control children, who had been admitted to the Clinical Hospital University of Chile and to the Child Surgery Department of the Santa María Clinic in Santiago, Chile, for minor traumas or elective surgery. Blood samples were drawn from children after written consent was obtained from their parents before admission to the clinics.
2.2. Materials
Culture media were purchased from Invitrogen (Carlsbad, CA). Na 125I was obtained from Perkin-Elmer Life Sciences (Boston, MA). The 21-amino acid peptides, KVNNSSLIGLGYTQTLKP GIKC (Lys235-Lys255) of VDAC1 (P1) and MIAAQLLAYYFTELKDDQVKKC (Met1-Lys21) of hexokinase I (P2), were obtained from Genemed Synthesis, Inc. (San Francisco, CA). A carboxy-terminal L-Cysteine residue was added to facilitate conjugation to keyhole limpet hemocyanin (KLH). Staurosporine was purchased from Sigma (St. Louis, MO).
2.3. Proteins
Human brain HK-I was produced in Escherichia coli and purified as previously described (Gonzalez-Gronow et al., 2007). Human liver glucokinase (HK-IV) and VDAC were produced in Escherichia coli and purified from clones obtained from Genecopeia (Germantown, MD) as previously described (Brocklehurst et al., 2004; Shi et al., 2003). Western blot dipsticks of human normal tissue lysates from the caudate nucleus, cerebellum, hippocampus, hypothalamus and brain cortex were purchased from Protein Biotechnologies (Ramona, CA). Radioiodination of proteins was carried out by the method reported by Markwell (1982). Incorporation of the 125I label was 2 × 107 cpm/nmol protein. Radioactivity was measured in a Pharmacia-LKB Biotechnology 1272 γ-radiation counter.
2.4 Antibodies
The anti-HK-I or anti-VDAC IgGs were sequentially purified by immunoadsorption of the flow through IgG fraction to recombinant VDAC or HK-I proteins conjugated to carboxyhexyl-Sepharose. Alkaline phosphatase-conjugated secondary antibody against human IgG was purchased from Sigma (St. Louis, MO). IRDye 800 DX-conjugated affinity purified anti-human IgG was purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA). Alexa Fluor 488 (AF488)-conjugated to goat anti-human IgG was purchased from Molecular Probes, Inc. (Eugene, OR).
2.5. SDS-PAGE and immunoblotting
Recombinant VDAC, HK-I and HK-IV were analyzed on 4–20% polyacrylamide gels (1.2 mm thick, 14 × 10 cm) containing 0.1% SDS under reducing conditions. A discontinuous Laemli buffer system was used (Laemli, 1970). Transfer of proteins from the gels to nitrocellulose membranes was performed by western blot (Towbin et al., 1979). The molecular weights were assessed using a set of dye-conjugated Mr markers (Fermentas Life Sciences, Glen Burnie, MD). The membranes were thoroughly rinsed with PBS and then incubated with 3% BSA in PBS for 1 h at room temperature to block non-conjugated areas. Each serum (100 μl) in 5 ml PBS was singly incubated with membranes containing transferred proteins overnight at 22 °C, followed by 3 rinses in PBS. Next, the membranes were incubated with a 1:800 dilution of an IRDye 800 DX-conjugated affinity-purified anti-human IgG in blocking buffer for 60 min at 22 °C. At this stage, the membranes were kept under low light conditions to protect the IR-conjugated antibody. After rinsing the membranes three times with PBS, imaging of the blots was performed using the LI-COR Odyssey System from LI-COR Biotechnologies (Lincoln, NE).
2.6. Mass spectrometric analysis
Normal human cerebellar proteins were separated on SDS-PAGE gels, and the protein band in the 30 kDa size range was excised and digested in situ with trypsin. A portion (1/5) of the sample was analyzed by matrix-assisted laser desorption ionization (MALDI-TOF), and the obtained mass spectrometric peptide maps were used to identify the protein using the Mascot search engine (Matrix Science Inc. Boston, MA) and the UniProtKB/Swiss-Prot Data Bank, release 57.6 of Jul-2009.
2.7. Cell culture
Human neuroblastoma SK-N-SH cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in MEM culture medium containing 2mM L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate and 10% fetal bovine serum (FBS), all purchased from GIBCO-Invitrogen (Carlsbad, CA).
2.8. Immunofluorescence microscopy
SK-N-SH cells were plated at 5 × 105 cells/ml on glass coverslips and allowed to adhere overnight. Cells were incubated for 1 h at 4 °C in PBS containing 2% bovine serum albumin (BSA), 0.2 mg/ml goat IgG, and 0.01% NaN3 (staining buffer), followed by incubations with anti-GRP78 or anti-VDAC IgGs (2 μg/ml) for 1 h at 4°C. Cells were washed in PBS and incubated for 1 h with an AF488-conjugated goat anti-human IgG before washing and fixing in 4% paraformaldehyde. Immunofluorescence microscopy was performed using an Olympus BX-60 microscope (Olympus, Lake Success, NY).
2.9. Flow cytometry
SK-N-SH cells were detached from the culture flasks (75 cm2) by incubation for 5 min at 37 °C with Ca2+ and Mg2+-free phosphate-buffered saline (PBS) containing 4 mM EDTA and pelleted. The cells (1 × 106/ml) were washed with PBS before resuspension in ice-cold staining buffer. The cell suspensions (500 μl) were incubated 30 min with human anti-GRP78 or anti-VDAC IgGs (10 μg/ml). An equivalent quantity of non-immune human IgG was used as an isotype control. At this time, the cells were washed, pelleted, and resuspended in 500 μl of ice-cold staining buffer. The cell suspensions were incubated for 30 min in the dark with AF488-conjugated goat anti-human IgG. The cells were washed twice with ice-cold staining buffer, resuspended in the same buffer and stored in the dark at 4 °C for 10 min until analysis. Staining with propidium iodide (2 μg/ml) was performed immediately prior to flow cytometric analysis to exclude dead cells. Flow cytometry was conducted using the Guava EasyCyte Plus system (Guava Technologies, Hayward, CA). Data analysis and histogram preparation was performed using FlowJo® 8.6.3 software (Treestar Inc., Ashland, OR).
2.10. Human neuroblastoma SK-N-SH cell proliferation assays
SK-N-SH cells, suspended in MEM culture medium containing 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 5% FBS at a density of 1 × 105 cells/ml, were plated in 96-well culture plates (0.1 ml/well) containing increasing concentrations of autistic anti-GRP78, anti-VDAC (Lys235-Lys255), or anti-HK-I (Met1-Lys21) IgGs at a final volume of 0.2 ml/well. Cell proliferation was determined at 48 h using a BrdU labeling and colorimetric immunoassay detection method (Roche Molecular Biochemicals, Indianapolis, IN). Results were expressed as absorbance at 372 nm (reference wavelength: 492 nm). Control cell proliferation was determined in the presence of non-immune human IgG added at the same concentrations as autistic IgGs.
2.11. SK-N-SH cell membrane voltage-dependent anion channel NADH-ferricyanide reductase activity
The VDAC NADH-ferricyanide reductase activity was measured in SK-N-SH cells grown to confluency in 96-well plates. Cells were incubated for 1 h in 200 μl PBS containing 250 μM β-NADH and 250 μM potassium ferricyanide. At this time, 100 μl aliquots of reaction mixture were mixed with 100 μl of PBS containing 0.5 mM bathophenantroline disulfonic acid, a chelator of ferrous ions, forming a chromogenic complex with photometric absorbance at 535 nm (Avron and Shavit, 1963). Activity was expressed as nmol/min/ml, calculated from a calibration curve constructed with different concentrations of potassium ferrocyanide. All experiments were done in triplicate.
2.12. Enzyme-linked immunosorbent assay (ELISA) procedure
We determined antibody titers against VDAC, HK-I and HK-IV in the sera of patients with autism and of control subjects. These three proteins, at a concentration of 5 μg/ml in 0.1 M sodium carbonate, pH 9.5, containing 0.01 % NaN3, were used (200 μl/well) to coat 96-well polystyrene flat-bottom ELISA plates. Plates were incubated overnight at room temperature and then washed three times with PBS containing 0.05% Tween 80 (PBS-Tween). Plates were then incubated for 2 h at room temperature with 2% BSA to block nonspecific binding. Plates were again washed with PBS-Tween, and then serum samples diluted 1:500 in PBS-Tween were added to triplicate wells and incubated for 2 h at room temperature. At this time, the plates were washed three times with PBS-Tween and then alkaline phosphatase-conjugated goat anti-human IgG diluted 1:1000 in PBS-Tween was added to each well. The plates were incubated for an additional 2 h at room temperature. After washing three times with PBS-Tween, the plates were incubated with the alkaline phosphatase substrate (1 mg/ml p-nitrophenylphosphate) in 0.1 M glycine, 1mM MgCl2, and 1 mM ZnCl2 at pH 10.4. Absorbance was monitored at a wavelength of 405nm using an Anthos Labtec Kinetic Plate reader. Bound IgG was expressed as μg/ml serum. Concentrations of specific IgGs were calculated from a calibration curve constructed with purified human IgG.
2.13. Caspase-3/7 activity assay
Caspase 3/7 activity was assayed with the Caspase-Glo® 3/7 assay system purchased from Promega (Madison, WI) under conditions suggested by the manufacturer. The measurements were performed in a Thermo Fisher Scientific Luminoskan-Ascent luminometer (Milford, MA) and expressed as relative light units. All experiments were done in triplicate.
2.14. Statistics
The levels of autoantibodies in sera from autistic children and matched controls were evaluated by a Student’s t test using the program SYSTAT for Windows: Statistics, Version 11 (Systat).
3. Results
3.1. Detection of autoantibodies in serum from autistic children
Thirty-four children with autistic disorder were included in the study. Table 1 shows the demographics and baseline characteristics of the patients (94.11% males) and their controls (91.17% males). We found no statistically significant differences between the demographic characteristics of the two groups. The average age when the first autistic symptoms were detected was 19.72 ± 9.17 months. Most patients had a very high autistic score according to the CARS, ADI-R and ADOS tests. Western immunoblot analyses of the caudate nucleus, cerebellum, hippocampus, hypothalamus and brain cortex identified numerous antigen-antibody bands in all subjects and very little reactivity in control serum (Fig. 1). The reactivity of the autistic sera with brain tissue was consistently observed in all tested sera (data not shown). However, only caudate nucleus and cerebellum lysates consistently showed a prominent protein with an apparent molecular mass of about 33 kDa.
Table 1.
Baseline Demographic and Disease Characteristics of 34 autistic patients and their controls.
| Autistic patients | Controls | P | |
|---|---|---|---|
| Age, years. Mean ± SE | 9.06 ± 3.07 | 9 ± 3.82 | N.S. |
| Age, years. Median (range) | 9.0 (5–14) | 8 (1–14) | N.S. |
| Gender, n | |||
| Male | 32 | 31 | N.S. |
| Female | 2 | 3 | N.S. |
| CARS. Mean ± SE | |||
| Nonautistic, score <30, n (%) | 0 | ||
| Mild/moderate score 31–36, n (%) | 3 (9.68) | ||
| Severe, score 37–60, n (%) | 29 (90.32) | ||
| ADI-R social interaction | 27.42 ± 1.71 | ||
| ADI-R verbal communication | 13.57 ± 0.78 | ||
| ADI-R repetitive behaviors | 6.71 ± 2.2 | ||
| ADOS Social Interaction. Mean ± SE | 12.4 ± 1.21 | ||
| ADOS Communication. Mean ± SE | 7.26 ± 1.27 | ||
| ADOS Stereotyped behaviors. Mean ± SE | 3.8 ± 1.97 | ||
N.S. = non significant
Figure 1.
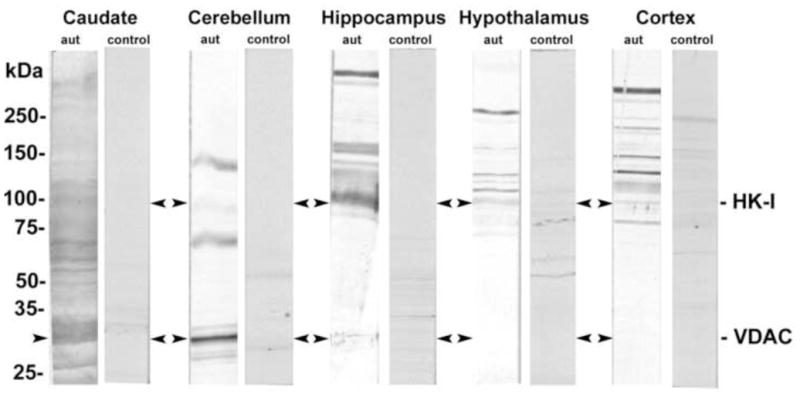
Detection of autoantibodies to normal brain tissue lysates in serum of autistic children. Brain tissue lysates from the caudate nucleus, cerebellum, hippocampus, hypothalamus, and brain cortex were run on 4–20% PAGE and then transferred to nitrocellulose membranes. The membranes were singly incubated with autistic or control sera (Dil. 1:100).
3.2. Peptide mass fingerprinting of autoantibody binding site
Proteins from normal human brain cerebellum lysate (50 μg) were separated, under reducing conditions, in a 4–20% SDS-PAGE gel. A protein band in the 33 kDa size range was digested with trypsin in situ (Matsui et al., 1997). The resulting peptides were extracted and the mass of approximately 12 peptides (Table 2) was determined using MALDI-TOF analysis, which provided a unique signature for protein identification by peptide mass searches. The 33 kDa protein was identified as VDAC.
Table 2.
MALDI-TOF analysis of a 35 kDa peptide from human brain cerebellum*
| Peptide mass, Monoisotopic, Da | ||
|---|---|---|
| Sequence | Measured | Calculated |
| SENGLEFTSSGSANTETTK | 1958.67 | 1958.87 |
| VTGSLETK | 833.39 | 833.45 |
| WTEYGLTFTEK | 1373.45 | 1373.65 |
| WNTDNTLGTEITVEDQLAR | 2174.71 | 2175.05 |
| LTFDSSFSPNTGK | 1399.42 | 1399.66 |
| LTFDSSFSPNTGKK | 1527.53 | 1527.76 |
| VTQSNFAVGYK | 1212.39 | 1212.61 |
| TDEFQLHTNVNDGTEFGGSIYQK | 2598.83 | 2599.18 |
| KLETAVNLAWTAGNSNTR | 1944.76 | 1945.00 |
| YQIDPDACFSAK | 1427.38 | 1427.64 |
| VNNSSLIGLGYTQTLKPGIK | 2102.86 | 2102.17 |
| LTLSALLDGK | 1029.48 | 1029.61 |
A 35 kDa protein band from a 4–20% SDS/PAGE was excised from the gel, and then digested in situ with trypsin. Then 1/5 of the sample was analyzed by MALDI. The obtained mass spectroscopic peptide map was used to identify VDAC1 in the UniProtKB/Swiss-Prot Data Bank, release 57.6.
3.3. Western blots of purified proteins
The existence of autoantibodies to VDAC, HK-I, and hexokinase-IV (HK-IV) was assessed in serum from three autistic patients and one randomly selected control participant. We chose HK-IV as a control because this liver hexokinase lacks the N-terminal 15 amino acid sequence extension normally found in brain HK-I (Schwab and Wilson, 1989). The staining pattern suggested the presence of autoantibodies to VDAC and HK-I in the serum of autistic children (Figs. 2A, 2B) and the absence of autoantibodies to HK-IV (Fig. 2C).
Figure 2.
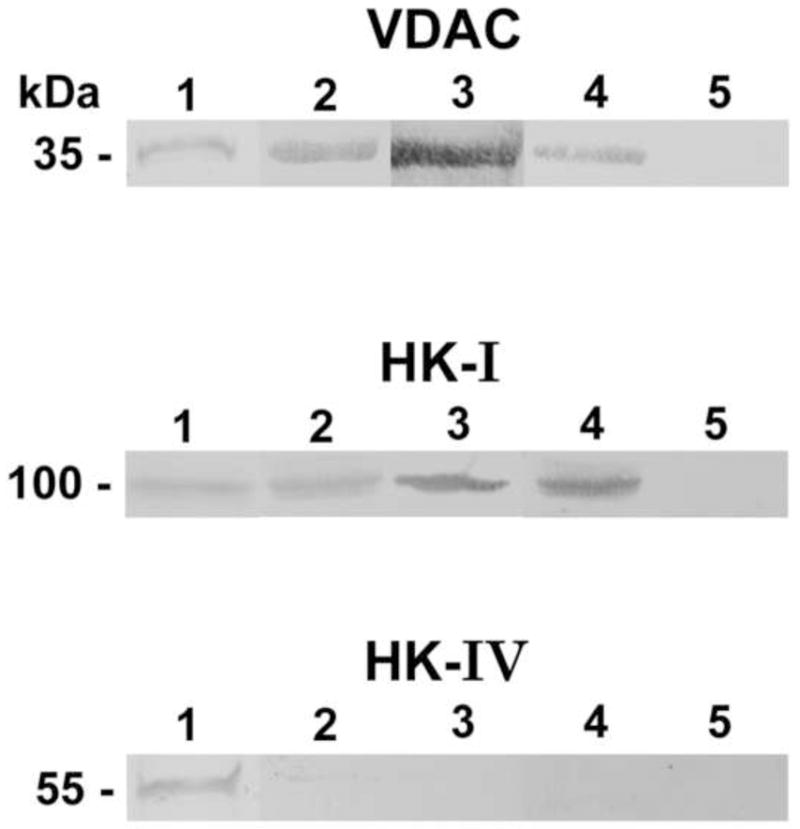
Blot binding assay of serum autoantibodies from autistic children and controls (Dil. 1:100 to immobilized recombinant human VDAC, human HK-I, and human HK-IV). Lane 1, Coomassie Brilliant Blue protein stains of the recombinant protein. Lanes 2–4, blots incubated individually with serum from three autistic children. Lane 5, blots incubated with control serum.
3.4. ELISA results
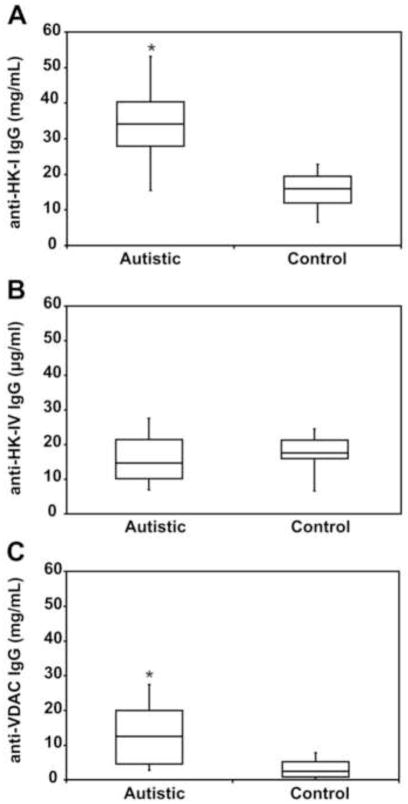
Serum levels of autoantibodies to VDAC, HK-I, and hexokinase-IV (HK-IV) in each sample (n=34) were assessed by ELISA (Fig. 3). In autistic children, the median concentrations of anti-HK-I, anti-HK-IV, and anti-VDAC were 34.87 ± 12.53, 15.83 ± 6.43, and 17.30 ± 8.43, respectively. In control children, median titers of the same autoantibodies were 18.29 ± 10.79, 16.98 ± 7.31, and 4.20 ± 2.67, respectively. Comparison of the data obtained with each antigen shows statistically significant differences between the autoantibody levels of the two groups (p<0.001), with the exception of anti-HK-IV autoantibody levels, which are similar in both groups; these findings suggest an autoimmune response targeted specifically against the N-terminal extension of brain HK-I. No correlation was found between clinical scores (CARS, ADI-R and ADOS) and antibody titers in these patients, possibly because their high clinical scores show very little value dispersion (Table 1).
Figure 3.
Serum titers of IgG antibody against HK-I, HK-IV and VDAC in autistic children (n=34) (●) and age-matched control children (n=34) (○). Each value represents the mean of experiments done in triplicate. * Significantly different from controls (P<0.001).
3.5. Purification of autistic anti-VDAC and HK-I IgG and evaluation of their binding to Human neuroblastoma SK-N-SH cells by immunofluorescence microscopy and flow cytometry
We pooled serum from all autistic children (20 ml) to purify the autoantibodies. Anti-VDAC IgG was purified by immunoaffinity chromatography on a resin containing recombinant human VDAC covalently attached to Sepharose 4B. Because the autistic serum contained autoantibodies against the HK-I isozyme and these antibodies did not show significant reactivity against the HK-IV isozyme, the autoantibody was purified on a resin containing the 21 amino acid peptide Met1-Lys21 from HK-I covalently attached to aminohexyl-Sepharose. Immunofluorescence microscopic studies of non-permeabilized SK-N-SH cells incubated with autistic anti-VDAC IgG (Fig. 4A) showed surface expression of VDAC on these cells. These results were confirmed by flow cytometric analysis with the autistic anti-VDAC IgG (Fig. 4B).
Figure 4.
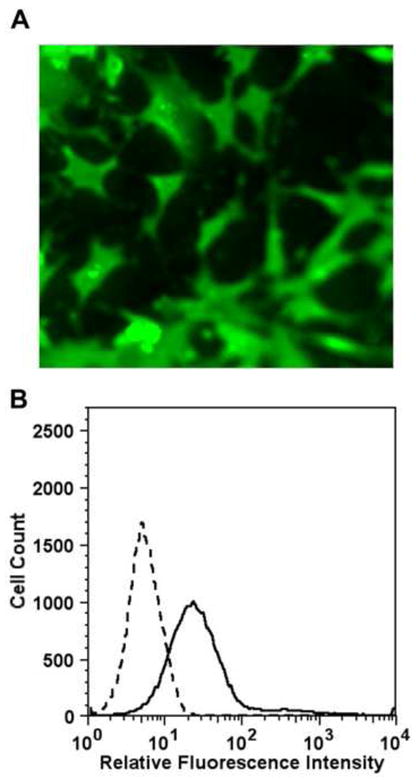
Binding of isolated autistic VDAC autoantibodies to human neuroblastoma SK-N-SK cells. (A) Immunofluorescence microscopy of non-permeabilized SK-N-SH cells incubated with anti-VDAC IgG. (B) Flow cytometric analysis of anti-VDAC IgG binding to SK-N-SH cells. The solid lines represent cells incubated with autoantibodies directed against VDAC. The dashed lines represent binding of isotype control.
3.6 Binding of autistic autoantibodies to immobilized human recombinant VDAC and HK-I
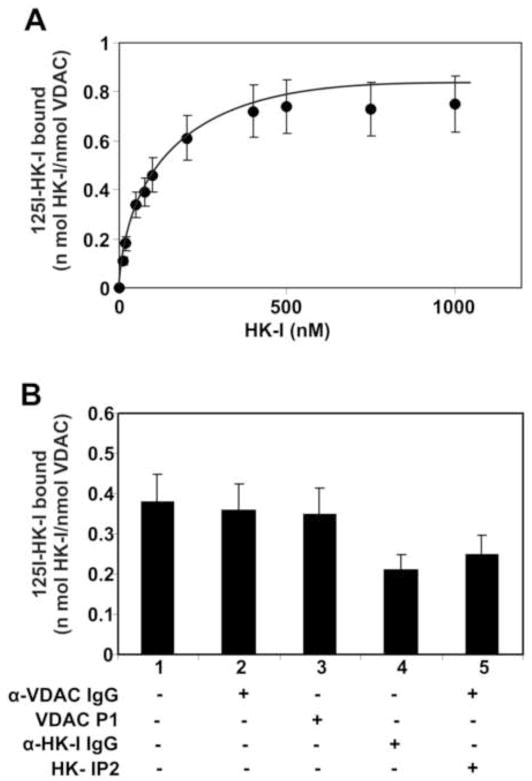
First, we assessed the capacity of HK-I to bind immobilized recombinant VDAC (Fig. 5A). [125I]-labeled HK-I binds to this protein in a dose-dependent manner with high affinity (Kd of 38.41 ± 13.48). Next, we investigated the capacity of the autistic anti-VDAC or anti-HK-IgGs to interfere with [125I]-labeled HK-I binding to immobilized VDAC (Fig. 5B). We also used the VDAC peptide Lys235-Lys255 (P1) and the HK-I peptide Met1-Lys21 (P2) as competitors of HK-I binding to VDAC. The anti-VDAC IgG or P1 do not compete for binding of HK-I, whereas both the autistic anti-HK-I IgG or P2 function as inhibitors, thereby confirming that HK-I binds to VDAC via its N-terminal Met1-Lys21 P2 segment to a region that is not affected by either the anti-VDAC IgG or VDAC P1 and suggesting that this region is not involved in HK-I binding.
Figure 5.
(A) Binding of HK-I to immobilized recombinant VDAC. Increasing concentrations of [125I]-labeled HK-I were added to VDAC immobilized on 96-strip-well tissue culture plates. The data are represented as mean ± S.D. from experiments performed in triplicate. (B) Inhibition of binding of HK-I to immobilized VDAC. Plates were incubated with a single concentration of [125I]-labeled HK-I (100 nM) and a single concentration (500 nM) of autistic anti-VDAC IgG, VDAC P1, anti-HK-I IgG or HK-I P2. The data are represented as mean ± S.D. from experiments performed in triplicate
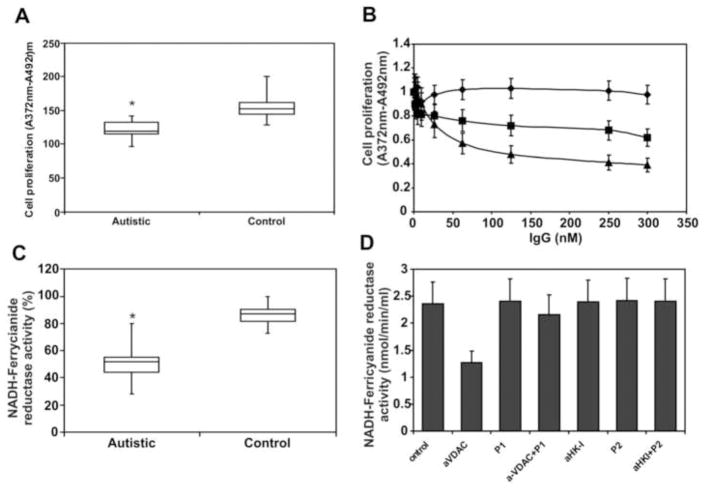
3.7. Effect of autistic autoantibodies on cell proliferation and NADH-ferricyanide reductase activity of VDAC
VDAC expressed on the cell surface functions as a membrane NADH-ferricyanide reductase (Baker et al., 2004). For this reason, we tested the capacity of whole autistic serum or purified autoantibodies to affect either cell proliferation or NADH-ferricyanide reductase activity of human neuroblastoma SK-N-SH cells. Whole autistic serum inhibits cell proliferation at a significant level (P<0.001) (Fig. 6A). Both anti-VDAC and anti-HK-I IgGs are able to inhibit cell proliferation, whereas a control IgG does not affect cell proliferation (Fig.6B). Whole autistic serum also inhibits NADH-ferricyanide reductase activity to a significant degree (P<0.001) (Fig. 6C), but only anti-VDAC IgG inhibits this activity (Fig. 6D), thereby suggesting that these antibodies may inhibit cell proliferation via independent mechanisms. Also, VDAC P1 is able to inhibit the effect of the autistic antibody, thereby suggesting that the VDAC Lys235-Lys255 amino acid sequence is a target of this autoantibody.
Figure 6.
(A) Effect of whole autistic serum (n =34) and isolated autistic autoantibodies on SK-N-SH cell proliferation. Cells in 96-well culture plates (1 × 104 per well) were incubated with 5 μl serum for 48 h in a final volume of 200 μl RPMI 1640 culture medium containing 5% FBS. (B) Increasing amounts of anti-VDAC IgG (▲), anti-HK-I IgG (■), or non-immune control IgG (◆) for 48 h in RPMI 1640 culture medium containing 5% FBS. (C) Effect of whole autistic serum (n=34) on NADH-ferricyanide reductase activity of SK-N-SH cells grown to confluency in 96-well culture plates. (D) NADH-ferricyanide reductase activity of cell-surface VDAC was measured in SK-N-SH cells grown to confluency in 96-well culture plates. Cells were incubated with a single concentration (200 nM) of anti-VDAC, VDAC P1, anti-VDAC + P1, anti-HK-I, HK-I P2, anti-HK-I + P2, or non-immune control IgG in cell culture medium for 2 h prior to the assay. * Significantly different from controls (P<0.001).
3.8. Effect of autistic autoantibodies to VDAC or HK-I on apoptosis of human neuroblastoma SK-N-SH cells
We assessed the capacity of whole autistic serum or isolated anti-VDAC or anti-HK-I IgGs to induce apoptosis on SK-N-SH cells. The extent of apoptosis was evaluated by measuring caspase-3/7 activity. Whole serum from these patients induces apoptosis to a significant degree (P<0.0001) (Fig. 7A). Apoptosis is induced by anti-VDAC IgG; however, this effect is inhibited when the antibody is incubated with VDAC P1 (Fig. 7B), thereby suggesting a direct interaction between this antibody and its target VDAC on the cell surface. Next, we evaluated the effect of anti-HK-I IgG. For these experiments, we had to consider that HK-I prevents cell apoptosis via a mechanism that induces closure of VDAC (Azoulay-Zohar et al., 2004) and also protects cells against death in model of apoptosis induced by either TNF-α (Gonzalez-Gronow et al., 2007) or staurosporine (Shimizu et al., 2001). We chose staurosporine (1.5 μM) to induce apoptosis of SK-N-SH cells in the presence of HK-I (1 μM) or HK-IV (1 μM). HK-I is able to protect these cells from apoptosis induced by staurosporine, but HK-IV, which does not bind to VDAC, elicited no protection (Fig. 7C). When SK-N-SH cells were incubated with staurosporine (1.5 μM) and HK-I (1 μM), in the presence of the autistic anti-HK-I IgG (1 μM), the protective effect of HK-I against cell death induced by staurosporine was abolished (Fig. 7D). As demonstrated above, binding of this antibody to the amino-terminal (Met1-Lys21) region of HK-I prevents its binding to VDAC, thereby blocking the protective effect of HK-I against cell death.
Figure 7.
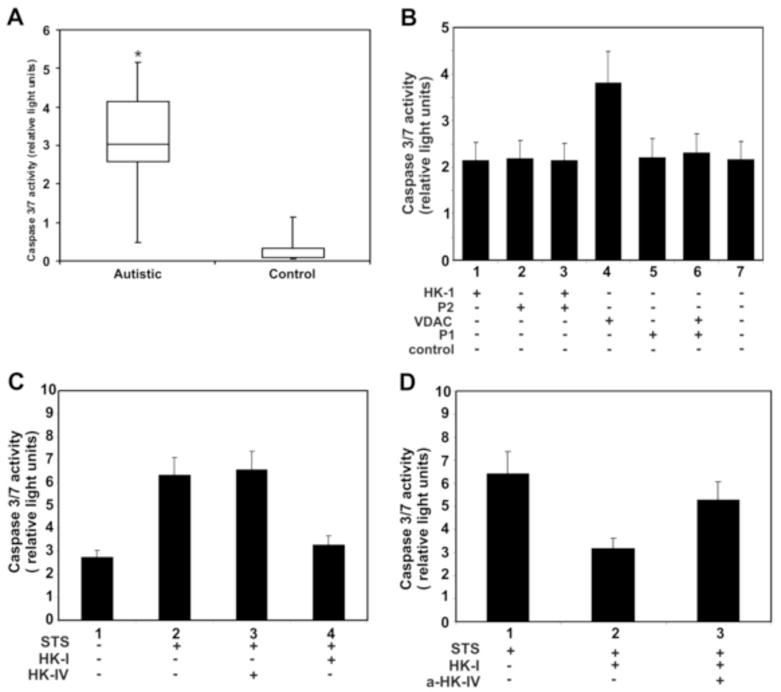
(A) Effect of whole autistic serum (n=34) on apoptosis of SK-N-SH cells grown to confluency in 96-well culture plates. (B) Effect of autoantibodies to HK-I and VDAC an their competing peptides on apoptosis of SK-N-SH cells grown to confluency in 96-well culture plates. (C) Effect of HK-I or HK-IV on staurosporine induced apoptosis of SK-N-SH cells. Cells were incubated with staurosporine (1.5 μM) in the presence of a single concentration (1 μM) of HK-I or HK-IV in cell culture medium for 6 h prior to the caspase 3/7 activity assay. (D) Effect of anti-HK-I IgG on the protective function of HK-I on staurosporine induced apoptosis. Cells were incubated with staurosporine (1.5 μM) and HK-I (1 μM) in the presence of isolated autistic anti-HK-I IgG (1 μM). * Significantly different from controls (P<0.001).
4. Discussion
There is increasing information suggesting that aberrant immune activity during neurodevelopment may be linked to the neurologic dysfunction characteristic of autism (Ashwood et al., 2006). Several reports have described the presence of autoantibodies to neurologic antigens in autistic patients ( Singh et al., 1993, 1997; Vojdani et al., 2002, 2003, 2004). For this study, we selected autistic patients with high clinical diagnostic scores. Although most reports show that the gender ratio (male:female) in autistic children ranges between 2:1 to 4:1, in our group, 32 out of 34 patients were male. This discrepancy can be partially explained by the fact that parents of 6 female patients declined to participate in the study, while parents of only 2 male patients declined to participate. Furthermore, most of our patients represent the current mixed Chilean population, which descends from both aboriginal peoples (Amerindians of Asian origin) and Spanish conquerors of European origin, who arrived in the country in the latter part of the 16th century. Chilean patients have different genes of susceptibility to autoimmune diseases, such as rheumatoid arthritis, Vogt-Koyanagi-Harada disease, and ankylosing spondylitis, than patients with either isolated Amerindian or European ancestry. Thus, our autistic patients may have different genetic backgrounds than patients from other countries. (Massardo L, et al., 1990; Cifuentes L, et al., 2004).
We investigated the immune response in these patients against VDAC, a key receptor in the brain, which is thought to prevent ischemic damage to brain tissue (Perez-Velazquez et al., 2003) due to its capacity to close when it binds HK-I (Azoulay-Zohar et al., 2004). VDAC also functions as a NADH-ferricyanide reductase on the plasma membrane, where it is critical to maintain cellular redox homeostasis (Baker et al., 2004). We found that both whole serum and purified anti-VDAC IgG from autistic serum have the capacity to decrease human neuroblastoma SK-N-SH cell proliferation, presumably via a mechanism that involves inhibition of NADH-ferricyanide reductase activity in these cells. This antibody binds to a primary structural sequence (Lys235-Lys255) of VDAC, in the vicinity of the Cys232 residue, one of the cysteine residues critical for the function of an NADH dehydrogenase (Baker et al., 2004). Because this activity is involved in the regulation of apoptosis (Lawen et al., 2005), the autistic anti-VDAC antibody appears to affect this mechanism. Whole autistic sera have been previously demonstrated to inhibit the growth and differentiation of cultured neuronal progenitor cells (Mazur-Kolecka et al., 2007), but no attempt was made by these investigators to identify the factors in autistic sera involved in these abnormalities.
The antibody against HK-I blocks the binding of HK-I to VDAC and abolishes the protective effect of this enzyme in preventing cell death. HK-I is a specific brain enzyme that shares with HK-II a highly homologous 15 amino acid N-terminal sequence, which is absent in HK-III and HK-IV typically found in the liver (Trayer, 1981). This peptide includes the amino acid sequence MIAAQLLAYYFTELK (Met1-Lys15), which serves HK-I as the anchoring region to brain VDAC (Oudard et al., 2004). Because autoantibody titers against HK-IV in the autistic population were similar to those of the control population, our results suggest that the 15 amino acid N-terminal extension of brain HK-I is a critical target of autoimmunity in autistic children.
Several investigators have reported anti-VDAC antibodies that prevent cell damage in brain tissue (Perez-Velazquez et al., 2003). Two antibodies against different segments of VDAC, including PTYADLGKSARDVFT (Pro4–Thre18) and SPNTGKKNAKIKTGY (Ser101–Tyr118), were found to attenuate ischemia-induced mitochondrial depolarization and deterioration of the neuronal electrophysiologic properties (Perez-Velazquez et al., 2003). In addition, antibodies against the sequence of VDAC including PNTGKKNAKIKTGYKREH (Pro102-His122) and GYQMNFETAKSRVTQ (Gly152-Gln166) significantly inhibit staurosporine-induced apoptosis in HeLa cells (Shimizu et al., 2001). It is important to mention that none of these antibodies react with epitopes that affect either the cysteine residues or the NADH binding domain critical for the NADH-ferrycianide reductase activity of VDAC (Baker et al., 2004). However, the anti-VDAC autoantibody found in autistic children discussed in this study has the opposite effect, presumably as a result of its capacity to inhibit this enzymatic activity.
Reports in the literature demonstrate that the caudate nucleus is enlarged (Langen et al., 2007), whereas the cerebellar vermis and its components are smaller, in subjects with autism (Hashimoto et al.,1995). The report by Hashimoto et al. emphasized the fact that abnormalities in autism are due to hypoplasia secondary to an early insult rather than a progressive degenerative process. These investigators suggest that environmental factors acting in the prenatal or perinatal periods are risk factors that may play a role in the onset of autism. In this context, exposure to antigens that may induce autoantibodies to VDAC and HK-I may play a role by selectively targeting cells in the cerebellum, thereby causing hypoplasia of this structure. The inhibition of SK-N-SH cell proliferation with whole autistic serum suggests such a possibility; however, in addition to these two autoantibodies, there are many other, still unidentified, autoantibodies that may participate in such a phenomenon. Inhibition of NADH-ferricyanide reductase activity in cells treated with whole serum suggests that anti-VDAC autoantibodies may be involved in changes of the redox state that may cause a decrease in cell proliferation leading to hypoplasia. Although isolated anti-VDAC IgG causes apoptosis of SK-N-SH cells, it is possible that some other autoantibodies may also be involved in cerebellar hypoplasia. These studies are part of an ongoing project, and they will be the subject of a future report by our laboratory.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute, NIH grant HL-24066
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem. 2008;283:13482–13490. doi: 10.1074/jbc.M708216200. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Avron M, Shavit N. A sensistive and simple method for determination of ferrocyanide. Anal Biochem. 1963;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Lane DJR, Ly JD, DePinto V, Lawen A. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. J Biol Chem. 2004;279:4811–4819. doi: 10.1074/jbc.M311020200. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Jr, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, DeLorey TM, Tierney E. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Brocklehurst KJ, Davies RA, Agius L. Differences in regulatory properties between human and rat glucokinase regulatory protein. Biochem J. 2004;378:693–697. doi: 10.1042/BJ20031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes L, Morales, Sepúlveda D, Jorquera H, Acuña M. DYS19 and DYS199 loci in a Chilean population of mixed ancestry. Am J Phys Anthropol. 2004;125:85–89. doi: 10.1002/ajpa.10380. [DOI] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979;279:643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Messina A, Accardi R, Aiello R, Guarino F, Tommasello MF, Tommasino M, Tasco G, Casadio R, Benz R, De Giorgi F, Ichas F, Baker M, Lawen A. New functions of an old protein: the eukaryotic porin or voltage dependent anion selective channel (VDAC) Ital J Biochem. 2003;52:17–24. [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Elinder F, Akanda N, Tofighi R, Shimizu S, Tsujimoto Y, Orrenius S, Ceccatelli S. Opening of plasma membrane voltage-dependent anion channels (VDAC) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell Death and Differentiation. 2005;12:1134–1140. doi: 10.1038/sj.cdd.4401646. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M, Cuchacovich M, Llanos C, Urzua C, Gawdi G, Pizzo SV. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–11431. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M, Kaczowka SJ, Payne S, Wang F, Gawdi G, Pizzo SV. Plasminogen structural domains exhibit different functions when associated with cell surface GRP78 or the voltage-dependent anion channel. J Biol Chem. 2007;282:32811–32820. doi: 10.1074/jbc.M703342200. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25:1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- Kern JK. The possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Medical Hypotheses. 2002;59:255–260. doi: 10.1016/s0306-9877(02)00212-8. [DOI] [PubMed] [Google Scholar]
- Laemli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJMC, Van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lawen A, Ly JD, Lane DJR, Zarschler K, Messina A, De Pinto V. Voltage-dependent anion-selective channel 1 (VDAC1), a mitochondrial protein, rediscovered as a novel enzyme in the plasma membrane. Int J Biochem & Cell Biol. 2005;37:277–282. doi: 10.1016/j.biocel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Lecouteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism diagnostic interview—a standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A. Autism diagnostic interview-revised—a revised version of a diagnostic interview for caregivers and individuals with possible developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Massardo L, Jacobelli S, Rodríguez L, Rivero S, González A, Marchetti R. Weak association between HLA-DR4 and rheumatoid arthritis in Chilean patients. Ann Rheum Dis. 1990;49:290–292. doi: 10.1136/ard.49.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui NM, Smith DM, Clauser KR, Fichmann J, Andrews LE, Sullivan CM, Burlingame AL, Epstein LB. Immobilized pH gradient two-dimensional gel electrophoresis and mass spectrometric identification of cytokine-regulated proteins in ME-180 cervical carcinoma cells. Electrophoresis. 1997;18:409–417. doi: 10.1002/elps.1150180315. [DOI] [PubMed] [Google Scholar]
- Markwell MA. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982;125:427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mazur-Kolecka B, Cohen IL, Jenkins EC, Kaczmarski W, Flory M, Frackowiak J. Altered development of neuronal progenitor cells after stimulation with autistic blood sera. Brain Research. 2007;1168:11–20. doi: 10.1016/j.brainres.2007.06.084. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Dawson TM, Verma A, Gurley D, Colombini M, Snyder SH. Mitochondrial voltage-dependent anion channel. J Biol Chem. 1993;268:23289–23296. [PubMed] [Google Scholar]
- Money J, Bobrow NA, Clarke FC. Autism and autoimmune disease: a family study. J Autism Child Schizophr. 1971;1:146–160. doi: 10.1007/BF01537954. [DOI] [PubMed] [Google Scholar]
- Oudard S, Miccoli L, Beurdeley-Thomas A, Butrillaux B, Poupon MF. Homophilic anchorage of brain hexokinase to mitochondria porins revealed by specific peptide antibody cross recognition. Bull Cancer. 2004;91:E184–E200. [PubMed] [Google Scholar]
- Perez-Velazquez JL, Kokarovtseva L, Weisspapir M, Frantseva M. Anti-porin antibodies prevent excitotoxic and ischemic damage to brain tissue. J Neurotrauma. 2003;20:633–747. doi: 10.1089/089771503322144554. [DOI] [PubMed] [Google Scholar]
- Raymond GV, Bauman ML, Kemper TL. Hippocampus in autism: a Golgi analysis. Acta Neuropathol. 2003;91:117–119. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- Roncero I, Alvarez E, Vázques P, Blázquez E. Functional glucokinase isoforms are expressed in rat brain. J Neurochem. 2000;74:1848–1857. doi: 10.1046/j.1471-4159.2000.0741848.x. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Schwab DA, Wilson JE. Complete amino acid sequence of rat brain hexokinase, deduced from the cloned cDNA, and proposed structure of a mammalian hexokinase. Proc Nat Acad Sci USA. 1989;86:2563–2567. doi: 10.1073/pnas.86.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Jiang C, Chen Q, Tang H. One-step on-column affinity refolding purification and functional analysis of recombinant human VDAC1. Biochem Biophys Res Commun. 2003;303:475–482. doi: 10.1016/s0006-291x(03)00359-0. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol. 2001;152:237–250. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer IP. Structure, function and evolution of the mammalian hexokinases. Biochem Soc Trans. 1981;9:23–25. doi: 10.1042/bst0090023. [DOI] [PubMed] [Google Scholar]
- Trottier G, Srivastava L, Walker CD. Biology of infantile autism: a review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci. 1999;24:103–115. [PMC free article] [PubMed] [Google Scholar]
- Vojdani A, Bazargan M, Vojdani E, Samadi J, Nourian AA, Eghbalieh N, Cooper EL. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin Diagnost Lab Immunol. 2004;11:515–524. doi: 10.1128/CDLI.11.3.515-524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A, Campbell AW, Anyanwu E, Kashanian A, Bock K, Vojdani E. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J Neuroimmunol. 2002;129:168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003;16:189–199. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]