Abstract
Novel paclitaxel-mimicking alkaloids were designed and synthesized based on a bioactive conformation of paclitaxel, i.e., REDOR-Taxol. The alkaloid 2 bearing a 5-7-6 tricyclic scaffold mimics REDOR-Taxol best among the compounds designed and was found to be the most potent compound against several drug-sensitive and drug-resistant human cancer cell lines. MD simulation study on the paclitaxel mimics 1 and 2 as well as REDOR-Taxol bound to the 1JFF tubulin structure was quite informative to evaluate the level of mimicking. The MD simulation study clearly distinguishes the 5-6-6 and 5-7-6 tricyclic scaffolds, and also shows substantial difference in the conformational stability of the tubulin-bound structures between 2 and REDOR-Taxol. The latter may account for the large difference in potency, and provides critical information for possible improvement in the future design of paclitaxel mimics.
1. Introduction
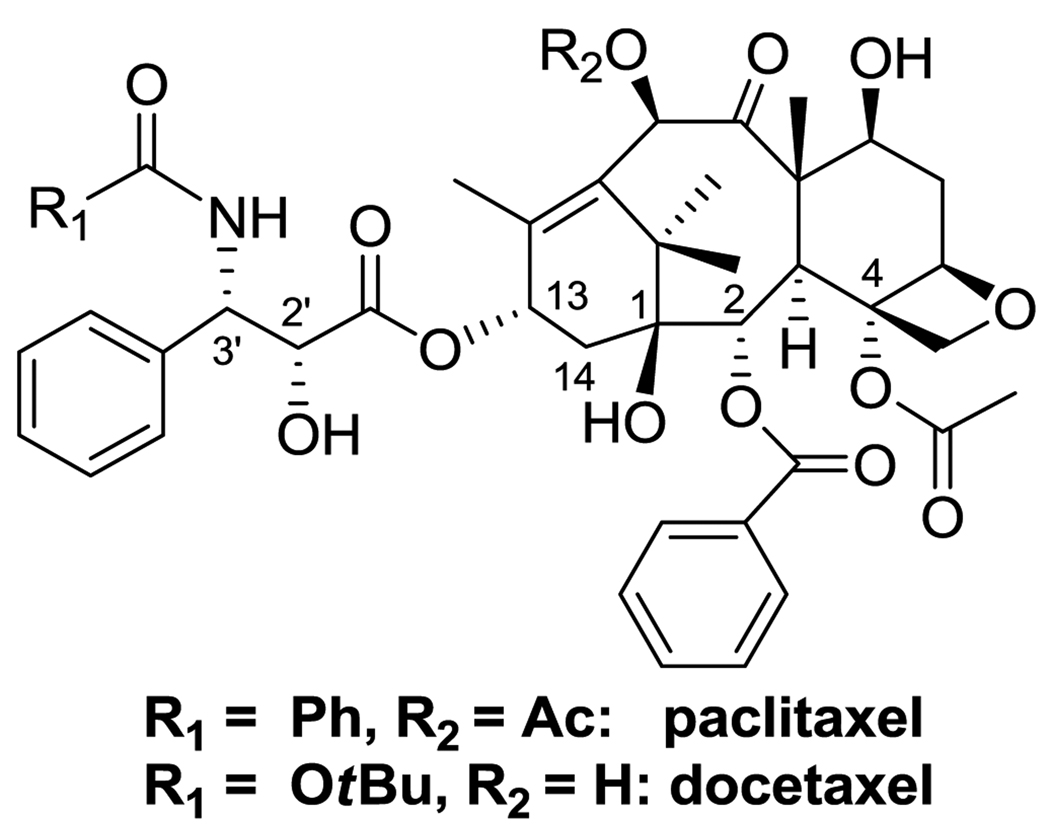
Cancer is the first leading cause of death for people under the age of 85 in the U.S.1–2 Paclitaxel (Taxol®) and docetaxel (Taxotere®) currently serve as the most widely used drugs in the fight against cancer among a variety of chemotherapeutic agents (Figure 1).3–4 The drugs bind to the β-tubulin subunit, accelerate the polymerization of tubulin and stabilize the resultant microtubules, which leads to apoptosis through cell-signaling cascade.5–6 New generation taxoids with much higher activities have been developed through structure-activity relationship (SAR) studies.7–11
Figure 1.
Paclitaxel and docetaxel
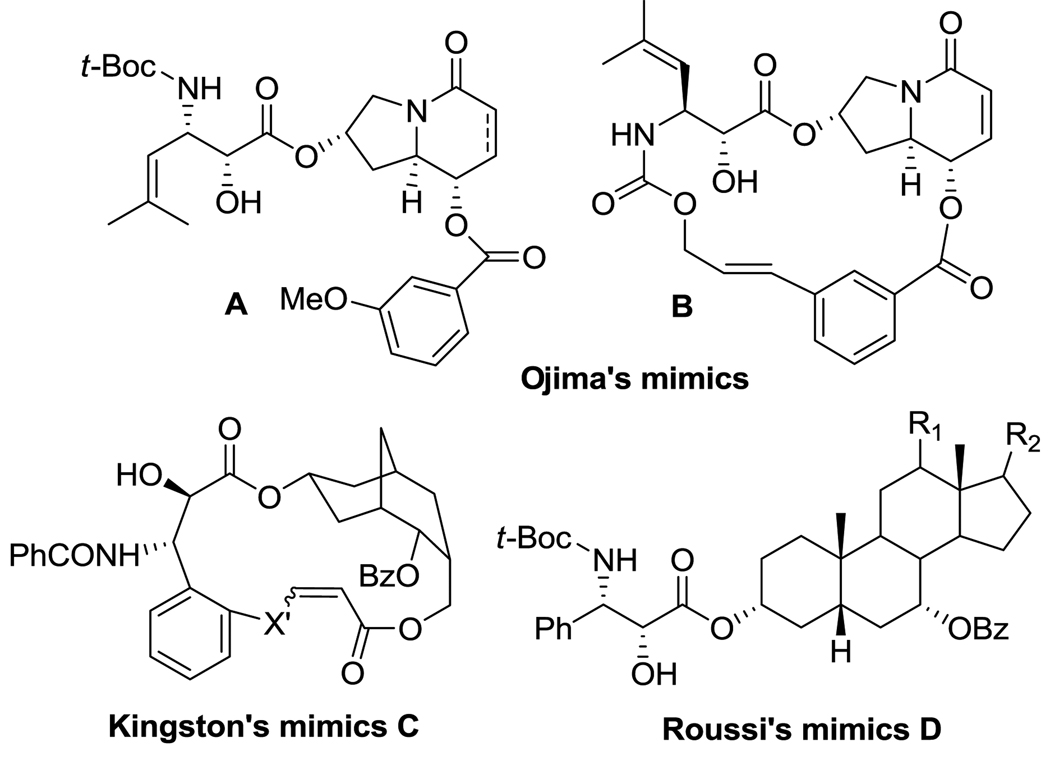
Since the paclitaxel structure is highly complex, especially its baccatin component, it would be exciting if we can design and develop paclitaxel mimics with much simpler structure, retaining potent anticancer activity.12–13 Thus, some pioneering efforts along this line have been made by a couple of research groups. In 2004, Ojima and coworkers reported four paclitaxel mimics with an indolizidine alkaloid scaffold based on their pharmacophore model (Figure 2).14 Two hydroxyl groups were designed to mimic the C2 and C13 hydroxyl groups (atom-atom distance and dihedral angle) of the baccatin III skeleton. Their mimics showed substantially reduced but still good cytotoxicity (IC50 = single~double digit µM) against human breast cancer cell lines, but did not promote tubulin polymerization at the concentrations examined.14 In 2005, Génard, Gérrite and coworkers synthesized eight steroidal compounds bearing the phenylisoerine and benzoate side-chains to mimic the “T-form” docetaxel (Figure 2).15 Their mimics only showed four orders of magnitude lower activity than docetaxel and did not show any inhibitory activity for microtubule disassembly.15 Unexpectedly, however, two compounds showed inhibitory activity for microtubule assembly.15 In 2006, Kingston and coworkers reported five macrocyclic paclitaxel-mimics (Figure 2) based on a highly potent conformationally restrained macrocyclic paclitaxel analog, which was designed to mimic the T-Taxol structure.16–17 Their mimics showed fairly good cytotoxicity (IC50 = 11 ~ 18 µM) against a human ovarian cancer cell line A2780 as well as weak, but recognizable activity in promoting tubulin polymerization and stabilizing microtubules.16–17 We report here the design, synthesis and biological evaluation of novel paclitaxel mimics, bearing tricyclic alkaloid scaffolds, mimicking the REDOR-Taxol13, 18–19 structure.
Figure 2.
Previously reported paclixel mimics
2. Design and synthesis of novel paclitaxel mimics
We set out to design novel paclitaxel mimics through molecular modeling analysis of tricyclic alkaloid skeletons by adding an acetoxymethylbenzene moiety to the previously reported indolizidine scaffold, mentioned above. The acetoxymethyl moiety was intended to mimic the acetoxy group of paclitaxel at the C4 position. The molecular design and analysis of the novel paclitaxel mimics were carried out using the REDOR-Taxol structure,13, 18–19 which was proposed based on the analysis of the tubulin-bound bioactive conformation of paclitaxel. The REDOR-Taxol structure has been successfully used for the design and synthesis of highly active conformatinoally rigidified macrocyclic taxoids.13, 18
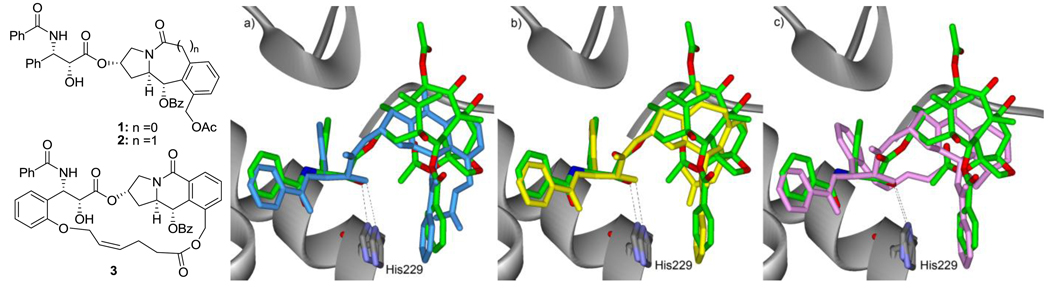
First, the designed tricyclic alkaloids with an N-benzoylphenylisoserine side chain were analyzed by molecular mechanics (MM) energy-minimization (InsightII 2000) at the binding site of paclitaxel in β-tubulin (1TUB20). The binding structures were compared with the REDOR-Taxol structure and the ones showing good overlays were selected. Mimics 1 and 2 have three ester groups in the tricyclic ring systems to mimic the C2, C4 and C13 groups of the baccatin III skeleton. Macrocyclic mimic 3 was also designed to rigidify the N-benzoylphenylisoserine and acetoxy moieties. These three mimics showed good overlay with the REDOR-Taxol structure in the 1TUB β-tubulin, as illustrated in Figure 3.
Figure 3.
Overlays of 1 (cyan) (a), 2 (yellow) (b), 3 (magenta) (c), and REDOR-Taxol (green) in the 1TUB β-tubulin.
3. Synthesis of paclitaxel-mimicking alkaloids
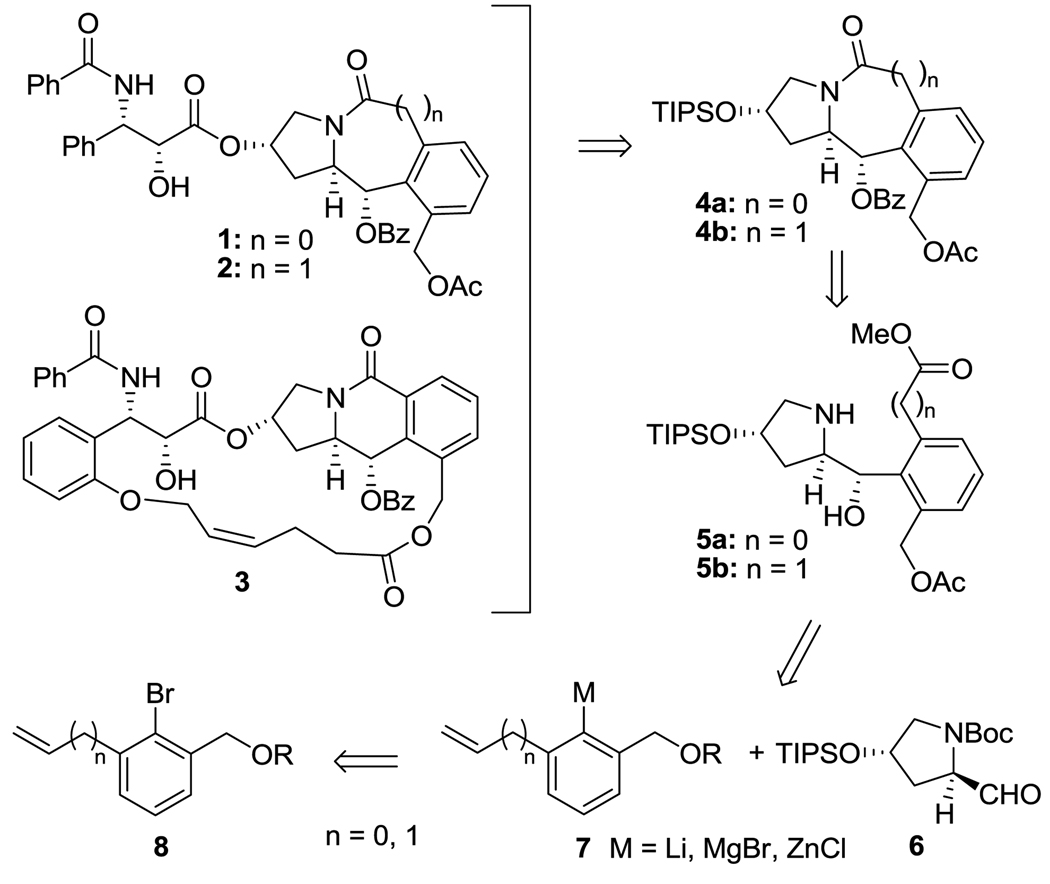
As Scheme 1 shows, the retrosynthetic analysis indicates that (i) compounds 1 and 2 can be obtained by introducing the phenylisoserine moiety to the tricyclic alkaloid scaffold 4, and (ii) compound 3 can be synthesized by introducing two olefinic side chains to 4 followed by ring-closing metathesis (RCM). Tricyclic alkaloid scaffold 4 can be prepared by the coupling reaction of lithium or Grignard species 7 with protected enantiopure prolinal 6, followed by lactamization and acylations.
Scheme 1.
Retrosynthesis of paclitaxel-mimicking alkaloids 1–3
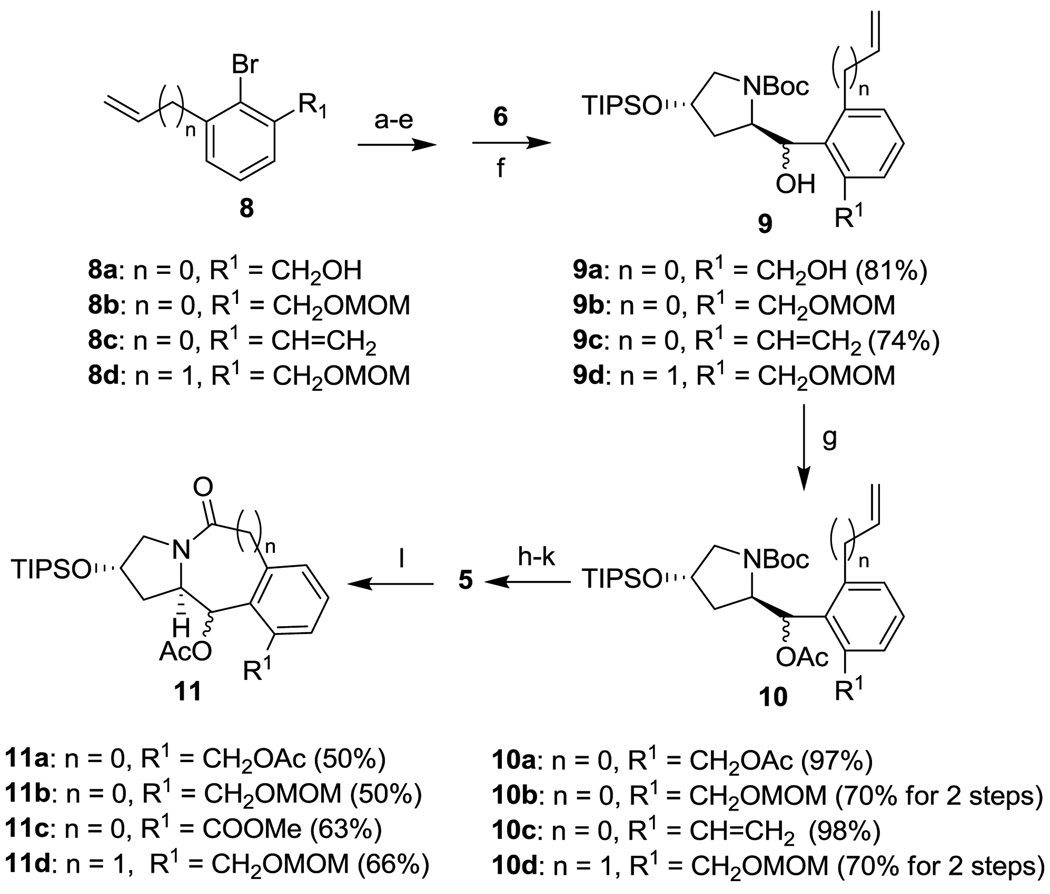
Preliminary study indicated that the coupling reaction of 7 with 6 was very sensitive to the bulkiness of the groups at the 2 and 6 positions of 7, and only small groups were tolerated in this coupling. After screening of various 2,6-substituents, bromobenzene 8a with a vinyl group serving as a hydroxymethyl synthon was selected for the synthesis of 4a via 5a (n = 0) (Scheme 2). An aryllithium species 7a (n = 0; R = Li) was generated by treating 8a (n = 0; R = H) with 2.2 equivalents of n-BuLi. The coupling of 7a with 6 in the presence of HMPA afforded the desired product 9a in 81% yield, and the subsequent acetylation of hydroxyl groups gave 10a in 97% yield. Since the diastereomers of 9a and 10a were not separable by HPLC analysis, the determination of diastereomer ratio (d.r.) had to wait after lactamization. The vinyl moiety of 10a was subjected to ozonolysis, oxidization, esterification, N-deprotection and lactamization to afford the desired 5-6-6 tricyclic scaffold 11a in 50% yield for 5 steps. Diastereomers, 11a-α and 11a-β, were separated by column chromatography. It was found that the d.r. was 2:1 with the undesirable diastereomer 11a-β as the major product (entry 1, Table 1) although the Garner model14, 21 predicted that 11a-α should be the major product. The stereochemistry of the acetoxy group in 11a-α as well as 11a-β was unambiguously assigned by NOE analysis.
Scheme 2.
Synthesis of tricyclic alkaloid scaffold 11. Reagents and conditions: (a) – (f) see Table 1; (g) Ac2O (1.5 ~ 3.0 eq), Et3N (2.5 ~ 5.0 eq), DMAP (0.2 eq), CH2Cl2, 0 °C-rt, overnight; (h) O3, CH2Cl2, −78 °C; Me2S, r.t., 3 h; (i) NaClO2 (8 eq), NaH2PO4 (8 eq), acetone, H2O, r.t., 40 min; (j) CH2N2, ether, r.t., 10 min; (k) CF3COOH, anisole (0.3 eq), CH2Cl2, 0 °C - r.t., 1 h; (l) NaHCO3, H2O, EtOAc, r.t., overnight.
Table 1.
Diastereoselectivity of the coupling of 8 with 6
| Entry | Substrate | Conditions | 11-α: 11-β |
|---|---|---|---|
| 1 | 8a | a, b, f | 1:2 |
| 2 | 8b | c, b, f | 3:4 |
| 3 | 8b | d, f | NA |
| 4 | 8b | a, e, b, f | NA |
| 5 | 8c | c, b, f | 1:1 |
| 6 | 8c | d, f | 3:2 |
| 7 | 8d | c, b, f | 1:10 |
Reagents and conditions: (a) n-BuLi (2.2 eq), THF, −78 °C, 1 h; (b) HMPA, −78 °C, 1 h (c) n-BuLi (1.1 eq), THF, −78 °C, 1 h; (d) Mg (1.2 eq), BrCH2CH2Br, THF, reflux, 2 h; (e) ZnCl2 (0.5 eq), THF, 0 °C, 1 h; (f) 6 (1.0 eq), −78 °C-r.t, overnight. NA = not applicable.
In the hope of possible improvement of diastereoselectivity in the coupling reaction of 8 with 6, 8b and 8c were employed under different coupling conditions (Scheme 2). Results are summarized in Table 1. Bromo(vinyl)benzene 8b with a MOM-protected hydroxymethyl group reacted with 6 to give 10b via 9b in good yield (70% for 2 steps), and 10b was subjected to the same protocol as that for 10a to afford 11b in 50% overall yield for five steps.22 The d.r. was found to be 3:4 in favor of 11b-β (entry 2). Next, 8b was successfully converted to potentially more selective Grignard species (entry 3) and zinc species (entry 4) by standard procedures.23–24 However, the attempted coupling reaction did not yield any desired product.
2,6-Divinylbromobenzene 8c, bearing no oxygen to coordinate to lithium, was converted to the corresponding aryllithium species25 and subjected to the coupling reaction with 6 to afford the product 9c in 74% yield. The desired product 11c (R1 = CO2Me) was obtained in good yield via 10c, using the same 6-step protocol as that for 9a. The d.r. was 1:1. The coupling reaction using the Grignard species generated from 8c afforded 9c in 74% yield, which was converted to 11c with a 3:2 d.r. in favor of the desirable 11c-α (entry 6).
The 5-7-6 tricyclic scaffold 11d was synthesized in a similar manner from 8d (n = 1, R1 = CH2O-MOM) (Scheme 2). The coupling of the aryllithium species generated from 8d with 6 gave 9c in 74% yield. The subsequesnt acetylation afforded 10d in 96% yield, which was converted to the 5-7-6 tricylclic scaffold 11d in 66% overall yield through the same 5-step protocol as that for 10a (Scheme 2). The d.r. was substantially increased to 10:1, but in favor of 11d-β. Since this stereochemistry can be inverted through the Mitsunobu reaction, further optimization of the coupling conditions was not pursued.
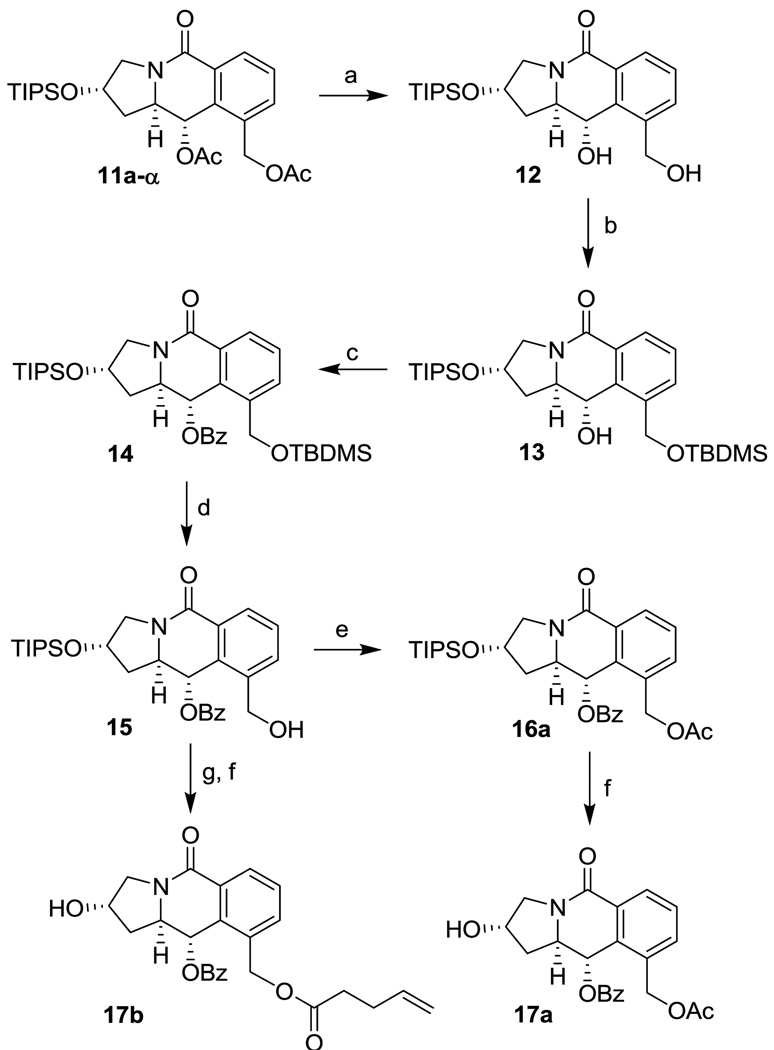
Enantiopure tricyclic scaffold 11a-α was converted to the coupling-ready 5-6-6 tricyclic scaffolds 17a and 17b as illustrated in Scheme 3. Basic hydrolysis of the two acetate groups of 11a-α gave 12 in high yield, and the subsequent selective protection of the primary alcohol of 12 with TBDMSCl in the presence of imidazole proceeded with very high selectivity, affording 13 in 99% yield.26 Acylation of the secondary alcohol of 13 using 6 equivalents of benzoyl chloride gave 14 in quantitative yield. Acidic deprotection of TBDMS group afforded 15 in 95% yield.27 Acylation of the primary alcohol of 15 with acetic anhydride gave 16a and the subsequent deprotection of the TIPS group with HF/pyridine afforded 17a quantitatively. In the same manner, the acylation of 15 with 4-pentenoyl chloride, NEt3, DMAP followed by deprotection with HF/pyridine gave 17b in quantitative yield.
Scheme 3.
Synthesis of tricyclic scaffolds 17a and 17b. Reagents and conditions: (a) K2CO3 (2.5 eq), MeOH, H2O, r.t. 30 min (84%); (b) TBDMSCl (1.5 eq), Imidazole (4.0 eq), DMF, r.t., 30 min (99%); (c) BzCl (6.0 eq), Et3N (8.0 eq), DMAP (0.05 eq), CH2Cl2, r.t., overnight (100%); (d) 0.1 N HCl, EtOH, r.t., 2.5 h (95%); (e) Ac2O (2.0 eq), Et3N (4.0 eq), DMAP (0.03 eq), CH2Cl2, r.t., overnight (100%); (f) HF/Py, CH3CN, Py, r.t., overnight (100%); (g) 4-pentenoyl chloride (2 eq), Et3N (4 eq), DMAP (0.03 eq), CH2Cl2, r.t., 1.5 h (100%).
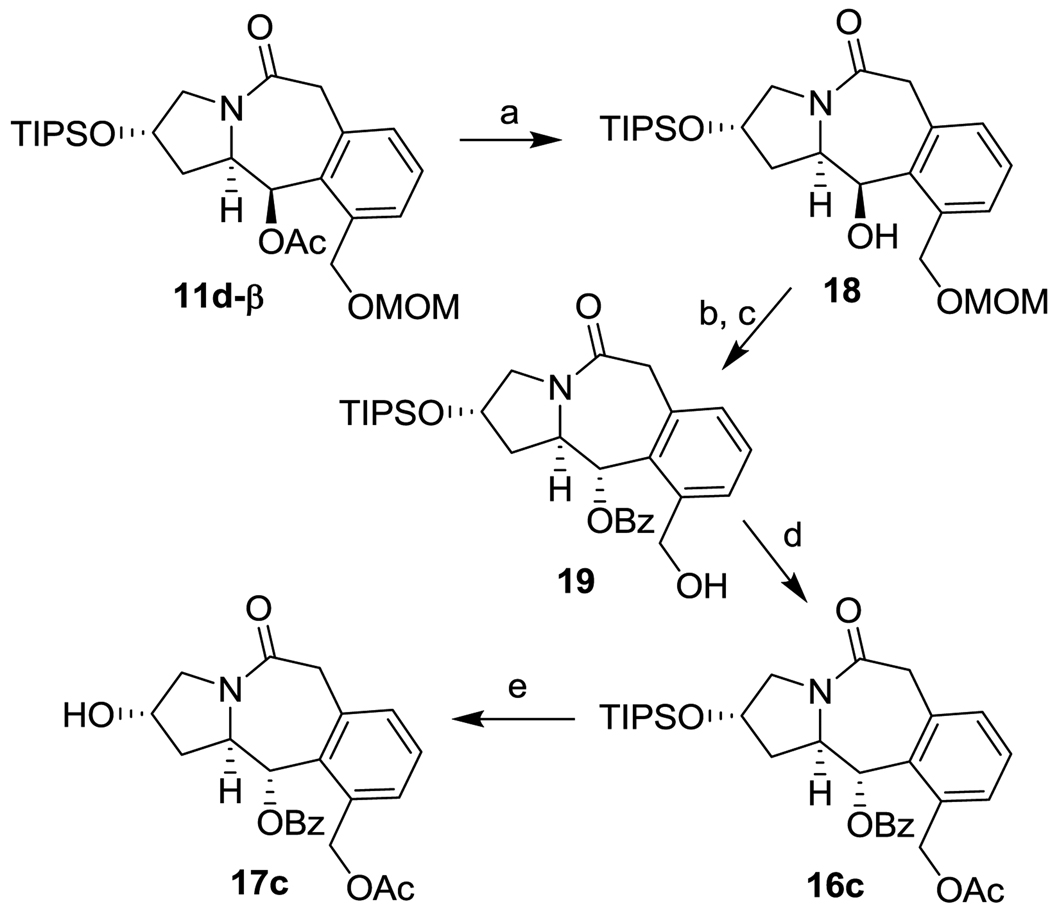
For the synthesis of enantiopure 5-7-6 tricyclic scaffold 17c, 11d-β was deacetylated to give 18 first, and then, 18 was subjected to the Mitsunobu reaction with benzoic acid in the presence of DIAD and PPh3 (Scheme 4). The conversion of this reaction was only 35% in refluxing THF for 3 days. Nevertheless, the desired α-benzoate 19 was obtained in 63% yield (based on 35% conversion) for 2 steps, after deprotection of the MOM group. Acetylation and the subsequent deprotection of the TIPS group with HF/pyridine gave coupling-ready 17c in high yield. The inversion of configuration at the chiral center bearing the benzoate moiety was confirmed by NOE analysis.
Scheme 4.
Synthesis of tricyclic scaffold 17c. Reagents and conditions: (a) K2CO3 (2.5 eq), MeOH, H2O, r.t. 30 min (81%); (b) DIAD (1.1 eq), PhCOOH (1.2 eq), PPh3 (1.1 eq), THF, reflux, 3 days; (c) anisole, CF3COOH, CH2Cl2, rt, 2 h (63% for 2 steps); (d) Ac2O (2 eq), Et3N (3 eq), DMAP (0.2 eq), CH2Cl2, 0 °C-rt, overnight (88%); (e) HF/Py, CH3CN, Py, r.t., overnight (86%).
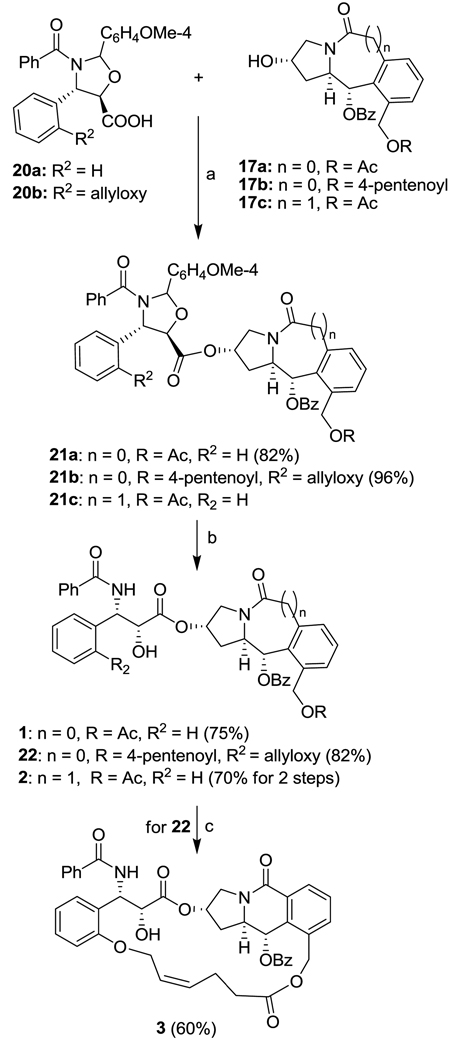
Tricyclic scaffolds 17a and 17c were coupled with oxazolidine acid 20a (R2 = H), which was prepared by the literature method,28–29 in the presence of EDC and DMAP in CH2Cl2 to give 21a and 21b. Deprotection of 21a and 21b with p-toluenesulfonic acid (p-TsOH) in methanol afforded the designed paclitaxel-mimicking alkaloids 1 and 2 in good yields (Scheme 5).
Scheme 5.
Synthesis of paclitaxel mimics 1–3. Reagents and conditions: (a) 20 (1.5 eq), EDC (2 eq), DMAP (0.5 eq), CH2Cl2, r.t., overnight; (b) p-TSA (0.2 eq), MeOH, r.t., 10 h; (c) Cl2Ru (=CHPh)(PCy3)2 (0.2 eq), CH2Cl2, reflux, 2 days.
For the synthesis of the macrocyclic paclitaxel-mimicking alkaloid 3, 20b was coupled with 17b, followed by deprotection to give the final precursor, diene 22 in high yield in the same manner as that described above. Finally, 22 was subjected to ring-closing metathesis (RCM) using the first-generation Grubbs catalyst. The reaction was very slow and not completed after 2 days in refluxing dichloromethane. Nevertheless, the designed paclitaxel mimic 3 was obtained in 60% yield.
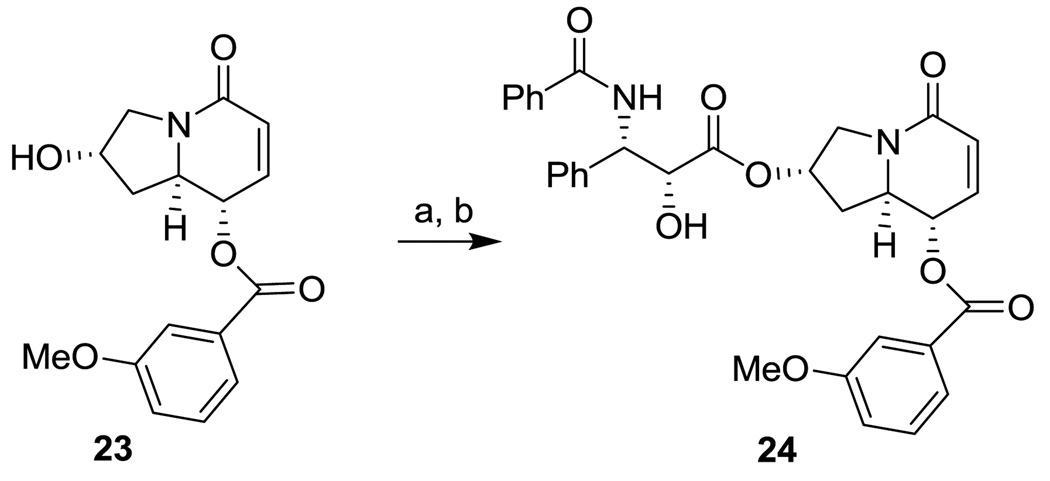
In order to examine the difference between the tricyclic alkaloid scaffolds and the bicyclic alkaloid scaffold that we reported previously,14 paclitaxel mimic 24 was also synthesized through coupling of 2314 with oxazolidine carboxylic acid 20a in the same manner as that described above (Scheme 6).
Scheme 6.
Reagents and conditions: (a) 20a (1.5 eq), EDC (2 eq), DMAP (0.5 eq), CH2Cl2, r.t., overnight; (b) p-TSA (0.2 eq), MeOH, r.t., 10 h, (76% for two steps).
4. Biological activity of novel paclitaxel mimics
Novel paclitaxel mimics, thus obtained, were evaluated for their cytotoxicities against drug-sensitive and drug-resistant human cancer cell lines and compared with those of previously reported paclitaxel mimics. Results are summarized in Table 2. As Table 2 shows, paclitaxel-mimic 2 bearing the 5-7-6 tricyclic scaffold is the most potent among the novel paclitaxel-mimicking alkaloids synthesized, exhibiting a single micromolar IC50 values against drug-sensitive human breast cancer cell lines, MCF7-S, LCC6-WT, human ovarian cancer cell line, A2780, and human pancreatic cancer cell line, CFPAC-1 in spite of its drastically simplified structure (entry 6). The macrocyclic mimic 3, bearing a 5-6-6 tricyclic scaffold, is the least potent among the newly synthesized paclitaxel-mimicking alkaloids. Nevertheless, mimic 3 shows IC50 value (14.8 µM), comparable to Kingston’s mimics (IC50 = 11.3~20 µM)14, 17 against A2780 ovarian cancer cell line (entry 7). The polycyclic alkaloid scaffold structures have critical effect on the potency of the mimics (entries 5, 6 and 8), i.e., the potency increases in the order, 24 (5–6 bicyclic) < 1 (5-6-6 tricyclic) < 2 (5-7-6 tricyclic), wherein 2 is substantially more potent than 1. Mimics 1 and 2 also possess an acetoxy group, mimicking the acetoxy group at the C4 position of paclitaxel, which might be contributing to better potency than 24. Based on the overlay, 2 is better mimicking paclitaxel than 1 for this acetoxyl group.
Table 2.
In vitro cytotoxicities of paclitaxel mimics (IC50, µM)a
| Entry | Compounds | MCF7-Sb | NCI/ADRc | LCC6-WTd | LCC6-MDRe | A2780f | CFPAC-1g |
|---|---|---|---|---|---|---|---|
| 1 | Paclitaxel | 0.0020 ± 0.0002 | 0.410 ± 0.045 | 0.0030 ± 0.0003 | 0.379 ± 0.022 | 0.020 ± 0.002 | 0.047± 0.007 |
| 2 | Mimic Ah | 5.70 ± 0.26 | 8.70 ± 0.18 | 6.68 ± 0.17 | 10.00 ± 0.56 | N.D. | N.D. |
| 3 | Mimic Bh | 8.10 ± 0.22 | 13.0 ± 0.6 | 10.5 ± 0.8 | >20 | N.D. | N.D. |
| 4 | Mimic Ci | N.A. | N.A. | N.A. | N.A. | 10.9±1.5 ~ 20±2 |
N.A. |
| 5 | 1 | 39.6 ± 4.0 | > 100 | 36.3 ± 3.5 | > 100 | 7.80 ± 0.13 | > 100 |
| 6 | 2 | 8.31 ± 0.75 | 41.5 ± 4.5 | 4.93 ± 0.39 | 16.8 ± 1.9 | 3.81 ± 0.33 | 6.16 ± 0.71 |
| 7 | 3 | > 100 | > 100 | > 100 | > 100 | 14.8 ± 1.5 | > 100 |
| 8 | 24 | 45.9 ± 0.40 | > 100 | 42.8 ± 0.26 | > 100 | N.D | > 100 |
Concentration of compound which inhibits 50% (IC50, µM) of the growth of human tumor cell line after 72 h drug exposure.
MCF7-S: human breast carcinoma cell line (Pgp−).
NCI/ADR: multi-drug resistant human ovarian carcinoma cell line (Pgp+).
LCC6-WT: human breast carcinoma cell line (Pgp−).
LCC6-MDR: mdr1 transduced cell line (Pgp+).
A2780: human ovarian carcinoma cell line.
CFPAC-1: human pancreatic carcinoma cell line.
Reference 14.
Reference 17.
The results also show clear difference between N-benzoylphenylisoserine, i.e., paclitaxel’s C13 side chain, and N-t-Boc-3-(2-methylprop-2-enyl)isoserine, a C13 side chain of second- and third-generation taxoids,10 with regard to their effect on the potency of paclitaxel mimics (entries 2 and 8), i.e. the potencies of mimic A is one order of magnitude higher than those of 24, wherein the only difference is the structure of the isoserine side chain.
The resistance factor, i.e., R-factor = IC50 (drug-resistant cell line)/IC50(drug-sensitive cell line), is an excellent indicator for the sensitivity of cytotoxic agents against MDR phenotype (P-glycoprotein overexpression, Pgp+). The R-factors of paclitaxel for the MCF-7 vs. NCI/ADR and LCC6-WT vs. LCC6-ADR are 205 and 126, respectively (entry 1). In contrast, the R-factors of 2 for the same pair of cell lines are 5.0 and 3.4, respectively (entry 6), whereas those of A are 1.5 for both pairs of cell lines (entry 2). Accordingly, mimics 2 and A are significantly less affected by the P-glycoprotein efflux pump and the use of non-aromatic isoserine side chain is found to be beneficial to further increase the potency against MDR cancer cell lines.
For the binding of mimics 2 to microtubules, a preliminary study has been done through competitive displacement of Flutax-230 using TR-NOESY analysis.31 The critical concentration of tubulin required for microtubule assembly was determined to be 3.7 µM, while DMSO (vehicle) was 3.3 µM. This might suggest that mimic 2 inhibits tubulin polymerization through low affinity interaction. Due to low water solubility of mimic 2, accurate binding constant has not been determined. Nevertheless, the result observed for Flutax-2 displacement indicates that the binding constant (Kb) would be in 50–100 µM range. Unexpected inhibitory activity was also observed for a T-Taxol mimic (mimic D, Figure 2).15 These results may imply that there are compounds, which interact with the paclitaxel binding site in β-tubulin, but destabilize tubulin nucleates/oligomers. The study on the binding ability of mimic 2 to tubulin will be reported elsewhere.
5. Molecular modeling analysis
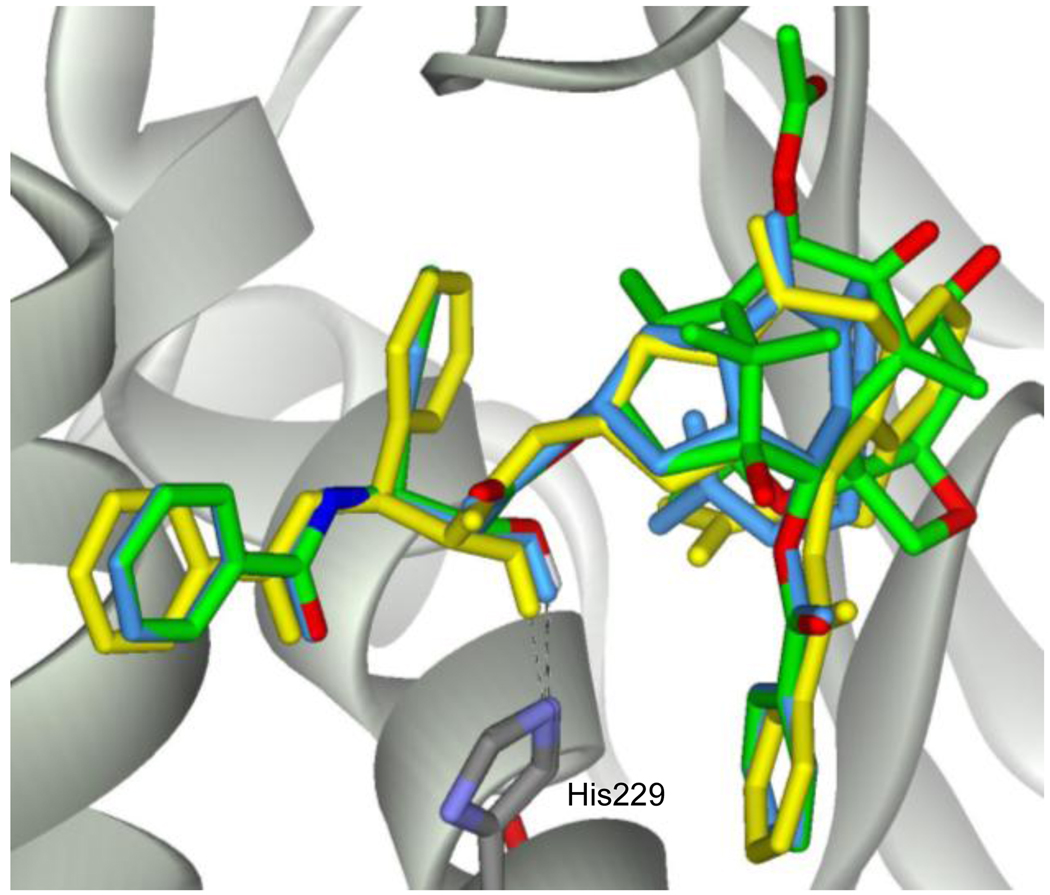
When we first designed paclitaxel mimics 1, 2 and 3, the REDOR-Taxol structure in the 1TUB20 tubulin was employed, as shown in Figure 3. Since then, the REDOR-Taxol structure was refined using the higher-resolution 1JFF32 β-tubulin.18–19 Accordingly, we performed molecular modeling as well as molecular dynamics (MD) studies on the mimics 1 and 2 in comparison with the REDOR-Taxol structure in the 1JFF tubulin. The overlay of the energy minimized structures of 1 and 2 in the 1JFF tubulin, using the InsightII 2000 program (CVFF force field), with the REDOR-Taxol-1JFF structure is shown in Figure 5.
Figure 5.
Overlay of paclitaxel (REDOR-Taxol-1JFF) (green), 1 (cyan) and 2 (yellow) in 1JFF
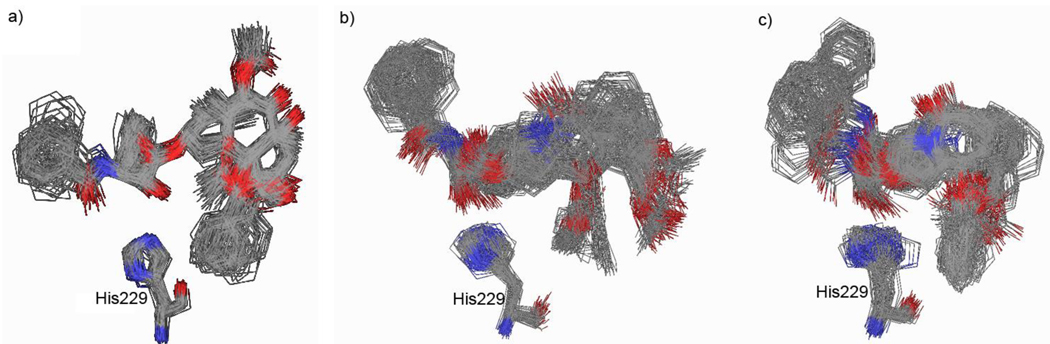
The critical H-bond between the phenylisoserine moiety’s C2’-OH of 1 (1.8 Å) or 2 (2.1 Å) and His229 was very stable during the energy minimization. As Figure 5 shows, the three structures have very good matching for the 3’-phenyl, 3’N-benzoyl, and 2-benzoate groups, as well as even the 4-acetoxyl group, although the oxetane and the northern part of the baccatin structure are missing. Nevertheless, 1 and 2 only exhibit much lower potency than paclitaxel. To obtain some insight into this observation, we carried out the MD simulations of 1 and 2 in 1JFF using the Macromodel program (MMFF94 force field33) and compared their conformational stability with that of REDOR-Taxol-1JFF. In the MD simulations, all atoms farther than 10 Å from the binding site were fixed, following our previously reported protocol,19 and the stability of the C2’-OH--N(His229) H-bond was monitored during the whole simulation in each case. The overlays of 200 snapshots for each (sampled every 0.25 ps) of the conformations of 1, 2 and paclitaxel are shown in Figure 6. As Figure 6 shows, the critical C2’-OH--N(His229) H-bond is stable in all three cases (average distance: 2.1 ± 0.4 Å for 1; 2.3 ± 0.7 Å for 2; 2.0 ± 0.2 Å for REDOR-Taxol-1JFF). However, the conformation of 1 is very flexible, i.e., unstable (Figure 6b), and substantially deviates from that of REDOR-Taxol (Figure 6a), especially the phenyl moiety as well as the acetoxy group, which is supposed to mimic the same group at the C4 position of paclitaxel. As Figure 6c indicates, 2 mimics REDOR-Taxol much better than 1, but the 3’-phenyl and 3’N-benzoyl groups, especially the latter, move around in a wide range, showing conformational instability. The MD simulation analysis may indicate that the conformational instability of the paclitaxel mimics needs to be addressed to improve their potency, besides structural over-simplification.
Figure 6.
MD simulation of paclitaxel (REDOR-Taxol-1JFF) (a), 1 (b) and 2 (c) in 1JFF (50 ps)
In summary, novel paclitaxel-mimicking alkaloids were synthesized based on the computational design, mimicking the REDOR-Taxol structure. The alkaloid 2 bearing a 5-7-6 tricyclic scaffold mimics REDOR-Taxol best among the compounds designed and was found to be the most potent compound against several drug-sensitive and drug-resistant human cancer cell lines. An MD simulation study on the paclitaxel mimics 1 and 2 as well as REDOR-Taxol in the 1JFF tubulin was quite informative to evaluate the level of mimicking, as compared to static energy minimized structures of these mimics. For example, the MD simulation study clearly distinguishes the difference between the 5-6-6 and 5-7-6 tricyclic scaffolds, and also substantial difference in the conformational stability of the tubulin-bound structures between 2 and REDOR-Taxol. The latter may account for the large difference in potency, and provides critical information for possible improvement in the future design of paclitaxel mimics. The comparison of potency with the previously reported paclitaxel mimics has disclosed a considerable enhancement in potency by introducing non-aromatic isoserine side chain, especially against MDR cancer cell lines. Thus, it is anticipated that the paclitaxel mimics, combining 5-7-6 tricyclic scaffolds and a non-aromatic isoserine side chains, would be considerably more potent than the mimics 2 and A, exhibiting IC50 values less than 1 µM against several cancer cell lines examined in this study. Further studies along this line are actively in progress in these laboratories.
5. Experimental
5.1. Chemistry
5.1.1. General Methods
1H and 13C NMR spectra were measured on a Varian 300, 400, 500 or 600 MHz NMR spectrometer. Melting points were measured on a Thomas Hoover Capillary melting point apparatus and are uncorrected. Specific optical rotations were measured on a Perkin-Elmer Model 241 polarimeter. TLC was performed on Merck DC-alufolien with Kieselgel 60F-254 and column chromatography was carried out on silica gel 60 (Merck; 230–400 mesh ASTM). Purity was determined with a Waters HPLC assembly consisting of dual Waters 515 HPLC pumps, a PC workstation running Millennium 32, and a Waters 996 PDA detector, using a Phenomenex Curosil-B column, employing CH3CN/water (2/3) as the solvent system with a flow rate of 1 mL/min. High-resolution mass spectra were obtained at the Mass Spectrometry Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL.
5.1.2. Materials
The chemicals were purchased from Aldrich-Sigma Co. and used as received or purified before use by standard methods. Tetrahydrofuran (THF) was freshly distilled from sodium metal and benzophenone. Dichloromethane was distilled immediately prior to use under nitrogen from calcium hydride. N,N-Dimethylformamide (DMF) was distilled over 4A molecular sieves under reduced pressure. 4-(N,N-Dimethylamino)pyridine (DMAP) was uses as received. The preparation of 8a–d is described in the Supplementary data.
5.1.3. (3S,5R)-1-(tert-Butoxycarbonyl)-5-[hydroxy(2-ethenyl-6-hydroxymethylphenyl)methyl]-3-triisopropylsiloxy-pyrrolidine (9a)
Aryl bromide 8a (94 mg, 0.44 mmol) was desolved in dry THF (4 mL) under N2 and 2.5 M n-BuLi in hexane (0.39 mL, 0.97 mmol) was added at −78 °C. The resulting solution was stirred for 1 h. Then, HMPA (0.09 mL) was added and the solution was stirred for 1 h. To this solution, a solution of aldehyde 6 (127 mg, 0.34 mmol) in dry THF (2.8 mL) was added dropwise. The resulting mixture was allowed to slowly warm up to room temperature with stirring overnight. Then, the reaction was quenched with a saturated NH4Cl solution (20 mL) and the reaction mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure, and the residue was purified by flash chromatography on silica gel (hexanes/EtOAc = 8/1–4/1) as eluent to give 9a (139 mg, 81% yield) as white solid: mp 38–40 °C; 1H NMR (400 MHz, CDCl3) δ 0.96 (m, 21 H), 1.32–1.44 (m, 9H), 1.74 (m, 1 H), 2.19 (m, 1 H), 3.34 (m, 1 H), 3.67 (m, 1 H), 4.31 (m, 1 H), 4.58 (m, 1 H), 4.76 (m, 1 H), 4.96 (m, 1 H), 5.10 (m, 1 H), 5.42 (m, 2 H), 7.03 (m, 1 H), 7.28 (m, 3 H); 13C NMR (100.5 MHz, CDCl3) δ 12.8, 18.5, 28.9, 39.4, 56.5, 57.1, 65.5, 70.9, 80.2, 81.3, 116.5, 117.7, 127.4, 127.7, 131.2, 136.5, 136.8, 140.2, 158.7. HRMS calcd. for C28H47NO5SiNa+ 528.3121, found 528.3116 (Δ = −1.0 ppm).
5.1.4. 1-(tert-Butoxycarbonyl)-2-[(2,6-diethenylphenyl)-hydroxymethyl]-4-triisopropylsiloxypyrrolidine (9c)
A mixture of 8c (257 mg, 1.22 mmol) and Mg turnings (35 mg, 1.47 mmol) in THF (12 mL) was added 1 drop of 1,2-dibromoethane and the mixture was heated at reflux for 2 h. Then, the resulting Grignard reagent solution was cooled to room temperature. Aldehyde 6 (226 mg, 0.61 mmol) was dissolved in THF (7 mL) and cooled down to −78 °C. The Grignard reagent generated above (1.22 mmol, 2.0 equiv) was added to the aldehyde solution by cannula and the reaction mixture was stirred overnight. The reaction was quenched with saturated aqueous NH4Cl solution (20 mL) and extracted with CH2Cl2 (20 mL × 3). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (17:1) as the eluent to afford 9c (227 mg, 74% yield) as colorless oil: 1H NMR (400 MHz, CDCl3) δ 0.96 (m, 21 H), 1.32–1.44 (m, 9H), 1.74 (m, 1 H), 2.19 (m, 1 H), 3.31 (m, 1 H), 3.57 (m, 1 H), 3.85 (m, 1 H), 4.51 (m, 1 H), 4.98 (m, 1 H), 5.12 (m, 2 H), 5.41 (m, 2 H), 6.96–7.74 (m, 5 H); 13C NMR (100.5 MHz, CDCl3) δ 12.0, 18.0, 28.2, 36.8, 39.6, 55.6, 63.8, 70.0, 74.1, 81.0, 114.9, 127.4, 127.9, 128.1, 136.5, 136.4, 137.9, 158.8. HRMS calcd. for C29H46NO4SiH+ 502.3353, found 502.3373 (Δ = 4.0 ppm).
5.1.5. (3S,5R)-5-[Acetoxy(2-acetoxymethyl-6-ethenylphenyl)methyl]-1-(tert-butoxycarbonyl)-3-triisopropylsiloxypyrrolidine (10a)
To a solution of 9a (134 mg, 0.26 mmol) and DMAP (4.8 mg, 0.06 mmol) in CH2Cl2 (2.0 mL) was added triethylamine (0.41 mL, 1.4 mmol) and acetic anhydride (0.27 mL, 0.84 mmol) at 0 °C. The reaction mixture was stirred overnight, quenched with saturated aqueous NH4Cl solution (20 mL) and extracted with CH2Cl2 (30 mL × 3). The organic layer was dried over anhydrous MgSO4 and the solvent was removed under reduced pressure to afford a liquid residue. The residue was purified by silica gel column chromatography on silica gel using hexanes:EtOAc (8:1) as the eluent to afford 10a (150 mg, 97% yield) as colorless oil: 1H NMR (400 MHz, CDCl3) δ 1.04 (m, 21 H), 1.28–1.43 (m, 9 H), 1.70 (m, 1 H), 2.06–2.10 (m, 7 H), 3.40 (m, 1 H), 3.62 (m,1 H), 4.57 (m, 1 H), 4.79 (m, 1 H), 5.31 (m, 3 H), 5.54 (m, 2 H), 6.03 (m, 1 H), 7.17–7.52 (m, 4 H); 13C NMR (100.5 MHz, CDCl3) δ 12.1, 17.9, 20.9, 28.0, 28.4, 38.3, 55.0, 58.5, 64.3, 71.0, 73.9, 79.5, 116.6, 128.2, 128.7, 129.9, 132.3, 134.2, 136.5, 139.4, 154.6, 170.3, 170.7. HRMS calcd. for C32H51NO7SiNa+ 612.3333, found 612.3329 (Δ = −0.6 ppm).
5.1.6. (3S,5R)-2-[Acetoxy(2-methoxymethoxymethyl-6-ethenyl-phenyl)methyl]-1-(tert-butoxycarbonyl)-3-triisopropylsiloxypyrrolidine (10b)
Colorless oil; 70% yield for 2 steps; 1H NMR (400 MHz, CDCl3) δ 1.02 (m, 21 H), 1.28–1.43 (m, 9 H), 2.06–2.10 (m, 5 H), 3.40 (m, 4 H), 3.59 (m, 1 H), 4.57 (m, 2 H), 4.79 (m, 4 H), 5.28 (d, J = 10.8 Hz, 1 H), 5.51 (d, J = 17.6, 1 H), 6.02–6.22 (m, 1 H), 7.17–7.52 (m, 4 H); 13C NMR (100.5 MHz, CDCl3) δ 12.1, 17.9, 20.9, 28.0, 28.4, 38.3, 54.7, 55.0, 58.6, 66.6, 70.2, 72.7, 79.7, 95.4, 116.0, 127.2, 127.5, 128.9, 129.1, 133.5, 136.4, 136.8, 137.0, 138.6, 154.4, 169.5. HRMS calcd. for C32H53NO7SiH+ 592.3670, found 592.3663 (Δ = −1.2 ppm).
5.1.7. (3S,5R)-5-[Acetoxy(2,6-diethenylphenyl)methyl]-1-(tert-butoxycarbonyl)-3-triisopropylsiloxypyrrolidine (10c)
Colorless oil; 98% yield; 1H NMR (400 MHz, CDCl3) δ 1.09 (m, 21 H), 1.28–1.47 (m, 9 H), 1.66 (m, 1 H), 1.90–2.10 (m, 4 H), 3.48 (m, 2 H), 4.43 (m, 1 H), 4.79 (m, 1 H), 5.23 (m, 2 H), 5.47 (m, 2 H), 6.43 (m, 1 H), 7.27–7.74 (m, 5 H). 13C NMR (100.5 MHz, CDCl3) δ 12.7, 18.5, 21.1, 28.7, 37.5, 55.5, 59.4, 71.5, 73.9, 79.4, 116.6, 128.2, 128.7, 129.5, 132.8, 137.4, 139.4, 155.8, 169.4. HRMS calcd. for C31H48NO5SiH+ 544.3458, found 544.3472 (Δ = 2.6 ppm).
5.1.8. (3S,5R)-5-[Acetoxy(2-allyl-6-methoxymethoxymethylphenyl) methyl]-1-(tert-butoxycarbonyl)-3-triisopropylsiloxypyrrolidine (10d)
Colorless oil; 70% yield for 2 steps; 1H NMR (400 MHz, CDCl3) δ 1.03 (m, 21 H), 1.28–1.43 (m, 9 H), 1.66 (m, 1 H), 2.05 (m, 4 H), 3.42 (s, 3 H), 3.65 (m, 4 H), 4.70 (m, 6 H), 5.05 (m, 2 H), 5.97 (m, 1 H), 6.10 (m, 1 H), 7.11–7.31 (m, 3 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.8, 21.0, 27.7, 37.6, 38.4, 55.2, 58.6, 66.9, 70.7, 72.5, 73.4, 79.7, 95.4, 115.9, 127.7, 128.1, 130.3, 133.5, 134.1, 137.1, 139.2, 154.5, 169.6. 1HRMS calcd. for C33H55NO7SiH+ 606.3826, found 606.3832 (Δ = 1.0 ppm).
5.1.9. (2S,10S/R,10aR)-10-Acetoxy-9-acetoxymethyl-2-triisopropylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-5-one (11a)
Nitrogen gas was bubbled into a solution of 10a (167 mg, 0.28 mmol) in CH2Cl2 (15 mL) at −78 °C for 3 min. Then, O3 gas was bubbled into the solution till the color of the solution turned blue (8 min), and N2 was bubbled into the solution for another 3 min until the blue color disappeared. Dimethyl sulfide (0.1 mL, 1.4 mmol) was added to the solution and the reaction mixture was allowed to warm to room temperature. The reaction mixture was stirred at room temperature for 3 h. The solvents were removed in vacuo and the resulting crude aldehyde was used in the next step without further purification.
Sodium chlorite (200 mg, 2.24 mmol) was added to a solution of the aldehyde obtained (~0.28 mmol) and sodium phosphate (monobasic) (350 mg, 2.24 mmol) in acetone/water (1:1, 2.4 mL). The reaction mixture was stirred at room temperature for 1 h and quenched with ethyl acetate (60 mL). The organic layer was washed with hydrochloric acid (1 N, 10 mL), Na2S2O3 (10%, 10 mL × 2), brine (10 mL), dried over MgSO4, filtered, and concentrated. The resulting crude acid was used in the next step without further purification.
A solution of potassium hydroxide (4 g) in water (8 mL) and ethanol (32 mL) was added to a solution of diazald® (4 g) in ether (60 mL) at 0 °C. The mixture was stirred for 10 min at 0 °C and distilled to afford a yellow ether solution. The diazomethane solution in ether was added to the solution of the acid obtained (~ 0.28 mmol) in ether (12 mL) until the yellow color did not disappear. The mixture was stirred for 10 min and the reaction was quenched with acetic acid. The solvents were removed in vacuo and the resulting crude methyl ester was used in the next step without further purification.
To a solution of crude ester obtained (~ 0.28 mmol) in CH2Cl2 (1.5 mL) at 0 °C was added trifluoroacetic acid (1.5 mL). The mixture was stirred at 0 °C for 30 min and room temperature for 30 min. The solvent and the acid were removed under reduced pressure and the residue was used in next step without further purification.
To a solution of the residue in ethyl acetate (14 mL) was added saturated aqueous sodium bicarbonate (14 mL). The mixture was stirred vigorously overnight and the reaction was quenched with ethyl acetate (50 mL). The reaction mixture was washed with water (10 mL) saturated aqueous ammonium chloride (10 mL), water (10 mL) and brine (10 mL). The organic layer was dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (3:1~2:1) as the eluent to afford 11a-α and 11a-β (1:2) in 50% yield for 5 steps.
5.1.9.1. (2S,10S,10aR)-10-Acetoxy-9-acetoxymethyl-2-triisopropylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-5-one (11a-α)
Colorless oil; 17% for 5 steps; [α]D22 −80.4 (c 1.1, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.09 (m, 21 H), 2.05 (s, 3 H), 2.06 (m, 2 H), 2.21 (s, 3 H), 3.80 (d, J = 3.2 Hz, 2 H), 4.15 (td, J = 10.8, 5.6 Hz, 1 H), 4.61 (s, 1 H), 5.09 (d, J = 12.8 Hz, 1 H), 5.33 (d, J = 12.8 Hz, 1H), 6.38 (d, J = 12.8, 1 H), 7.43 (t, J = 7.6 Hz, 1 H), 7.50 (d, J = 1.2 Hz, 1 H), 8.15 (dd, J = 7.6, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 12.2, 18.2, 20.8, 21.1, 42.2, 55.3, 58.9, 65.8, 69.5, 73.1, 128.6, 129.2, 130.8, 132.9, 134.2, 135.5, 162.3, 170.4, 171.1. HRMS calcd. for C26H39NO6SiH+ 490.2625, found 490.2621 (Δ = −0.8 ppm).
5.1.9.2. (2S,10R,10aR)-10-Acetoxy-9-acetoxymethyl-2-triisopropylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-5-one (11a-β)
Colorless oil; 33% yield for five steps; [α]D22 −137 (c 0.9, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.09 (m, 21 H), 1.83 (td, J = 12.4, 4.0 Hz, 1 H), 2.02 (s, 3 H), 2.07 (s, 3 H), 2.13 (ddd, J = 18.4, 7.2, 4.8 Hz, 1 H), 3.72 (d, J = 13.2 Hz, 1 H), 3.80 (dd, J = 12.8, 4.4, 1 H), 4.31 (ddd, J = 10.8, 5.6, 2.4 Hz, 1 H), 4.66 (t, J = 3.6 Hz, 1 H), 5.17 (d, J = 12.4 Hz, 1 H), 5.28 (d, J = 12.8 Hz, 1 H), 6.40 (d, J = 2.4 Hz, 1 H), 7.55 (m, 2 H), 8.18 (dd, J = 7.2, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 12.0, 17.9, 20.8, 20.9, 37.8, 54.6, 57.5, 63.1, 63.7, 69.5, 128.5, 129.8, 131.5, 133.5, 133.8, 162.0, 170.0, 170.4. HRMS calcd. for C26H39NO6SiH+ 490.2625, found 490.2618 (Δ = −1.4 ppm).
5.1.10. (2S,10S/R,10aR)-10-Acetoxy-9-methoxymethoxymethyl-2-triiso-propylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]iso-quinolin-5-one (11b)
Colorless oil (α:β = 3:4 by 1HNMR); 50% yield for 5 steps; 11bα: 1H NMR (400 MHz, CDCl3) δ 1.06 (m, 21 H), 2.11 (m, 2 H), 2.21 (s, 3 H), 3.31 (s, 3 H), 3.79 (m, 2 H), 4.14 (td, J = 10.8, 5.6 Hz, 1 H), 4.61 (m, 6 H), 4.80 (d, J = 12.8 Hz, 1 H), 6.32 (d, J = 10.4, 1 H), 7.40 (t, J = 7.6 Hz, 1 H), 7.53 (d, J = 7.6 Hz, 1 H), 8.10 (dd, J = 8.0, 1.6 Hz, 1 H). HRMS calcd. for C26H41NO6SiH+ 492.2781, found 492.2775 (Δ = −1.2 ppm).
5.1.11. (2S,10S/R,10aR)-10-Acetoxy-9-methoxycarbonyl-2-triisopropyl-siloxy-1,2,3,5,10,10a-hexahydropyrrolo-[1,2-b]iso-quinolin-5-one (11c)
Colorless oil (α:β = 3:2 by 1HNMR); 63% yield for 5 steps; 1H NMR (400 MHz, CDCl3) δ 1.04 (m, 21 H), 1.89 (td, J = 12.8, 4.0, 0.5 H), 1.98 (s, 1.5 H), 2.16 (m, 2 H), 3.77 (m, 2 H), 3.84 (s, 1.5 H), 3.92 (s, 1.5 H), 4.07 (m, 0.5 H), 4.30 (ddd, J = 11.2, 5.6, 2.4 Hz, 0.5 H), 4.64 (m, 1 H), 6.40 (d, J = 10.8, 0.5 H), 6.74 (d, J = 2.4 Hz, 0.5 H), 7.49 (t, J = 8.4 Hz, 0.5 H), 7.58 (t, J = 7.6 Hz, 0.5 H), 7.77 (dd, J = 8.0, 1.6 Hz, 0.5 H), 8.04 (dd, J = 8.0, 1.6 Hz, 0.5 Hz), 8.24 (dd, J = 8.0, 1.6 Hz, 0.5 H), 8.36 (dd, J = 7.6, 1.2 Hz, 0.5 H); 13C NMR (100.5 MHz, CDCl3) δ 12.3, 12.5, 17.9, 20.6, 20.8, 38.2, 42.2, 52.4, 52.6, 54.7, 54.9, 57.6, 58.9, 64.6, 69.2, 69.5, 71.8, 128.3, 129.4, 130.1, 130.2, 130.3, 130.8, 131.7, 131.9, 132.9, 133.5, 134.9, 136.7, 161.5, 161.7, 166.9, 168.6, 169.7, 170.1. HRMS calcd. for C26H41NO6SiH+ 492.2781, found 492.2775 (Δ = −1.2 ppm).
5.1.11.1. (2S,11S,11aR)-11-acetoxy-10-(methoxymethoxymethyl)-2-triisopropylsiloxy-2,3,11,11a-tetrahydro-1H-benzo[d]pyrrolo[1,2-a]azepin-5(6H)-one (11d-α)
Colorless oil; 63% yield for 5 steps; [α]D22 −26 (c 1.8, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.01 (m, 21 H), 1.99 (m, 2 H), 2.07 (s, 3 H), 3.36 (s, 3 H), 3.46 (d, J = 13.2 Hz, 1 H), 3.75 (d, J = 17.2 Hz, 1 H), 3.98 (dd, J = 12.8, 5.6 Hz, 1 H), 4.18 (dd, J = 10.8, 6.0 Hz, 1 H), 4.38 (d, J = 17.2 Hz, 1 H), 4.45 (t, J = 3.6 Hz, 1 H), 4.50 (d, J = 11.6 Hz, 1 H), 4.61 (dd, J = 12.8, 6.4 Hz, 2 H), 5.05 (d, J = 11.6 Hz, 1 H), 6.43 (s, 1 H), 7.14 (dd, J = 6.0, 2.4 Hz, 1 H), 7.25 (m, 2 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.9, 20.9, 40.9, 43.8, 55.4, 57.9, 59.8, 67.5, 68.9, 95.3, 128.9, 129.4, 131.2, 134.6, 135.6, 135.7, 167.5, 170.2. HRMS calcd. for C27H43NO6SiH+ 506.2938, found 506.2928 (Δ = −2.0 ppm).
5.1.11.2. (2S,11R,11aR)-11-acetoxy-10-(methoxymethoxymethyl)-2-triisopropylsiloxy-2,3,11,11a-tetrahydro-1H-benzo[d]pyrrolo[1,2-a]azepin-5(6H)-one (11d-β)
Colorless oil; 6% yield for 5 steps; [α]D22 +62 (c 0.2, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.05 (m, 21 H), 2.02 (dt, J = 13.6, 2.8 Hz, 1 H), 2.06 (s, 3 H), 2.26 (dd, J = 10.0, 4.0 Hz, 1 H), 3.38 (s, 3 H), 3.38 (d, J = 11.2 Hz, 1 H), 3.47 (dd, J = 10.4, 3.6 Hz, 1 H), 3.52 (d, J = 10.0 Hz, 1 H), 4.36 (m, 2 H), 4.46 (d, J = 10.0 Hz, 1 H), 4.52 (d, J = 9.6 Hz, 1 H), 4.65 (dd, J = 10.8, 5.2 Hz, 2 H), 4.84 (d, J = 9.6 Hz, 1 H), 6.41 (d, J = 2.4 Hz, 1 H), 7.26 (m, 3 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.8, 21.1, 41.9, 42.7, 55.4, 55.9, 61.6, 67.3, 67.9 70.9, 95.6, 129.2, 129.3, 129.9, 131.9, 137.5, 137.6, 169.9, 170.6. HRMS calcd. for C27H43NO6SiH+ 506.2938, found 506.2928 (Δ = −2.0 ppm).
5.1.12. (2S,10S,10aR)-10-Hydroxy-9-hydroxymethyl-2-triisopropyl-siloxy-2,3,10,10a-tetrahydro-1H-pyrrolo-[1,2-b]iso-quinolin-5-one (12)
To a solution of 11a-α (133 mg, 0.27 mmol) in methanol (10.9 mL) and water (5.5 mL) was added potassium carbonate (115 mg, 0.70 mmol). The mixture was stirred at room temperature for 35 min. Then, the reaction mixture was quenched with ethyl acetate (50 mL) and the organic layer was washed with saturated aqueous NH4Cl solution (10 mL), water (10 mL × 2) and brine (10 mL). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using 3% methanol in chloroform as the elant to afford 12 as a white solid (92 mg, 84% yield): mp 153–155 °C; [α]D22 −86 (c 1.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.06 (m, 21 H), 1.85 (ddd, J = 14.8, 10.8, 4.0 Hz, 1 H), 2.48 (dd, J = 12.8, 5.6 Hz, 1 H), 3.68 (d, J = 13.6 Hz, 1 H), 3.72 (dd, J = 13.2, 4.4 Hz, 1 H), 4.02 (td, J = 16.4, 5.2 Hz, 1 H), 4.61 (t, J = 3.6 Hz, 1 H), 4.71 (t, J = 12.4 Hz, 1 H), 4.83 (m, 2 H), 5.79 (bs, 1 H), 7.22 (m, 2 H), 7.78 (dd, J = 7.6, 3.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.9, 42.3, 55.3, 59.8, 65.1, 69.4, 73.3 127.5, 127.8, 129.5, 133.8, 137.3, 139.7, 162.9. HRMS: m/e calcd for C22H35NO4SiH+: 406.2414, found: 406.2411 (Δ = −0.6 ppm).
5.1.13. (2S,10S,10aR)-9-(tert-Butyldimethylsiloxymethyl)-10-hydroxy-2-triisopropylsiloxy-2,3,10,10a-tetrahydro-1H-pyrrolo-[1,2-b]isoquinolin-5-one (13)
To a solution of 12 (90 mg, 0.22 mmol) and imidazole (60 mg, 0.88 mmol) in dry DMF (0.6 mL) was added t-butyldimethylsilyl chloride (0.29 mmol in 1.2 mL DMF) dropwise via syringe at 0 °C. The reaction mixture was stirred for 35 min at room temperature and quenched with saturated aqueous NH4Cl solution (10 mL). The reaction mixture was extracted with EtOAc (20 mL × 3), washed with water (10 mL × 2), and brine (10 mL), dried over MgSO4 and concentrated in vacuo. The crude product was purified on a silica gel column using hexanes:EtOAc (2:1) as the eluent to give 13 as a white solid (113 mg, 99% yield): mp 183–184 °C; [α]D22 −110 (c 2.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ −0.01 (s, 3 H), 0.09 (s, 3 H), 0.87 (s, 9 H), 1.04 (m, 21 H), 1.93 (td, J = 12.8, 4.0 Hz, 1 H), 2.51 (dd, J = 12.8, 5.6 Hz, 1 H), 3.72 (d, J = 12.8 Hz, 1 H), 3.84 (dd, J = 12.8, 4.4 Hz, 1 H), 4.09 (td, J = 10.8, 5.6 Hz, 1 H), 4.64 (s, 1 H), 4.74 (d, J = 12.8 Hz, 1 H), 4.88 (dd, J = 10.8, 3.6, 1 H), 5.18 (d, J = 12.8 Hz, 1 H), 5.61 (d, J = 4.4 Hz, 1 H), 7.28 (m, 2 H), 8.04 (dd, J = 7.2, 2.0 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ −5.4, −5.1, 12.0, 17.9, 18.1, 25.6, 42.5, 55.2, 59.6, 66.2, 69.6, 73.8, 127.3, 128.3, 130.4, 132.7, 136.1, 139.9, 162.5. HRMS: m/e calcd for C28H49NO4Si2H+: 520.3278, found: 520.3279 (Δ = 0.1 ppm).
5.1.14. (2S,10S,10aR)-10-Benzoyloxy-9-(tert-butyldimethylsiloxymethyl)-2-triisopropylsiloxy-1,2,3,5,10,10a-hexahydro-pyrrolo[1,2-b]isoquinolin-5-one (14)
To a solution of 13 (60 mg, 0.115 mmol) and DMAP (4.8 mg, 0.06 mmol) in CH2Cl2 (1.0 mL) was added triethylamine (0.13 mL, 0.92 mmol) and benzoyl chloride (0.1 mL, 0.69 mmol) at 0 °C. The reaction mixture was stirred overnight, quenched with saturated aqueous NH4Cl solution (20 mL) and extracted with CH2Cl2 (30 mL × 3). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure to afford a liquid residue. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (6:1) as the eluent to afford 14 (73 mg, 100%) as colorless oil: [α]D22 –110 (c 1.3, CHCl3); 1H NMR (400 MHz, CDCl3) δ −0.22 (s, 3 H), 0.21 (s, 3 H), 0.75 (s, 9 H), 1.02 (m, 21 H), 2.15 (m, 2 H), 3.85 (m, 2 H), 4.30 (td, J = 10.8, 6.0 Hz, 1 H), 4.63 (s, 1 H), 4.67 (d, J = 14.4 Hz, 1 H), 4.76 (d, J = 14.0, 1 H), 6.60 (d, J = 10.8 Hz, 1 H), 7.48 (m, 3 H), 7.64 (m, 2 H), 8.13 (m, 3 H); 13C NMR (100.5 MHz, CDCl3) δ −5.7, −5.8, 12.0, 17.9, 18.2, 25.7, 41.9, 55.1, 58.7, 63.2, 69.3, 73.2, 127.2, 128.3, 128.7, 129.2, 129.7, 129.8, 130.7, 133.7, 133.8, 138.9, 162.6, 165.5. HRMS: m/e calcd for C35H53NO5Si2H+: 624.3541, found: 624.3516 (Δ = −3.9 ppm).
5.1.15. (2S,10S,10aR)-10-Benzoyloxy-2-triisopropylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-5-one (15)
To a solution of 14 (134 mg, 0.21 mmol) in ethanol (10 mL) was added 0.5 N HCl in ethanol (2 mL). The mixture was stirred at room temperature for 2.5 h. Then, the reaction mixture was quenched with saturated aqueous NaHCO3 solution (20 mL) and extracted with CH2Cl2 (30 mL × 3). The organic layer was washed with water (10 mL) and brine (10 mL). The organic layer was dried over anhydrous Na2SO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (2:1) as the eluent to afford 15 as a white solid (102 mg, 95% yield): mp 95–98 °C; [α]D22 −130 (c 1.3, CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.98 (m, 21 H), 2.17 (m, 2 H), 2.68 (bs, 1 H), 3.75 (d, J = 12.8 Hz, 1 H), 3.88 (dd, J = 12.8, 4.4 Hz, 1 H), 4.30 (td, J = 10.8, 6.0 Hz, 1 H), 4.55 (d, J = 13.6Hz, 1 H), 4.62 (d, J = 13.6, 1 H), 4.66 (s, 1 H), 6.53 (d, J = 10.8 Hz, 1 H), 7.32 (t, J = 8.0 Hz, 1 H), 7.49 (t, J = 8.0 Hz, 2 H), 7.61 (m, 2 H), 7.86 (dd, J = 7.6, 1.2 Hz, 1 H), 8.10 (dd, J = 8.4, 1.2 Hz, 2 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.9, 42.0, 55.1, 58.8, 63.1, 69.2, 73.0, 127.5, 128.4, 128.7, 129.1, 129.8, 132.1, 133.7, 134.1, 138.9, 162.7, 165.9. HRMS: m/e calcd for C29H39NO5SiH+: 510.2676, found: 510.2672 (Δ = −0.7 ppm).
5.1.16. (2S,10S,10aR)-9-Acetoxymethyl-10-benzoyloxy-2-triisopropyl-siloxy-1,2,3,5,10,10a-hexahydropyrrolo-[1,2-b]iso-quinolin-5-one (16a)
To a solution of 15 (32 mg, 0.06 mmol) and DMAP (1 mg, 0.005 mmol) in CH2Cl2 (0.5 mL) was added triethylamine (0.08 mL, 0.24 mmol) and acetic anhydride (0.05 mL, 0.12 mmol) at 0 °C. The reaction mixture was stirred overnight, quenched with saturated aqueous NH4Cl solution (20 mL) and extracted with CH2Cl2 (20 mL × 3). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (2:1) as the eluent to afford 16a (35 mg, 100%) as a colorless oil: [α]D22 −164 (c 14.1, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.05 (m, 21 H), 1.89 (s, 3 H), 2.17 (m, 2 H), 3.83 (td, J = 12.8, 3.6 Hz, 1 H), 4.32 (dd, J = 16.4, 9.2 Hz, 1 H), 4.63 (d, J = 2.0 Hz, 1 H), 5.02 (d, J = 12.8, 1 H), 5.20 (d, J = 12.4 Hz, 1 H), 6.70 (d, J = 10.8 Hz, 1 H), 7.48 (m, 4 H), 7.64 (ddd, J = 8.8, 2.4, 1.2 Hz, 1 H), 8.10 (dd, J = 8.4, 1.2 Hz, 2 H), 8.20 (dd, J = 6.8, 2.4 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.9, 20.4, 41.9, 55.1, 58.8, 65.3, 69.3, 73.1, 128.5, 128.7, 129.0, 129.1, 129.7, 130.6, 133.0, 133.8, 134.3, 135.7, 162.2, 165.7, 170.3. HRMS: m/e calcd for C31H41NO6SiH+: 552.2781, found: 552.2781 (Δ = −0.1 ppm).
5.1.17. (2S,10S,10aR)-9-Acetoxymethyl-10-benzoyloxy-2-hydroxyl-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-5-one (17a)
To a solution of 16a (31 mg, 0.056 mmol) in CH3CN (0.6 mL) and pyridine (0.6 mL) was added HF-pyridine (70:30, 0.31 ml) and the mixture was stirred overnight. The reaction mixture was diluted with EtOAc (40 mL) and washed with saturated aqueous NaHCO3 solution (10 mL × 2), CuSO4 solution (10 mL × 3), water (10 mL × 3) and brine (3 mL). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography using hexanes:EtOAc (1:4) as the eluent to afford 17a as white solid (23 mg, 100% yield): mp 76–77 °C; [α]D22 −239 (c 10.3, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.90 (s, 3 H), 2.24 (m, 2 H), 3.83 (dd, J = 11.2, 3.2 Hz, 2 H), 4.32 (td, J = 8.4, 4.4 Hz, 1 H), 4.64 (t, J = 3.2 Hz, 1 H), 5.04 (d, J = 10.0, 1 H), 5.21 (d, J = 10.4 Hz, 1 H), 6.70 (d, J = 8.4 Hz, 1 H), 7.48 (m, 4 H), 7.64 (t, J = 6.0 Hz, 1 H), 8.10 (d, J = 6.0 Hz, 2 H), 8.18 (dd, J = 6.0, 1.2 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 20.6, 41.2, 54.8, 58.9, 65.6, 68.8, 73.4, 128.8, 129.0, 129.1, 129.2, 130.0, 130.7, 133.4, 134.1, 134.6, 135.9, 162.6, 165.9, 170.6. HRMS: m/e calcd for C22H21NO6H+: 396.1447, found: 396.1436 (Δ = −2.8 ppm).
5.1.18. (2S,10S,10aR)-10-Benzoyloxy-9-pent-4-enoyloxymethyl-2-triiso-propylsiloxy-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]iso-quinolin-5-one (16b)
Colorless oil; 100% yield; [α]D22 −158 (c 2.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.00 (m, 21 H), 2.22 (m, 6 H), 3.83 (m, 2 H), 4.32 (dd, J = 18.0, 8.8 Hz, 1 H), 4.63 (d, J = 2.0 Hz, 1 H), 4.92 (dd, J = 10.4, 1.6 Hz, 1 H), 4.98 (t, J = 5.6 Hz, 1 H), 5.06 (d, J = 12.8, 1 H), 5.22 (d, J = 12.8 Hz, 1 H), 5.72 (m, 1 H), 6.68 (d, J = 10.8 Hz, 1 H), 7.49 (m, 4 H), 7.64 (t, J = 7.6 Hz, 1 H), 8.10 (dd, J = 8.4, 1.2 Hz, 2 H), 8.20 (dd, J = 7.2, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 11.9, 17.9, 28.5, 32.9, 41.9, 55.1, 58.9, 65.1, 69.3, 73.2, 115.4, 128.4, 128.7, 128.9, 129.0, 129.7, 130.6, 133.2, 133.8, 134.3, 135.7, 136.5, 162.2, 165.7, 172.3. HRMS: m/e calcd for C34H45NO6SiH+: 592.3094, found: 592.3077 (Δ = −2.9 ppm).
5.1.19. (2S,10S,10aR)-10-Benzoyloxy-2-hydroxy-9-pent-4-enoyloxy-methyl-1,2,3,5,10,10a-hexahydropyrrolo-[1,2-b]iso-quinolin-5-one (17b)
White solid; 97% yield; mp 50–52 °C; [α]D22 −227 (c 1.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 2.22 (m, 6 H), 3.85 (d, J = 2.4 Hz, 2 H), 4.33 (td, J = 10.4, 5.6 Hz, 1 H), 4.61 (t, J = 2.4 Hz, 1 H), 4.92 (dd, J = 10.4, 1.6 Hz, 1 H), 4.95 (dd, J = 13.2, 1.6 Hz, 1 H), 5.06 (d, J = 12.8 Hz, 1 H), 5.21 (d, J = 12.8 Hz, 1 H), 5.70 (m, 1 H), 6.68 (d, J = 10.8 Hz, 1 H), 7.48 (m, 4 H), 7.63 (t, J = 7.3 Hz, 1 H), 8.08 (d, J = 8.4, 1.2 Hz, 2 H), 8.14 (dd, J = 7.2, 1.2 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 28.5, 32.9, 40.9, 54.5, 58.7, 65.1, 68.4, 73.1, 115.5, 128.5, 128.7, 128.8, 128.9, 129.8, 130.4, 133.3, 133.9, 134.3, 135.7, 136.5, 162.6, 165.6, 172.3. HRMS: m/e calcd for C25H25NO6H+: 436.1760, found: 436.1750 (Δ = −2.3 ppm).
5.1.20. (2S,11R,11aR)-11-hydroxy-10-(methoxymethoxymethyl)-2-triisopropylsiloxy-2,3,11,11a-tetrahydro-1H-benzo[d]pyrrolo[1,2-a]azepin-5(6H)-one (18)
To a solution of 11d (260 mg, 0.51 mmol) in methanol (20 mL) and water (10 mL) was added potassium carbonate (180 mg, 1.02 mmol). The mixture was stirred at room temperature for 30 min. Then, the reaction mixture was quenched with ethyl acetate and the organic layer was washed with saturated aqueous NH4Cl solution (20 mL), water (10 mL × 2) and brine (10 mL). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (3:1) as the eluent to afford 18 as a white solid (189 mg, 81% yield): mp 104–105 °C; 1H NMR (400 MHz, CDCl3) δ 1.05 (m, 21 H), 2.20 (m, 1 H), 2.54 (m, 1 H), 3.35 (d, J = 14.0 Hz, 1 H), 3.40 (s, 3 H), 3.42 (d, J = 5.2 Hz, 1 H), 3.64 (dd, J = 11.6, 3.6 Hz, 1 H), 4.36 (d, J = 14.0 Hz, 1 H), 4.42 (dd, J = 15.6, 6.4 Hz, 1 H), 4.52 (d, J = 4.4 Hz, 1 H), 4.56 (d, J = 11.2 Hz, 1 H), 4.71 (dd, J = 11.6, 6.4 Hz, 2 H), 4.82 (d, J = 2.8 Hz, 1 H), 4.85 (s, 1 H), 7.26 (m, 3 H); 13C NMR (100.5 MHz, CDCl3) δ 12.0, 17.9, 43.0, 43.7, 53.9, 55.8, 60.5, 68.5, 69.4, 73.1, 95.6, 128.2, 130.9, 131.9, 133.7, 136.9, 138.6, 172.2. HRMS: m/e calcd for C25H41NO5SiH+: 464.2832, found: 464.2837 (Δ = 1.1 ppm).
5.1.21. (2S,11S,11aR)-10-(acetoxymethyl)-11-benzoyloxy-2-triisopropylsiloxy-2,3,11,11a-tetrahydro-1H-benzo[d]pyrrolo[1,2-a]azepin-5(6H)-one (16c)
To a solution of 18 (125 mg, 0.27 mmol), benzoic acid (40 mg, 0.32 mmol) and triphenylphosphine (78 mg, 0.30 mmol) in THF (1 mL) was added diisopropyl azodicarboxylate (0.060 mL, 0.30 mmol). The mixture was stirred overnight and refluxed for 3 days. Solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel using hexanes:EtOAc (5:1) to afford the corresponding Mitsunobu coupling product, (2S,11R,11aR)-11-benzoyloxy-10-(methoxy-methoxymethyl)-2-triisopropylsiloxy-2,3,11,11a-tetra-hydro-1H-benzo[d]pyrrolo[1,2-a]-azepin-5(6H)-one (47 mg, conversion 36%) accompanied by impurity and the starting material 18 (80 mg).
To a solution of the Mitsunobu coupling product thus obtained and anisole (0.2 mL) in CH2Cl2 (2.0 mL) was added trifluoroacetic acid (2.0 mL) at 0 °C. The reaction mixture was stirred 2 h at room temperature and diluted with ethyl acetate (50 mL). The organic layer was washed by saturated NaHCO3 solution (10 mL × 2) and NaCl (10 ml), dried over anhydrous MgSO4, and solvent was removed under reduced pressure to afford a liquid residue. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (2:1) as the eluent to afford 19 (32 mg, 63% yield in 2 steps) as colorless oil.
To a solution of 19 (22 mg, 0.042 mmol) and DMAP (1 mg, 0.005 mmol) in CH2Cl2 (0.5 mL) was added triethylamine (0.04 mL, 0.12 mmol) and acetic anhydride (0.03 mL, 0.08 mmol) at 0 °C. The reaction mixture was stirred overnight, quenched with saturated aqueous NH4Cl solution (20 mL) and extracted with CH2Cl2 (20 mL × 3). The organic layer was dried over anhydrous MgSO4 and solvent was removed under reduced pressure to afford a liquid residue. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (2:1) as the eluent to afford 16c (21 mg, 80% yield) as colorless oil: [α]D22 +5.5 (c 0.5, CHCl3); 1H NMR (600 MHz, CDCl3) δ 1.02 (m, 21 H), 1.95 (s, 3 H), 2.18 (m, 2 H), 3.55 (d, J = 13.2 Hz, 1 H), 3.92 (d, J = 17.8 Hz, 1 H), 4.04 (d, J = 13.2, 5.4 Hz, 1 H), 4.35 (dd, J = 10.2, 6.0 Hz, 1 H), 4.39 (d, J = 18.0 Hz, 1 H), 4.46 (s, 1 H), 4.25 (d, J = 12.6 Hz, 1 H), 5.57 (d, J = 12.0 Hz, 1 H), 5.59 (s, 1 H), 7.19 (d, J = 7.2 Hz, 1 H), 7.29 (t, J = 7.2 Hz, 1 H), 7.33 (d, J = 7.2 Hz, 1 H), 7.43 (t, J = 7.2 Hz, 2 H), 7.57 (t, J = 7.8 Hz, 1 H), 7.99 (d, J = 7.8 Hz, 2 H); 13C NMR (125.7 MHz, CDCl3) δ 12.1, 18.1, 29.9, 41.1, 44.3, 57.8, 60.0, 64.8, 67.9, 69.9, 128.9, 129.3, 129.5, 130.0, 130.5, 132.2, 133.9, 134.7, 134.8, 135.5, 165.8, 167.9, 170.5. HRMS: m/e calcd for C32H43NO6SiH+: 566.2938, found: 566.2955 (Δ = 3.0 ppm).
5.1.21. (2S,11S,11aR)-10-(acetoxymethyl)-11-benzoyloxy-2-hydroxy-2,3,11,11a-tetrahydro-1H-benzo[d]-pyrrolo[1,2-a]azepin-5(6H)-one (17c)
White solid; 86% yield; 1H NMR (500 MHz, CDCl3) δ 2.00 (s, 3 H), 2.20 (m, 2 H), 3.64 (d, J = 13.5 Hz, 1 H), 3.93 (d, J = 17.5 Hz, 1 H), 3.98 (m, 1 H), 4.40 (m, 2 H), 4.48 (s, 1 H), 5.26 (d, J = 12.5, 1 H), 5.58 (d, J = 12.5 Hz, 1 H), 6.61 (s, 1 H), 7.18 (d, J = 7.0 Hz, 1 H), 7.29 (t, J = 8.0 Hz, 1 H), 7.34 (d, J = 7.5 Hz ,1 H), 7.45 (t, J = 8.0 Hz, 2 H), 7.59 (d, J = 7.5 Hz, 1 H), 7.99 (d, J = 7.5 Hz, 2 H); 13C NMR (125.7 MHz, CDCl3) δ 20.8, 39.8, 44.0, 57.1, 59.5, 64.5, 67.0, 69.5, 128.7, 129.1, 129.3, 129.7, 129.8, 130.0, 131.8, 133.7, 134.3, 134.5, 135.2, 165.6, 167.9, 170.3. HRMS: m/e calcd for C23H23NO6H+: 410.1604, found: 410.1594 (Δ = −2.4 ppm).
5.1.22. (4S,5R)-2-(4-Methoxyphenyl)-3-benzoyl-4-phenyloxazolidine-5-carboxylic acid (20a)
White solid; 67% yield; 1H NMR (400 MHz, CDCl3) δ 3.82 (s, 3 H), 4.41 (s, 1 H), 4.90 (s, 1 H), 5.48 (s, 1 H), 6.85 (d, J = 8.4 Hz, 2 H), 6.90 (m, 1 H), 7.21–7.39 (m, 11 H); 13C NMR (100.5 MHz, CDCl3) δ 55.0, 55.6, 72.5, 114.3, 127.0, 127.1, 127.3, 128.1, 128.3, 128.3, 128.7, 128.8, 130.0, 132.0, 132.3, 133.2, 138.1, 164.6, 174.0, 190.9. HRMS: m/e calcd for C24H22NO5H+: 404.1498, found: 404.1495 (Δ = −0.7 ppm).
5.1.23. (4S,5R)-4-(2-Allyloxyphenyl)-3-benzoyl-2-(4-methoxyphenyl)oxazolidine-5-carboxylic acid (20b)
White solid; 88% yield; mp 47–49 °C; 1H NMR (400 MHz, CDCl3) δ 3.83 (s, 3 H), 4.47 (dd, J = 12.8, 3.6 Hz, 1 H), 4.55 (dd, J = 13.2, 5.2 Hz, 1 H), 4.91 (s, 1 H), 5.18 (d, J = 10.8 Hz, 1 H), 5.25 (d, J = 17.2 Hz, 1 H), 5.59 (s, 1 H), 5.92 (m, 1 H), 6.88 (m, 4 H), 7.03 (m, 1 H), 7.20 (m, 3 H), 7.28 (m, 4 H), 7.59 (s, 2 H), 8.71 (bs, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 55.2, 60.5, 68.9, 81.4, 90.7, 111.6, 113.3, 117.7, 120.4, 127.0, 128.2, 128.6, 129.0, 129.2, 129.9, 130.7, 132.7, 135.5, 154.9, 159.9, 170.4. HRMS: m/e calcd for C27H25NO6H+: 460.1760, found: 460.1754 (Δ = −1.3 ppm).
5.1.24. (2S,10S,10aR)-9-Acetoxymethyl-10-benzoyloxy-5-oxo-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-2-yl (4’S,5’R)-3’-benzoyl-2’-(4-methoxyphenyl)-4’-phenyloxazolidine-5’-carboxylate (21a)
To a solution of 17a (20 mg, 0.053 mmol), 20a (21 mg, 0.053 mmol) and DMAP (3.5 mg, 0.026 mmol) in CH2Cl2 (0.5 mL) was added EDC (22 mg, 0.11 mmol) and the reaction mixture was stirred overnight. The mixture was then quenched with EtOAc (50 mL) and washed with water (10 mL × 2) and brine (10 mL). The organic layer was dried over anhydrous MgSO4, filtered, and solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (1:2) as the eluent to afford 21a as a white solid (27 mg, 82% yield based on 85% conversion): mp 109–111 °C; 1H NMR (400 MHz, CDCl3) δ 1.90 (s, 3 H), 2.32 (dd, J = 14.0, 5.2 Hz, 1 H), 2.42 (td, J = 10.8, 4.4 Hz, 1 H), 3.80 (s, 3 H), 3.89 (d, J = 14.8 Hz, 1 H), 4.05 (d, J = 14.4, 4.8, 1 H), 4.17 (td, J = 10.8, 5.6 Hz, 1H), 4.81 (d, J = 2.0 Hz, 1 H), 5.05 (d, J = 11.6 Hz, 1 H), 5.22 (d, J = 12.8 Hz, 1 H), 5.36 (bs, 1 H), 5.55 (t, J = 4.0 Hz, 1 H), 6.71 (d, J = 10.8 Hz, 1 H), 6.81 (d, J = 8.4 Hz, 2 H), 7.25 (m, 11 H), 7.47 (m, 4), 7.63 (t, J = 7.6 Hz, 1 H), 8.12 (d, J = 7.2 Hz, 2 H), 8.21 (dd, J = 7.2, 2.0 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 20.4, 38.3, 51.8, 55.3, 58.8, 65.4, 72.9, 73.0, 113.5, 127.0, 127.1, 128.1, 128.2, 128.6, 128.7, 128.7, 128.8, 129.0, 129.8, 129.8, 130.2, 130.7, 133.3, 134.0, 134.6, 135.2, 135.4, 159.9, 162.1, 165.6, 169.3, 170.2. HRMS: m/e calcd for C46H40N2O10H+: 781.2761, found: 781.2789 (Δ = 3.6 ppm).
5.1.25. (2S,10S,10aR)-9-Acetoxymethyl-10-benzoyloxy-5-oxo-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-2-yl (2’S,3’R)-3’-benzoylamino-2’-hydroxy-3’-phenylpropanoate (1)
To a solution of 21a (26 mg, 0.03 mmol) in methanol (0.5 mL) was added p-toluenesulfonic acid (1.5 mg, 0.006 mmol). After stirring the mixture overnight, the solvent was removed and the residue purified by column chromatography on silica gel using hexanes:EtOAc = 1:2 as the eluent to afford 1 as a white solid (16 mg, 75% yield): mp 116–118 °C; [α]D22 −135 (c 2.7, CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.90 (s, 3 H), 2.37 (td, J = 8.8, 3.2 Hz, 1 H), 2.48 (dd, J = 11.2, 4.4 Hz, 1 H), 3.18 (d, J = 2.8 Hz, 1 H), 3.99 (dd, J = 11.2, 3.6 Hz, 1 H), 4.03 (d, J = 12.0 Hz, 1 H), 4.44 (td, J = 8.8, 4.4 Hz, 1 H), 4.62 (s, 1 H), 5.05 (d, J = 10.0 Hz, 1 H), 5.22 (d, J = 10.4 Hz, 1 H), 5.59 (t, J = 3.2 Hz, 1 H), 5.69 (d, J = 7.6 Hz, 1 H), 6.71 (d, J = 9.2 Hz, 1 H), 6.74 (d, J = 7.2 Hz, 1 H), 7.17 (t, J = 6.0 Hz, 1 H), 7.41 (m, 11 H), 7.61 (t, J = 6.0 Hz, 1 H), 8.13 (d, J = 6.0 Hz, 2 H), 8.20 (dd, J = 5.6, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 20.3, 38.1, 51.5, 54.4, 58.7, 65.5, 73.2, 73.6, 126.8, 126.9, 128.0, 128.4, 128.5, 128.7, 128.7, 128.9, 128.9, 130.0, 130.3, 131.5, 133.2, 133.6, 133.7, 134.5, 136.0, 138.4, 162.5, 165.7, 166.7, 170.2, 172.2. HRMS: m/e calcd for C38H34N2O9H+: 663.2343, found: 663.2361 (Δ = 2.8 ppm).
5.1.26. (2S,10S,10aR)-10-Benzoyloxy-5-oxo-9-pent-4-enoyloxymethyl-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-2-yl (4’S,5’R)-4’-(2-allyloxyphenyl)-3’-benzoyl-2’-(4-methoxyphenyl)oxazolidine-5-carboxylate (21b)
White solid; 96% yield; mp 73–75 °C; [α]D22 −145 (c 2.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 2.27 (m, 5 H), 2.42 (m, 1 H), 3.81 (s, 3 H), 3.86 (d, J = 14.4 Hz, 1 H), 4.05 (dd, J = 14.4, 4.8, 1 H), 4.25 (td, J = 10.8, 5.6, Hz, 1 H), 4.39 (m, 2 H), 4.80 (s, 1 H), 4.93 (dd, J = 10.0, 0.8 Hz, 1 H), 4.96 (dd, J = 17.6, 1.6 Hz, 1 H), 5.03 (d, J = 10.4 Hz, 1 H), 5.08 (s, 1 H), 5.11 (d, J = 4.8 Hz, 1 H), 5.24 (d, J = 12.8 Hz, 1 H0, 5.43 (s, 1 H), 5.55 (t, J = 4.8 Hz, 1 H), 5.74 (m, 2 H), 6.71 (d, J = 10.4 Hz, 1 H), 6.76 (d, J = 8.4 Hz, 1 H), 6.81 (m, 3 H), 6.94 (s, 1 H), 7.08 (m, 3 H), 7.19 (m, 5 H), 7.50 (m, 6 H), 7.64 (t, J = 7.2 Hz, 1 H), 8.13 (d, J = 7.6 Hz, 2 H), 8.22 (dd, J = 7.6, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 28.5, 32.9, 38.4, 51.9, 55.2, 58.8, 65.2, 68.8, 72.4, 73.0, 111.5, 113.3, 115.5, 117.7, 120.5, 126.9, 128.2, 128.7, 128.7, 128.8, 128.8, 128.9, 129.2, 129.9, 130.2, 132.5, 133.4, 133.9, 134.6, 135.3, 136.5, 154.7, 159.8, 162.0, 165.5, 169.3, 172.3. HRMS: m/e calcd for C52H48N2O11H+: 877.3336, found: 877.3322 (Δ = −1.6 ppm).
5.1.27. (2S,10S,10aR)-10-Benzoyloxy-5-oxo-9-pent-4-enoyloxymethyl-1,2,3,5,10,10a-hexahydropyrrolo[1,2-b]isoquinolin-2-yl (2’S,3’R)-3’-(2-allyloxyphenyl)-3’-benzoylamino-2’-hydroxypropanoate] (22)
White solid; 82% yield; mp 110–112 °C; [α]D22 −139 (c 1.6, CHCl3); 1H NMR (400 MHz, CDCl3) δ 2.27 (m, 6 H), 3.34 (bs, 1 H), 3.95 (m, 2 H), 4.29 (d, J = 8.0 Hz, 1 H), 4.33 (d, J = 8.0 Hz, 1 H), 4.51 (d, J = 4.8 Hz, 2 H), 4.65 (d, J = 3.2 Hz, 1 H), 4.92 (dd, J = 9.2, 1.6 Hz, 1 H), 4.96 (dd, J = 17.2, 1.6 Hz, 1 H), 5.09 (d, J = 12.8 Hz, 1 H), 5.21 (dd, J = 12.0, 1.2 Hz, 1 H), 5.23 (dd, J = 19.6, 12.8 Hz, 1 H), 5.48 (dd, J = 25.2, 1.6 Hz, 1 H), 5.66 (d, J = 6.0 Hz, 1 H), 5.73 (m 1 H), 5.98 (m, 2 H0, 6.67 (d, J = 10.8 Hz, 1 H), 6.78 (d, J = 8.0 Hz, 1 H), 6.87 (t, J = 7.2 Hz, 1 H), 7.19 (m, 5 H), 7.38 (t, J = 11.6 Hz, 1 H), 7.45 (m, 6 H), 7.62 (t, J = 7.2 Hz, 1 H), 8.13 (d, J = 7.2 Hz, 2 H), 8.18 (dd, J = 7.6, 1.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 28.5, 32.9, 38.2, 51.6, 52.0, 58.8, 65.3, 68.7, 72.9, 73.2, 111.8, 115.5, 117.7, 120.9, 126.1, 126.8, 127.8, 128.4, 128.5, 128.7, 128.8, 128.9, 129.2, 130.0, 130.3, 131.4, 132.5, 133.3, 133.7, 133.9, 134.5, 135.9, 136.5, 155.5, 162.3, 165.6, 166.7, 172.1, 172.2. HRMS: m/e calcd for C44H42N2O10H+: 759.2918, found: 759.2893 (Δ = −3.3 ppm).
5.1.28. (2S,11S,11aR)-10-(acetoxymethyl)-11-benzoyloxy-2-hydroxy-2,3,11,11a-tetrahydro-1H-benzo[d]-pyrrolo-5(6H)-oxo-[1,2-a]azepin-2-yl (2’S,3’R)-3’-benzoylamino-2’-hydroxy-3’-phenylpropanoate (2)
To a solution of 17c (11 mg, 0.027 mmol), 20a (12 mg, 0.03 mmol) and DMAP (3.2 mg, 0.027 mmol) in CH2Cl2 (0.5 mL) was added EDC (22 mg, 0.11 mmol) and the mixture was stirred for 1 day. The reaction mixture was then quenched with EtOAc (50 mL) and washed with water (10 mL × 2) and brine (10 mL). The organic layer was dried over anhydrous MgSO4, filtered, and then solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel using hexanes:EtOAc (1:1) as the eluent to afford the coupling product 21c as a white solid (11 mg, 92% based on 50% conversion).
To a solution of 21c (11 mg, 0.014 mmol) in methanol (0.5 mL) was added p-TSA (0.5 mg, 0.003 mmol). After stirring overnight, the solvent was removed and the residue purified by column chromatography on silica gel using hexanes:EtOAc (1/2) as the eluent to afford 2 as a white solid (7 mg, 74% yield for 2 steps): mp 130–132 °C; 1H NMR (500 MHz, CDCl3) δ 1.95 (s, 1 H), 2.27 (td, J = 11.0, 5.0 Hz, 1 H), 2.40 (dd, J = 13.5, 6.0 Hz, 1 H), 3.27 (d, J = 4.0 Hz, 1 H), 3.79 (d, J = 14.0 Hz, 1 H), 4.02 (d, J = 17.0, 1 H), 4. 04 (dd, J =14.5, 5.0 Hz, 1 H), 4.40 (dd, J = 17.0 Hz, 1 H), 4.50 (dd, J = 11.0, 5.5 Hz, 1 H), 4.65 (dd, J = 4.2, 1.2 Hz, 1 H), 5.39 (d, J = 9.0 Hz, 1 H), 5.41 (d, J = 12.5 Hz, 1 H), 5.49 (d, J = 12.5 Hz, 1 H), 5.7 4 (d, J = 7.5 Hz, 1 H), 6.64 (s, 1 H), 6.86 (d, J = 9.0 Hz, 1 H), 7.21 (d, J = 8.0 Hz, 1 H), 7.26 (m, 4 H), 7.43 (m, 6 H), 7.55 (m, 3 H), 7.75 (d, J = 9.0 Hz, 2 H), 7.98 (d, J = 7.5 Hz, 2 H); 13C NMR (100.5 MHz, CDCl3) δ 22.6, 36.8, 44.0, 53.7, 54.4, 59.6, 64.2, 69.1, 72.6, 73.1, 126.8, 127.0, 128.0, 128.7, 128.8, 129.0, 129.4, 129.7, 129.8, 131.7, 131.9, 133.7, 133.8, 134.2, 134.5, 135.0, 138.2, 165.4, 166.9, 168.1, 170.5, 172.4. HRMS: m/e calcd for C39H36N2O9H+: 677.2499, found: 677.2502 (Δ = 0.4 ppm).
5.1.29. Macrocyclic paclitaxel mimic 3
To a solution of 22 (17 mg, 0.02 mmol) in CH2Cl2 (12 mL) was added Cl2Ru(=CHPh)(PCy3)2 (3 mg, 0.004 mmol) in CH2Cl2 (0.2 mL). The mixture was stirred at room temperature for 3 days and reflux for 2 days. The solvent was removed under reduced pressure. The residue was passed through a short silica gel column (hexanes:EtOAc = 1:1.5) to remove the catalyst and then afford 3 (8 mg, 66% yield based on 71% conversion) as a white solid, as well as the starting material (5 mg).
3: 1H NMR (400 MHz, CDCl3) δ 2.30 (m, 5 H), 2.78 (dd, J = 10.4, 3.6 Hz, 1 H), 3.32 (d, J = 2.8 Hz, 1 H), 3.82 (dd, J = 10.0, 1.2 Hz, 1 H), 3.96 (m, 2 H), 4.36 (dd, J = 11.2, 2.4 Hz, 1 H), 4.52 (s, 1 H), 4.59 (m, 1 H), 4.77 (d, J = 9.2 Hz, 1 H), 5.44 (d, J = 9.2 Hz, 1 H), 5.56 (s, 2 H), 6.14 (dd, J = 7.2, 1.6 Hz, 1 H), 6.72 (d, J = 7.6 Hz, 1 H), 6.75 (d, J = 9.6 Hz, 1 H), 6.78 (d, J = 6.4 Hz,1 H), 6.96 (t, J = 6.4 Hz, 1 H), 7.24 (m, 1 H), 7.42 (m, 3 H), 7.46 (m, 4 H), 7.59 (m, 3 H), 7.74 (dd, J = 6.0, 1.2 Hz, 1 H), 8.21 (dd, J = 6.4, 1.2 Hz, 1 H), 8.36 (dd, J = 6.0, 0.4 Hz, 2 H); 13C NMR (100.5 MHz, CDCl3) δ 28.2, 33.6, 36.9, 49.2, 51.9, 59.1, 64.1, 68.4, 72.9, 73.0, 73.3, 112.4, 121.0, 125.8, 127.0, 127.5, 128.1, 128.4, 128.5, 128.6, 128.6, 129.2, 129.2, 129.6, 130.5, 130.6, 130.7, 131.6, 131.8, 133.8, 134.0, 137.0, 138.8, 154.8, 162.2, 166.7, 171.8, 172.6. HRMS: m/e calcd for C42H38N2O10H+: 731.2605, found: 731.2597 (Δ = −1.1 ppm).
5.1.30. (2S,8S,8aR)-8-(3-Methoxybenzoyloxy-6,7-didehydroindolizidin-5-one-2-yl (2’R,3’S)-2’-hydroxy-3’-phenyl-3’-benzylaminopropionate (24)
White solid; 76% yield for 2 steps; mp 78–80 °C; 1H NMR (400 MHz, CDCl3) δ 2.09 (td, J = 10.8, 4.4 Hz, 1 H), 2.47 (dd, J = 14.0, 5.6 Hz, 1 H), 3.54 (br s, 1 H), 3.82 (s, 3 H), 3.83 (m, 2 H), 4.33 (td, J = 11.2, 5.3, Hz, 1 H), 4.64 (d, J = 2.0 Hz, 1 H), 5.11 (d, J = 3.6 Hz, 1 H), 5.75 (m, 2 H), 6.01 (dd, J = 10.4, 2.8 Hz, 1 H), 6.53 (dd, J = 10.0, 1.6 Hz, 1 H), 7.13 (dd, J = 8.0, 2.0 Hz, 1 H), 7.19–7.41 (m, 6 H), 7.45 (d, J = 7.6 Hz, 2 H), 7.55 (d, J = 7.2 Hz, 1 H), 7.59 (t, J = 2.0 Hz, 1 H), 7.71 (d, J = 7.6 Hz, 1 H); 13C NMR (100.5 MHz, CDCl3) δ 31.5, 38.2, 50.8, 54.5, 55.4, 55.4, 58.3, 73.0, 114.4, 120.2, 122.5, 125.7, 126.9, 126.9, 128.0, 128.5, 128.8, 129.6, 130.3, 131.6, 133.8, 140.6, 159.6, 162.4, 165.7, 166.8, 172.2. HRMS: m/e calcd for C32H30N2O8Na+: 593.1900, found: 593.1893 (Δ = −1.2 ppm).
5.2. In vitro cell growth inhibition assay
Tumor cell growth inhibition was determined according to the method established by Skehan et al.34 Human cancer cell lines, LCC6-WT (Pgp−), MCF-7 (Pgp−), LCC6-MDR (Pgp+), NCI/ADR (Pgp+), A2780 (Pgp−) and HT-29 (Pgp−) were plated at a density of 400–2,000 cells/well in 96-well plates and allowed to attach overnight. These cell lines were maintained in RPMI-1640 medium supplemented with 5% fetal bovine serum and 5% Nu serum (Collaborative Biomedical Product, MA). Paclitaxel mimics were dissolved in DMSO and further diluted with RPMI-1640 medium. Triplicate wells were exposed to various treatments. After 72 h incubation, 100 µL of ice-cold 50% trichloroacetic acid (TCA) was added to each well, and the samples were incubated for 1 h at 4 °C. Plates were then washed five times with water to remove TCA and serum proteins, and 50 µL of 0.4% sulforhodamine B (SRB) was added to each well. Following a 5-min incubation, plates were rinsed five times with 0.1% acetic acid and air-dried. The dye was then solubilized with 10 mM Tris base (pH 10.5) for 5 min on a gyratory shaker. Optical density was measured at 570 nm. The IC50 values were then calculated by fitting the concentration-effect curve data with the sigmoid-Emax model using nonlinear regression, weighted by the reciprocal of the square of the predicted effect.35
5.3. Computational methods
5.3.1. Construction of molecular complexes
Paclitaxel mimics 1 and 2 were manually docked into the paclitaxel binding site of the REDOR-Taxol-1JFF structure18–19 using the InsightII 2000 program (CVFF force field) by overlaying three hydroxyl groups of each mimic with those of the paclitaxel molecule (C13, C2, and C4 hydroxyl groups) with the REDOR-Taxol-ITUB13, 18 conformation. The resulting molecular complex was energy-minimized in 5,000 steps or until the maximum derivative became <0.001 kcal/Å by means of the conjugate gradients method using the CVFF force field and the distance-dependent dielectric. The backbone of the protein was fixed during the energy minimization. In the same manner, the molecular complex of paclitaxel mimic 3 was obtained. The overlays of mimics 1, 2 and 3 with REDOR-Taxol-1TUB are shown in Figure 3.
The molecular complexes of 1 and 2 in the 1JFF tubulin32 were constructed using the same protocol as that described above except for employing REDOR-Taxol-1JFF18–19 in place of REDOR-Taxol-1TUB. Since there are differences in the protein structure between 1TUB and 1JFF, the mimics’ structures underwent small changes, but the critical H-bond between the C2’-OH and His227 was very stable during the energy minimization. After the energy minimization, the snapshots were overlaid by superimposing the backbones of the proteins. The overlays are shown in Figure 5. The mimics 1 and 2 showed very good overlays with REDOR-Taxol-1JFF.
5.3.2. MD simulations of mimics 1 and 2 in 1JFF
To examine the stability of the structures of mimics 1 and 2, molecular dynamics (MD) simulation was performed using the Macromodel program (MMFF94 force field).33 The molecular complexes, after 1000-step energy minimization (MMFF94), were used for the MD simulations with a 10 Å sphere around the binding site at 300K with the time step of 0.5 fs for 50 ps in a generalized Born with surface area term (GBSA) continuum solvent description of water solvation.36 Within the 10 Å sphere of the binding site, the ligand and the protein were allowed to move, while all atoms outside the sphere were frozen in order to maintain the overall integrity of the protein. The structures were sampled every 0.25 ps and overlaid by the backbones of the protein. The overlays of 200 snapshots for each of the mimics 1 and 2 are shown in Figure 6 in comparison with those of REROR-Taxol-1JFF.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA103314 and GM42798 to I.O.; CA 73872 to R.J.B). The authors are grateful to Dr. J. Fernando Díaz and his laboratory at the Centro de Investigaciones Biológicas, Madrid, Spain for obtaining preliminary results on the TR-NOESY analysis for binding of mimic 2 to tubulin, as well as the determination of the critical concentration of tubulin for polymerization in the presence of mimic 2. L.S. and I.O. also thank Ms. Rebecca Rowehl for her valuable help at the Cell Culture and Hybridoma Facility at State University of New York at Stony Brook.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at
References
- 1.Jemal A, Ward E, Hao Y, Thun M. J. Am. Med. Assoc. 2005;294:1255. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun Michael J. CA Cancer J Clin. 2006;56:106. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK. Ann. Rev. Med. 1997;48:353. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 4.Suffness ME. Taxol Science and Applications. Boca Raton: CRC Press; 1995. p. 426. [Google Scholar]
- 5.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 6.Schiff PB, Horwitz SB. Proc. Natl. Acad. Sci. USA. 1980;77:1561. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojima I, Wang T, Delaloge F. Tetrahedron Lett. 1998;39:3663. [Google Scholar]
- 8.Kingston DGI. J. Nat. Prod. 2000;63:726. doi: 10.1021/np000064n. [DOI] [PubMed] [Google Scholar]
- 9.Kingston DGI. Chem. Commun. 2001:867. [Google Scholar]
- 10.Ojima I, Chen J, Sun L, Borella CP, Wang W, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St.Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. J. Med. Chem. 2008;51:3203. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojima I, Das M. J. Nat. Prod. 2009;72:554. doi: 10.1021/np8006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingston DGI, Bane S, Snyder JP. Cell cycle. 2005;4:279. [PubMed] [Google Scholar]
- 13.Geney R, Sun L, Pera P, Bernacki Ralph J, Xia S, Horwitz Susan B, Simmerling Carlos L, Ojima I. Chem. Biol. 2005;12:339. doi: 10.1016/j.chembiol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Geng X, Geney R, Pera P, Bernacki RJ, Ojima I. Bioorg. Med. Chem. Lett. 2004;14:3491. doi: 10.1016/j.bmcl.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Roussi F, Ngo QA, Thoret S, Gueritte F, Guenard D. Eur. J. Org. Chem. 2005:3952. [Google Scholar]
- 16.Ganesh T, Guza RC, Bane S, Ravindra R, Shanker N, Lakdawala AS, Snyder JP, Kingston DGI. Proc. Natl. Acad. Sci. USA. 2004;101:10006. doi: 10.1073/pnas.0403459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh T, Norris A, Sharma S, Bane S, Alcaraz AA, Snyder JP, Kingston DGI. Bioorg. Med. Chem. 2006;14:3447. doi: 10.1016/j.bmc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Geng X, Geney R, Li Y, Simmerling C, Li Z, Lauher JW, Xia S, Howtizs SB, Veith JM, Pera P, Bernackic RJ, Ojima I. J. Org. Chem. 2008;73:9584. doi: 10.1021/jo801713q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Simmerling C, Ojima I. ChemMedChem. 2009;4:719. doi: 10.1002/cmdc.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogales E, Wolf SG, Downing KH. Nature. 1998;391:199. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 21.Garner P, Ramakanth S. J. Org. Chem. 1986;51:2609. [Google Scholar]
- 22.Hosoya T, Takashiro E, Matsumoto T, Suzuki K. J. Am. Chem. Soc. 1994;116:1004. [Google Scholar]
- 23.Kaiser F, Schwink L, Velder J, Schmalz H-G. Tetrahedron. 2003;59:3201. [Google Scholar]
- 24.Krasovskiy A, Malakhov V, Gavryushin A, Knochel P. Angew. Chem. Int. Ed. 2006;45:6040. doi: 10.1002/anie.200601450. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser F, Schmalz H-G. Tetrahedron. 2003;59:7345. [Google Scholar]
- 26.Park H, Hepperle M, Boge TC, Himes RH, Georg GI. J. Med. Chem. 1996;39:2705. doi: 10.1021/jm960142x. [DOI] [PubMed] [Google Scholar]
- 27.Cunico RF, Bedell L. J. Org. Chem. 1980;45:4797. [Google Scholar]
- 28.Shiina I, Saitoh K, Frechard-Ortuno I, Mukaiyama T. Chem. Lett. 1998:3. [Google Scholar]
- 29.Mukaiyama T, Shiina I, Iwadare H, Saitoh M, Nishimura T, Ohkawa N, Sakoh H, Nishimura K, Tani Y-I, Hasegawa M, Yamada K, Saitoh K. Chem.--Eur. J. 1999;5:121. [Google Scholar]
- 30.Diaz JF, Strobe R, Engelborghs Y, Souto AA, Andreu JM. J. Biol. Chem. 2000;275:26265. doi: 10.1074/jbc.M003120200. [DOI] [PubMed] [Google Scholar]
- 31.Matesanz R, Barasoain I, Yang C-G, Wang L, Li X, de Ines C, Coderch C, Gago F, Barbero JJ, Andreu JM, Fang W-S, Diaz JF. Chem. Biol. 2008;15:573. doi: 10.1016/j.chembiol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Lowe J, Li H, Downing KH, Nogales E. J. Mol. Bol. 2001;313:1045. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 33.Halgren TA. J. Computat. Chem. 1996;17:490. [Google Scholar]
- 34.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. J. Nat. Cancer Inst. 1990;82:1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 35.Motulsky HJ, Ransnas LA. Faseb J. 1987;1:365. [PubMed] [Google Scholar]
- 36.Still WC, Tempczyk A, Hawley RC, Hendrickson T. J. Am. Chem. Soc. 1990;112:6127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.