Abstract
Background
Distraction enterogenesis is a novel method for increasing small bowel length by the application of linearly directed forces. However, the magnitude of distractive forces that human and animal small bowel can safely withstand is unknown.
Methods
Acute ex-vivo force-displacement curves for human (n=5) and pig (n=6) small intestine (with and without mesentery) were made by applying increasing amounts of distractive forces to bowel immersed in normal saline (39°C). Progressive load was applied until gross disruption of the tissue was detected, or the applied force reached 1000 gram-force (gf). Histology was used to detect evidence of load-induced damage. In-vivo blood flow to pig bowel with distractive loads (30–200gf) was measured by laser Doppler.
Results
The relationship between the level of force and degree of displacement was linear. The presence of a mesentery increased stiffness of pig bowel, but did not affect human bowel. Gross tissue disruption in pig and human tissue was seen at forces between 235 and 295 gf respectively. However, in grossly undamaged areas, histology was unchanged even after application of higher loads. With in-vivo testing, mesenteric blood flow was present up to 200 gf; however, blood flow to the bowel wall was reduced to undetectable levels at loads exceeding 100 gf.
Conclusions
While whole bowel tissue may tolerate greater applied loads, blood flow to the bowel wall was compromised at loads over 100 gf, suggesting that any higher forces place the bowel at risk for ischemia. These measurements will help guide the clinical application of distraction enterogenesis.
Keywords: Distraction enterogenesis, short bowel syndrome, mechanical properties of small bowel, force displacement
INTRODUCTION
Short bowel syndrome (SBS) is a highly morbid condition in which patients have insufficient small bowel length for proper nutritional absorption. Patients with SBS require supplemental parenteral nutrition, and often suffer from complications such as cholestatic liver disease, central line infection, and bacterial overgrowth[1]. While advancements have been made in the treatment of SBS, such as the advent of new intestinal lengthening procedures (like serial transverse enteroplasty[2]) and improvements in survival after intestinal transplantation[3], SBS remains a very difficult disease to treat.
The prognosis of SBS patients is known to be dependent on remaining bowel length[1]. In fact, patients with less than 10% of predicted small bowel length have little to no chance of weaning off of parenteral nutrition. It therefore stands to reason that therapies to increase the length of small bowel hold promise in improving the treatment of SBS patients. Recently, other groups have successfully achieved small bowel lengthening in a rat model, and showed that its histology was not compromised and enzymatic functions increased[4, 5]. Our group successfully induced longitudinal growth in porcine small bowel[6]. Through the application of mechanical distractive forces, a 45% increase in longitudinal bowel length was achieved over a 10 day period. The lengthened intestine showed increased rates of epithelial cell proliferation, consistent with true growth due to mechanotransductive mechanisms. This bowel also demonstrated intact epithelial barrier function as well as transmucosal uptake of nutrient substrate. Such a rapid form of intestinal growth may offer a unique approach to treat SBS.
What is unknown, however, is what amount of distractive forces can be safely applied during the induction of bowel growth. Several factors may limit the amount of force that can be applied. The strain of distractive mechanical forces could cause gross or microscopic damage to one or more layers of the intestinal wall (mucosa, muscularis or serosa) or the mesentery. Furthermore, even if the bowel wall and mesentery are able to withstand high levels of forces, intestinal blood flow may be compromised when it is stretched too far.
Yamada describes various mechanical strengths of human tissues including the small bowel[7]. However, measurements such as tensile strength and expansive strength, were obtained from strips of tissue 10-mm long and 3-mm wide. Egorov, et al also described properties of the human gastrotintestinal tract, but again, their data was based on tissue cut into segments[8]. Such intestinal strips provide a sound basis for testing local tissue strength, but whole segments of intestine more than likely have different physical properties. For example, strips of intestine necessarily disrupt the integrity of the circular and longitudinal muscles. Gregersen’s group has published several papers on biomechanical properties of the rodent gastrointestinal tract, including bowel that was growing under the influence of EGF[9, 10]. However, to our knowledge, there are no reports on the mechanical properties of whole pig bowel and human bowel in terms of the magnitude of longitudinal forces they can withstand before suffering gross or microscopic damage.
This paper presents the characterization of the mechanical properties of whole bowel in both pigs and humans, as well as the effect of increasing loads on mesenteric and mucosal blood flow. Such data will provide useful safety guidelines for translating distraction enterogenesis to a clinical setting.
METHODS
All animal experiments were conducted with approval from the University Committee on the Use and Care of Animals (protocol number 09026). Human specimens were obtained with permission from donor families through Gift of Life Michigan. An exemption from regulated status was obtained from the Institutional Review Board at the University of Michigan (HUM00023670).
Procurement of Samples
Pig intestinal samples were obtained from 6 female Yorkshire pigs between 20 and 40 kilograms. After each pig was killed (via pentobarbital overdose), at least 30 cm of proximal jejunum with its mesentery was resected. Harvested bowel was located between 30 and 50 cm distal to the ligament of Treitz. Human small bowel samples (n=5, age 17 to 65 years) were obtained from organ donors. After organs for transplant were procured, a 30–50 cm length of bowel was resected starting from 30 cm distal to the ligament of Treitz. Both types of intestinal samples were placed in RPMI 1640 (Invitrogen, Carlsbad, CA) on ice until they were studied.
Ex-vivo Application of Acute Distractive Forces
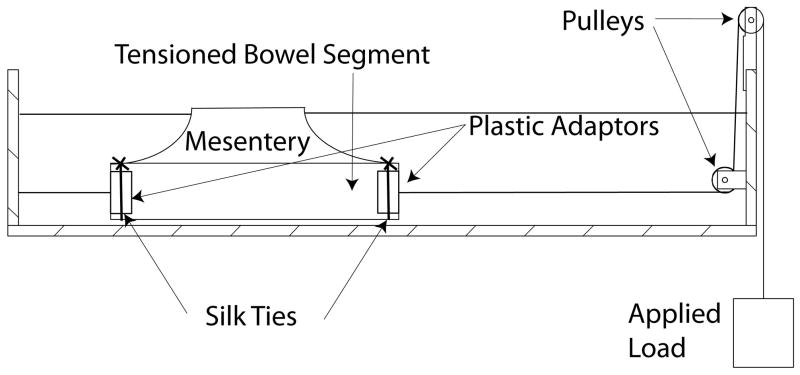
Bowel segments ranging between 10 to 15 cm in length (with or without its mesentery) were subjected to longitudinal distractive loads using the setup depicted in Figure 1. The bowel was tied to plastic adaptors on both ends using silk ties. One adaptor was fixed to a hook on one wall of a water bath, and the other end was attached to weights through a pulley system. The length of the bowel was zeroed at 35 gram-force (gf), as this was the weight of the apparatus on the opposite weighted end. Forces were then applied in 20 to 40 gram increments every 2 minutes, up to tissue failure or up to a maximum of 1000 gf. This time period was selected as longer durations between increments of forces did not appreciably affect stretched length. Throughout the application of forces, the bowel was immersed in a 0.9% saline bath maintained at 35–40°C to best approximate physiologic conditions. Tissue failure was defined as a grossly visible tear in the mesentery or bowel wall. At tissue failure, full thickness samples from grossly undamaged areas of the bowel were collected for histologic analysis. Force-displacement curves were generated based on the percentage increase in length compared to the starting length of the bowel. From this curve, the stiffness per unit length of the bowel (defined as (applied load/displacement) per original length of bowel) with and without its mesentery was calculated using linear regression on GraphPad Prism software version 5 (GraphPad software inc., La Jolla, CA).
Figure 1.
Layout of setup used to test tissue disruption of full-thickness bowel. An end piece with holes to allow water through is tied by sutures to the ends of the cut intestinal segment.
Histology
The collected full-thickness samples were fixed in zinc-formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin. The histology of the tissue was scored at the level of the submucosa, muscularis and serosa according to the scale in Table 1. The condition of the mucosa was not part of the evaluation because the mucosa is not a major contributor of tensile strength[8]. Furthermore, the mucosal layer was recognized to be severely compromised by sitting in a warm saline water bath for up to 2 hours.
Table 1.
Histologic Scoring
| Submucosa | Normal (0) |
| Microscopic tears seen (2) | |
| Muscularis | Normal (0) |
| Thinning of the muscularis without tearing (1) | |
| Microscopic tears seen (2) | |
| Serosa | Normal (0) |
| Microscopic tears seen (2) |
Measurement of Mesenteric Blood Flow
After anesthetizing female Yorkshire pigs (mean weight 22 kg, n=3), the abdominal cavity was opened and the proximal jejunum 50 cm distal to the ligament of Treitz was identified. An enterotomy was created, and a 3 cm segment of full-thickness bowel was harvested. Through the enterotomy, a 17cm long radio-controlled intestinal lengthening device (described in the next section) was inserted into the jejunal segment proximal to the enterotomy. The intestine over both ends of the device was tied off with silk ties, removing it from intestinal continuity, but taking care not to compromise the mesentery. Loads on the bowel were increased at 30 gram intervals up to a maximum of 200 gf using the lengthening device. Blood flow to the isolated bowel segment and mesentery were measured using a Lisca PIM II Laser Doppler Perfusion Imager (Perimed AB, Stockholm, Sweden), placed 11cm above the bowel segment of interest. Blood flow patterns were analyzed using LDPIwin 2.3 software (Perimed AB) as previously described[11]. After completing the measurement, full thickness intestinal samples were taken from the middle and ends of the lengthened segment for histologic analysis.
Lengthening device
A custom expansion device was created that expands 1/32 inch (0.8mm) in a linear direction with each actuation, which is generated by a ratcheting mechanism employing a shape memory alloy (SMA). To avoid additional cables entering and exiting the bowel, the device was controlled using a wire transceiver. A force-transducer was positioned on one end of the device to allow for accurate measurements of applied loads to the bowel and a Hall sensor monitored the displacement. The power to heat the SMA wire and power the control, measurement and radio electronics was provided by a 4V internal battery. A detailed description of the lengthening device is provided in Utter et al[12].
Statistics
Results are expressed as the mean ± standard deviation. Differences in histologic scores were evaluated using the Mann-Whitney U-test. Comparison of stiffness was done using the Wilcoxon matched rank test with p < 0.05 considered significant.
RESULTS
Load Displacement
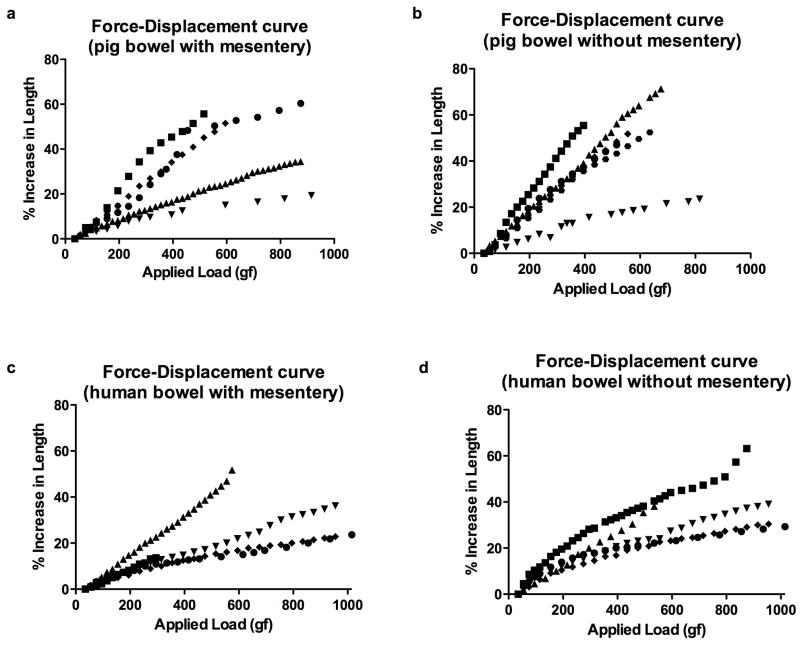
The force-displacement curves for pig specimens are shown in Figure 2a and 2b. The mean stiffness for pig bowel with its mesentery was greater than without its mesentery (17±12 gf/%displacement with mesentery vs. 13±9 gf/%displacement without mesentery; p = 0.03). Pig bowel without its mesentery failed between 395 and 815 gf. Three out of 6 pig samples with mesentery remained intact at 1000gf (upper limit measured), the mesentery tore on one sample at 235gf and the bowel wall on the other two failed at 515 and 595 gf.
Figure 2.
Force-displacement curves for pig bowel with (a) and without (b) mesentery, and human bowel with (c) and without (d) mesentery. Each symbol represents a different sample.
Similar graphs for the human specimens are shown in Figure 2c and 2d. Similar to the porcine samples, the mean bowel stiffness was greater with the mesentery; however, the difference was not statistically significant in human samples (26±11 gf/%displacement with mesentery vs. 20±7 gf/%displacement without mesentery; p = 0.13). Tissue failure for human bowel occurred in 2 out of 5 samples with the mesentery (mesenteric tears were seen at 295gf and 575gf), and 2 out of 5 samples without the mesentery (gross bowel wall tears seen at 575gf and 875gf).
Histology
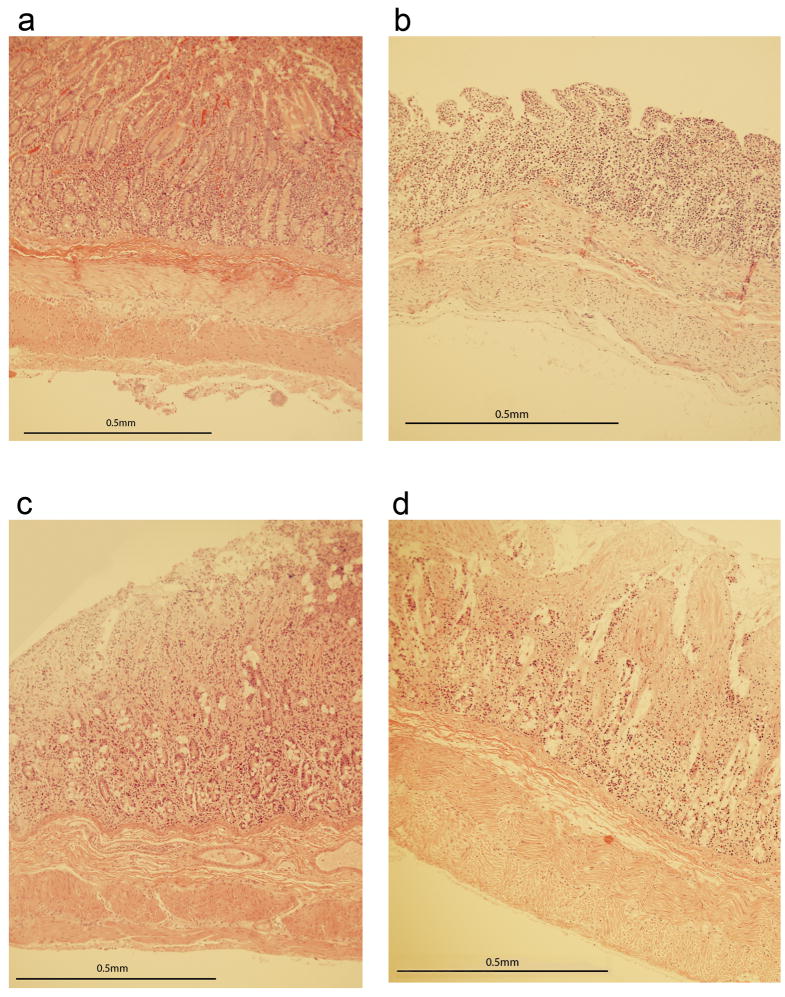
In order to evaluate for any microscopic damage, histologic samples were collected either at 1000gf or when failure occurred. Intestinal samples in these sections were taken at least 2 cm away from the grossly disrupted portion of the bowel. An unstretched segment of bowel was used as a negative control. As expected, the mean score of pig control segments was 0. The mean score after stretching was 0.3±0.6 (p = 1.0). Representative samples of pig intestinal histology before and after stretching are shown in Figure 3a and 3b.
Figure 3.
a. Sample control pig bowel. b. Sample stretched pig bowel. Note that despite the edema and loss of mucosal architecture, the serosa, muscularis and submucosa are still intact. c. Sample control human bowel. d. Sample stretched human bowel. Villus architecture distortion and submucosal edema is seen, but no microscopic tears are seen in the serosa, muscularis or submucosa.
Similar histologic comparison was performed with human bowel. The histologic scores of the control and stretched segments were 0.4±0.9 and 0.8±1.1 respectively (p = 0.6). Representative sections are shown in Figure 3c and 3d.
Mesenteric Blood Flow
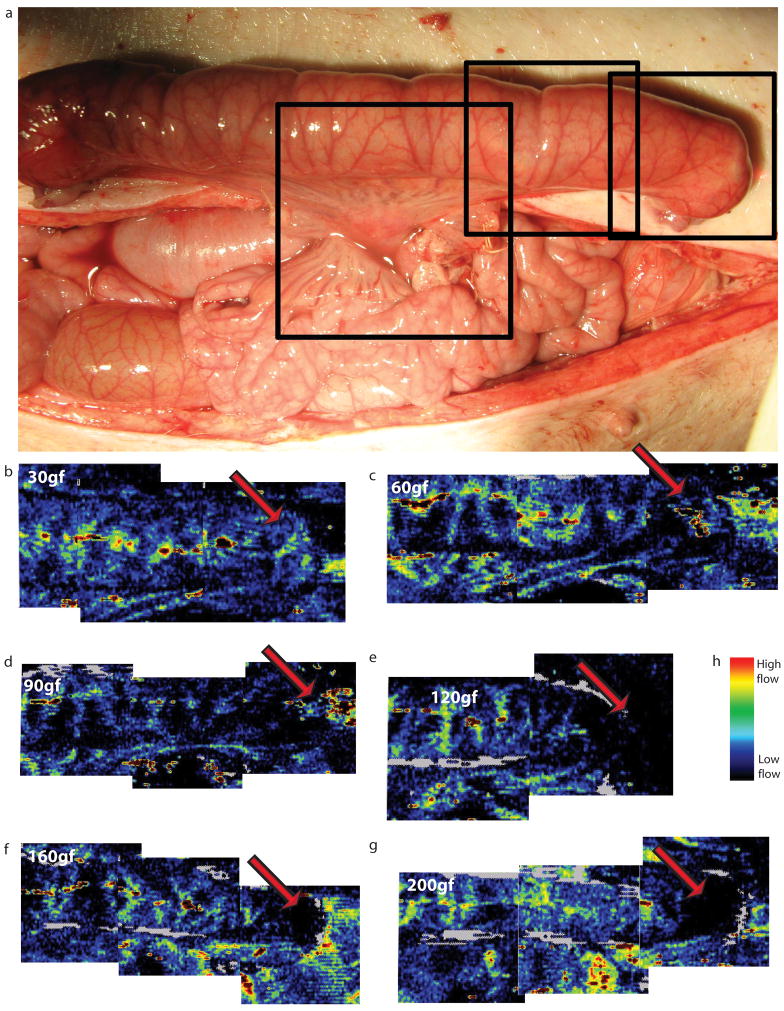
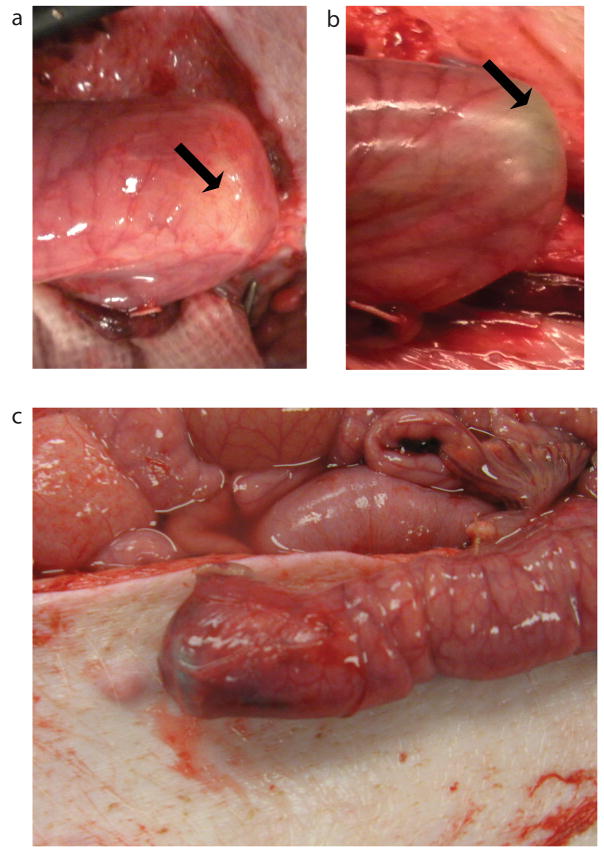
In addition to testing the load profile of the bowel to determine levels of loads that cause damage, tests were conducted to determine load levels that adversely effected blood flow. To assess blood flow through the mesentery supplying the bowel as well as the bowel itself, the laser Doppler device was set up to capture blood flow as shown in Figure 4a. Merged images between 30 and 200 gf are shown in Figure 4b–g. Blood flow in the mesentery is present in all visible parts of the mesentery up to 200 gf. However, blood flow near the end of the bowel segment, where the device pushes against the bowel wall (corresponding to arrows in figure 4b–g), was compromised at forces exceeding 100 gf (compare Figure 4d at 90 gf and Figure 4e at 120 gf). This observation is also grossly apparent at the end cap of where the device abuts the bowel. At 90 gf (Figure 5a), intestinal blood vessels are visible, however there is blanching at 120 gf (Figure 5b). In a different experiment, a serosal tear was seen at 120 gf near the device end cap (Figure 5c).
Figure 4.
a. Layout of pig bowel with the device implanted. The black boxes represent the approximate areas that were scanned by one sweep of the laser Doppler machine. b–g. Merged laser Doppler images of bowel under 30, 60, 90, 120, 160 and 200gf. The red arrow shows the point of maximal pressure on the mucosa. Blue represents low flow velocity, red represents high flow velocity. Note on e, f and g that there is no visible blood flow around the red arrows.
Figure 5.
a. Pig jejunum under 90gf of distractive force. b. Pig jejunum under 120gf of distractive force. The arrows correspond to the same location as the red arrows in Figure 4b–g. c. Mesenteric tear at the end of the bowel seen at 120gf.
Discussion
Distraction-induced enterogenesis is a novel and useful method with potential for increasing absolute bowel length in the treatment of short bowel syndrome. This study addressed a critical question needed to translate this concept safely to the clinical setting – specifically, what loads can be applied to small bowel without causing damage?
All the visible damage to the bowel was noted at the gross level with loads starting as early as 235 gf for pigs and 295 gf for human. This failure was localized and there were no significant histological changes observed at the microscopic level just 2 cm away from these injured areas. There are several explanations for this phenomenon. It is possible that there were subtle microscopic changes that were obscured by the edema caused by bowel being in the saline bath. The physical properties of the bowel may be such that with application of an acute load over a time period less than 2 hours, the original histology is restored. A more likely explanation is that the loads are not evenly distributed throughout the entire bowel segment. Stress builds up in the weakest sections that fail before the microstructure within the other segments begin to experience stresses at levels which adversely affect it. We also noted a fairly large variability in the tissue failure points in our human specimens. It could be that this was due to variability in patient age and the amount of fat in the mesentery, as well as potential underlying patient pathology.
It was noted that pig small intestine with its mesentery attached was significantly more stiff than small intestine alone. This indicates that the mesenteric structure is another force resisting distractive forces on the intestine. It is also notable that in humans, the difference in stiffness between the samples with and without mesentery was not as great as that in the pig. The most likely explanation is that the human mesentery had a much higher fat content, and lesser connective tissue density; and therefore was less stiff than the pig mesentery. The main significance of finding the differences in stiffness is that any device that attempts to grow bowel in-vivo may need to account for distractive forces on the mesentery.
A primary concern with any therapy that endeavors to increase tissue size is to assure that there will be sufficient blood supply to maintain the expanded tissue. Typical human systolic blood pressure is 120 mmHg, and the surface area of the end cap of the implantable device is 2.4 cm2. Since pressure is force per unit area, after converting units, 120 mmHg of pressure corresponds to 391 gf over the area of the end cap. This sets an estimate for the theoretical maximum force that could be applied in-vivo without compromising inflow. However, such high forces will likely compromise venous outflow and capillary circulation. True to these expectations, we saw that mesenteric blood flow was observed even at 200 gf (i.e. the pig systolic pressure was greater than 61 mmHg). However, there was also a distinct lack of capillary flow at the end-cap of the device at forces exceeding 100 gf (31 mmHg). Known normal human capillary pressure is between 8–35 mmHg[13, 14], which would mean that our findings in the pig are relevant to future human applications.
In conclusion, we showed that from a structural standpoint, pig and human bowel are able withstand relatively high forces (in excess of 500 gf in most of the samples tested). However, in-vivo testing showed that blood flow to the bowel wall would be compromised at forces as low as 100 gf over 2.4 cm2 (equivalent of 31 mmHg), indicating that the physiology of the blood supply is the limiting factor in determining a safe load to place on small bowel. Such work helps to lay out the groundwork for developing a clinical device for applying distractive forces to the small bowel for the purposes of growing intestinal length.
Acknowledgments
We would like to thank Dr. Daniel Myers for allowing us to use the laser Doppler equipment and Gail Rising for her excellent care of the pigs while under anesthesia. The work was supported by NIH 1R21DK075413-01A1, The Hartwell Foundation Biomedical Research Award, and FD003787-01. Funding for this project was also provided by the Center for Organogenesis (T32HD007505, for EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spencer AU, Neaga A, West B, et al. Pediatric Short Bowel Syndome: Redifining Predictors of Success. Annals of Surgery. 2005;242:403–412. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. Journal of Pediatric Surgery. 2003;38:425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 3.Vianna RM, Mangus RS, Tector AJ. Current status of small bowel and multivisceral transplantation. Advances in Surgery. 2008;42:129–150. doi: 10.1016/j.yasu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Chang PC, Mendoza J, Park J, et al. Sustainability of mechanically lengthened bowel in rats. Journal of Pediatric Surgery. 2006;41:2019–2022. doi: 10.1016/j.jpedsurg.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Puapong DP, Wu BM, Lam MM, et al. Distension enterogenesis: increasing the size and function of small intestine. Journal of Pediatric Surgery. 2006;41:763–767. doi: 10.1016/j.jpedsurg.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Spencer AU, Sun X, El-Sawaf M, et al. Enterogenesis in a clinically feasible model of mechanical small-bowel lengthening. Surgery. 2006;140:212–220. doi: 10.1016/j.surg.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada H. Strength of Biological Materials. Baltimore, MD: The Williams and Wilkins Company; 1970. [Google Scholar]
- 8.Egorov VI, Schastlivtsev IV, Prut EV, et al. Mechanical properties of the human gastrointestinal tract. Journal of Biomechanics. 2002;35:1417–1425. doi: 10.1016/s0021-9290(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 9.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterology Motility. 1996;8:277–297. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 10.Vinter-Jensen L, Duch BU, Petersen JAK, et al. Systemic treatment with epidermal growth factor in the rat. Biomechanical properties of hte growing small intestine. Regulatory Peptides. 1996;61:135–142. doi: 10.1016/0167-0115(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 11.Spencer AU, Yang H, Haxhija EQ, et al. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci. 2007;52:1060–1070. doi: 10.1007/s10620-006-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utter B, Brei D, Luntz J, et al. Preliminary in vivo experimental validation of SMA based bowel extender for short bowel syndrome: Conference on Smart Materials, Adaptive Structures and Intelligent Systems; Oxnard, CA. 2009. [Google Scholar]
- 13.Eichna LW, Bordley J. Capillary blood pressure in man. Comparison of direct and indirect methods of measurement. Journal of Clinical Investigation. 1939;18:695–704. doi: 10.1172/JCI101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichna LW. Capillary blood pressure in man. Direct measurements in the digits during arterial hypertension induced by paredrinol sulfate. Journal of Clinical Investigation. 1942;21:731–734. doi: 10.1172/JCI101348. [DOI] [PMC free article] [PubMed] [Google Scholar]