Abstract
Polychlorinated biphenyls (PCBs) can be metabolized to hydroxylated polychlorinated biphenyls (OH-PCBs) as reported in a number of animal studies. However, there are few studies on OH-PCBs in vivo in whole plants. In order to explore the formation of OH-PCBs in whole plants in detail, poplars (Populus deltoides × nigra, DN34) were exposed to 3,3′,4,4′-tetrachlorobiphenyl (CB 77) in hydroponic solution. Poplars are widely used in phytoremediation applications and the complete genome has been sequenced. In this research, a HPLC-MS method was developed to directly determine the hydroxylated metabolites of CB77 (OH-CB77s), avoiding the experimental errors introduced by derivatization pretreatments required by gas chromatography-based methods. Three potential hydroxylated metabolites of CB 77, including 6-hydroxy-3,3′,4,4′-tetrachlorobiphenyl (6OH-CB77), 5-hydroxy-3,3′,4,4′-tetrachlorobiphenyl (5OH-CB77) and 4′-hydroxy-3,3′,4,5′-tetrachlorobiphenyl (4′OH-CB79), were determined in poplar tissues. The major product, 6OH-CB77, was detected in the roots, bottom bark, bottom wood, middle bark and middle wood for the whole poplar plants, but the minor product, 5OH-CB77, was detected only in the poplar roots. The concentration of 6OH-CB77 was about 10 times greater than that of 5OH-CB77 in the roots. However, the major mammalian metabolite, 4′OH-CB79 was not detected in any of the samples. The results suggest that the hydroxylated metabolic pathway of CB77 is via an epoxide intermediate in poplar.
Keywords: 3,3′,4,4′-tetrachlorobiphenyl; hydroxylated metabolite; poplar; pathway
1. Introduction
Polychlorinated biphenyls (PCBs) are manmade persistent organic mixtures used in electrical equipment worldwide due to their high degree of chemical stability. When released in the environment, PCBs are persistent and accumulate in the food chain, giving rise to human health effects (Soechitram et al., 2004). Hydroxylated PCBs (OH-PCBs) have no known anthropogenic source and many OH-PCBs have been found in different environmental samples, including humans (Bergman et al., 1994; Newsome and Davies, 1996; Sandau et al., 2000), bears (Letcher et al., 2005), cetaceans (Kunisue et al., 2007), fish (Melancon et al., 1976; Buckman et al., 2006), bird eggs (Berger et al., 2004) and blood of mammals and birds (Kunisue and Tanabe, 2009). Moreover, there are very high proportions of total OH-PCBs to total PCBs by mass, reaching 11% and 33% in Inuits and southern Quebec people in pooled samples of whole blood, respectively (Sandau et al., 2000).
The OH-PCBs are thought to be generated from the metabolic transformation of PCBs by cytochrome P450 monooxygenases (Verreault et al., 2009). Unlike more lipophilic organic compounds, OH-PCBs are more readily excreted from living organisms due to their higher polarity and water solubility. Therefore, PCBs are thought to be detoxified by metabolic hydroxylation (Yoshimura et al., 1987). However, some OH-PCBs are more toxic to organisms than their parent compounds. For example, it has been reported that OH-PCBs have a higher affinity to bind to transthyretin, carrier of the thyroid hormone thyroxine (Brouwer et al., 1990). Furthermore, metabolites of PCBs can bind to other proteins (Darnerud et al., 1996; Tampal et al., 2003), DNA and lipids (Morck et al., 2002), and influence their biological functions. Mortensen et al. (2007) found both agonist or antagonist interactions of OH-PCBs with hormone receptors (HRs) or hormone receptor mediated responses, which can decrease AhRa and ARNT transcript levels, and CYP1A1, UGT and GST gene expressions. Thus, it is important to study hydroxylated metabolites and metabolic pathways of PCBs in plants and animals. Up to now, hydroxylated metabolites of 3,3′,4,4′-tetrachlorobiphenyl (OH-CB77s) were detected in vivo in fish, rat and other mammals after the exposure of 3,3′,4,4′-tetrachlorobiphenyl (CB77). Specific OH-CB77s, including 2-hydroxy-3,3′,4,4′-tetrachlorobiphenyl (2OH-CB77), 4′-hydroxy-3,3′,4,5′-tetrachlorobiphenyl (4′OH-CB79) and 5-hydroxy-3,3′,4,4′-tetrachlorobiphenyl (5OH-CB77) were detected in the catfish (Doi et al. 2006). Darnerud et al. (1986; 1996) found that 2OH-CB77, 4′OH-CB79 and 5OH-CB77 were detected in fetuses after injection of CB77 into pregnant mice.
Plants, such as poplar, have been extensively used in the remediation of organic pollutants and have some analogous metabolic pathways to mammals known as the “green-liver” model of metabolism to transform and degrade xenobiotic contaminants (Sandermann, 1994; Coleman, et al., 1997). However, there are few papers on the hydroxylation of PCBs in vivo in plants. Studies of the metabolism of PCBs by plant cell tissue cultures found some hydroxylated metabolites of PCBs (Kucerova et al., 2000). The metabolisms of ten different congeners of PCBs were tested in cell cultures of 12 different plant species (Wilken et al., 1995). The result showed that metabolism of defined PCB congeners was strongly dependent on the plant species. One dihydroxylated and six different monohydroxylated compounds were detected in studies with 14C-PCB1 (2-chlorobiphenyl) in soybean cultures. Poplars have been shown to uptake and translocate some lower chlorinated PCBs (Liu and Schnoor, 2008). However, there were not detailed metabolic studies of PCBs in poplar plants. Very little is known about hydroxylated metabolism of PCBs in whole plants except for the initial study by Liu et al. (2009) which detected only one OH-PCB metabolite of CB77 in poplar roots.
CB77 is a dioxin-like congener with relatively high toxicity. Therefore, CB77 was selected as a model to study its metabolism in hydroponic exposure studies with whole poplar plants. In the present paper, a high performance liquid chromatography-electrospray mass spectrometry (HPLC-MS) method was developed for the direct detection of OH-CB77s in poplar plants. This method was employed to investigate the formation and distribution of OH-CB77s in whole poplar plants and to elucidate possible metabolic pathways.
2. Experimental Section
2.1. Chemicals
The standard of CB77 was obtained from Accustandard Inc., USA. The possible hydroxylated metabolites of CB77 (4′OH-CB79, 5OH-CB77, 6OH-CB77; 98% purity or better) were synthesized according to the published procedures (Bauer et al., 1995; Lehmler and Robertson, 2001). A stock solution of CB77 was prepared at 1 mg mL−1 in acetone. Stock solutions of 4′OH-CB79, 5OH-CB77 and 6OH-CB77 were prepared at 1 mg mL−1 in acetonitrile. Working solutions were prepared by gradual dilution of their stock solutions. All standards and their solutions were stored in amber glass vials at 4 °C.
Florisil (60-100 mesh, Acros Organics) was activated at 450 °C for 12 h and then deactivated using 1% (w/w) water. Methyl-tert butyl ether (MTBE) (HPLC grade), dichloromethane (HPLC grade), hexane (pesticide grade), anhydrous sodium sulfate, and sodium hydroxide (98.6%) were obtained from Fisher Scientific. Methanol (HPLC grade solvent) was from Acros Organics. The deionized water (18.3 MΩ) was produced from an ultrapure water system (Barnstead International, Dubuque, IA, USA). All other chemicals and reagents were of analytical reagent grade or better in this experiment.
2.2. Hydroponic Setup
The exposure setup is similar to our previous experiments (Liu and Schnoor, 2008; Liu, et al., 2009; Zhai, et al., 2010). In brief, cuttings of poplar tree (Populus deltoides × nigra, DN34) were fit snugly the hole of screw cap and then the interface of the cap and the cutting was sealed using 100% silicone sealant. The poplar cuttings were hydroponically grown in half strength Hoagland nutrient solution (Epstein, 1972) under fluorescent lighting. The healthy whole poplar plants were selected for the exposed experiments in conical flasks after 25 days. The glass screw-top conical flasks (500 mL) with a sampling port sealed with screw cap, were used as the exposure reactors. Hoagland nutrient solution (400 mL), prepared from autoclaved de-ionized water and a suitable amount of CB77 were added to each autoclaved reactor. The starting concentration of CB77 in each reactor was 10 μg L−1. In order to elucidate the metabolism of CB77 in whole poplar, a variety of relative reference “controls” were tested with the following rationale: Blank plant control- three whole, intact plants without CB77 (contamination control); Solution control- three growth media solutions with CB77 but without poplar plants (abiotic reaction control); Autoclaved plant control- three autoclaved whole plants with CB77 (microorganism control); Dead plant control- three wilted, dead plants exposed for 4 days with CB77 (inactive plant control); Alcohol washed plant control – three whole plants whose roots were washed with 70% ethanol solution and then rinsed with de-ionized water with CB77 (another microorganism control); Whole poplar plant- whole, growing, intact plant with CB77 (treatment). All reactors were wrapped with aluminum foil to keep the roots in a dark environment and to eliminate photolysis of CB77. The temperature was maintained at 23±1°C during the exposure period, and the photoperiod was set at 16 h a day under fluorescent lighting. Autoclaved de-ionized water was injected into the reactors through the sampling port to replace the transpiration losses according to the weight of the reactors (gravimetric determination). The plants grew vigorously and transpired approximately 40 mL d−1.
According to the reported method (Liu and Schnoor, 2008), each reactor specimen was divided into samples of hydroponic solution, root, bottom bark, bottom wood, middle bark, middle wood, top bark, top wood and leaf in order to investigate the translocation and distribution of hydroxylated metabolites of CB77 in different parts of the poplar plants after 15 days exposure. Roots and leaves were ground in liquid nitrogen with a ceramic mortar and pestle. Other parts of the plant were cut into very small pieces in order to efficiently extract the target compounds. All equipment was rinsed with acetone between samples.
2.3. Extraction and cleanup
The extraction and cleanup procedure for OH-CB77s was modified from a method developed for plasma (Bergman et al., 1994). In brief, 0.5 mL of 37% HCl and 5 mL of 2-propanol were added to the hydroponic solutions (400 mL) and then these solutions were extracted twice with 50 mL of hexane/MTBE (1:1, v/v) for 30 minutes. The poplar tissue samples (approximately 2.0-4.0 g) were mixed with 2 mL of 37% HCl and 5 mL of 2-propanol, homogenized and extracted with 3 mL of hexane/MTBE (1:1, v/v) g−1 of sample and shaken vigorously overnight. The organic extract was transferred after centrifugation. A second extraction was performed for half an hour. The combined extracts were evaporated to dryness using rotary evaporator and re-dissolved in 1 mL of hexane. Then, 500 μL of NaOH solution (0.5 M in 50% ethanol) was added in hexane phase to partition hexane phase. The hexane phase was removed. The alkaline solution was re-extracted by another 1 mL of hexane. The combined hexane extract contained PCBs and any other neutral metabolites (neutral fraction), while OH-PCBs were kept in the alkaline phase. The alkaline solution was acidified with 125 μL HCl (2 M) and extracted twice with 1 mL of hexane/MTBE (9:1, v/v). The OH-CB77 fraction was cleaned up by a florisil column: The solution, root and wood samples were cleaned up with the small column (0.5 g anhydrous Na2SO4 on top, 1.0 g of florisil at the bottom). This column was activated with 3 mL of dichloromethane (DCM)/hexane/methanol (50:45:5) and then with 5 mL DCM/hexane/methanol of for elution; The bark and leaf samples were cleaned up with the large column (2.0 g anhydrous Na2SO4 on top, 5.0 g of florisil at the bottom). The column was activated with 3 mL of dichloromethane (DCM)/hexane/methanol and then with 7 mL DCM/hexane/methanol of for elution of OH-CB77s. The eluates were evaporated to dryness and dissolved in 1 mL of acetonitrile for HPLC-MS analysis after filtration using a 0.4 μm membrane.
2.4. Instrument
Qualitative and quantitative analysis of hydroxylated metabolites of CB77 were performed with an Agilent 1100 Series LC/MSD. Samples were separated by an Agilent Zorbax 80A Extended C18 column (2.1×150 mm, 5 μm) with a mobile phase flow rate of 0.2 mL min−1 at room temperature. The flow phase was acetonitrile and water with a ratio of 65:35. The injection volume was 20 μL. The negative ionization mode of electrospray mass spectrometry (ESI (−)-MS) was utilized. Other analytical parameters were as follows: SIM Ion Mass: 307; Fragmentor: 115V; Capillary Voltage: 3500V; Gain: 7.00; Drying Gas Flow: 10 L min−1; Nebulizer Pressure: 35psi; Drying Gas Temperature: 250°C.
3. Results
3.1. Analytical performance of OH-CB77 by HPLC-MS
A rapid HPLC-MS method was firstly developed to measure three of the most likely hydroxylated metabolites of CB77 (4′OH-CB79, 5OH-CB77 and 6OH-CB77) without a derivatization step. With the instrumental parameters mentioned above, 6OH-CB77 could be completely separated from 4′OH-CB77 and 5OH-CB77. However, 4′OH-CB79 and 5OH-CB77 could not be separated completely, but this did not influence the quantification of both peaks. The elution sequence of these three compounds was 5OH-CB77, 4′OH-CB79 and 6OH-CB77.
The HPLC-MS method provides good analytical performance. Linear calibration curves, based on peak areas to concentrations, were obtained in the range of 2.0-100 ng mL−1 of the three hydroxylated CB77s with correlation coefficients of 0.9999, 0.9996 and 1.0000 for 4′OH-CB79, 5OH-CB77 and 6OH-CB77, respectively. The relative standard deviations measured at 10 ng mL−1 level were in the range of 0.8-1.6% (n=5). The calculated detection limits (S/N=3) of this HPLC-MS method for 4′OH-PCB79, 5OH-PCB77 and 6OH-PCB77 were 0.150, 0.135, 0.040 ng mL−1, respectively. Furthermore, the good recoveries of these three OH-CB77s were obtained in the leaf, wood, bark and root with spiking masses of 4′OH-PCB79, 5OH-PCB77 and 6OH-PCB77, respectively (table 1). Therefore, this HPLC-MS method was employed in the following exposure experiment.
Table 1.
Percent recoveries of OH-CB77s in hydroponic solution and in different parts of poplar blanks spiked with the following masses: 4′OH-CB79 36 ng, 5OH-CB77 36 ng and 6OH-CB77 18 ng (%, n = 3)
| Compound | Hydroponic solution |
Root | Wood | Bark | Leaf |
|---|---|---|---|---|---|
| 4′OH-CB79 | 102.5±4.1 | 90.3±3.8 | 108.8±11.5 | 81.3±5.5 | 70.7±3.4 |
| 5OH-CB77 | 100.3±3.1 | 83.3±2.9 | 107.5±2.0 | 75.9±3.3 | 68.1±1.4 |
| 6OH-CB77 | 88.6±3.3 | 77.6±3.4 | 97.8±2.0 | 85.1±2.2 | 73.4±2.3 |
3.2. Hydroxylated metabolites of CB77 in hydroponic solutions
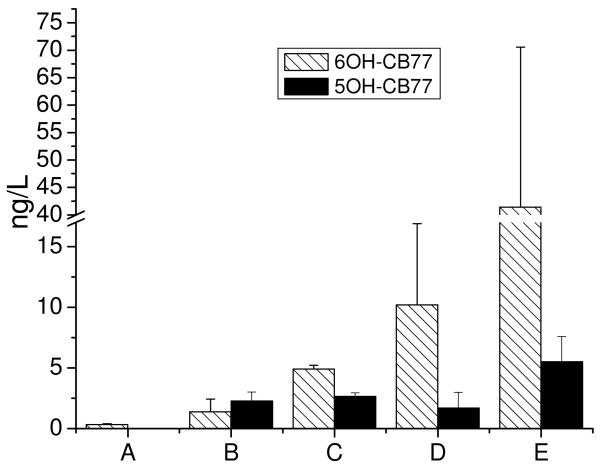
The possible formation and release of hydroxylated metabolites of CB77 into solution were monitored after 15 days of exposure to poplars by CB77. In general, hydroxylated metabolites of CB77 were not detected in hydroponic solutions of the blank plant controls, which indicate that the reactors were not contaminated inadvertently during the experimental procedures. Furthermore, the presence of 4′OH-CB79 was not detected in any of the solutions. However, trace amounts of 6OH-CB77 and 5OH-CB77 were detected in the solutions of other controls and whole plants (Figure 1). Concentrations of 6OH-CB77 and 5OH-CB77 increased progressively in solution from the solution control to the autoclaved plant control, the dead plant control, the alcohol-washed plant control and the whole plant. The highest concentrations of 6OH-CB77 and 5OH-CB77 were detected in whole poplar solutions at concentrations of 41.43±29.14 and 5.51±2.07 ng L−1, respectively. In the solution controls 6OH-CB77 was barely detected, which suggested trace amount of 6OH-CB77 be possibly produced due to hydrolysis. In the solution from the autoclaved plant control and the dead plant control, small amounts of 6OH-CB77 and 5OH-CB77 were detected, even though solutions and reactors were autoclaved and microorganisms were decreased before CB77 exposure. The concentrations of 6OH-CB77 and 5OH-CB77 in the solutions of the dead plant controls were a little higher than those in the solutions of autoclaved plant controls, likely due to greater microbial activity suspected in the vicinity of dead plant roots than tissues that were fully inactivated by autoclaving. These control samples suggest that small amounts of OH-CB77s are produced by microorganisms in the reactor solution.
Fig.1.
A comparison of 6OH-CB77 and 5OH-CB77 in the hydroponic solutions (n=3) after 15 days exposure to a spiked concentration of 10 μg L−1 CB77. (A) solution control; (B) autoclaved plant control; (C) dead plant control; (D) alcohol-washed plant control; (E) whole plant
Slightly lower concentrations of 6OH-CB77 and 5OH-CB77 were detected in the solutions of poplars washed with 70% alcohol than those in the solutions of whole poplars. There are two reasons that may explain this phenomenon: one is that poplar roots can metabolize CB77 to produce 6OH-CB77 and 5OH-CB77, and some fractions of 6OH-CB77 and 5OH-CB77 are released from the roots into solution. The other is roots washed with alcohol appeared injured and likely reduced the root's capacity to produce 6OH-CB77 and 5OH-CB77. Likewise, microorganisms in the vicinity of roots are likely to be affected by the 70% alcohol treatment which could decrease metabolism of CB77. As a result, the concentrations of 6OH-CB77 and 5OH-CB77 in the solution of alcohol-washed poplar plants were less than those in whole poplar trees. The concentration difference between all the controls and whole poplar plants suggested that poplar plants were the major cause of the metabolism of CB77 to OH-CB77s, even though there was some evidence that 6OH-CB77 and 5OH-CB77 may be partly produced by microorganisms in some solutions.
3.3 Hydroxylated metabolites of CB77 in different parts of poplar trees
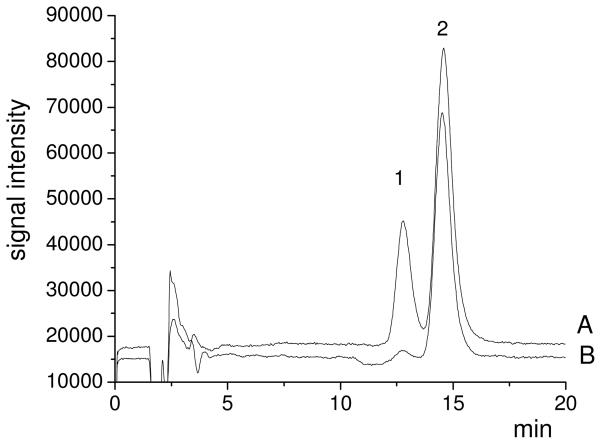
Liu and Schnoor (2008) have reported in detail that whole poplar plants can uptake and translocate CB77 from hydroponic solution. Therefore, only the plant metabolism of CB77 and distribution of hydroxylated metabolites were studied in this paper. The distribution of hydroxylated metabolites of CB77 in different parts of the poplar plants was analyzed in order to elucidate the transformation of CB77 and translocation of hydroxylated metabolites in the poplar plants. Results show that 6OH-CB77 and 5OH-CB77 were detected in the roots of the whole poplar plants (figure 2). The distribution and concentration of 6OH-CB77 are shown in Figure 3. However, 4′OH-CB79 was not detected in any part of the poplar (root, stem, or leaves); 5OH-CB77 and 6OH-CB77 were not detected in the blank plant controls, autoclaved poplar controls and dead poplar controls.
Fig.2.
A comparison of chromatograms of standards (A) and root samples of whole plant (B). (1) 5OH-CB77; (2) 6OH-CB77
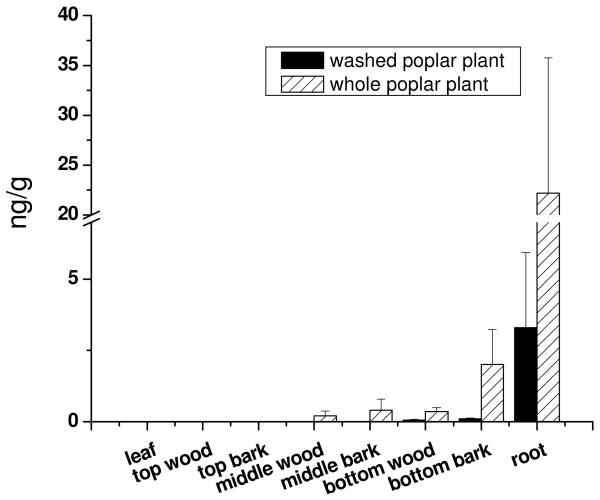
Fig.3.
A comparison of concentrations of 6OH-CB77 in different parts of the whole poplar plants (n=3) after 15 days exposure to a spiked concentration of 10 μg L−1 CB77. There were not hydroxylated metabolites of PCBs detected in the leaf, top wood and top bark. No hydroxylated metabolites of PCBs were detected in the controls.
The metabolite 6OH-CB77 was detected in the root, bottom bark, bottom wood, middle bark and middle wood of whole poplars. First, CB77 was bound to root tissues and was then translocated to other portions of the plant (wood, bark and leaves), and the distribution of its metabolites, OH-CB77s, also was translocated in a similar fashion. Among the different parts of the plant, the highest concentration of 6OH-CB77 was detected in the roots, reaching 22.19±13.56 ng g−1, which is about 535 times greater than the concentration of 6OH-CB77 in solution. Apparently, CB77 was metabolized in the root and expelled or desorbed to the hydroponic solution and translocated to other parts of the poplar. The concentrations of 6OH-CB77 in the bark were a little higher than those in the wood which may have two reasons. First, the bark has a strong affinity for PCBs (Hermansont and Hites, 1990) and the bark is near the active transport paths for water and nutrition. Second, the bark can absorb 6OH-CB77 from the reactor solution and diffuse it along the bark above the solution.
The compound 6OH-CB77 was also detected in the root, the bottom bark and the bottom wood of alcohol-washed poplar plant roots. But the concentrations of 6OH-CB77 in the alcohol-washed plants were lower than those in the whole poplar. Similar to CB77 (Liu and Schnoor, 2008), 6OH-CB77 was detected only in the roots (and not in the top bark, top wood or leaves) for whole poplars and alcohol-washed poplar plants, suggesting that CB77 and hydroxylated 6OH-CB77 are not easily to translocate in the plant, over the duration of exposure (15 days).
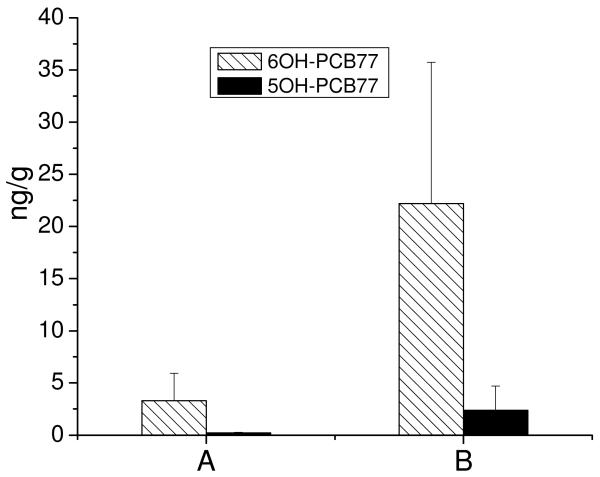
The compound 5OH-CB77 was detected only in the root of the whole poplars and alcohol-washed plants (Figure 4) at concentrations of 2.39±2.33 ng g−1 and 0.22±0.05 ng g−1, respectively. The ratio of 6OH-CB77 and 5OH-CB77 was approximately 10:1. The amount of 5OH-CB77 was much less than that of 6OH-CB77 and the detection limit of 5OH-CB77 was also lower than that of 6OH-CB77 which might explain the fact that 5OH-CB77 was not detected in other parts of the whole poplar plants.
Fig.4.
Comparison of 6OH-CB77 and 5OH-CB77 (n=3) in the root samples after 15 days exposure to a spiked concentration of 10 μg L−1 CB77. (A) alcohol-washed plant; (B) the whole plant
Liu et al. (2009) detected 6OH-CB77 only in the root of poplar trees. This was the only product detected, and the concentration of 5OH-CB77 was apparently below the detection limit of gas chromatographic method with derivatization. Based on the literature and the results from poplar exposure to CB77 in this study, CB77 is metabolized primarily to 6OH-CB77 and 5OH-CB77 (10:1 ratio), and microorganisms in the root zone would appear to play a small role in their production.
4. Discussion
4.1. Advantage of LC-MS
Analytical methods play an important role in the study of hydroxylated metabolites of PCBs and their metabolic pathways. Gas chromatography-based methods are presently the most common methods for the determination of OH-PCBs in environmental matrices. However, OH-PCBs can not be detected by GC-based methods because of their lower volatility. They must be derivatized using different derivatization reagents, such as diazomethane, acetyl, trifluoroacetyl, or pentafluoropropionyl analogues (Preston and Allen, 1980; Abraham et al., 1997; Berger et al., 2004) before the analysis. The derivatization procedure is a time-consuming step and introduces errors due to the derivatization efficiency. This newly developed LC-MS method can detect OH-CB77s directly, save a lot of derivatization time and decrease the errors in the process of pretreatment. Therefore, there were two OH-CB77s to be detected in whole poplar plants in this work by LC-MS; only one OH-CB77 was detected by GC-MS (Liu et al., 2009).
4.2. Comparison of hydroxylated metabolites of CB77 in different species
In order to elucidate the various OH-CB77s produced by different species from CB77, a comparison of OH-CB77s in different studies was compiled (table 2). As shown in table 2, animal studies yielded four hydroxylated metabolites with 4′OH-CB79 and 5OH-CB77 as major products. For cultures of plants and whole poplar, part of 2OH-CB77, 5OH-CB77 and 6OH-CB77 were generally found, and 6OH-CB77 was the major product. Figure 4 shows that the concentration of 6OH-CB77 is about 10 times as much as that of 5OH-CB77 in the roots of alcohol-washed poplars and the whole poplar plants. The different speciation and ratios of hydroxylated metabolites of PCBs might be due differences in the enzyme biochemistry in different species. The reactivity of different substrates with these nonspecific monoxygenases varies considerably (Verreault et al., 2009). In addition, the activities and specificities of cytochrome monoxygenases differ widely in different tissues and different species. Therefore, such differences are reflected in differing speciation and ratios of oxidized products formed by metabolism of a particular compound, such as CB77. Electronic and steric factors can lead to uneven isomerization of expoxide intermediates.
Table 2.
Comparison of hydroxylated metabolites of CB77 in different species
| Species | OH-CB77s | Literature Reference |
|---|---|---|
| Rat | 2OH-CB77, 4′OH-CB79 (major), | Yoshimura et al., 1987; |
| 5OH-CB77 (major) and 6OH-CB77 | Koga et al., 1989 | |
| Fish | 2OH-CB77, 4′OH-CB79 (major), | White et al., 1997; |
| 5OH-CB77 (major) and 6OH-CB77 | Doi et al. 2006 | |
| Chicken | 2OH-CB77, 4′OH-CB79 and 5OH-CB77 | Klasson-Wehler et al., 1990 |
| Cultivars of tomato and Paul's Scarlet rose |
2OH-CB77 and 6OH-CB77 (major) | Bock, 1999 |
| Cultivar of lettuce | 2OH-CB77, 5OH-CB77 and 6OH-CB77 (major) |
Bock, 1999 |
| Whole poplar | 5OH-CB77 and 6OH-CB77 (major) | This work |
4.3. Metabolic pathways
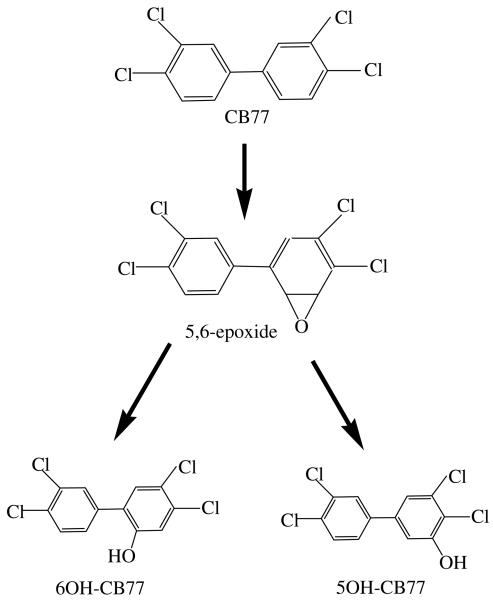
There are two reported hydroxylation pathways for the hydroxylation of the aromatic ring of PCBs (Jerina et al., 1974). The first pathway is to form the hydroxylated metabolites via an epoxide intermediate. The second one is to directly introduce the hydroxyl substitution to a carbon on the aromatic ring. But the most widely accepted theory of hydroxylation of PCB congeners proceeds by the epoxide mechanism (Jerina et al., 1974; Guengerich, 2003). The epoxide mechanism was also supported by the hydroxylated metabolites of 4-monochlorobiphenyl (CB3) in whole poplar plants in our previous work (Zhai et al., 2010). Furthermore, there is some biochemical evidence supporting the epoxide mechanism. For example, cytochrome P-450 dependent monoxygenases can oxidize PCBs to produce hydroxylated metabolites via an epoxide intermediate (Ishida et al., 1991). In this work, 6OH-CB77 and 5OH-CB77 was detected in the roots of alcohol-washed poplar and the whole poplar. Thus, the hydroxylated metabolites of CB77 from CB77 suggest the function of cytochrome P-450 isoenzymes via an epoxide intermediate (White et al., 1997). In vitro studies also indicated the involvement of P450 in scup, and CYP1A in the study of CB77 metabolism by fish, but in vivo data indicated the possible involvement of other enzyme(s) as well. The compounds 4′OH-CB79, 5OH-CB77 and 6OH-CB77 could be the rearrangement products of 4,5- and 5,6-epoxides. Furthermore, CYP1A could metabolize CB77 to the 5,6-oxide, whereas other CYPs may produce the 4,5-oxide (White et al., 1997). The two hydroxylated metabolites of CB77 in this work are likely produced via a 5,6-epoxide intermediate (Figure 5). We suggest that this epoxide intermediate was subsequently isomerized to form 6OH-CB77 and 5OH-CB77. The major metabolic product is 6OH-CB77 likely due to electronic and steric factors. An NIH shift to produce 4′OH-CB79 was not observed in this exposure study on poplar, although 4′OH-CB79 is the major mammalian product.
Fig.5.
Proposed hydroxylated metabolic pathway of CB77 in the whole poplar plant.
5. Conclusions
The metabolic production and distribution of hydroxylated metabolites of CB77 were studied in exposed whole intact poplar plants in hydroponic studies by HPLC-MS. Results suggest that CB77 was hydroxylated in vivo in the poplar plants, and the products were consistent with a proposed epoxide intermediate pathway. This paper confirms the previously reported metabolism of CB77 to 6OH-CB77 in whole intact poplars by Liu et al. (2009); it reports the discovery of a new metabolite 5OH-CB77 and proposes an enzymatic pathway through epoxidation; and it utilizes a new methodology for LC-MS analysis of hydroxyl-metabolites in hydroponic solution and through extraction of plant tissues.
Acknowledgment
This work was supported by the Iowa Superfund Basic Research Program (SBRP), National Institute of Environmental Health Science, Grant Number P42ES13661. We thank Collin Just, Richard Meggo and Cassie Krahe, Civil Environmental Engineering, University of Iowa for supporting the analytical method and the sample collection. We also thank the Center for Global and Regional Environmental Research (CGRER) at the University of Iowa for support of Zhai and Schnoor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham VM, Lynn BC., Jr. Determination of hydroxylated polychlorinated biphenyls by ion trap gas chromatography-tandem mass spectrometry. J. Chromatogr. A. 1997;790:131–141. doi: 10.1016/s0021-9673(97)00745-0. [DOI] [PubMed] [Google Scholar]
- Bauer U, Amaro AR, Robertson LW. A new strategy for the synthesis of polychlorinated biphenyl metabolites. Chem. Res. Toxicol. 1995;8:92–95. doi: 10.1021/tx00043a012. [DOI] [PubMed] [Google Scholar]
- Berger U, Herzke D, Sandanger TM. Two trace analytical methods for determination of hydroxylated PCBs and other halogenated phenolic compounds in eggs from Norwegian birds of prey. Anal. Chem. 2004;76:441–452. doi: 10.1021/ac0348672. [DOI] [PubMed] [Google Scholar]
- Bergman A, Kiasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ. Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C. Untersuchungen des Metabolismus von 3,3′,4,4′-tetrchlorbiphenyl (PCB77) und 2,2′,5-trichlorbiphenyl (PCB 18) in ausgewaehlten pflanzlichen in-vitro-systemen. landbauforschung voelkenrode. Sonderheft. 1999;207:1–106. [Google Scholar]
- Brouwer A, Klasson-Wehler E, Bokdam MM. Competitive inhibition of thyroxin binding to transthyretin by monohydroxy metabolites of 3,4,3′4′-tetrachlorobiphenyl. Chemosphere. 1990;20:1257–1262. [Google Scholar]
- Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006;78:176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Coleman J, Blake-Kalff M, Davies E. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997;2:144–151. [Google Scholar]
- Darnerud PO, Brandt I, Klasson-Wehler E, Bergman A, Dargy R, Dencker L, Sperber GO. 3,3′,4,4′-Tetrachloro[14C]biphenyl in pregnant mice: enrichment of phenol and methyl sulphone metabolites in late gestational fetuses. Xenobiotica. 1986;16:295–306. doi: 10.3109/00498258609043532. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Morseb D, Klasson-Wehlerc E, Browverb A. Binding of a 3,3′,4,4′-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicol. 1996;106:105–114. doi: 10.1016/0300-483x(95)03169-g. [DOI] [PubMed] [Google Scholar]
- Doi AM, Lou Z, Holmes E, Venugopal CS, Nyagode B, James MO, Kleinow KM. Intestinal bioavailability and biotransformation of 3,3′,4,4′-tetrachlorobiphenyl (CB 77) in in situ preparations of channel catfish following dietary induction of CYP1A, Aquat. Toxicol. 2006;77:33–42. doi: 10.1016/j.aquatox.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Epstein E. Mineral nutrition of plants: principles and perspectives. John Wiley & Sons; New York: 1972. [Google Scholar]
- Guengerich FP. Cytochrome P450 oxidations in the generation of reactive electrophiles: epoxidation and related reactions. Arch. Biochem. Biophys. 2003;409:59–71. doi: 10.1016/s0003-9861(02)00415-0. [DOI] [PubMed] [Google Scholar]
- Hermansont MH, Hites RA. Polychlorinated biphenyls in tree bark. Environ. Sci. Technol. 1990;24:666–671. [Google Scholar]
- Ishida C, Koga N, Hanioka N, SAEKI HK, Yoshimura H. Metabolism in vitro of 3,4,3′,4′- and 2,5,2′,5′- tetrachlorobiphenyl by rat liver microsomes and highly purified cytochrome P-450. J. Pharmacobio. Dyn. 1991;14:276–284. doi: 10.1248/bpb1978.14.276. [DOI] [PubMed] [Google Scholar]
- Jerina DM, Yagi H, Daly JW. Arene oxide: a new aspect of drug metabolism. Science. 1974;185:573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Klasson-Wehler E, Brunstrom B, Rannug U, Bergman A. 3,3′,4,4′-tetrachlorobiphenyl: metabolism by the chick embryo in ovo and toxicity of hydroxylated metabolites. Chem. Biol. Interact. 1990;73:121–132. doi: 10.1016/0009-2797(90)90112-z. [DOI] [PubMed] [Google Scholar]
- Koga N, Beppu M, Ishida C, Yoshimura H. Further studies on metabolism in vivo of 3,4,3′,4′-tetrachlorobiphenyl in rats: identification of minor metabolites in rat faeces. Xenobiotica. 1989;19:1307–1318. doi: 10.3109/00498258909043182. [DOI] [PubMed] [Google Scholar]
- Kucerova P, Mackova M, Chroma L, Burkhard J, Triska J, Demnerova K, Macek T. Metabolism of polychlorinated biphenyls by Solanum nigrum hairy root clone SNC-9O and analysis of transformation products. Plant and Soil. 2000;225:109–115. [Google Scholar]
- Kunisue T, Sakiyama T, Yamada TK, Takahashi S, Tanabe S. Occurrence of hydroxylated polychlorinated biphenyls in the brain of cetaceans stranded along the Japanese coast. Mar. Pollut. Bullet. 2007;54:963–973. doi: 10.1016/j.marpolbul.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kunisue T, Tanabe S. Hydroxylated polychlorinated biphenyls (OH-PCBs) in the blood of mammals and birds from Japan: lower chlorinated OH-PCBs and profiles. Chemosphere. 2009;74:950–961. doi: 10.1016/j.chemosphere.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere. 2001;45:1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Li HX, Chu SG. Determination of hydroxylated polychlorinated biphenyls (HO-PCBs) in blood plasma by high-performance liquid chromatography-electrospray ionization-tandem quadrupole mass spectrometry. J. Anal. Toxicol. 2005;29:209–216. doi: 10.1093/jat/29.4.209. [DOI] [PubMed] [Google Scholar]
- Liu J, Schnoor JL. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in the whole hybrid poplar plants after hydroponic exposure. Chemosphere. 2008;73:1608–1616. doi: 10.1016/j.chemosphere.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu D, Jiang G, Schnoor JL. In Vivo biotransformation of 3,3′,4,4′-tetrachlorobiphenyl by whole plants-poplars and switchgrass. Environ. Sci. Technol. 2009;43:7503–7509. doi: 10.1021/es901244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon MJ, Jr., Lech JJ. Isolation and identification of a polar metabolite of tetrachlorobiphenyl from bile of rainbow trout exposed to 14C-tetrachlorobiphenyl. Bullet. Environ. Contam. Toxicol. 1976;15:181–188. doi: 10.1007/BF01685158. [DOI] [PubMed] [Google Scholar]
- Morck A, Larsen G, Klasson-Wehler E. Covalent binding of PCB metabolites to lipids: route of formation and characterization. Xenobiotica. 2002;32:625–640. doi: 10.1080/00498250210130573. [DOI] [PubMed] [Google Scholar]
- Mortensen AS, Braathen M, Sandvik M, Arukwe A. Effects of hydroxy-polychlorinated biphenyl (OH-PCB) congeners on the xenobiotic biotransformation gene expression patterns in primary culture of Atlantic salmon (Salmo salar) hepatocytes. Ecotoxicol. Environ. Saf. 2007;68:351–360. doi: 10.1016/j.ecoenv.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Newsome WH, Davies D. Determination of PCB metabolites in canadian human milk. Chemosphere. 1996;33:559–565. doi: 10.1016/0045-6535(96)00199-3. [DOI] [PubMed] [Google Scholar]
- Preston BD, Allen JR. 2,2′,5,5′-tetrachlorobiphenyl: Isolation and identification of metaboliltes generated by rat liver microsomes. Drug metabol. Dispos. 1980;4:197–204. [PubMed] [Google Scholar]
- Sandermann H. Higher-plant metabolism of xenobiotics – the green liver concept. Pharmacogenetics and Genomics. 1994;4:225–241. doi: 10.1097/00008571-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom1 RJ. Analysis of hydroxylated metabolites of PCBs (OH-PCB s) and other chlorinated phenolic compounds in whole blood from Canadian Inuit. Environ. Health Perspect. 2000;108:611–616. doi: 10.1289/ehp.00108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechitram SD, Athanasiadou M, Hovander L, Bergman A, Sa PJJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ. Health Perspect. 2004;112:1208–1212. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampal N, Myers S, Robertson LW. Binding of polychlorinated biphenyls/metabolites to hemoglobin. Toxicol. Lett. 2003;142:53–60. doi: 10.1016/s0378-4274(02)00484-8. [DOI] [PubMed] [Google Scholar]
- Verreault J, Letcher RJ, Sonne C, Dietz R. In vitro metabolism of polychlorinated biphenyls and cytochrome P450 monooxygenase activities in dietary-exposed Greenland sledge dogs. Comp. Biochem. Physiol. C. 2009;150:91–100. doi: 10.1016/j.cbpc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- White RD, Shea D, Stegeman JJ. Metabolism of the aryl hydrocarbon receptor agonist 3,4,3′,4′-tetrachlorobiphenyl by the marine fish scup (stenotomus chrysops) in vivo and in vitro. Drug Metabol. Dispos. 1997;25:564–572. [PubMed] [Google Scholar]
- Wilken A, Bock C, Bokern M, Harms H. Metabolism of different PCB congeners in plant cell cultures. Environ. Toxicol. Chem. 1995;14:2017–2022. [Google Scholar]
- Yoshimura H, Yonemoto Y, Yamada H, Koga N, Oguri K, Saeki S. Metabolism in vivo of 3,4,3′,4′-tetrachlorobiphenyl and toxicological assessment of the metabolites in rats. Xenobiotica. 1987;17:897–910. doi: 10.3109/00498258709044189. [DOI] [PubMed] [Google Scholar]
- Zhai G, Lehmler H, Schnoor JL. Hydroxylated metabolites of 4-monochlorobiphenyl and its metabolic pathway in whole poplar plants. Environ. Sci. Technol. 2010;44:3901–3907. doi: 10.1021/es100230m. [DOI] [PMC free article] [PubMed] [Google Scholar]