Abstract
Purpose of review
This review discusses the role of enteroendocrine cells of the gastrointestinal tract as chemoreceptors that sense lumen contents and induce changes in gastrointestinal function and food intake through the release of signaling substances acting on a variety of targets locally or at a distance.
Recent findings
Recent evidence supports the concept that chemosensing in the gut involves G protein-coupled receptors and effectors that are known to mediate gustatory signals in the oral cavity. These include sweet-taste and bitter-taste receptors, and their associated G proteins, which are expressed in the gastrointestinal mucosa, including selected populations of enteroendocrine cells. In addition, taste receptor agonists elicit a secretory response in enteroendocrine cells in vitro and in animals in vivo, and induce neuronal activation.
Summary
Taste-signaling molecules expressed in the gastrointestinal mucosa might participate in the functional detection of nutrients and harmful substances in the lumen and prepare the gut to absorb them or initiate a protective response. They might also participate in the control of food intake through the activation of gut–brain neural pathways. These findings provide a new dimension to unraveling the regulatory circuits initiated by luminal contents of the gastrointestinal tract.
Keywords: afferent neurons, chemoreception, gustducin, peptides, taste receptors
Introduction
Chemosensing of potentially beneficial or harmful substances in the lumen by the gastrointestinal mucosa triggers various gastrointestinal functions including secretion, motility and regulation of blood flow that are essential for digestion and absorption of nutrients, but that are also important for the initiation of protective responses, including vomiting and food aversive behaviour, through the activation of hormonal and neuronal pathways.
Enteroendocrine cells are likely to be the first level of integration of information from the gut lumen, acting as specialized transducers of luminal factors, releasing signaling molecules that activate nerve fibers or other local or distant targets [1–3]. The neurons that detect luminal contents comprise extrinsic afferent neurons (mostly vagal) and intrinsic afferent neurons whose cell bodies are part of the enteric nervous system, which generate reflexes affecting motility, blood flow, and water and electrolytes secretion [4]. This paper reviews the current notion on chemosensing of luminal contents and focuses on recent discoveries of chemosensory signaling molecules in enteroendocrine cells of the gastrointestinal mucosa which, upon activation, might initiate a cascade of events inducing digestion and absorption of nutrients or neutralization and expulsion of drugs, toxins and microorganisms.
The gastrointestinal endocrine system
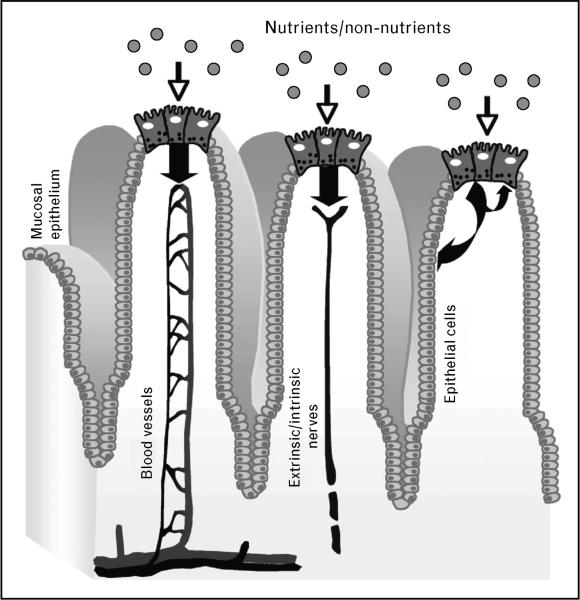
Enteroendocrine cells are specialized epithelial cells dispersed among mucosal cells of the gastrointestinal tract that represent less than 1% of the entire gut epithelial population. They constitute the largest endocrine organ of the human body, which produces and secretes a variety of hormones or signaling molecules, including gastrin (G cells), ghrelin (P or X cells), somatostatin (D cells), cholecystokinin (CCK) (I cells), serotonin (enterochromaffin cells), glucose-dependent insulinotropic peptide (GIP) (K cells), glucagon-like peptides (GLPs) and peptide YY (PYY) (L cells). Gastrointestinal peptides are derived from different genes and are expressed in multiple forms due to alternative splicing or differential processing [5–8]. Enteroendocrine cells can be distinguished in ‘open cells’ with microvilli extending to the lumen, and ‘closed cells’ that do not reach the lumen. Their secretory products are accumulated in secretory granules and secreted upon stimulation by exocytosis at the basolateral membrane into the interstitial space, where they can act locally or on distant targets through the bloodstream [1–3,5,7]. In this respect, enteroendocrine cells can be regarded as primary chemoreceptors, capable of responding to luminal constituents by releasing secretory products that activate neuronal pathways, nearby cells or distant targets through different mechanisms (Fig. 1). This model is particularly suitable for the ‘open cells’ that reach the luminal surface. ‘Closed cells’, however, can be regulated by luminal content indirectly through neural and humoral mechanisms [3].
Figure 1. Possible pathways involved in nutrient sensing by enteroendocrine cells.
Putative sites of chemosensing are localized on the cell surface. When the luminal content (nutrients and non-nutrients) comes in contact with enteroendocrine cells, it induces release of hormones that enter blood vessels or signaling molecules that activate extrinsic or intrinsic afferent neurons thereby sending neuronal messages to the central nervous system and to enteric neurons. Released molecules can also act directly on adjacent cells, including other enteroendocrine cells and other types of epithelial cells like brush cells.
Luminal amino acids and calcium are the major chemicals signaling the release of gastrin from G cells (‘open cells’) of the gastric antrum and pylorus [6]. Gastrin enters the systemic circulation and induces release of histamine via CCK-2 receptors expressed by enterochromaffin-like cells. Histamine stimulates acid secretion from parietal cells, which are also targets of gastrin [9]. Somatostatin released by ‘closed’ D cells of the gastric corpus upon stimulation by intestinal hormones and neurotransmitters, inhibits acid secretion by acting directly on parietal cells and indirectly via inhibition of histamine release. By contrast, ‘open’ D cells of the pylorus can be directly activated by luminal content.
‘Open’ I cells of the upper small intestine produce CCK in response to saturated fat, long-chain fatty acids, amino acids and small peptides from protein digestion [2,10]. CCK, in turn, triggers the release of digestive enzymes from the pancreas and the emptying of bile salts from the gallbladder into the duodenum, thus inducing protein and fat digestion. CCK release also regulates gastrointestinal functions, including inhibition of gastric emptying and food intake through activation of CCK-1 receptors on vagal afferent fibers innervating the gut [11]. These effects can be modulated by orexigenic peptides; orexin and ghrelin inhibit, whereas leptin potentiates CCK effects on vagal neurons [2]. Interestingly, ghrelin is the only peptide that increases food intake [8].
The primary nutrients that induce release of PYY from L cells of the distal ileum and colon are lipids and carbohydrates that can act directly on L cells or indirectly by neurohumoral signals deriving from the proximal gut, for example, via GIP released by the K cells [12]. PYY plays an important role in the inhibitory feedback mechanism regulating nutrient transit, known as the ileal brake, by inhibiting gastrointestinal motility and secretion and reducing gastric emptying [12]. There has been increasing interest in PYY3–36 for its inhibition of food intake and induction of aversive food response [13,14], even though there are controversies about the food intake effect [15]. GLP1 is also released by L cells and is involved in the regulation of ileal brake induced by peptones; together with GIP, it mediates food-stimulated, glucose-dependent secretion of insulin from pancreatic β cells [16,17]. PYY, GLP1 and GIP have been implicated in the pathogenesis of metabolic disorders, including obesity and type 2 diabetes [18]. PYY and GLP1, together with CCK, have been regarded as satiety signals [8]. Carbohydrates are likely to induce release of serotonin from enterochromaffin cells into the lamina propria where it acts in a paracrine fashion on distinct receptors located on nerve endings [19].
G protein-coupled receptors as sensors of luminal contents
The cellular and neural pathways that mediate the biological responses to luminal molecules are still poorly understood. Recent evidence supports a role for members of the G protein-coupled receptor (GPCR) superfamily as sensors of luminal contents which, upon stimulation, initiate functional responses through the activation of G protein signaling cascades. The background for this hypothesis resides in the identification of the molecular transduction mechanisms operating in the mouth. Nutrients and nonnutrients are initially detected in the mouth by interacting with different transduction elements, including ion channels, ligand-gated channels, transporters and GPCRs that are expressed in the apical membranes of specialized epithelial cells, known as taste receptor cells. These cells, localized in taste buds of the oral epithelium, transmit taste information to the brain as changes in neural activity through afferent gustatory fibers [20]. This detection process might influence ingestive behavior by stimulating secretions that facilitate digestion and absorption or by inducing a behavior that protects from potentially harmful substances. The sensors detecting sweet and bitter sensations and amino acids have now been identified and characterized as unrelated GPCR families–the T1R and T2R taste receptors families [21–23]. The T1R family comprises three distinct members that heterodimerize to sense sweetness (T1R2 and T1R3) and amino acids (T1R1 and T1R3), whereas the T2R family includes numerous divergent GPCRs that act as broadly tuned bitter sensors [21,24]. Taste receptors interact with specific Gα subunits, including α-gustducin, a taste-specific signaling protein with a prominent role in bitter taste [25,26]. Not all bitter-sensitive taste cells contain α-gustducin, however. In addition, α-gustducin knockout mice have reduced but not abolished response to bitter compounds, indicating the existence of other G-protein α-subunits in bitter taste transduction, including α-transducin and Gi. Other transducers associated with taste signaling include phospholipase Cβ2 and transient receptor potential channel type 5, a calcium-activated cation channel [24]. Several of these proteins, including α-gustducin, are also involved in sweet-taste transduction [20,24]. Coupling of T1R and T2R taste receptors with signaling proteins activates transduction pathways through different effector systems, including cAMP and inositol phosphate P3, leading to intracellular Ca2+ increase and transmitter release [20,24].
Some of the key molecules implicated in taste signaling are expressed in the gastrointestinal mucosa and enteroendocrine cell lines, providing strong support to the hypothesis that transduction mechanisms identified in the oral epithelium also operate in the gut. Multiple T2R transcripts have been detected in the mucosa of the mouse and rat gastrointestinal tract and human colon, some of which have known ligands, like mT2R138 and hT2R38, the phenylthiocarbamide receptor, and mT2R108 and hT2R47, the denatonium benzoate receptor [27–29]. The sweet taste receptors, T1R2 and T1R3, have also been described in rodent and human intestine [30,31•,32••]. In addition, transcripts for α-gustducin have been reported in rat and mouse gastrointestinal mucosa [29,30,31•,32••,33,34]. Immunoreactivity for α-gustducin or α-transducin 2 has been localized to the mucosa of mouse stomach and duodenum [29], and α-gustducin cells have been observed in the rat intestinal mucosa and pancreatic duct [35,36], and in human gastric and intestinal mucosa [27]. Finally, co-expression of α-gustducin with T1R2 and T1R3 has been described in the mouse duodenum, further supporting the role of this G protein in taste sensation [32••].
Localization of α-gustducin to subpopulations of enteroendocrine cells
Different epithelial cell populations are likely to utilize gustducin signaling in the gastrointestinal tract. These include enteroendocrine cells, as indicated by the colocalization of α-gustducin immunoreactivity with markers for enteroendocrine cells like chromogranin A [27,29,34,37]. Alpha-gustducin immunoreactivity was localized to different types of enteroendocrine cells, including ‘open’ cells like those producing PYY, GLP1, GIP [27,32••,38••,39] and CCK (unpublished results) in rodents and humans, and ‘closed’ cells like those containing serotonin in the mouse jejunum [39], but not in the human colon [27]. Alpha-gustducin immunoreactivity has also been reported in a subset of cells containing villin, or the lectin Ulex europaeus agglutinin, in the rat and mouse gastrointestinal tract [35–37,39], but not in the human gastrointestinal tract where α-gustducin was observed in enteroendocrine cells but not in other epithelial cells [27,32••]. Brush cells are a population of specialized epithelial cells that are scattered within the epithelial lining of the gastrointestinal tract, and are characterized by the lack of intracellular secretory vesicles [3]. Their function is unknown, but it has been suggested that they are involved in chemosensory functions, based on their morphology and expression of α-gustducin.
Functional significance of taste-signaling elements in enteroendocrine cells
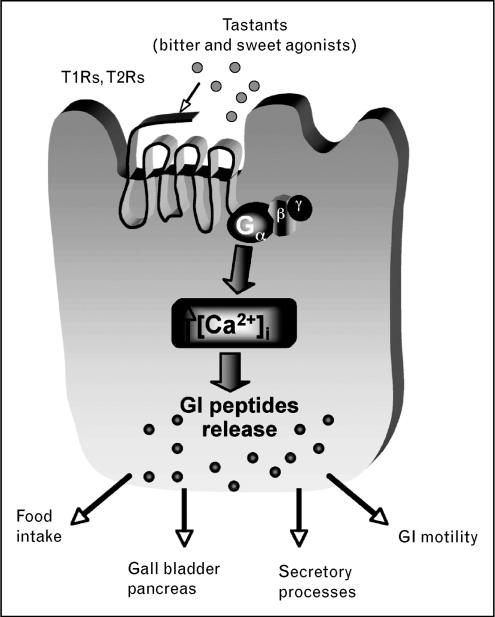
The expression of taste-signaling molecules in the gastrointestinal tract provides the foundation for the hypothesis that GPCR-mediating sensations of sweet and bitter in the tongue function as luminal sensors for nutrients or non-nutrients, thus participating in the chemosensory processes responsible for the induction of functional responses. Several additional lines of evidence support this hypothesis. These include the finding that bitter agonists induce an increase in intracellular Ca2+ in enteroendocrine cell lines expressing bitter-taste receptors and G α-proteins, but not in cell lines that do not express these molecules [29,40•], providing indirect evidence for the functionality of bitter-taste receptors outside the tongue. It is reasonable to postulate that activation of bitter-taste receptor signaling molecules expressed in enteroendocrine cells by luminal content (nutrients, drugs and toxins) induces increase in intracellular Ca2+ that triggers release of peptides like PYY, GLP1 or CCK, as suggested by their colocalization with α-gustducin [27], and by the observation that bitter agonists induce release of CCK in intestinal endocrine cell lines [40•] (Fig. 2).
Figure 2. Postulated mechanism involving sweet (T1R) and bitter (T2R) taste receptors on enteroendocrine cells.
Taste receptors couple to G proteins upon activation to induce intracellular Ca2+ increase resulting in release of peptides, which regulate a variety of gastrointestinal functions, including action on organs associated with the gut like the gallbladder and pancreas, via neuronal or humoral pathways to induce digestion and absorption or protection from harmful substances. Released peptides can also control food intake through the gut–brain axis.
Additional support for a functional role of taste-signaling pathways in the gut is provided by recent studies in vivo showing that the deletion of α-gustducin prevents GLP-1 release in response to intragastric administration of carbohydrates, and impairs insulin release with consequent hyperglycemia [38••]. Since L cells that produce GLP-1 express sweet-taste receptors and α-gustducin, these findings are an indication that glucose-induced GLP-1 secretion from L cells is mediated by gustducin-coupled sweet-taste receptors. Furthermore, the deletion of the α-gustducin gene abolishes the sodium-dependent glucose transporter isoforms 1 (SGLT1) response induced by dietary sugars or sweeteners, emphasizing the importance of T1R3 and the α-gustducin system in the maintenance of glucose homeostasis through the regulation of the sodium–glucose cotransporter SGLT1 [32••]. This is consonant with the proposal that sweet-taste receptors in enteroendocrine cells sense luminal glucose concentrations. Finally, sweet-receptor ligands have been reported to increase the expression of the glucose transporter GLUT2 in perfused rat jejunum, which might be due to the activation of sweet-taste receptors expressed by epithelial cells together with α-gustducin and transducin [31•]. Taken together, these findings support a role of taste receptors and their signaling molecules in mediating physiological responses induced by luminal contents.
The current concept is that luminal contents activate GPCRs on enteroendocrine cells, resulting in the activation of distinct signal transduction pathways through different effector systems, ultimately leading to Ca2+ increase and peptides release (Fig. 2). Once released, peptides could enter the circulation to reach peripheral targets acting as classical hormones or activate neuronal pathways, including intrinsic and extrinsic (predominantly vagal) afferent neurons, to induce a functional response (Figs 1 and 2). Indirect evidence that bitter-taste receptors’ stimulation might induce neuronal activation through extrinsic vagal pathways derives from the observation that intragastric administration of bitter-taste ligands induces expression of the immediate-early gene product, c-fos, a neural activity marker, in the nucleus of the solitary tract, where most of upper gastrointestinal vagal afferents terminate (H.E. Raybould, C. Sternini, unpublished observation). Ingested substances could also be sensed by other intestinal epithelial cells (Fig. 1), including the brush cells, which have been shown to express G α-proteins [36]. Sensing of luminal content by these cells could trigger release of nitric oxide, since brush cells have been reported to contain nitric oxide synthase, which in turn could activate adjacent enteroendocrine cells or neuronal processes innervating the villi [3,36].
Conclusion
The recognition that several GPCRs and G proteins that respond to bitter and sweet compounds and amino acids are expressed by gastrointestinal epithelial cells has provided strong support for the concept that the gastrointestinal mucosa lining is equipped with a chemosensory machinery that resembles the one operating in the lingual epithelium. GPCRs are envisaged to function as sensors, which couple to G proteins upon activation by distinct luminal contents, and through second messengers and ion channels lead to intracellular Ca2+ increase, the key signal that triggers transmitter release (Fig. 2). This signaling cascade is responsible for orchestrating appropriate responses to specific nutrients or harmful substances, thus preparing the gut to absorb them or initiate a protective response including vomiting and aversive behavior. As a result of the presence of taste-signaling molecules in gastrointestinal mucosa, and given that bitter and sweet tastants induce peptide (CCK, GLP1) release from enteroendocrine cell lines and in vivo, it is reasonable to propose that enteroendocrine cells act as sites of ‘taste’ in different regions of the gastrointestinal tract. Enteroendocrine cells expressing these taste-signaling molecules produce and release brain–gut peptides that are involved in the regulation of gastrointestinal secretion and motility, food intake and satiety, and glucose homeostasis, including CCK, GIP, PYY and GLP1. Modulation of these peptides’ secretion in gastrointestinal ‘taste cells’ might provide novel approaches to develop therapeutic agents for the treatment of aberrant conditions ranging from feeding disorders, obesity, and impairment of glucose homeostasis to intoxication and inflammation, which might be triggered by luminal contents.
Acknowledgements
The work discussed in this paper was supported by National Institute of Health (NIH) Grants DK54155, Stein Oppenheimer award and UCLA Academic Senate Research Grant to C.S., and DK55003, DK56930 and DK41301 to E.R.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 111).
- 1.Buchan AM. Nutrient tasting and signaling mechanisms in the gut. III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–G1107. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- 2.Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. [PubMed] [Google Scholar]
- 3.Hofer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 5.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 6.Dockray GJ, Varro A, Dimaline R. Gastric endocrine cells: gene expression, processing, and targeting of active products. Physiol Rev. 1996;76:767–798. doi: 10.1152/physrev.1996.76.3.767. [DOI] [PubMed] [Google Scholar]
- 7.Dockray G. Gut endocrine secretions and their relevance to satiety. Curr Opin Pharmacol. 2004;4:557–560. doi: 10.1016/j.coph.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood) 2001;226:963–977. doi: 10.1177/153537020122601102. [DOI] [PubMed] [Google Scholar]
- 11.Glatzle J, Wang Y, Adelson DW, et al. Chylomicron components activate duodenal vagal afferents via a cholecystokinin. A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550:657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiller RC, Trotman IF, Higgins BE, et al. The ileal brake: inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 14.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab. 2005;1:159–168. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Tschop M, Castaneda TR, Joost HG, et al. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:1. doi: 10.1038/nature02665. page following 165; discussion 2 page following 165. [DOI] [PubMed] [Google Scholar]
- 16.Gautier JF, Fetita S, Sobngwi E, Salaun-Martin C. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005;31:233–242. doi: 10.1016/s1262-3636(07)70190-8. [DOI] [PubMed] [Google Scholar]
- 17.Giralt M, Vergara P. Glucagonlike peptide-1 (GLP-1) participation in ileal brake induced by intraluminal peptones in rat. Dig Dis Sci. 1999;44:322–329. doi: 10.1023/a:1026654417697. [DOI] [PubMed] [Google Scholar]
- 18.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raybould HE, Glatzle J, Robin C, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 20.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 21.Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Staszewski L, Xu H, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler E, Hoon MA, Mueller KL, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 24.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 25.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 27.Rozengurt N, Wu S, Chen MC, et al. Co-localization of the {alpha} subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 28.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 29.Wu SV, Rozengurt N, Yang M, et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 31•.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [This study supports the functionality of sweet taste receptors in the gut by showing the increase of glucose transporter in response to sweet taste ligands, which is likely due to the activation of sweet taste receptors expressed in the gut] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [This study provides evidence that sweet taste receptor molecules act as luminal sensors for sugars and sweeteners supporting their participation in physiological responses that can affect carbohydrate absorption and regulation of insulin release] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 34.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 35.Hofer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 36.Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and {alpha}-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 38••.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [This study supports the functional significance of taste receptor signaling molecules in the gut by showing that enteroendocrine cells might act as sensors for lumen glucose with the same mechanisms as taste cells in the mouth] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland K, Young RL, Cooper NJ, et al. Phenotypic characterization of taste cells of the mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–G1428. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 40•.Chen MC, Wu V, Reeve JR, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–C739. doi: 10.1152/ajpcell.00003.2006. [This article provides the first indirect evidence for a function of bitter taste receptors in enteroendocrine cell lines by showing [Ca2+]i and CCK release in response to bitter taste agonists] [DOI] [PubMed] [Google Scholar]