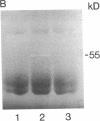
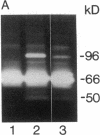
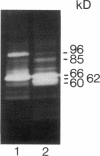
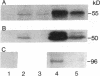
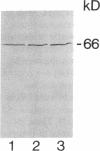
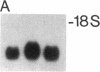
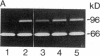
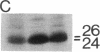
Abstract
Heparin inhibits the migration and proliferation of arterial smooth muscle cells and modifies the extracellular matrix. These effects may be the result of heparin's effects on proteinases that degrade the matrix. We have previously reported that heparin inhibits the induction of tissue-type plasminogen activator and interstitial collagenase mRNA. We have investigated the possibility that heparin affects other members of the matrix metalloproteinase family. Phorbol ester increased the levels of mRNA of collagenase, 92-kD gelatinase and stromelysin as well as the synthesis of these proteins. These effects were inhibited by heparin, but not by other glycosaminoglycans, in a dose-dependent manner. The induction of these matrix metalloproteinases was also inhibited by staurosporine and pretreatment with phorbol ester indicating the involvement of the protein kinase C pathway. In contrast, the 72-kD gelatinase was expressed constitutively and was not affected by phorbol ester or heparin. Tissue inhibitor of metalloproteinase-1 was expressed constitutively and was slightly increased by phorbol ester. It was not affected by heparin. Thus, heparin inhibits the production of four proteinases (tissue plasminogen activator, collagenase, stromelysin and 92-kD gelatinase) that form an interdependent system capable of degrading all the major components of the extracellular matrix.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. M., Werb Z. Targeted disruption of the tissue inhibitor of metalloproteinases gene increases the invasive behavior of primitive mesenchymal cells derived from embryonic stem cells in vitro. J Cell Biol. 1992 Aug;118(3):727–739. doi: 10.1083/jcb.118.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Au Y. P., Kenagy R. D., Clowes A. W. Heparin selectively inhibits the transcription of tissue-type plasminogen activator in primate arterial smooth muscle cells during mitogenesis. J Biol Chem. 1992 Feb 15;267(5):3438–3444. [PubMed] [Google Scholar]
- Au Y. P., Montgomery K. F., Clowes A. W. Heparin inhibits collagenase gene expression mediated by phorbol ester-responsive element in primate arterial smooth muscle cells. Circ Res. 1992 May;70(5):1062–1069. doi: 10.1161/01.res.70.5.1062. [DOI] [PubMed] [Google Scholar]
- Auble D. T., Brinckerhoff C. E. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991 May 7;30(18):4629–4635. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Scheinbuks J., Radek J., Osikowicz G., Feder S., Quarfoot A. J. Immunological identification and comparison of plasminogen activator forms in cultured normal human endothelial cells and smooth muscle cells. Thromb Res. 1981 Dec 1;24(5-6):495–504. doi: 10.1016/0049-3848(81)90085-2. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Campbell C. E., Flenniken A. M., Skup D., Williams B. R. Identification of a serum- and phorbol ester-responsive element in the murine tissue inhibitor of metalloproteinase gene. J Biol Chem. 1991 Apr 15;266(11):7199–7206. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Pukac L. A., Caleb B. L., Wright T. C., Jr, Karnovsky M. J. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J Cell Biol. 1989 Dec;109(6 Pt 1):3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wong K., Herman B., Hoover R. L., Albertini D. F., Wright T. C., Caleb B. L., Karnovsky M. J. Binding and internalization of heparin by vascular smooth muscle cells. J Cell Physiol. 1985 Jul;124(1):13–20. doi: 10.1002/jcp.1041240104. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. IV. Heparin inhibits rat smooth muscle mitogenesis and migration. Circ Res. 1986 Jun;58(6):839–845. doi: 10.1161/01.res.58.6.839. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Kirkman T. R., Jackson C. L., Au Y. P., Kenagy R. Heparin inhibits the expression of tissue-type plasminogen activator by smooth muscle cells in injured rat carotid artery. Circ Res. 1992 Jun;70(6):1128–1136. doi: 10.1161/01.res.70.6.1128. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Cochran D. L., Castellot J. J., Jr, Robinson J. M., Karnovsky M. J. Heparin modulates the secretion of a major excreted protein-like molecule by vascular smooth muscle cells. Biochim Biophys Acta. 1988 Nov 17;967(2):289–295. doi: 10.1016/0304-4165(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Degen S. J., Rajput B., Reich E. The human tissue plasminogen activator gene. J Biol Chem. 1986 May 25;261(15):6972–6985. [PubMed] [Google Scholar]
- Desrochers P. E., Jeffrey J. J., Weiss S. J. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. 1991 Jun;87(6):2258–2265. doi: 10.1172/JCI115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager G., Camejo G., Bondjers G. Heparin-like glycosaminoglycans influence growth and phenotype of human arterial smooth muscle cells in vitro. I. Evidence for reversible binding and inactivation of the platelet-derived growth factor by heparin. In Vitro Cell Dev Biol. 1992 Mar;28A(3 Pt 1):168–175. doi: 10.1007/BF02631087. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. V., Ledbetter S. R. Secretion of stromelysin by cultured dermal papilla cells: differential regulation by growth factors and functional role in mitogen-induced cell proliferation. J Cell Physiol. 1992 Apr;151(1):41–49. doi: 10.1002/jcp.1041510108. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990 Jul;9(7):2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J. M., Maffrand J. P. Effect of pentosan polysulphate, standard heparin and related compounds on protein kinase C activity. Biochim Biophys Acta. 1991 Feb 19;1091(3):432–441. [PubMed] [Google Scholar]
- Hipps D. S., Hembry R. M., Docherty A. J., Reynolds J. J., Murphy G. Purification and characterization of human 72-kDa gelatinase (type IV collagenase). Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblasts. Biol Chem Hoppe Seyler. 1991 Apr;372(4):287–296. doi: 10.1515/bchm3.1991.372.1.287. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Tryggvason K. Completion of the primary structure of the human type IV collagenase preproenzyme and assignment of the gene (CLG4) to the q21 region of chromosome 16. Genomics. 1990 Mar;6(3):554–559. doi: 10.1016/0888-7543(90)90486-e. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Ishihara M., Conrad H. E. Correlations between heparan sulfate metabolism and hepatoma growth. J Cell Physiol. 1989 Mar;138(3):467–476. doi: 10.1002/jcp.1041380305. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lohi J., Tuuttila A., Tryggvason K., Vartio T. Proteolytic processing of the 72,000-Da type IV collagenase by urokinase plasminogen activator. Exp Cell Res. 1992 Oct;202(2):471–476. doi: 10.1016/0014-4827(92)90101-d. [DOI] [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Endogenous receptor-bound urokinase mediates tissue invasion of human monocytes. J Immunol. 1989 Oct 15;143(8):2634–2639. [PubMed] [Google Scholar]
- Kishi J., Hayakawa T. Synthesis of latent collagenase and collagenase inhibitor by bovine aortic medial explants and cultured medial smooth muscle cells. Connect Tissue Res. 1989;19(1):63–76. doi: 10.3109/03008208909016815. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Lindner V., Olson N. E., Clowes A. W., Reidy M. A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992 Nov;90(5):2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N. Does heparin stimulate fibrinolysis? Br J Haematol. 1990 Oct;76(2):163–167. doi: 10.1111/j.1365-2141.1990.tb07866.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray H. F., Parrott D. P., Bowyer D. E. A standardised method of culturing aortic explants, suitable for the study of factors affecting the phenotypic modulation, migration and proliferation of aortic smooth muscle cells. Atherosclerosis. 1991 Feb;86(2-3):227–237. doi: 10.1016/0021-9150(91)90219-s. [DOI] [PubMed] [Google Scholar]
- Medcalf R. L., Rüegg M., Schleuning W. D. A DNA motif related to the cAMP-responsive element and an exon-located activator protein-2 binding site in the human tissue-type plasminogen activator gene promoter cooperate in basal expression and convey activation by phorbol ester and cAMP. J Biol Chem. 1990 Aug 25;265(24):14618–14626. [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Atkinson S., Ward R., Gavrilovic J., Reynolds J. J. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992 Dec 4;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Enghild J. J., Suzuki K., Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990 Jun 19;29(24):5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pelletier J. P., Faure M. P., DiBattista J. A., Wilhelm S., Visco D., Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- Pukac L. A., Castellot J. J., Jr, Wright T. C., Jr, Caleb B. L., Karnovsky M. J. Heparin inhibits c-fos and c-myc mRNA expression in vascular smooth muscle cells. Cell Regul. 1990 Apr;1(5):435–443. doi: 10.1091/mbc.1.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukac L. A., Ottlinger M. E., Karnovsky M. J. Heparin suppresses specific second messenger pathways for protooncogene expression in rat vascular smooth muscle cells. J Biol Chem. 1992 Feb 25;267(6):3707–3711. [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Heparin-like molecules regulate the number of epidermal growth factor receptors on vascular smooth muscle cells. J Cell Physiol. 1988 Jul;136(1):23–32. doi: 10.1002/jcp.1041360104. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Kindy M. S., Brown K. E., Rosenberg R. D., Sonenshein G. E. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989 Apr 25;264(12):6990–6995. [PubMed] [Google Scholar]
- Reith A., Rucklidge G. J. Invasion of brain tissue by primary glioma: evidence for the involvement of urokinase-type plasminogen activator as an activator of type IV collagenase. Biochem Biophys Res Commun. 1992 Jul 15;186(1):348–354. doi: 10.1016/s0006-291x(05)80814-9. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Tsuboi R., Mignatti P. The role of proteases in matrix breakdown during cellular invasion. Am Rev Respir Dis. 1989 Oct;140(4):1112–1113. doi: 10.1164/ajrccm/140.4.1112. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Southgate K. M., Davies M., Booth R. F., Newby A. C. Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem J. 1992 Nov 15;288(Pt 1):93–99. doi: 10.1042/bj2880093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti G., van Leeuwen R. T., Quax P. H., Maseri A., Kluft C. Cultured rat aortic vascular smooth muscle cells digest naturally produced extracellular matrix. Involvement of plasminogen-dependent and plasminogen-independent pathways. Circ Res. 1992 Aug;71(2):385–392. doi: 10.1161/01.res.71.2.385. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sudbeck B. D., Eisen A. Z., Welgus H. G., Parks W. C. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992 Oct;99(4):497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Huhtala P., Tuuttila A., Chow L., Keski-Oja J., Lohi J. Structure and expression of type IV collagenase genes. Cell Differ Dev. 1990 Dec 2;32(3):307–312. doi: 10.1016/0922-3371(90)90044-w. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori E. N., Bair M. J., Bauer E. A., Amento E. P. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-gamma. J Biol Chem. 1991 Dec 5;266(34):23477–23482. [PubMed] [Google Scholar]
- Weiss J. B., Hill C. R., McLaughlin B., Elstow S. Potentiating effect of heparin in the activation of procollagenase by a low-Mr angiogenesis factor. FEBS Lett. 1983 Oct 31;163(1):62–65. doi: 10.1016/0014-5793(83)81163-6. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Wright T. C., Jr, Pukac L. A., Castellot J. J., Jr, Karnovsky M. J., Levine R. A., Kim-Park H. Y., Campisi J. Heparin suppresses the induction of c-fos and c-myc mRNA in murine fibroblasts by selective inhibition of a protein kinase C-dependent pathway. Proc Natl Acad Sci U S A. 1989 May;86(9):3199–3203. doi: 10.1073/pnas.86.9.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi H., Sasaguri Y., Sugama K., Morimatsu M., Nagase H. Production of tissue collagenase (matrix metalloproteinase 1) by human aortic smooth muscle cells in response to platelet-derived growth factor. Atherosclerosis. 1991 Dec;91(3):207–216. doi: 10.1016/0021-9150(91)90168-3. [DOI] [PubMed] [Google Scholar]
- Zehr B. D., Savin T. J., Hall R. E. A one-step, low background coomassie staining procedure for polyacrylamide gels. Anal Biochem. 1989 Oct;182(1):157–159. doi: 10.1016/0003-2697(89)90734-3. [DOI] [PubMed] [Google Scholar]