Abstract
The amazing repertoire of glycoconjugates present on bacterial cell surfaces includes lipopolysaccharides, capsular polysaccharides, lipooligosaccharides, exopolysaccharides, and glycoproteins. While the former are constituents of Gram-negative cells, we review here the cell surface S-layer glycoproteins of Gram-positive bacteria. S-layer glycoproteins have the unique feature of self-assembling into 2D lattices providing a display matrix for glycans with periodicity at the nanometer scale. Typically, bacterial S-layer glycans are O-glycosidically linked to serine, threonine, or tyrosine residues, and they rely on a much wider variety of constituents, glycosidic linkage types, and structures than their eukaryotic counterparts. As the S-layer glycome of several bacteria is unravelling, a picture of how S-layer glycoproteins are biosynthesized is evolving. X-ray crystallography experiments allowed first insights into the catalysis mechanism of selected enzymes. In the future, it will be exciting to fully exploit the S-layer glycome for glycoengineering purposes and to link it to the bacterial interactome.
1. Introduction
1.1. The Sweet Cell Surface of Bacteria at a Glance
Nature has equipped prokaryotic organisms from almost all phylogenetic branches with an amazing repertoire of components from its glycodiversification tool box, adding to the “sweetness” of their cell surface. The “sweetness” is derived from different kinds of polysaccharides, such as capsules or exopolysaccharides as well as glycoconjugates, such as lipopolysaccharides, lipooligosaccharides, and glycoproteins, all of which may additionally carry noncarbohydrate modifications. The complex type of biosynthesis of these prokaryotic carbohydrate components is truly amazing, and despite the tremendous progress in molecular biology it is still very difficult to see at present how the sequence of enzymatic reactions involved in the controlled biosynthesis of carbohydrate chains is regulated. Thus, it is reasonable to believe that the cellular sugar coat serves an important biological function. Prokaryotic glycoconjugates derive most of their structural diversity from the identities of their unusual sugar moieties. The addition of sugars to a nonglycosylated biomolecule changes its size and shape and this is likely to affect the access of proteolytic enzymes. Further, it will influence factors such as solubility, heat stability as well as many physical and chemical properties. Based on these properties, cell surface glycosylation may protect the prokaryotic cell from desiccation and other environmental stresses, contribute to the surface charge of the cell, facilitate adherence of bacteria to solid substrates or influence biofilm formation. Glycosylation of bacterial cell surfaces is furthermore emerging as a critical factor in symbiosis, pathogenesis, cell-cell interactions, and immune evasion. In addition, the presence of and the access to microbial glycoconjugates play a crucial role in the emerging field of biotechnology. For all of these reasons, it is desirable to fully understand the biochemical processes leading to the formation of prokaryotic glycoconjugates [1–4].
1.2. Bacterial S-Layer Glycoproteins
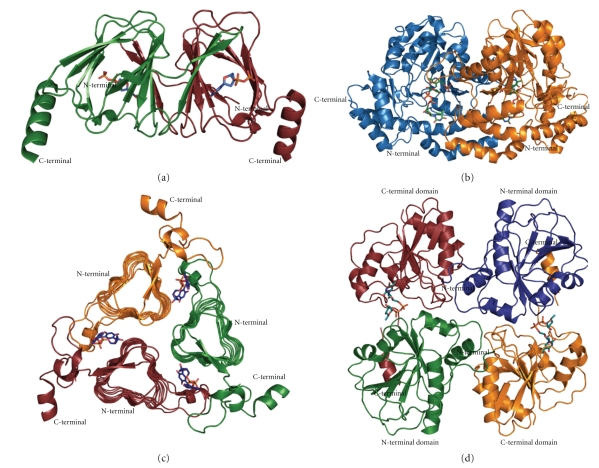
Out of the components contributing to the cellular sugar coat, surface layer (S-layer) glycoproteins are a specific group. In the 1970s, S-layer glycoproteins were the first prokaryotic glycoproteins ever described [5, 6]. Since then, they have attracted considerable interest by the research community. This is not only because of their native potential for modification with a great variety of rare glycan structures (see below)—and, thus, representing ideal model systems for studying bacterial glycosylation—but also because of their unique self-assembly features. On the bacterial cell surface, but also in vitro, S-layer glycoproteins are characteristically aligned in 2D arrays, thereby periodically displaying the attached glycans to the ambient environment with nanometer-scaled accuracy (“nanolattice”) (Figure 1). High-resolution electron microscopy studies revealed that S-layer nanolattices can have oblique (p1, p2), square (p4), or hexagonal (p3, p6) symmetry [7].
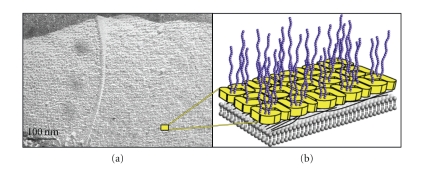
Figure 1.
(a) Electron micrograph of the oblique S-layer glycoprotein lattice as observed on the cell surface of G. stearothermophilus NRS 2004/3a upon freeze-etching and platinum-carbon shadowing. Bar, 100 nm. (b) Inset, schematic representation of the cell wall illustrating the S-layer glycoproteins, with the S-layer glycan chains protruding into the exterior environment. Colour code: yellow S-layer protein; blue S-layer glycan chains; grey cytoplasmic membrane; black peptidoglycan.
S-layers, in general, are present as common outermost structures of the prokaryotic cell envelope. Molecular masses of S-layer proteins typically range from 40 to 170 kDa [7, 8], and glycosylation is the most frequent posttranslational modification these proteins undergo. Other covalent modifications include lipid attachment, phosphorylation, and methylation [9–11]. S-layer glycoproteins occur both in the domains of Archaea and Bacteria. While investigation of S-layer protein glycosylation of haloarchaea, which was initiated by the group of Strominger [5] and extended by Sumper and Wieland [12], is currently extensively being performed by the group of Eichler [2], our research focus is on the S-layer protein glycosylation of Gram-positive bacteria. There, the S-layer nanolattice assembles on the surface of the rigid peptidoglycan layer [13], to which it is attached via a “nonclassical” secondary cell wall polysaccharide [14, 15]. S-layer glycoprotein synthesis and assembly resembles a very efficient system. With an average bacterial generation time of ~20 minutes, at least 500 copies of a single glycoprotein species have to be synthesized per second, translocated to the cell surface, and incorporated into the existing S-layer nanolattice [7, 16, 17]. This corresponds to ~20% of the total protein synthesis effort of a bacterium being devoted to S-layer production. Recent data indicate that S-layer glycosylation lags behind protein biosynthesis [18].
2. Composition and Structure of Bacterial S-Layer Glycoproteins
2.1. Bacteria with S-Layer Glycosylation
Although no precise function could be attributed to bacterial S-layer glycoprotein glycans so far, an enormous biosynthesis effort is undertaken by various bacteria from different phylogenetic branches to glycosylate their S-layer proteins (see below). Thus, it is conceivable that glycosylation adds significantly to the potential functional spectrum of S-layer proteins [3, 12]. Besides Gram-positive bacteria and archaea, for which S-layer glycosylation has been confirmed by various research groups in the past, only recently there were first reports on Gram-negative bacteria possessing an S-layer glycoprotein layer; these are the pathogenic species Tannerella forsythia [19] and presumably Parabacteroides distasonis [20]. Linking S-layer protein glycosylation to pathogenicity is shedding new light into a potential in vivo function of S-layer glycans.
About 15 different S-layer glycoprotein glycan structures have been fully or partially elucidated so far, and there are currently more than 25 further reports on glycan modifications of S-layer proteins according to biochemical evidence (Table 1). The degree of glycosylation of S-layer proteins generally varies between 1% and 10% (w/w).
Table 1.
Overview on S-layer glycoprotein-covered bacteria.
| Organism | Gene designation/Accession number | Lattice symmetrya/Lattice dimensions (nm)/Molecular mass (kD) | Biochemical evidence | Reference |
|---|---|---|---|---|
| Aneurinibacillus thermoaerophilus, strain L420-91T | satA/AY395578 slg cluster/AY442352 | S/10.0/116 | O-glycan structure Linkage region | [3, 18, 21] |
| Aneurinibacillus thermoaerophilus DSM 10155/G+ | satB/AY395579 | S/10.0/153 | O-glycan structure Linkage region | [18, 22, 23] |
| Bacillus smithii, several strains | −/− | O /~11.2 and 10.3/132–138 | Detection of glycoses | [24] |
| Bacillus sp., several strains | −/− | O,S,H/5–23/66–255 | SDS-PAGEb, PASc staining | [25] |
| Bacillus thuringiensis, several strains | c r y020/− | −/−/65–130 | SDS-PAGEb, PASc staining | [26] |
| Campylobacter rectus, strain ATCC 33238 | slp/AB001876 | H/−/150 | Western blot | [27] |
| Deinococcus radiodurans, strain Sark | HPI gene/M17895 | H/18/107 | SDS-PAGEb, PASc staining, Monosaccharide analysis | [28, 29] |
| Deinococcus radiodurans, strain R1 | hpi/DR2508, slpA/DR1185 | H/−/99(37) | SDS-PAGEb, PASc staining | [30] |
| Geobacillus stearothermophilus, strain ATCC 12980T | sbsC/AF055578 dTDP-L-Rha operon/AY278519 | O/9.5 and 5.2/122 | Detection of L-rhamnose | [31, 32] |
| Geobacillus stearothermophilus ATCC 12980 variant | sbsD/AF228338 | O/−/92d–175 | SDS-PAGEb, PASc staining, Monosaccharide analysis | [33] |
| Geobacillus stearothermophilus, strain NRS 2004/3a | sgs /AF328862 slg cluster/AF328862 | O/11.6 and 9.4/93d –170 | O-glycan structure Linkage region Biosynthesis | [32, 34, 35] |
| Geobacillus stearothermophilus, strain L32-65 | sgsF/DQ414249 dTDP-L-Rha operon/AY278518 | O/11.9 and 8.6/96 | Detection of L-rhamnose | [32] |
| Geobacillus tepidamans, strain GS5-97T | sgtA/AY883421 slg cluster/ AY883421 | O/11.2 and 7.9/106–166 | O-glycan structure Linkage region | [36–38] |
| Hyphomonas jannaschiana, strain ATCC 33884 | −/− | −/−/29 + 116 | SDS- PAGEb, PASc staining | [39] |
| Lactobacillus buchneri, strain 41021/251 | −/− | O/6.1 and 5.4/53 | O-glycan structureLinkage region | [40] |
| Lactobacillus helveticus, strain ATCC 12046 | −/− | O/9.6 and 4.5/43.5 | SDS-PAGEb, PASc staining | [41] |
| Lactobacillus kefir JCM 5818 and several other strains | −/− | −/−/69 | SDS- PAGEb, PASc staining Mass spectrometry | [42, 43] |
| Methylomicrobium alcaliphilum | −/− | H/36/10–45 | SDS- PAGEb Monosaccharide analysis | [44] |
| Paenibacillus alvei, strain CCM 2051T | spaA/FJ751775 | O/10.0 and 7.9/240, 160, 105d | O-glycan structure Linkage region Biosynthesis | [45–48] |
| Parabacteroides distasonis | DgpA, DgpC/− | −/−/140, 90 | SDS-PAGEb, PASc staining Monosaccharide analysis | [20] |
| Sulfobacillus thermosulfidooxidans | −/− | −/−/− | Monosaccharide analysis | [49] |
| Synechococcus sp., strain WH8102 | swmA/AF056046 | O/12/− | SDS- PAGEb, PASc staining | [50, 51] |
| Tannerella forsythia, strain ATCC 43037 | tfsA + tfsB/AY423857 | −/−/200 + 210 | SDS- PAGEb, PASc staining | [19, 52, 53] |
| Thermoanaerobacter kivui, strain DSM 2030 | slp/M31069 | H/19 /~90 | SDS- PAGEb, Deglycosylation | [54] |
| Thermoanaerobacter thermohydrosulfuricus, strain L111-69 | surA/AJ401026 sttA/FJ656210 | H/13.9/130 | O-glycan structure Linkage region | [55, 56] |
| Thermoanaerobacter thermohydrosulfuricus, strain S102-70 | −/− | H/16.3/94 | O-glycan structure Linkage region | [57, 58] |
| Thermoanaerobacter thermohydrosulfuricus, strains L77-66 and L92-71 | −/− | H/14.3/82d, 90–200 | O-glycan structure Deglycosylation | [59] |
| Thermoanaerobacterium thermosaccharolyticum, strain E207-71 | slg cluster/AY422724 | S /~12/83d–210 | O-glycan structure | [18, 60] |
| Thermoanaerobacterium thermosaccharolyticum, strain D120-70 | −/− | S/11/80d–170 | O-glycan structure Linkage region | [61] |
| Thermoanaerobacterium thermosulfurigenes, strain EM1 | −/− | −/−/83d–190 | SDS- PAGEb, PASc staining Monosaccharide analysis | [62] |
Abbreviations: a O, oblique (p1, p2); S, square (p4); H, hexagonal (p3, p6); b SDS-PAGE, sodiumdodecylsulfate polysaccharide gel electrophoresis; c PAS, periodic acid-Schiff staining; d deglycosylated protein.
From the accumulated data it is evident that S-layer protein glycosylation cannot serve as a criterion for taxonomic typing at the genus level. However, S-layer glycans are useful tools for typing strains. It is interesting to note that the S-layer glycans present in fresh isolates may change or even be lost after prolonged cultivation of the bacteria in rich synthetic growth media. This supports the assumption that S-layer glycans provide a selection advantage for the bacteria in the competitive natural habitat.
2.2. Structural Repertoire of Bacterial S-Layer Glycans
Prokaryotic organisms are capable of forming both S-layer protein attached N- and O-glycans. While the former have so far been exclusively found in the archaeal domain [2], bacterial S-layer O-glycans from various members of the Bacillaceae family have been quite extensively studied during the past two decades in our laboratory [3, 63, 64].
In addition to more common sugars present in eukaryotic glycans, such as β-D-Glcp, α-D-Galp, β-D-Galp, β-D-Galf, α-D-Manp, α-D-GlcpNAc, α-D-GalpNAc, and β-D-GalpNAc, some S-layer glycans include rare sugars such as α-D-Rhap, β-L-Rhap, α-D-Fuc, β-D-ManpNAc, α-D-FucpNAc, β-D-QuipNAc, or β-D-glycero-D-manno-Hepp, some of which are typical constituents of lipopolysaccharides (LPS) of Gram-negative bacteria [65]. Strain specifically, two to six of these sugars, build up the repeating units of the S-layer glycoprotein glycans. The repeating units may be linear (e.g., Geobacillus stearothermophilus NRS 2004/3a, Figure 2) or branched (e.g., Paenibacillus alvei CCM 2051T, Figure 2) and are polymerized to various degrees (usually between 15 and 50 repeats) to comprise long-chain S-layer heterosaccharides [10, 66]. The Gaussian distribution of S-layer glycan chain lengths is a result of the different degrees of polymerization of individual repeating units and is reflected in the well-known phenomenon of glycan microheterogeneity. Additionally, some S-layer glycans are modified with noncarbohydrate substituents, such as phosphoglyceric acid [46], O-methyl-groups (2-O-Me, 3-O-Me) [34, 67], α-(R)-N-acetylmuramic acid, or N-acetylglucosamine [37]. Interestingly, the latter residues are present as capping elements of the terminal sugar residue at the nonreducing end of the respective S-layer glycan chain, which may imply their function as termination signal for chain elongation as was also reported for LPS O-antigen biosynthesis [65, 68]. Most of the investigated S-layer glycans are linked via a variable adaptor saccharide, which frequently contains α1,3-linked L-rhamnose residues, to specific O-glycosylation sites of the S-layer protein. Besides the amino acids serine and threonine, which are preserved as O-glycosylation sites through all domains of life, in S-layer proteins the chemically more stable O-glycosidic linkage to tyrosine is additionally present [48]. Identified carbohydrate-protein linkage types include β-Glc→Tyr [58, 61], β-Gal→Tyr [46, 55, 56], β-Gal→Thr/Ser [34], β-GalNAc→Thr/Ser [67, 69], and α-Glc → Ser [40]. Commonly, S-layer proteins are glycosylated at multiple sites, which may be either clustered or distributed over the whole protein. So far, between two and four glycosylation sites have been identified per individual S-layer protein. While the structure of the S-layer glycan is conserved for a given S-layer protein, there is obvious variation in the protein primary sequence surrounding the glycosylated amino acid. Currently it is under investigation, to which extent the different glycosylation sites are occupied in the mature glycoprotein.
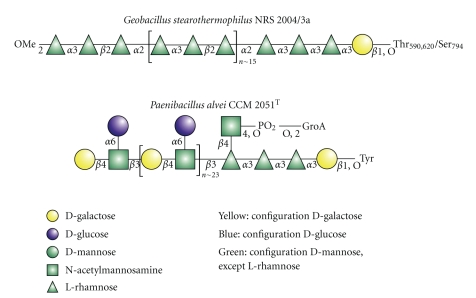
Figure 2.
S-layer glycan structures of G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T. Symbols for representation of the glycan structures are according to the Consortium for Functional Glycomics (http://web.mit.edu/glycomics/consortium/whatsnew.shtml).
In most instances, the overall architecture of S-layer glycoprotein glycans resembles that of LPS of Gram-negative bacteria [70]. Either category of cell surface glycoconjugate possesses a tripartite scheme. This scheme comprises a glycan chain built of a variable number of repeating units that is linked via a variable adaptor saccharide to a specific compound of the bacterial cell wall, which is the S-layer protein moiety in the case of S-layer glycoproteins and lipid A-core in the case of LPS.
In the following (Section 3), the S-layer glycoprotein glycans of G. stearothermophilus NRS 2004/3a (Figure 2) and P. alvei CCM 2051T (Figure 2) will be discussed as showcases to illustrate very recent data on the biosynthesis machinery governing S-layer protein glycosylation. The S-layer glycan of the thermophilic bacterium G. stearothermophilus NRS 2004/3a is a poly-L-rhamnan structure, consisting of 13 to 18 [→2)-α-L-Rhap-(1→3)-β-L-Rhap-(1→2)-α-L-Rhap-(1→] trisaccharide repeating units, which is O-glycosidically linked via an adaptor saccharide built of two to three α1,3-linked L-rhamnose residues and a β-D-Gal residue to the amino acids Thr590, Thr620, and Ser794 of the S-layer protein precursor SgsE [34, 71]. As a specific feature, the terminal rhamnose residue is 2-O-methylated. In the Gram-positive, mesophilic bacterium P. alvei CCM 2051T, a branched S-layer polysaccharide consisting of 22 to 25 [→3)-β-D-Galp-(1[α-D-Glcp-(1→6)] →4)-β-D-ManpNAc-(1→] repeating units is O-glycosidically linked via an adaptor with the structure -[GroA-2→OPO2→4-β-D-ManpNAc-(1→4)] →3)-α-L-Rhap-(1→3)-α-L-Rhap-(1→3)-α-L-Rhap-(1→3)-β-D-Galp-(1→ to at least two specific tyrosine residues of the S-layer protein SpaA [46, 48].
Based on the p2 symmetry of the S-layer nanolattices of G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T and assuming that each S-layer protein subunit is modified with two glycan chains, an average-sized cell of either bacterium displays a total number of ~7,000 glycan chains on the cell surface. This indicates that S-layer protein biosynthesis and export must be highly coordinated with S-layer glycan biosynthesis. Regulation mechanisms interlinking these events are still to be investigated.
3. Building S-Layer Glycans—Insights into the Biosynthesis Machinery behind S-Layer Glycosylation
3.1. The Genetic Basis for S-Layer Protein Glycosylation
For a long time, the understanding of the genetic basis for S-layer protein glycosylation was lagging behind the structural analyses, because of the lack of suitable molecular tools. An important milestone was accomplished with the identification of the first S-layer glycosylation (slg) gene cluster in the bacterium G. stearothermophilus NRS 2004/3a [18]. Over the past five years, several slg gene clusters have been identified, sequenced, and characterized. This is in agreement with the frequently encountered strategy of encoding bacterial polysaccharide biosynthesis routes (e.g., LPS O-antigens) by chromosomal operons or gene clusters [72]. While investigation of polysaccharide gene clusters in Gram-negative bacteria began some 30 years ago [73, 74], research on comparable gene clusters of Gram-positive bacteria (e.g., S-layer glycans, exopolysaccharides) has advanced only more recently.
3.1.1. Identification of slg Gene Clusters
Currently, most data for slg gene clusters is available from the bacteria G. stearothermophilus NRS 2004/3a (GenBank AF328862) [18], P. alvei CCM 2051T (GenBank HM011508) [47], Geobacillus tepidamans GS5-97T (GenBank AY883421) [38] as well as from Aneurinibacillus thermoaerophilus strains L420-91T (GenBank AY442352), and DSM 10155/G+ (GenBank AF324836) [18]. In addition, a partial slg gene cluster sequence is available from Thermoanaerobacterium thermosaccharolyticum E207-71 (GenBank AY422724) [18].
Slg gene clusters have been originally identified based on the presence of L-rhamnose in the respective S-layer glycans and the knowledge that the rmlABCD genes involved in biosynthesis of L-rhamnose are highly conserved among Gram-positive and Gram-negative bacteria [75]. Degenerate primers designed for motifs found in RmlA and RmlB allowed the detection of rml operons in the surveyed strains, which served as an entry point for identifying complete slg gene clusters by chromosome walking. All these slg gene clusters contain consecutively arranged genes for glycosyltransferases, glycan processing enzymes, and membrane transporter components. Interestingly, for biosynthesizing the complete S-layer glycans, these genes work in concert with house-keeping functions. For instance, in G. stearothermophilus NRS 2004/3a, genes for the biosynthesis of nucleotide-activated L-rhamnose (rmlABCD) are present, but the cluster does not code for the biosynthesis of the precursor of the galactose linkage sugar (UDP-Gal) [32]. Unlike the situation in G. stearothermophilus NRS 2004/3a, the slg locus of P. alvei CCM 2051T includes genes for the biosynthesis of the nucleotide-activated S-layer glycan constituents glycerol, galactose, glucose, and L-rhamnose, but lacks genes coding for enzymes involved in the biosynthesis of nucleotide-activated N-acetylmannosamine, which is also part of the S-layer glycan repeating unit [48].
3.1.2. Genetic Organization of slg Gene Clusters
For comparing slg gene clusters with other known bacterial polysaccharide gene clusters, several features can be taken into account, including the percentage G+C content, a characteristic order of genes, and the type of flanking genes [65, 76, 77].
Despite the low number of slg gene clusters that are available so far, some organizational principles can be inferred as follows: (i) glycosyltransferase genes responsible for the formation of the S-layer glycan repeating unit, such as wsaE and wsaF in G. stearothermophilus NRS 2004/3a (Figure 3) or wsfC and wsfE in P. alvei CCM 2051T (Figure 3), are found in the central region of the slg gene clusters; (ii) the ABC-transporter genes wzm and wzt are located upstream of these genes, either immediately or separated by only one open reading frame (ORF); (iii) the rmlABCD genes and most other genes required for biosynthesis of specific nucleotide-activated glycoses are found downstream of the central region.
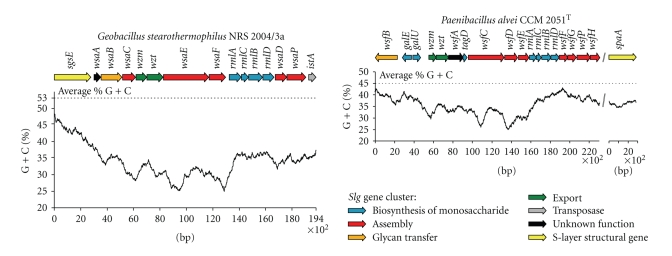
Figure 3.
Schematic representation of the slg gene clusters of the two model bacteria G. stearothermophilus NRS 2004/3a and P. alvei CCM2051T. Individual stages of the S-layer glycosylation process are colour coded as follows: blue, monosaccharide biosynthesis; red, glycan assembly; orange, glycan transfer; green, export; grey, transposase; black, unknown function; yellow, S-layer protein. The corresponding percentage G+C base composition is given below each slg gene cluster map. Designation of ORFs in alphabetical order is as follows: rmlA, glucose-1-phosphate thymidylyltransferase; rmlB, dTDP-D-glucose 4,6-dehydratase; rmlC, dTDP-dehydrorhamnose 3,5-epimerase; rmlD, dTDP-dehydrorhamnose reductase; sgsE, S-layer protein precursor of G. stearothermophilus NRS 2004/3a; spaA, S-layer protein precursor of P. alvei CCM 2051T; tagD, putative glycerol-3-phosphate cytidyltransferase; wsaA, TPR-repeat containing protein; wsaB, putative oligosaccharyl:protein transferase; wsaC, α1,3-rhamnosyltransferase; wsaD, α1,3-rhamnosyltransferase; wsaE, α1,2-rhamnosyltransferase, α1,3-rhamnosyltransferase, 2-O-methyltransferase; wsaF, β1,2-rhamnosyltransferase; wsaP, UDP-Gal:phosphoryl-polyprenol Gal-1-phosphate transferase; wsfA, asparagine synthase homologue; wsfC, putative CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, putative β1,4-galactosyltransferase, putative β1,3-N-acetylmannosamine transferase; wsfD, putative α1,6-glucosyltransferase; wsfE, putative β1,4-N-acetylmannosamine transferase; wsfF, putative α1,3-rhamnosyltransferase; wsfG, putative α1,3-rhamnosyltransferase; wsfH, putative UDP-Glc:phosphoryl-polyprenol Glc-1-phosphate transferase; wsfP, UDP-Gal:phosphoryl-polyprenol Gal-1-phosphate transferase; wzm, ABC transporter integral membrane protein; wzt, ABC transporter nucleotide-binding protein.
The G + C content of the genes encoded in the examined slg gene clusters is mostly between 30% and 43%, which, in each case, is lower than the average percentage G+C content of the residual genome of the respective bacterium. This is, for instance, 45% for P. alvei CCM 2051T and 53% for G. stearothermophilus NRS 2004/3a [18, 48]. It is interesting to note that the genes in the central regions of slg gene clusters, which are the most strain specific ones, exhibit the lowest G + C contents. This compares to cps loci of Streptococcus pneumoniae, where serotype specific enzymes, especially wzx and wzy, are of lower G + C content, while the surrounding biosynthesis and regulatory genes within the cluster are of average G + C content [77]. In general, as has been also argued for LPS gene clusters, the low G + C content might indicate evolutionary acquirement of the glycosylation potential by lateral gene transfer [72].
Genes within the slg gene clusters are closely arranged, some have overlapping termination and initiation codons, like wzm, wzt, and wsaE in G. stearothermophilus NRS 2004/3a and wsfF and wsfG in P. alvei CCM 2051T [32, 48]. A tight organization and overlap of ORFs has been reported in O-polysaccharide clusters, too [76], for instance, in the rfb locus of S. enterica C1 [78] and in the O-antigen biosynthesis locus of Leptospira borgpetersenii serovar Hardjobovis [79]. Concerning the mode of transcription of slg gene clusters, two different scenarios have been found, so far. In G. stearothermophilus NRS 2004/3a, the gene cluster is a polycistronic unit, with all genes being orientated in the same direction. While in P. alvei CCM 2051T, the UDP-galactose synthesis genes galE and galU as well as the putative oligosaccharyl: S-layer protein O-transferase (O-OTase) gene wsfB are transcribed monocistronically, in addition to the larger polycistronic part [48], which, in this bacterium, implies the presence of an “slg gene locus” rather than of an “slg gene cluster”. Regarding the chromosomal location of the slg gene clusters, no preferential insertion site on the chromosome could be identified, so far. Also no JUMPstart sequence that is located upstream of several O-polysaccharide gene clusters has been found [80].
The S-layer structural genes encoding the target proteins for glycosylation are transcribed monocistronically and independently of the slg gene cluster or locus [32, 81]. Whereas the S-layer structural genes sgsE and sgtA of G. stearothermophilus NRS 2004/3a and G. tepidamans GS5-97T, respectively, are located in close vicinity to the corresponding slg gene clusters [32, 38], this is not the case for P. alvei CCM 2051T and different Aneurinibacillus thermoaerophilus strains, where a more distant chromosomal location has been reported [18, 48].
Despite the overall number of known slg gene clusters or loci is still too low for generalization, the principal organization compares well with other bacterial polysaccharide gene clusters. For instance, a survey on the exopolysaccharide (eps) gene clusters from diverse lactic acid bacteria revealed that they have an operon structure with a high coding density. The genes are orientated in one direction and are transcribed as a single RNA [82]. The sequence of the functions of the genes in all of the investigated eps gene clusters was found to be as follows: regulation, chain length determination, biosynthesis of the repeating unit, and export.
It is conceivable to assume that the principal potential for S-layer protein glycosylation is obviously more widespread among bacteria than was initially assumed. This can be deduced from the identification of catalytically active Rml enzymes in G. stearothermophilus strains with an S-layer glycosylation negative phenotype and devoid of other rhamnose-containing glycoconjugates [32]. The corresponding typical and active rml operons were found to be flanked by putative glycosyltransferases, which may be indicative of a kind of silent glycosylation gene cluster.
3.2. Proposal of a Biosynthesis Pathway for S-Layer Glycans
Over the past five years, major strides have been made in identifying bacterial genes whose products are involved in the S-layer protein O-glycosylation process.
For our research effort, G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T are serving as model organisms (compare with Figure 2). In G. stearothermophilus NRS 2004/3a, several genes from the S-layer glycosylation machinery have been cloned and their products functionally characterized by combined biochemical, NMR, and MS approaches [35]. For P. alvei CCM 2051T, mutants with deletions in specific genes from the S-layer glycosylation locus were constructed and the effects on the S-layer glycosylation patterns were monitored by MS [47, 48]. Either approach led to a proposal of a complete biosynthesis pathway of the S-layer glycoproteins. The combined picture resulting from those data is explained below, addressing the different biosynthesis stages of S-layer glycoprotein glycans—initiation, assembly, and transfer onto the protein—separately. A pictorial model of S-layer glycoprotein biosynthesis is given in Figure 4, exemplified with G. stearothermophilus NRS 2004/3a.
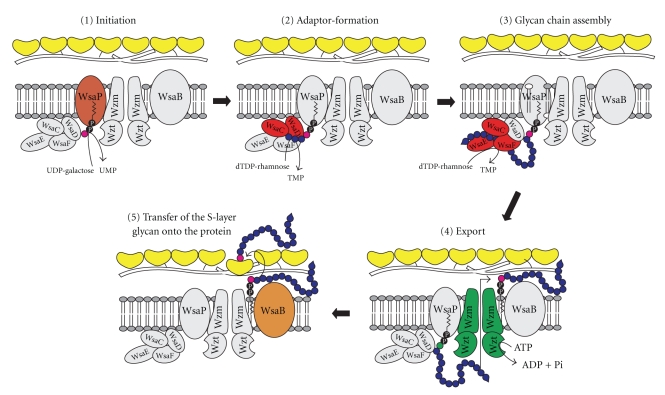
Figure 4.
Proposed model of S-layer glycoprotein glycan biosynthesis in G. stearothermophilus NRS 2004/3a. (1) Initiation: transfer of a Gal residue from UDP-α-D-Gal to a lipid carrier catalyzed by WsaP. (2) Adaptor formation: α1,3-linkage of a rhamnose residue from dTDP-β-L-Rha to the primer by the action of WsaD, followed by the transfer of one or two additional α1,3-linked rhamnoses by the action of WsaC. (3) Glycan chain assembly: formation of repeating unit-like structures by action of the rhamnosyltransferases WsaE and WsaF, whereby WsaE is forming the α1,2- and the α1,3-linkages, and WsaF is forming the α1,2-linkage. Shown is termination of chain growth by 2-O-methylation of the terminal repeating unit, catalyzed by the O-methyltransferase domain of WsaE. (4) Export: the Wzt component of the Wzm/Wzt ABC transporter system is predicted to be responsible for binding of the 2-O-methylated glycan chain and its subsequent export through the membrane. (5) Transfer of the S-layer glycan onto the protein: the final transfer of the completed S-layer glycan to the S-layer protein would be catalyzed by the oligosaccharyltransferase WsaB. This research was originally published in the Journal of Biological Chemistry [35]. The American Society for Biochemistry and Molecular Biology. For colour code see Figure 3; pink, linkage glycose.
3.2.1. Initiation
The initiation enzymes of S-layer glycoprotein glycan biosynthesis in G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T are the UDP-Gal:phosphoryl-polyprenol Gal-1-phosphate-transferases WsaP, and WsfP, respectively. WsaP was shown to transfer Gal-1-P from UDP-Gal to phosphoryl-polyprenol in vitro and to reconstitute the function of WbaP in WbaP-deficient strains of Escherichia coli and Salmonella enterica [83]. Deletion of wsfP in P. alvei CCM 2051T resulted in loss of S-layer glycosylation in this strain, while the original phenotype could be restored by expression of either WsfP or WsaP [47]. WsaP and WsfP share 60% identity and 75% similarity. They have been readily identified in the respective slg gene cluster, because both enzymes show high sequence homology to members of the poly-isopropyl-phosphate hexose-1-phosphate transferase (PHPT) family such as WbaP [84]. Either enzyme from the S-layer glycosylation pathway exhibits a distinct topology comprising five transmembrane domains and a large cytoplasmic C-terminus characteristic of PHPTs [18, 47, 83]. For WsaP, the functional domain was localized at the C-terminus of the enzyme [83], which is in agreement with the data presented for WbaP [85].
The involvement of WbaP homologues in the S-layer glycosylation pathway of G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T was rather unexpected, since in the corresponding slg gene clusters, the presence of the ABC-transporter constituents Wzm and Wzt is predicted. Consequently, in analogy to what is known from the well-investigated routes of LPS O-antigen biosynthesis [65], the initiation of S-layer glycan assembly should be catalyzed by a WecA homologue, which is a GlcNAc-1-phosphate transferase [65].
3.2.2. Assembly
S-layer glycans in G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T are assembled stepwise by the consecutive transfer of glycose residues from the nucleotide-activated forms to the lipid-bound growing glycan chain, with the individual transfer reactions being catalyzed by specific glycosyltransferases [35, 48]. This scheme implies glycan chain growth from the nonreducing end and, thus, resembles the ABC-transporter-dependent pathway of O-polysaccharide biosynthesis [65].
Assembly of the poly-L-rhamnan S-layer glycan of G. stearothermophilus NRS 2004/3a (compare with Figure 2) has been studied in different in vitro set-ups [35]. The slg gene cluster of this bacterium (Figure 3) encodes four rhamnosyltransferases, named WsaC through WsaF, which are involved in the biosynthesis of the S-layer glycan. The individual reactions catalyzed by each of the four enzymes have been elucidated in detail by their respective ability to transfer rhamnose from dTDP-β-L-Rha to different synthetic substrates resembling the different stages of the lipid-bound growing glycan chain [35]. Membrane-anchored WsaD was found to catalyze the initial rhamnosylation reaction which is the transfer of activated β-L-Rha to the lipid-bound galactose primer (compare with Section 3.2.1). Subsequently, WsaC adds one or two additional L-rhamnose residues, thereby completing the adaptor saccharide. The repeating units (individual repeating units can only be distinguished by the different types of glycosidic linkages between the rhamnose residues) are synthesized in a concerted action of the soluble proteins WsaE and WsaF. WsaF catalyzes the β1,2-linkage, while WsaE is a rather unusual trifunctional enzyme. It contains three active domains, two of which are glycosyltransferase domains responsible for the formation of the α1,3- and α1,2-linkage of the glycan repeating unit. N-terminally, a methyltransferase domain is located which was found to methylate an α1,3-linked rhamnose, indicating its role in the formation of the 2-O-methyl cap, and, hence, in termination of S-layer glycan chain elongation as discussed for the O8 and O9 antigens of E. coli [86]. The methylation reaction catalyzed by WsaE was shown to be independent of chain length in vitro. Consequently, an additional factor for the determination of glycan length is possibly required in vivo.
Insight into the assembly of the S-layer glycan of P. alvei CCM 2051T was gained through a systematic gene deletion approach of the individual genes encoded by the slg gene cluster of this bacterium [48] (compare with Figures 2 and 3). The biosynthesis pathway that was proposed based on the detailed analysis of the glycosylation pattern of the individual mutants corroborates the scenario described for G. stearothermophilus NRS 2004/3a, albeit additional information as to how a branched S-layer glycan may be created is unravelled. The first building block of the P. alvei CCM 2051T S-layer glycan is the adaptor saccharide with the structure -[GroA-2→OPO2→4-β-D-ManpNAc-(1→4)] →3)-α-L-Rhap-(1→3)-α-L-Rhap-(1→3)-α-L-Rhap-(1→3)-β-D-Galp-(1→] [46]. WsfG transfers the first L-rhamnose residue to the initial lipid-linked galactose obtained upon catalysis of WsfP (see Section 3.2.1). Subsequently, WsfF adds two more L-rhamnose residues. Both enzymes contain predicted transmembrane domains. The branching ManNAc residue is predicted to be transferred by WsfE, and the GroA-2-phosphate is likely to be added by the CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase domain of WsfC. WsfC contains two more active domains, most probably catalyzing the alternating transfer of D-galactose and D-ManNAc, thus synthesizing the repeating unit backbone [→3)-β-D-Galp-(1→4)-β-D-ManpNAc-(1→] of the S-layer glycan [45]. A branching glucose would be α1,6-linked to the repeating unit ManNAc residue via a separate mechanism that was shown to depend on the activity of WsfH and WsfD. According to our current interpretation, WsfH would transfer a single glucose residue from UDP-Glc to a separate lipid carrier, from which it would be further transferred to the S-layer glycan by the activity of WsfD. A comparable system is found for different E. coli serotypes [87] and in phage-encoded O-antigen modification systems [88]. There, an additional flippase is involved as link between cytoplasmically generated undecaprenyl phosphate-bound glucose and transfer to the periplasmic space. The absence of a flippase in the slg gene cluster might indicate that in P. alvei CCM 2051T, the transfer of the branching glucose occurs on the cytoplasmic side of the cytoplasmic membrane. However, an alternative transport mechanism for lipid-linked glucose cannot be ruled out.
3.2.3. Transfer of the S-Layer Glycan onto the Protein
The slg gene clusters from both G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T contain the integral membrane protein Wzm and the nucleotide-binding protein Wzt constituting an ABC transporter [89–91]. In analogy to the ABC-dependent pathway of O-polysaccharide synthesis, the ABC transporter would be responsible for the transport of complete lipid-bound S-layer glycans across the cytoplasmic membrane. Currently, it is being investigated, if the ABC transporters involved in S-layer glycan export have a certain chain-length preference and, if present, how it is specified [68].
The final protein glycosylation step, which is the transfer of the elongated glycan chain onto the S-layer protein, would most likely occur cosecretionally. It would be catalyzed by a specific O-OTase. The glycosylation sites on the G. stearothermophilus NRS 2004/3a S-layer protein precursor are the amino acids Thr590, Thr620, and Ser794 [34, 71], while in P. alvei CCM 2051T, at least two tyrosine residues are found to be glycosylated [46]. Interest in bacterial OTases has been rising over the past few years and much insight has been gained from studies on the pilin O-glycosyltransferases PilO of Pseudomonas aeruginosa and PglL of Neisseria meningitidis [92, 93] as well as on PglB of the N-glycosylation system of Campylobacter jejuni [94, 95]. The predicted O-OTases of G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T, WsaB and WsfB, respectively, both contain the O-antigen ligase domain Wzy_C, which is found in both PilO and PglL [96]. WsfB and PglL contain a C-terminally located tetratricopeptide (TPR) motif, which is described as mediator of protein-protein interactions [97–99]. While WsaB does not include a TPR motif, the ORF of wsaA located just upstream of wsaB encodes a 168-amino acid protein of otherwise unassigned function that contains a TPR motif. If WsaA in some way complements WsaB for the lack of a TPR motif is currently under investigation. Eventually, the signal sequence would be cleaved from the S-layer protein portion, and the mature S-layer glycoprotein would be deemed to 2D crystallization on the bacterial cell surface to form the S-layer glycoprotein nanolattice.
Summarizing, S-layer glycan biosyntheses in G. stearothermophilus NRS 2004/3a and P. alvei CCM 2051T follow similar pathways. The initiation enzymes are highly homologous Gal-1-phosphate transferases; the adaptor region of either glycan is synthesized by two membrane-bound glycosyltransferases. In both bacteria, a large cytoplasmic multidomain enzyme is involved in the formation of the repeating unit and has an additional function in catalyzing a further glycan modification (i.e., methylation and addition of glyceric acid phosphate, resp.). Multidomain enzymes are also found in O-polysaccharide synthesis pathways; an example is the multidomain mannosyltransferase WbdA found in E. coli serotypes O8 and O9a [100, 101]. Export across the cytoplasmic membrane involving a dedicated ABC transporter-dependent pathway and transfer of the completed S-layer glycan chain onto the protein O-glycosylation sites would follow identical routes. From this scenario it is obvious that different modules from the well-known LPS O-antigen biosynthesis pathways are used for S-layer glycoprotein glycan biosynthesis, albeit in new combination. This is clearly inferred from the involvement of a WbaP homologue, which is otherwise typical of the ABC transporter-independent pathway of O-polysaccharide biosynthesis [65].
4. Structure—Function Relationship of Selected Enzymes from S-Layer Glycosylation
During the past three years, several enzymes from S-layer glycosylation pathways have been crystallized, which allows, for the first time, insight into their reaction mechanisms.
4.1. Crystal Structure of the dTDP-4-Keto-6-Deoxy-D-Glucose-3,4-Ketoisomerase FdtA
The repeating unit of the glycan chain of the S-layer of the bacterium A. thermoaerophilus L420-91T is a hexasaccharide composed of four α-D-rhamnose residues and two 3-acetamido-3,6-dideoxy-α-D-galactose (Fucp3NAc) residues. The precursor of the latter component, dTDP-Fucp3NAc, is synthesized by the action of five enzymes from α-D-glucose-1-phosphate [102]. A crucial step in the biosynthesis is the conversion of the 4-keto substrate dTDP-4-keto-6-deoxyglucose into the 3-keto product dTDP-3-keto-6-deoxygalactose with accompanying inversion of the configuration about C-4′ of the hexose ring. This step is catalyzed by the isomerase FdtA [102].
The structure of FdtA was solved in complex with dTDP to a nominal resolution of 1.5 Å by Davis and coworkers [103]. The FdtA dimer has an almost jellyfish-like appearance with the sole α-helices representing the tentacles (Figure 5(a)). The overall structure of each subunit adopts a β-barrel motif formed by two layers of antiparallel β-sheets consisting of five β-strands each. The dimer is formed by domain swapping whereby β-strands two and three from one subunit form part of a β-sheet in the second subunit. The active site architecture of FdtA is characterized by a cluster of three histidine residues (His49, His51, and His95). His49 and His51 are strictly conserved among putative sugar isomerases in the database. The importance of these residues was further confirmed by site-directed mutagenesis and enzymatic assays. On the basis of the location of dTDP in the complex with FdtA, the substrate dTDP-4-keto-6-deoxyglucose was modelled into the active site cleft. This model places His49 and His51 near the hexose C-3′ oxygen and His95 near the hexose C-4′ oxygen. In the proposed mechanism, His49 would act as base by removing the hydrogen from the sugar C-3′ and shuttle it to the sugar C-4′ on the same side of the glycosyl group resulting in inversion of the configuration at C-4′. His51 might be involved in shuttling protons between the C-3′ and C-4′ oxygens. Sequence comparison with sugar isomerases revealed that enzymes that retain the configuration at C-4′ contain an arginine, and inverting isomerases contain a histidine at the position of His95 in FdtA. Overall, FdtA was the first structure in a new family of inverting sugar isomerases [103].
Figure 5.
Structure of a (a) dimer of FdtA in complex with dTDP (2PA7), (b) dimer of QdtB in complex with pyridoxal 5′ phosphates (PLP) and dTDP-3-keto-6-deoxy-D-glucose (3FRK), (c) trimer of QdtC in complex with CoA and dTDP-D-Quip3N (3FSB), (d) dimer of WsaF in complex with dTDP-L-Rha (2X0F). The individual subunits are colour-coded in (a) red and green, (b) blue and orange, and (c) red, green, and orange, in (d), in addition to the subunits, the domains are colour coded as follows: subunit 1: green/red, subunit 2: blue/orange. The protein chains are depicted as cartoon presentation. The ligands in all structures are shown as sticks. The figures were depicted using PyMOL (DeLano Scientific).
4.2. Crystal Structure of the Aminotransferase QdtB
3-acetamido-3,6-dideoxy-α-D-glucose (Quip3NAc) is an unusual sugar found in the O-antigens of some Gram-negative bacteria and in the S-layer glycoprotein glycans of Gram-positive bacteria, for example, Thermoanaerobacterium thermosaccharolyticum E207-71 [60]. It is synthesized as dTDP-linked sugar by five different enzymes starting from glucose 1-phosphate [104]. In step four, QdtB aminates dTDP-3-keto-6-deoxy-D-glucose at the C-3′ position of the hexose. The structure of QdtB was solved recently to 2.15 Å resolution [105]. QdtB is a dimer and shows homology to the aspartate aminotransferase superfamily. Each subunit contains ten β-strands, seven of which form a mostly parallel β-sheet and the other three fold into an antiparallel β-sheet (Figure 5(b)). These β-sheets are surrounded by eleven α-helices. The catalytic site is located between the two domains with the residues Tyr183, His309 and Tyr310 from one subunit, and Lys219 from the other subunit interacting with dTDP-3-keto-6-deoxy-D-glucose. A reaction intermediate could be trapped within the active site cleft in the structure, the Schiff base between C-4′ of pyridoxal 5′ phosphates (PLP) and the amino nitrogen of the sugar. Furthermore, the structure revealed a lack of a specific interaction between the protein and C-4′ hydroxyl group of the hexose, which could lead to relaxed substrate specificity. This was further confirmed by the ability of QdtB to aminate dTDP-3-keto-6-deoxy-D-galactose [105].
4.3. Crystal Structure of the N-Acetyltransferase QdtC
QdtC is a CoA-dependent N-acetyltransferase that catalyzes the last step in the Quip3NAc biosynthesis [104]. Interestingly, it can acetylate either dTDP-D-Quip3N or dTDP-D-Fucp3N. The structure of QdtC was determined in complex with CoA and either dTDP-D-Quip3N or dTDP-D-Fucp3N [106]. QdtC forms a physiological trimer. Each subunit interacts with both other subunits to form the trimer (Figure 5(c)). Each subunit consists of 32 β-strands that form a left-handed β-helix motif with 11 turns which is disrupted by two extended loops. The first one contains an α-helix and the second is involved in acetyl-CoA binding. The three active sites are located at the subunit-subunit interfaces. The acetyl-CoA binding sites are located between two subunits at one end of the trimer. Its phosphoryl groups point outwards toward the solvent, whereas the adenine ring, the pantothenate, and β-mercaptoethylamine are buried within the QdtC trimer. The adenine group of CoA interacts with Ala99 and the pantothenate with Ala180 and Gly198 from one subunit, respectively, whereas the 2-hydroxyl and the phosphoryl group of CoA interact with Thr204 and Lys205, respectively, and the β-mercaptoethylamine forms a hydrogen bond with Asp160 from the second subunit. Two subunits also contribute to the binding of the dTDP-sugars. Thr117 from one subunit forms a hydrogen bond to the O3 of 2-deoxyribose, whereas Tyr86 from the neighbouring subunit interacts with N3 of the thymidine ring. The pyrophosphoryl group forms interactions with one arginine from each of the subunits. The amino acid residues Glu141, Asn159, and Asp160 from one subunit and His134 from another subunit are responsible for anchoring the hexose moieties of the dTDP-sugars to the protein. Interestingly, the catalytic site shows only a slight movement in response to the different configuration at the C-4′-hydroxyl of dTDP-D-Quip3N and dTDP-D-Fucp3N.
Overall, QdtC shows structural homology to PglD, an N-acetyltransferase, but the nucleotide-linked sugars are accommodated differently in the active site [107, 108]. Although in PglD, His125, which is also conserved in His123 of QdtC, was described as general base in the reaction mechanism, site-directed mutagenesis of His123 and several other potential candidates, including His134, Glu141, Asp160, and Asn159, revealed only reduced activity. This is more likely the result from reduced hexose binding than from the loss of an enzymatic base. Thus, a new reaction mechanism was proposed by Thoden and coworkers [106], which does not involve a general base provided by the protein. dTDP-D-Quip3N would bind to the active site in an unprotonated form. One of the hydrogens on the sugar amino group is directed at the carboxamide group of Asn159, whereas the other is directed at the sulfur of acetyl-CoA. The lone pair of electrons on the nitrogens is directed to the carbonyl carbon of actyl-CoA. During the nucleophilic attack of the amino nitrogen, the bond between the carbonyl carbon and the sulfur of acetyl-CoA becomes longer and eventually breaks. Finally, the sulfur accepts a proton from the sugar amino group and by this serves as the catalytic base [106].
4.4. Crystal Structure of the Rhamnosyltransferase WsaF
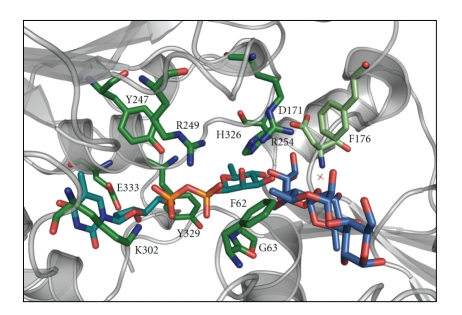
The S-layer glycoprotein glycan from G. stearothermophilus NRS 2004/3a is mainly composed of repeating units of three rhamnose sugars linked by α1,3-, α1,2-, and β1,2-linkages (compare with Figure 2). The formation of the β1,2-linkage is catalyzed by the rhamnosyltransferase WsaF [35]. Recently, the structure of WsaF could be solved to a resolution of 2.3 Å [109, 110]. The structure of WsaF shows a dimer with homology to the glycosyltransferase (GT) 4-family in the CAZy (Carbohydrate-Active EnZYme) database [111]. Each subunit consists of two domains with the typical GT-B-fold of two Rossmann-fold domains (β/α/β) and a cleft between the two domains, which includes the catalytic centre (Figure 5(d)). The N-terminal domain presumably binds the acceptor (the growing rhamnan chain) and the C-terminal domain binds the substrate (dTDP-β-L-rhamnose). The structure of WsaF bound to dTDP and dTDP-β-L-rhamnose, coupled to side-directed mutagenesis and biochemical analysis, identified the residues that underlie catalysis and substrate recognition. The dTDP forms interactions with Arg249, Lys302, and Tyr247, all of which have been shown by mutagenesis to be important or critical for enzyme function possibly by stabilizing the pyrophosphate leaving group. Arg254 is placed immediately adjacent to the site of glycosidic bond formation, where it could interact with both donor and substrate sugars as well as facilitate catalysis. Glu333 of WsaF binds to the O3 of 2-deoxyribose and corresponds to the second Glu of an EX7E motif found in the active site of all structures of the GT4-family. However, instead of the first Glu, which is interacting with the hexose moiety in the other members, a proline residue is found in WsaF. Pro325 is part of an unusual proline-rich motif, PHPSYPPLE, where the central tyrosine points towards the likely location of rhamnose. The motif is conserved among sequence homologues of WsaF in BLAST, including some putative rhamnosyltransferases and may be a characteristic signature for rhamnose usage. It should be noted that WsaF is the first structure of a rhamnosyltransferase published so far. The N-terminal domain is likely to function as the acceptor-binding region but is not conserved among the known structures of the GT4 family due to the very different nature of the acceptors. A model of the acceptor places it in a tunnel that is lined by Asp171 and Phe176 with the C-2′ of the acceptor rhamnose close to dTDP-Rha (Figure 6). However, the catalytic mechanism of WsaF, like in many other retaining glycosyltransferases, is still controversial, because of the absence of an amino acid that could serve as nucleophile in the classic double-displacement mechanism.
Figure 6.
Close-up view of WsaF with dTDP-β-L-Rha bound in the binding pocket and tri-rhamnose galactose modelled into the putative acceptor binding tunnel. Colour code: turquoise, dTDP-Rha; blue, tri-rhamnose galactose.
5. Concluding Remarks
Glycosylation of proteins accounts for much of the biological diversity generated at the proteome level. In this review, we focus on a special type of glycoproteins present on a wide variety of bacterial cell surfaces, namely on S-layer glycoproteins. Our model bacteria are members of the Bacillaceae family that are covered with a 2D crystalline nanolattice composed of individual S-layer O-glycoproteins and displaying long-chain S-layer glycans with nanometer-scale periodicity. Structure- and compositionwise, S-layer glycans are amazingly diverse and differ distinctly from eukaryotic glycoproteins. From the detailed comparison of the S-layer glycoprotein glycan biosynthesis pathways in G. stearothermophilus NRS 2004/3a and in P. alvei CCM 2051T, it can be concluded that these pathways are based on different modules known from established bacterial polysaccharide biosynthesis routes, but they are utilized in new combination.
Despite recent advances in deciphering the bacterial S-layer glycome, leading to the proposal of a complete biosynthesis pathway for bacterial S-layer O-glycoprotein glycans, many questions remain still open. In addition to identifying and functionally characterizing glycosyltransferases, especially branching enzymes and multidomain enzymes that are frequently encountered during S-layer protein glycosylation, the requirements for translocation of the S-layer glycan chains across the cytoplasmic membrane and the final event of glycan transfer onto the S-layer protein need to be defined. Particular efforts will also specifically address the details about tyrosine O-glycosylation, which is frequently found in S-layer glycoproteins. In the future, it will be exciting to fully exploit the S-layer glycome for glycoengineering purposes and to link it to the bacterial interactome.
Carbohydrate-active enzymes from S-layer protein glycosylation pathways, especially those from nucleotide sugar biosynthesis, offer interesting options for therapeutic intervention. For instance, the immediate source for L-rhamnose, a common component of the cell wall and the capsule of many pathogenic bacteria as well as of many S-layer glycans, is dTDP-L-Rha. Since to date, neither rhamnose nor the RmlABCD genes responsible for dTDP-L-Rha biosynthesis have been identified in humans, these enzymes are ideal targets for inhibiting rhamnose-mediated bacterial pathogenicity [75].
Other enzymes from the S-layer glycome can be beneficial for synthesis purposes because of their unusual specificities or thermostability. Thermostable Rml enzymes from A. thermoaerophilus DSM 10155 allow higher productivity of dTDP-L-Rha [112]. Other enzymes such as an isomerase derived from A. thermoaerophilus DSM 420-91T capable of synthesizing dTDP-3-keto-6-deoxygalactose from dTDP-4-keto-6-deoxyglucose [102] may be relevant for the synthesis of antibiotics that contain C-3-aminated deoxy-sugars, such as erythromycin or tylosin.
Since the S-layer glycome is connected with a molecular self-assembly system, our approach has a strong link to the field of nanobiotechnology, because means for organizing materials, such as biologically functional glycans, at the nanometer level are prime candidates for the fabrication of supramolecular structures and devices [113]. Nanobiotechnology applications of such devices that have additionally been tailored by glycoengineering may include the fields of receptor mimics, vaccine design, or drug delivery using carbohydrate recognition.
Acknowledgments
The authors would like to thank Andrea Scheberl for excellent technical assistance. This work was supported by the Austrian Science Fund FWF, projects P19047-B12, P20605-B12, P21954-B20 (to C. Schäffer), and P20745-B11 (to P. Messner). Zarschler and Ristl were supported by the Hochschuljubiläumsstiftung der Stadt Wien, Projects H-2229-2007 (to K. Zarschler) and H-1897-2008 (to R. Ristl).
References
- 1.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annual Review of Biochemistry. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Qarn M, Eichler J, Sharon N. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Current Opinion in Structural Biology. 2008;18(5):544–550. doi: 10.1016/j.sbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Messner P, Steiner K, Zarschler K, Schäffer C. S-layer nanoglycobiology of bacteria. Carbohydrate Research. 2008;343(12):1934–1951. doi: 10.1016/j.carres.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibodeaux CJ, Melançon CE, III, Liu H-W. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angewandte Chemie—International Edition. 2008;47(51):9814–9859. doi: 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mescher MF, Strominger JL. Purification and characterization of a prokaryotic glycoprotein from the cell envelope of Halobacterium salinarium . The Journal of Biological Chemistry. 1976;251(7):2005–2014. [PubMed] [Google Scholar]
- 6.Sleytr UB, Thorne KJI. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum . Journal of Bacteriology. 1976;126(1):377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B. S-Layers as a basic building block in a molecular construction kit. FEBS Journal. 2007;274(2):323–334. doi: 10.1111/j.1742-4658.2006.05606.x. [DOI] [PubMed] [Google Scholar]
- 8.Sleytr UB, Beveridge TJ. Bacterial S-layers. Trends in Microbiology. 1999;7(6):253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 9.Claus H, Aka E, Debaerdemaeker T, Evrard C, Declercq J-P, König H. Primary structure of selected archaeal mesophilic and extremely thermophilic outer surface layer proteins. Systematic and Applied Microbiology. 2002;25(1):3–12. doi: 10.1078/0723-2020-00100. [DOI] [PubMed] [Google Scholar]
- 10.Schäffer C, Messner P. Surface-layer glycoproteins: an example for the diversity of bacterial glycosylation with promising impacts on nanobiotechnology. Glycobiology. 2004;14(8):31R–42R. doi: 10.1093/glycob/cwh064. [DOI] [PubMed] [Google Scholar]
- 11.Eichler J, Adams MWW. Posttranslational protein modification in Archaea. Microbiology and Molecular Biology Reviews. 2005;69(3):393–425. doi: 10.1128/MMBR.69.3.393-425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumper M, Wieland FT. Bacterial glycoproteins. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins. Amsterdam, The Netherlands: Elsevier; 1995. pp. 455–473. [Google Scholar]
- 13.Sleytr UB, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers (S layers): from supramolecular cell structure to biomimetics and nanotechnology. Angewandte Chemie—International Edition. 1999;38(8):1034–1054. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Sára M. Conserved anchoring mechanisms between crystalline cell surface S-layer proteins and secondary cell wall polymers in Gram-positive bacteria? Trends in Microbiology. 2001;9(2):47–49. doi: 10.1016/s0966-842x(00)01905-3. [DOI] [PubMed] [Google Scholar]
- 15.Schäffer C, Messner P. The structure of secondary cell wall polymers: how Gram-positive bacteria stick their cell walls together. Microbiology. 2005;151(3):643–651. doi: 10.1099/mic.0.27749-0. [DOI] [PubMed] [Google Scholar]
- 16.Messner P, Sleytr UB. Crystalline bacterial cell-surface layers. Advances in Microbial Physiology. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- 17.Sára M, Sleytr UB. S-layer proteins. Journal of Bacteriology. 2000;182(4):859–868. doi: 10.1128/jb.182.4.859-868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny R, Pfoestl A, Messner P, Schäffer C. Genetic organization of chromosomal S-layer glycan biosynthesis loci of Bacillaceae. Glycoconjugate Journal. 2004;20(7-8):435–447. doi: 10.1023/B:GLYC.0000038290.74944.65. [DOI] [PubMed] [Google Scholar]
- 19.Lee S-W, Sabet M, Um H-S, Yang J, Kim HC, Zhu W. Identification and characterization of the genes encoding a unique surface (S-) layer of Tannerella forsythia . Gene. 2006;371(1):102–111. doi: 10.1016/j.gene.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CM, Coyne MJ, Bentley DL, Villa OF, Comstock LE. Phase-variable expression of a family of glycoproteins imparts a dynamic surface to a symbiont in its human intestinal ecosystem. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2413–2418. doi: 10.1073/pnas.0608797104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosma P, Neuninger C, Christian R, Schulz G, Messner P. Glycan structure of the S-layer glycoprotein of Bacillus sp. L420-91. Glycoconjugate Journal. 1995;12(1):99–107. doi: 10.1007/BF00731875. [DOI] [PubMed] [Google Scholar]
- 22.Kosma P, Wugeditsch T, Christian R, Zayni S, Messner P. Glycan structure of a heptose-containing S-layer glycoprotein of Bacillus thermoaerophilus . Glycobiology. 1995;5(8):791–796. doi: 10.1093/glycob/5.8.791. [DOI] [PubMed] [Google Scholar]
- 23.Meier-Stauffer K, Busse H-J, Rainey FA, et al. Description of Bacillus thermoaerophilus sp. nov., to include sugar beet isolates and Bacillus brevis ATCC 12990. International Journal of Systematic Bacteriology. 1996;46(2):532–541. [Google Scholar]
- 24.Messner P, Scheberl A, Schweigkofler W, et al. Taxonomic comparison of different thermophilic sugar beet isolates with glycosylated surface layer (S-Layer) proteins and their affiliation to Bacillus smithii . Systematic and Applied Microbiology. 1997;20(4):559–565. [Google Scholar]
- 25.Sidhu MS, Olsen I. S-layers of Bacillus species. Microbiology. 1997;143(4):1039–1052. doi: 10.1099/00221287-143-4-1039. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z, Yao B, Sun M, Yu Z. Protection of mice infected with Plasmodium berghei by Bacillus thuringiensis crystal proteins. Parasitology Research. 2004;92(1):53–57. doi: 10.1007/s00436-003-0990-7. [DOI] [PubMed] [Google Scholar]
- 27.Hinode D, Yokoyama M, Tanabe S, Yoshioka M, Nakamura R. Antigenic properties of the GroEL-like protein of Campylobacter rectus . Oral Microbiology and Immunology. 2002;17(1):16–21. doi: 10.1046/j.0902-0055.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 28.Peters J, Peters M, Lottspeich F, Schäfer W, Baumeister W. Nucleotide sequence analysis of the gene encoding the Deinococcus radiodurans surface protein, derived amino acid sequence, and complementary protein chemical studies. Journal of Bacteriology. 1987;169(11):5216–5223. doi: 10.1128/jb.169.11.5216-5223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller DJ, Baumeister W, Engel A. Conformational change of the hexagonally packed intermediate layer of Deinococcus radiodurans monitored by atomic force microscopy. Journal of Bacteriology. 1996;178(11):3025–3030. doi: 10.1128/jb.178.11.3025-3030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothfuss H, Lara JC, Schmid AK, Lidstrom ME. Involvement of the S-layer proteins Hpi and SlpA in the maintenance of cell envelope integrity in Deinococcus radiodurans R1. Microbiology. 2006;152(9):2779–2787. doi: 10.1099/mic.0.28971-0. [DOI] [PubMed] [Google Scholar]
- 31.Jarosch M, Egelseer EM, Mattanovich D, Sleytr UB, Sára M. S-layer gene sbsC of Bacillus stearothermaphilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli . Microbiology. 2000;146(2):273–281. doi: 10.1099/00221287-146-2-273. [DOI] [PubMed] [Google Scholar]
- 32.Novotny R, Schäffer C, Strauss J, Messner P. S-layer glycan-specific loci on the chromosome of Geobacillus stearothermophilus NRS 2004/3a and dTDP-L-rhamnose biosynthesis potential of G. stearothermophilus strains. Microbiology. 2004;150(4):953–965. doi: 10.1099/mic.0.26672-0. [DOI] [PubMed] [Google Scholar]
- 33.Egelseer EM, Danhorn T, Pleschberger M, Hotzy C, Sleytr UB, Sára M. Characterization of an S-layer glycoprotein produced in the course of S-layer variation of Bacillus stearothermophilus ATCC 12980 and sequencing and cloning of the sbsD gene encoding the protein moiety. Archives of Microbiology. 2001;177(1):70–80. doi: 10.1007/s00203-001-0363-5. [DOI] [PubMed] [Google Scholar]
- 34.Schäffer C, Wugeditsch T, Kählig H, Scheberl A, Zayni S, Messner P. The surface layer (S-layer) glycoprotein of Geobacillus stearothermophilus NRS 2004/3a. Analysis of its glycosylation. The Journal of Biological Chemistry. 2002;277(8):6230–6239. doi: 10.1074/jbc.M108873200. [DOI] [PubMed] [Google Scholar]
- 35.Steiner K, Novotny R, Werz DB, et al. Molecular basis of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus . The Journal of Biological Chemistry. 2008;283(30):21120–21133. doi: 10.1074/jbc.M801833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schäffer C, Franck WL, Scheberl A, Kosma P, McDermott TR, Messner P. Classification of isolates from locations in Austria and Yellowstone National Park as Geobacillus tepidamans sp. nov. International Journal of Systematic and Evolutionary Microbiology. 2004;54(6):2361–2368. doi: 10.1099/ijs.0.63227-0. [DOI] [PubMed] [Google Scholar]
- 37.Kählig H, Kolarich D, Zayni S, et al. N-acetylmuramic acid as capping element of α-D-fucose-containing S-layer glycoprotein glycans from Geobacillus tepidamans GS5-97T . The Journal of Biological Chemistry. 2005;280(21):20292–20299. doi: 10.1074/jbc.M501724200. [DOI] [PubMed] [Google Scholar]
- 38.Zayni S, Steiner K, Pföstl A, et al. The dTDP-4-dehydro-6-deoxyglucose reductase encoding fcd gene is part of the surface layer glycoprotein glycosylation gene cluster of Geobacillus tepidamans GS5-97T . Glycobiology. 2007;17(4):433–443. doi: 10.1093/glycob/cwl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen N, Weiner RM. Isolation and characterization of S-layer proteins from a vent prosthecate bacterium. Microbios. 1998;93(374):7–16. [PubMed] [Google Scholar]
- 40.Möschl A, Schäffer C, Sleytr UB, Messner P, Christian R, Schulz G. Characterization of the S-layer glycoproteins of two lactobacilli. In: Beveridge TJ, Koval SF, editors. Advances in Bacterial Paracrystalline Surface Layers. New York, NY, USA: Plenum Press; 1993. pp. 281–284. [Google Scholar]
- 41.Mozes N, Lortal S. X-ray photoelectron spectroscopy and biochemical analysis of the surface of Lactobacillus helveticus ATCC 12046. Microbiology. 1995;141(1):11–19. [Google Scholar]
- 42.Garrote GL, Delfederico L, Bibiloni R, et al. Lactobacilli isolated from kefir grains: evidence of the presence of S-layer proteins. Journal of Dairy Research. 2004;71(2):222–230. doi: 10.1017/s0022029904000160. [DOI] [PubMed] [Google Scholar]
- 43.Mobili P, Serradell MÁ, Trejo SA, Avilés Puigvert FX, Abraham AG, De Antoni GL. Heterogeneity of S-layer proteins from aggregating and non-aggregating LactoBacillus kefir strains. Antonie van Leeuwenhoek. 2009;95(4):363–372. doi: 10.1007/s10482-009-9322-y. [DOI] [PubMed] [Google Scholar]
- 44.Khmelenina VN, Kalyuzhnaya MG, Sakharovsky VG, Snzina NE, Trotsenko YA, Gottschalk G. Osmoadaptation in halophilic and alkaliphilic methanotrophs. Archives of Microbiology. 1999;172(5):321–329. doi: 10.1007/s002030050786. [DOI] [PubMed] [Google Scholar]
- 45.Altman E, Brisson J-R, Messner P, Sleytr UB. Chemical characterization of the regularly arranged surface layer glycoprotein of Bacillus alvei CCM 2051. Biochemistry and Cell Biology. 1990;69(1):72–78. doi: 10.1139/o91-010. [DOI] [PubMed] [Google Scholar]
- 46.Messner P, Christian R, Neuninger C, Schulz G. Similarity of “core” structures in two different glycans of tyrosine- linked eubacterial S-layer glycoproteins. Journal of Bacteriology. 1995;177(8):2188–2193. doi: 10.1128/jb.177.8.2188-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarschler K, Janesch B, Zayni S, Schäffer C, Messner P. Construction of a gene knockout system for application in Paenibacillus alvei CCM 2051T, exemplified by the S-layer glycan biosynthesis initiation enzyme WsfP. Applied and Environmental Microbiology. 2009;75(10):3077–3085. doi: 10.1128/AEM.00087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarschler K, Janesch B, Pabst M, Altmann F, Messner P, Schäffer C. Protein tyrosine O-glycosylation—a rather unexplored prokaryotic glycosylation system. Glycobiology. 2010;20(6):787–798. doi: 10.1093/glycob/cwq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Severina LO, Senyushkin AA, Karavaiko GI. The structure and chemical composition of the S-layer in representatives of the genus Sulfobacillus . Microbiology (Moscow) 1995;64(3):280–283. [Google Scholar]
- 50.Brahamsha B. An abundant cell-surface polypeptide is required for swimming by the nonflagellated marine cyanobacterium Synechococcus . Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6504–6509. doi: 10.1073/pnas.93.13.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarren J, Heuser J, Roth R, Yamada N, Martone M, Brahamsha B. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. Journal of Bacteriology. 2005;187(1):224–230. doi: 10.1128/JB.187.1.224-230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higuchi N, Murakami Y, Moriguchi K, Ohno N, Nakamura H, Yoshimura F. Localization of major, high molecular weight proteins in Bacteroides forsythus . Microbiology and Immunology. 2000;44(9):777–780. doi: 10.1111/j.1348-0421.2000.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 53.Sabet M, Lee S-W, Nauman RK, Sims T, Um H-S. The surface (S-) layer is a virulence factor of Bacteroides forsythus . Microbiology. 2003;149(12):3617–3627. doi: 10.1099/mic.0.26535-0. [DOI] [PubMed] [Google Scholar]
- 54.Peters J, Peters M, Lottspeich F, Baumeister W. S-layer protein gene of Acetogenium kivui: cloning and expression in Escherichia coli and determination of the nucleotide sequence. Journal of Bacteriology. 1989;171(11):6307–6315. doi: 10.1128/jb.171.11.6307-6315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christian R, Messner P, Weiner C, Sleytr UB, Schulz G. Structure of a glycan from the surface-layer glycoprotein of Clostridium thermohydrosulfuricum strain L111-69. Carbohydrate Research. 1988;176(1):160–163. doi: 10.1016/0008-6215(88)84069-2. [DOI] [PubMed] [Google Scholar]
- 56.Bock K, Schuster-Kolbe J, Altman E, et al. Primary structure of the O-glycosidically linked glycan chain of the crystalline surface layer glycoprotein of Thermoanaerobacter thermohydrosulfuricus L111-69. Galactosyl tyrosine as a novel linkage unit. The Journal of Biological Chemistry. 1994;269(10):7137–7144. [PubMed] [Google Scholar]
- 57.Messner P, Christian R, Kolbe J, Schulz G, Sleytr UB. Analysis of a novel linkage unit of O-linked carbohydrates from the crystalline surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. Journal of Bacteriology. 1992;174(7):2236–2240. doi: 10.1128/jb.174.7.2236-2240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christian R, Schulz G, Schuster-Kolbe J, et al. Complete structure of the tyrosine-linked saccharide moiety from the surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. Journal of Bacteriology. 1993;175(5):1250–1256. doi: 10.1128/jb.175.5.1250-1256.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altman E, Brisson J-R, Gagne SM, Kolbe J, Messner P, Sleytr UB. Structure of the glycan chain from the surface layer glycoprotein of Clostridium thermohydrosulfuricum L77-66. Biochimica et Biophysica Acta. 1992;1117(1):71–77. doi: 10.1016/0304-4165(92)90164-p. [DOI] [PubMed] [Google Scholar]
- 60.Altman E, Schäffer C, Brisson J-R, Messner P. Characterization of the glycan structure of a major glycopeptide from the surface layer glycoprotein of Clostridium thermosaccharolyticum E207-71. European Journal of Biochemistry. 1995;229(1):308–315. doi: 10.1111/j.1432-1033.1995.tb20470.x. [DOI] [PubMed] [Google Scholar]
- 61.Schäffer C, Dietrich K, Unger B, et al. A novel type of carbohydrate-protein linkage region in the tyrosine-bound S-layer glycan of Thermoanaerobacterium thermosaccharolyticum D120-70. European Journal of Biochemistry. 2000;267(17):5482–5492. doi: 10.1046/j.1432-1327.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 62.Brechtel E, Matuschek M, Hellberg A, Egelseer EM, Schmid R, Bahl H. Cell wall of Thermoanaerobacterium thermosulfurigenes EM1: isolation of its components and attachment of the xylanase XynA. Archives of Microbiology. 1999;171(3):159–165. doi: 10.1007/s002030050694. [DOI] [PubMed] [Google Scholar]
- 63.Messner P. Prokaryotic protein glycosylation is rapidly expanding from “curiosity” to “ubiquity”. ChemBioChem. 2009;10(13):2151–2154. doi: 10.1002/cbic.200900388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Messner P, Egelseer E, Sleytr UB, Schäffer C. Surface layer glycoproteins and secondary cell wall polymers. In: Moran AP, Brennan PJ, Holst O, von Itzstein M, editors. Microbial Glycobiology: Structures, Relevance and Applications. San Diego, Calif, USA: Academic Press, Elsevier; 2009. pp. 109–128. [Google Scholar]
- 65.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Messner P, Schäffer C. Prokaryotic glycoproteins. In: Herz W, Falk H, Kirby GW, editors. Progress in the Chemistry of Organic Natural Products. Vol. 85. Wien, Austria: Springer; 2003. pp. 51–124. [DOI] [PubMed] [Google Scholar]
- 67.Schäffer C, Müller N, Christian R, et al. Complete glycan structure of the S-layer glycoprotein of Aneurinibacillus thermoaerophilus GS4-97. Glycobiology. 1999;9(4):407–414. doi: 10.1093/glycob/9.4.407. [DOI] [PubMed] [Google Scholar]
- 68.Whitfield C. Polymerases: glycan chain-length control. Nature Chemical Biology. 2010;6(6):403–404. doi: 10.1038/nchembio.376. [DOI] [PubMed] [Google Scholar]
- 69.Wugeditsch T, Zachara NE, Puchberger M, Kosma P, Gooley AA, Messner P. Structural heterogeneity in the core oligosaccharide of the S-layer glycoprotein from Aneurinibacillus thermoaerophilus DSM 10155. Glycobiology. 1999;9(8):787–795. doi: 10.1093/glycob/9.8.787. [DOI] [PubMed] [Google Scholar]
- 70.Schäffer C, Wugeditsch T, Neuninger C, Messner P. Are S-layer glycoproteins and lipopolysaccharides related? Microbial Drug Resistance. 1996;2(1):17–23. doi: 10.1089/mdr.1996.2.17. [DOI] [PubMed] [Google Scholar]
- 71.Steiner K, Pohlentz G, Dreisewerd K, et al. New insights into the glycosylation of the surface layer protein SgsE from Geobacillus stearothermophilus NRS 2004/3a. Journal of Bacteriology. 2006;188(22):7914–7921. doi: 10.1128/JB.00802-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keenleyside W, Whitfield C. Genetics and biosynthesis of lipopolysaccharide O antigens. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. New York, NY, USA: Marcel Dekker; 1999. pp. 331–358. [Google Scholar]
- 73.Sutherland IW. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annual Review of Microbiology. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- 74.Whitfield C, Valvano MA. Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria. Advances in Microbial Physiology. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 75.Giraud M-F, Naismith JH. The rhamnose pathway. Current Opinion in Structural Biology. 2000;10(6):687–696. doi: 10.1016/s0959-440x(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 76.Samuel G, Reeves P. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydrate Research. 2003;338(23):2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Bentley SD, Aanensen DM, Mavroidi A, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genetics. 2006;2(3, article e31) doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee SJ, Romana LK, Reeves PR. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. Journal of General Microbiology. 1992;138(9):1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- 79.Kalambaheti T, Bulach DM, Rajakumar K, Adler B. Genetic organization of the lipopolysaccharide O-antigen biosynthetic locus of Leptospira borgpetersenii serovar Hardjobovis. Microbial Pathogenesis. 1999;27(2):105–117. doi: 10.1006/mpat.1999.0285. [DOI] [PubMed] [Google Scholar]
- 80.Hobbs M, Reeves PR. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Molecular Microbiology. 1994;12(5):855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 81.Novotny R, Berger H, Schinko T, Messner P, Schäffer C, Strauss J. A temperature-sensitive expression system based on the Geobacillus stearothermophilus NRS 2004/3a sgsE surface-layer gene promoter. Biotechnology and Applied Biochemistry. 2008;49(1):35–40. doi: 10.1042/BA20070083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jolly L, Stingele F. Molecular organization and functionality of exopolysaccharide gene clusters in lactic acid bacteria. International Dairy Journal. 2001;11(9):733–745. [Google Scholar]
- 83.Steiner K, Novotny R, Patel K, et al. Functional characterization of the initiation enzyme of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus NRS 2004/3a. Journal of Bacteriology. 2007;189(7):2590–2598. doi: 10.1128/JB.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Liu D, Reeves PR. C-terminal half of Salmonella enterica WbaP (RfbP) is the galactosyl-1-phosphate transferase domain catalyzing the first step of O-antigen synthesis. Journal of Bacteriology. 1996;178(9):2598–2604. doi: 10.1128/jb.178.9.2598-2604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saldías MS, Patel K, Marolda CL, Bittner M, Contreras I, Valvano MA. Distinct functional domains of the Salmonella enterica WbaP transferase that is involved in the initiation reaction for synthesis of the O antigen subunit. Microbiology. 2008;154(2):440–453. doi: 10.1099/mic.0.2007/013136-0. [DOI] [PubMed] [Google Scholar]
- 86.Clarke BR, Cuthbertson L, Whitfield C. Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. The Journal of Biological Chemistry. 2004;279(34):35709–35718. doi: 10.1074/jbc.M404738200. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Perepelov AV, Feng L, et al. A group of Escherichia coli and Salmonella enterica O antigens sharing a common backbone. Microbiology. 2007;153(7):2159–2167. doi: 10.1099/mic.0.2007/004192-0. [DOI] [PubMed] [Google Scholar]
- 88.Allison GE, Verma NK. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri . Trends in Microbiology. 2000;8(1):17–23. doi: 10.1016/s0966-842x(99)01646-7. [DOI] [PubMed] [Google Scholar]
- 89.Bronner D, Clarke BR, Whitfield C. Identification of an ATP-binding cassette transport system required for translocation of lipopolysaccharide O-antigen side-chains across the cytoplasmic membrane of Klebsiella pneumoniae serotype O1. Molecular Microbiology. 1994;14(3):505–519. doi: 10.1111/j.1365-2958.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 90.Cuthbertson L, Powers J, Whitfield C. The C-terminal domain of the nucleotide-binding domain protein Wzt determines substrate specificity in the ATP-binding cassette transporter for the lipopolysaccharide O-antigens in Escherichia coli serotypes O8 and O9a. The Journal of Biological Chemistry. 2005;280(34):30310–30319. doi: 10.1074/jbc.M504371200. [DOI] [PubMed] [Google Scholar]
- 91.Cuthbertson L, Kimber MS, Whitfield C. Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19529–19534. doi: 10.1073/pnas.0705709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faridmoayer A, Fentabil MA, Mills DC, Klassen JS, Feldman MF. Functional characterization of bacterial oligosaccharyltransferases involved in O-linked protein glycosylation. Journal of Bacteriology. 2007;189(22):8088–8098. doi: 10.1128/JB.01318-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faridmoayer A, Fentabil MA, Haurat MF, et al. Extreme substrate promiscuity of the Neisseria oligosaccharyl transferase involved in protein O-glycosylation. The Journal of Biological Chemistry. 2008;283(50):34596–34604. doi: 10.1074/jbc.M807113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wacker M, Feldman MF, Callewaert N, et al. Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):7088–7093. doi: 10.1073/pnas.0509207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maita N, Nyirenda J, Igura M, Kamishikiryo J, Kohda D. Comparative structural biology of eubacterial and archaeal oligosaccharyltransferases. The Journal of Biological Chemistry. 2010;285(7):4941–4950. doi: 10.1074/jbc.M109.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Power PM, Seib KL, Jennings MP. Pilin glycosylation in Neisseria meningitidis occurs by a similar pathway to wzy-dependent O-antigen biosynthesis in Escherichia coli . Biochemical and Biophysical Research Communications. 2006;347(4):904–908. doi: 10.1016/j.bbrc.2006.06.182. [DOI] [PubMed] [Google Scholar]
- 97.Blatch GL, Lässle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays. 1999;21(11):932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 98.D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends in Biochemical Sciences. 2003;28(12):655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Whitfield C, Mainprize IL. TPR motifs: hallmarks of a new polysaccharide export scaffold. Structure. 2010;18(2):151–153. doi: 10.1016/j.str.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Kido N, Sugiyama T, Yokochi T, Kobayashi H, Okawa Y. Synthesis of Escherichia coli O9a polysaccharide requires the participation of two domains of WbdA, a mannosyltransferase encoded within the w b ∗ gene cluster. Molecular Microbiology. 1998;27(6):1213–1221. doi: 10.1046/j.1365-2958.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- 101.Clarke BR, Greenfield LK, Bouwman C, Whitfield C. Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ATP-binding cassette transporter-dependent pathway. The Journal of Biological Chemistry. 2009;284(44):30662–30672. doi: 10.1074/jbc.M109.052878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pfoestl A, Hofinger A, Kosma P, Messner P. Biosynthesis of dTDP-3-acetamido-3,6-dideoxy-α-D-galactose in Aneurinibacillus thermoaerophilus L420-91T . The Journal of Biological Chemistry. 2003;278(29):26410–26417. doi: 10.1074/jbc.M300858200. [DOI] [PubMed] [Google Scholar]
- 103.Davis ML, Thoden JB, Holden HM. The X-ray structure of dTDP-4-keto-6-deoxy-D-glucose-3,4-ketoisomerase. The Journal of Biological Chemistry. 2007;282(26):19227–19236. doi: 10.1074/jbc.M702529200. [DOI] [PubMed] [Google Scholar]
- 104.Pföstl A, Zayni S, Hofinger A, Kosma P, Schäffer C, Messner P. Biosynthesis of dTDP-3-acetamido-3,6-dideoxy-α-D-glucose. Biochemical Journal. 2008;410(1):187–194. doi: 10.1042/BJ20071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thoden JB, Schäffer C, Messner P, Holden HM. Structural analysis of QdtB, an aminotransferase required for the biosynthesis of dTDP-3-acetamido-3,6-dideoxy-α-D-glucose. Biochemistry. 2009;48(7):1553–1561. doi: 10.1021/bi8022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thoden JB, Cook PD, Schäffer C, Messner P, Holden HM. Structural and functional studies of QdtC: an N-acetyltransferase required for the biosynthesis of dTDP-3-acetamido-3,6-dideoxy-R-D-glucose. Biochemistry. 2009;48(12):2699–2709. doi: 10.1021/bi802313n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rangarajan ES, Ruane KM, Sulea T, et al. Structure and active site residues of PglD, an N-acetyltransferase from the bacillosamine synthetic pathway required for N-glycan synthesis in Campylobacter jejuni . Biochemistry. 2008;47(7):1827–1836. doi: 10.1021/bi702032r. [DOI] [PubMed] [Google Scholar]
- 108.Olivier NB, Imperiali B. Crystal structure and catalytic mechanism of PglD from Campylobacter jejuni . The Journal of Biological Chemistry. 2008;283(41):27937–27946. doi: 10.1074/jbc.M801207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steiner K, Wojciechowska A, Schäffer C, Naismith JH. Purification, crystallization and preliminary crystallographic analysis of WsaF, an essential rhamnosyltransferase from Geobacillus stearothermophilus . Acta Crystallographica Section F. 2008;64(12):1163–1165. doi: 10.1107/S1744309108036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steiner K, Hagelueken G, Messner P, Schäffer C, Naismith JH. Structural basis of substrate binding in WsaF, a rhamnosyltransferase from Geobacillus stearothermophilus . Journal of Molecular Biology. 2010;397(2):436–447. doi: 10.1016/j.jmb.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyl transferases: structures, functions, and mechanisms. Annual Review of Biochemistry. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 112.Graninger M, Kneidinger B, Bruno K, Scheberl A, Messner P. Homologs of the Rml enzymes from Salmonella enterica are responsible for dTDP-β-L-rhamnose biosynthesis in the gram-positive thermophile Aneurinibacillus thermoaerophilus DSM 10155. Applied and Environmental Microbiology. 2002;68(8):3708–3715. doi: 10.1128/AEM.68.8.3708-3715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sleytr UB, Egelseer E-M, Ilk N, et al. Nanobiotechnological applications of S layers. In: König H, Claus H, Varma A, editors. Prokaryotic Cell Wall Compounds—Structure and Biochemistry. Heidelberg, Germany: Springer; 2010. pp. 459–481. [Google Scholar]