Abstract
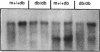
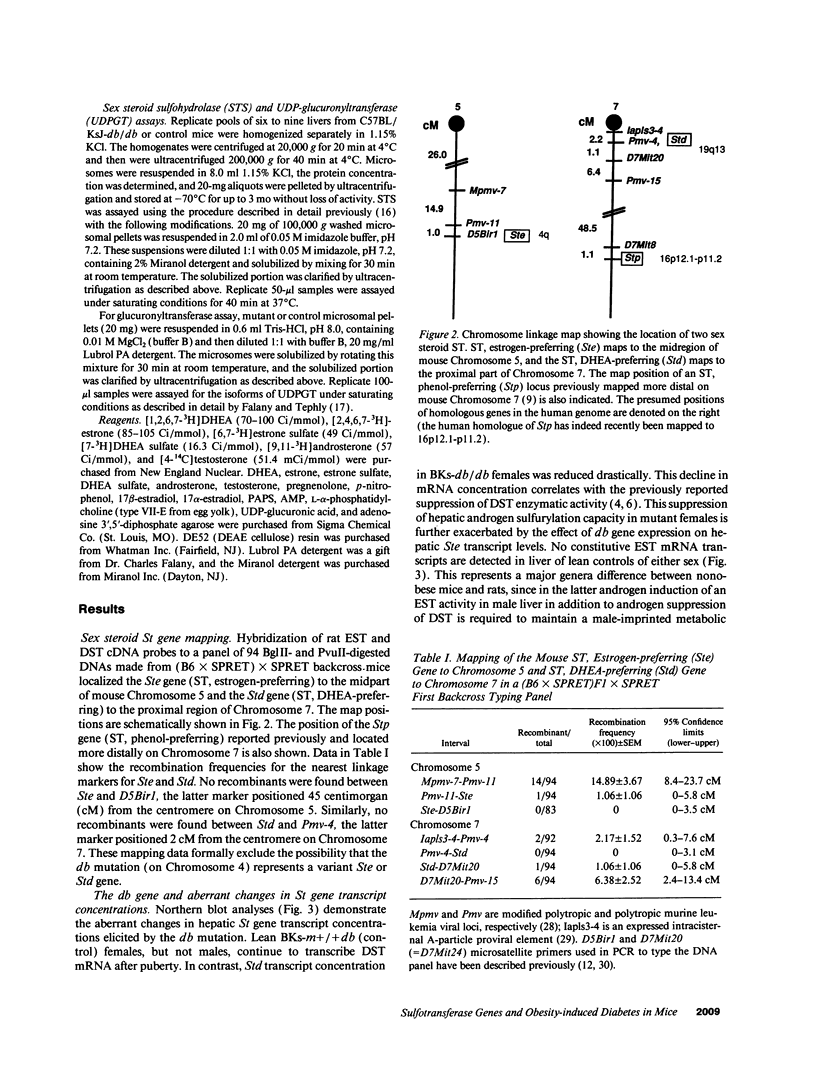
The diabetes (db) gene is a recessive obesity mutation in the mouse capable of producing diabetes only through interaction with heretofore undefined modifiers in the genetic background of certain inbred strains. Here we identify the genetic map locations of androgen and estrogen sulfotransferase genes important in maintaining the balance of active sex steroids in the liver. The Std locus encoding dehydroepiandrosterone sulfotransferase was mapped to proximal Chromosome 7, and the Ste locus encoding estrogen sulfotransferase was mapped to Chromosome 5. The db mutation in the diabetes-susceptible C57BL/KsJ strain aberrantly regulated mRNA transcript levels from these two loci. Hepatic Ste mRNA transcripts were increased from undetectable levels in normal males and females to high levels in db/db mice of both sexes. An anomalous suppression of Std transcription was observed in db/db females, but not in normal females. These reciprocal changes in mRNA concentrations in mutant females were reflected by an induction of a high affinity estrogen sulfotransferase activity and a concomitant loss of dehydroepiandrosterone sulfotransferase activity. These db gene-elicited effects were specific for the sex steroid sulfotransferases since other potential sex steroid metabolizing enzymes (phenol sulfotransferase, sex steroid sulfohydrolase, and UDP-glucuronyltransferase) were unaffected. These aberrant changes would virilize hepatic metabolism in females by increasing the ratio of active androgens to estrogens. In human females, non-insulin-dependent diabetes mellitus often develops when visceral obesity and hyperinsulinemia are associated with hyperandrogenization. This study demonstrates that background modifier genes interacting deleteriously with an obesity mutation are not necessarily defective alleles. Rather, some are functional genes whose regulation has been altered by pleiotropic effects of the obesity gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Schneider U., Thurston S. J. Fingerprinting genomes by use of PCR with primers that encode protein motifs or contain sequences that regulate gene expression. Mamm Genome. 1992;3(10):537–545. doi: 10.1007/BF00350618. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Androgens, the metabolic syndrome, and non-insulin-dependent diabetes mellitus. Ann N Y Acad Sci. 1993 Mar 15;676:242–252. doi: 10.1111/j.1749-6632.1993.tb38738.x. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Eicher E. M. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990 Nov-Dec;81(6):424–427. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia. 1974 Nov;10 (Suppl):607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- Comer K. A., Falany J. L., Falany C. N. Cloning and expression of human liver dehydroepiandrosterone sulphotransferase. Biochem J. 1993 Jan 1;289(Pt 1):233–240. doi: 10.1042/bj2890233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyan W. F., Song C. S., Kim D. S., Her S., Gallwitz W., Rao T. R., Slomczynska M., Chatterjee B., Roy A. K. Estrogen sulfotransferase of the rat liver: complementary DNA cloning and age- and sex-specific regulation of messenger RNA. Mol Endocrinol. 1992 Apr;6(4):589–597. doi: 10.1210/mend.6.4.1374839. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley T. P., Obermoeller R. D., Leiter E. H., Chapman H. D., Falany C. N., Deng Z., Siciliano M. J. Mapping of the phenol sulfotransferase gene (STP) to human chromosome 16p12.1-p11.2 and to mouse chromosome 7. Genomics. 1993 Nov;18(2):440–443. doi: 10.1006/geno.1993.1494. [DOI] [PubMed] [Google Scholar]
- Falany C. N. Molecular enzymology of human liver cytosolic sulfotransferases. Trends Pharmacol Sci. 1991 Jul;12(7):255–259. doi: 10.1016/0165-6147(91)90566-b. [DOI] [PubMed] [Google Scholar]
- Falany C. N., Tephly T. R. Separation, purification and characterization of three isoenzymes of UDP-glucuronyltransferase from rat liver microsomes. Arch Biochem Biophys. 1983 Nov;227(1):248–258. doi: 10.1016/0003-9861(83)90368-5. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K., Gaskins H. R., Leiter E. H. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes. 1991 Jul;40(7):842–849. doi: 10.2337/diab.40.7.842. [DOI] [PubMed] [Google Scholar]
- Hirshey S. J., Dooley T. P., Reardon I. M., Heinrikson R. L., Falany C. N. Sequence analysis, in vitro translation, and expression of the cDNA for rat liver minoxidil sulfotransferase. Mol Pharmacol. 1992 Aug;42(2):257–264. [PubMed] [Google Scholar]
- Kauffman F. C., Whittaker M., Anundi I., Thurman R. G. Futile cycling of a sulfate conjugate by isolated hepatocytes. Mol Pharmacol. 1991 Mar;39(3):414–420. [PubMed] [Google Scholar]
- Kissebah A. H., Peiris A. N. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989 Mar;5(2):83–109. doi: 10.1002/dmr.5610050202. [DOI] [PubMed] [Google Scholar]
- Krakower G. R., Meier D. A., Kissebah A. H. Female sex hormones, perinatal, and peripubertal androgenization on hepatocyte insulin dynamics in rats. Am J Physiol. 1993 Mar;264(3 Pt 1):E342–E347. doi: 10.1152/ajpendo.1993.264.3.E342. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Beamer W. G., Coleman D. L., Longcope C. Androgenic and estrogenic metabolites in serum of mice fed dehydroepiandrosterone: relationship to antihyperglycemic effects. Metabolism. 1987 Sep;36(9):863–869. doi: 10.1016/0026-0495(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Chapman H. D., Coleman D. L. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. V. Interaction between the db gene and hepatic sex steroid sulfotransferases correlates with gender-dependent susceptibility to hyperglycemia. Endocrinology. 1989 Feb;124(2):912–922. doi: 10.1210/endo-124-2-912. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Chapman H. D., Falany C. N. Synergism of obesity genes with hepatic steroid sulfotransferases to mediate diabetes in mice. Diabetes. 1991 Oct;40(10):1360–1363. doi: 10.2337/diab.40.10.1360. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Le P. H., Coleman D. L. Susceptibility to db gene and streptozotocin-induced diabetes in C57BL mice: control by gender-associated, MHC-unlinked traits. Immunogenetics. 1987;26(1-2):6–13. doi: 10.1007/BF00345448. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Obesity genes and diabetes induction in the mouse. Crit Rev Food Sci Nutr. 1993;33(4-5):333–338. doi: 10.1080/10408399309527629. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. 1989 Sep;3(11):2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- Lindstedt G., Lundberg P. A., Lapidus L., Lundgren H., Bengtsson C., Björntorp P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991 Jan;40(1):123–128. doi: 10.2337/diab.40.1.123. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Frankel W. N., Mietz J. A., Kuff E. L. Genomic mapping of intracisternal A-particle proviral elements. Mamm Genome. 1993;4(2):69–77. doi: 10.1007/BF00290429. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Rothenberg P., Shechter Y., Bonner-Weir S., Kahn C. R. Vanadate normalizes hyperglycemia in two mouse models of non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Apr;87(4):1286–1294. doi: 10.1172/JCI115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler J. E., Clore J. N., Blackard W. G. Dehydroepiandrosterone: the "missing link" between hyperinsulinemia and atherosclerosis? FASEB J. 1992 Sep;6(12):3073–3075. doi: 10.1096/fasebj.6.12.1387859. [DOI] [PubMed] [Google Scholar]
- Roy A. K. Regulation of steroid hormone action in target cells by specific hormone-inactivating enzymes. Proc Soc Exp Biol Med. 1992 Mar;199(3):265–272. doi: 10.3181/00379727-199-43356a. [DOI] [PubMed] [Google Scholar]
- Shafrir E. Animal models of non-insulin-dependent diabetes. Diabetes Metab Rev. 1992 Oct;8(3):179–208. doi: 10.1002/dmr.5610080302. [DOI] [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]
- van Weerden W. M., Bierings H. G., van Steenbrugge G. J., de Jong F. H., Schröder F. H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50(12):857–861. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]