Abstract
We describe acute myopathy following I-131 treatment for hyperthyroidism due to Graves Disease (GD) in an adolescent. A 15 year-old diagnosed with GD required treatment with radioactive iodine (I-131) therapy. Six weeks post I-131, he developed generalized muscle cramps. The CK was 19.800 U/L, the total thyroxine was 2.3 mcg/dL (29.6 nmol/L SI) and the estimated free thyroxine (EFT) was 0.5 ng/dL (6.4 pmol/L SI). The ALT was 112 U/L and AST was 364 U/L (normal <35 U/L). The muscle cramps and CK elevation normalized five months after initiation of thyroid replacement therapy. This observation shows that acute myopathy can rarely occur in pediatric patients with GD following treatment with I-131.
1. Introduction
Graves Disease (GD) is uncommon in children with an estimated incidence of 1 per 10,000 [1]. GD accounts for 95% of the cases of hyperthyroidism in children and is more frequent in those with a familial history of autoimmune thyroid disease [2]. Although pharmacological therapy with antithyroid drugs (ATDs) is the first-line therapy in pediatric GD, long-term remission rates of GD in children are less than 25% [3]. Thus, definitive treatment with radioactive iodine (RAI, I-131) or surgery is required for the majority of individuals with GD [3, 4]. When I-131 is used, the treatment goal is hypothyroidism which typically occurs 2–4 months after the administered dose [5, 6].
Muscle abnormalities can occur in hypothyroidism via mechanisms that are unclear [7]. Hypothyroidism-related myopathy has been observed in adults and children [8], with a wide spectrum of problems including myalgias, proximal myopathy, and muscle hypertrophy [7]. Elevation of serum creatine kinase (CK), associated with rhabdomyolysis and renal complications, has been reported [9–12]. Fifty seven to 90% hypothyroid individuals can have CK elevations [13, 14], a phenomenon not observed in hyperthyroidism [15].
Acute myopathy with onset during the acute hypothyroidism following treatment of GD is rare in adults [16]. Severe rhabdomyolysis, a more severe state where elevation of muscle enzymes is additionally associated with myoglobinuria, elevated creatinine, and renal failure, has also been observed in this setting [17, 18]. In children, myopathy with hypothyroidism is rare [19], and we are unaware of reports of acute myopathy following 131-I treatment in the pediatric population. We now describe acute myopathy following I-131 treatment for hyperthyroidism due to GD in an adolescent.
2. Case Report
A 15-year-old male was diagnosed with GD. At presentation, there was goiter (estimated 40–60 grams of thyroid tissue). The total thyroxine (T4) was 27.8 mcg/dL (357.8 nmol/L SI) (normal 4.5–12.5 mcg/dL), estimated free thyroxine (EFT) 6.6 ng/dL (85 pmol/L SI) (normal 1.1–2.2 ng/dL), thyrotropin-stimulating hormone (TSH) <0.01 uIU/mL (normal 0.35–4.35 uIU/mL); total triiodothyronine (T3) was 490 ng/dL (7.5 nmol/L SI) (normal 47–186 ng/dL) (Table 1).
Table 1.
Biochemical test results over time.
| T4 (5–10.6 mcg/dL) | EFT (1.1–2.2 ng/dL) | CK (24–195 U/L) | ALT (0–35 U/L) | AST (0–35 U/L) | |
|---|---|---|---|---|---|
| Diagnosis of GD | 27.8 | 6.6 | — | 20 | 27 |
| Myopathy onset | 2.3 | 0.5 | 19,800 | 242 | 112 |
| 5 months after 131-I | 10.5 | 2 | 69 | 14 | 16 |
T4, total thyroxine levels; EFT, estimated free thyroxine; CK, Creatine kinase; ALT, alanine aminotransferase; ALT, aspartate aminotransferase. Normal ranges are shown in parenthesis.
His past medical history was unremarkable. He was treated with 20 mg of methimazole (MMI) per day and atenolol. One month into treatment, he developed neutropenia. The MMI was discontinued, and the patient was treated with 15.2 mCi of 131-I five days later. The 123-I uptake prior to 131-I therapy was >95% in the neck. A 99-Tc scan showed diffuse uptake over the thyroid. At the time of treatment, the T4 was 19.5 mcg/dL (251 nmol/L SI), and the EFT was 6.5 ng/dL (83.6 pmol/L SI). Four weeks later, the goiter had decreased in size, the WBC was 3.1 · 103/uL, the absolute neutrophil count (ANC) was 2.3 · 103 cells/uL, and the T4 was 13.4 mcg/dL (172.4 nmol/L SI).
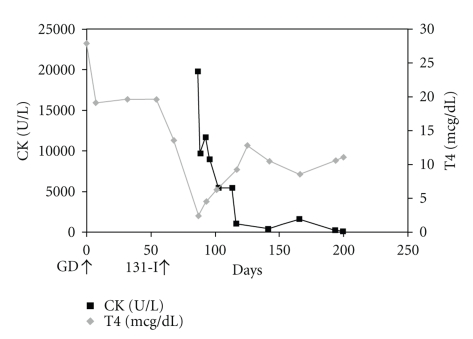
Six weeks after 131-I treatment, he developed sudden onset of generalized muscle cramps. The CK was 19.800 U/L (normal 24–195 U/L), with an increased CK-MB fraction of 13.9 ng/mL (normal <5 ng/mL). Troponin-I levels and an electrocardiogram were normal. The total T4 was 2.3 mcg/dL (29.6 nmol/L SI), and the EFT was 0.5 ng/dL (6.4 pmol/L SI). The ALT was 112 U/L, and AST was 364 U/L (normal <35 U/L). There was no evidence of myoglobinuria or electrolyte abnormalities. Renal function was normal. He received intravenous hydration and was placed on levothyroxine (125 mcg/day). He continued to have milder muscle cramps which resolved over the next three months. Neurological evaluation did not reveal an intrinsic myopathy. Five months after starting levothyroxine therapy, CK levels normalized (Figure 1).
Figure 1.
Serial changes in creatine kinase (CK) and total thyroxine (T4) levels. GD: onset of Graves Disease, 131-I: radioactive iodine treatment.
3. Discussion
Acute myopathy in GD was initially reported in an adult treated with 131-I, who presented with muscle cramps and CK elevation six weeks after treatment, a condition that resolved with the correction of the hypothyroid state [16]. Severe rhabdomyolysis was reported in a patient with papillary thyroid cancer two weeks after total thyroidectomy [17]. Adults have been reported to have developed either myopathy or rhabdomyolysis following the sudden onset of hypothyroidism [18, 20, 21]. The magnitude of CK elevations in these individuals was postulated to be related to the degree of hypothyroidism [14]; however, such correlations have not been found by others [22]. Acute myopathy has also been described in individuals undergoing GD treatment with thionamides [15, 18, 23]. CK elevations during medical treatment of GD, in the absence of a hypothyroid state in two children, have been reported too [19].
The pathogenesis of hypothyroid myopathy is not yet well understood. It has been suggested that thyroxine deficiency leads to an abnormal glycogenolysis [21], metabolic disturbances in mitochondrial oxidative metabolism and triglyceride turnover, which impairs muscle function [24]. Muscle biopsies performed in this setting reveal nonspecific changes, including type II fiber atrophy [25]. It has also been suggested that a reversible, acquired glycogen storage and mitochondrial disorder is a part of hypothyroid myopathy [13, 26].
Although a muscle biopsy was not performed, formal neurological evaluation did not reveal a potential for underlying myopathic condition. Following normalization of CK levels, they have remained normal even in the face of rigorous physical activity. Of note, the decline in T4 levels subsequent to 131-I therapy in our patient was also not unusual in comparison with that reported in pediatric patients [5]. Thus, at present, a specific cause for myopathy, other than acute onset hypothyroidism, cannot be discerned.
Over the past two decades of 131-I use in the treatment of GD in pediatric patients at our institution, this is the only individual to have developed this problem. Thus, we estimate the incidence of myopathy following 131-I therapy on GD to be less than 0.5% in children.
References
- 1.Williamson S, Greene SA. Incidence of thyrotoxicosis in childhood: a national population based study in the UK and Ireland. Clinical Endocrinology. 2010;72(3):358–363. doi: 10.1111/j.1365-2265.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaguelidou F, Carel JC, Léger J. Graves’ disease in childhood: advances in management with antithyroid drug therapy. Hormone Research. 2009;71(6):310–317. doi: 10.1159/000223414. [DOI] [PubMed] [Google Scholar]
- 3.Rivkees SA. The treatment of graves’ disease in children. Journal of Pediatric Endocrinology and Metabolism. 2006;19(9):1095–1111. doi: 10.1515/jpem.2006.19.9.1095. [DOI] [PubMed] [Google Scholar]
- 4.Rivkees SA, Dinauer C. Controversy in clinical endocrinology: an optimal treatment for pediatric Graves’ disease is radioiodine. Journal of Clinical Endocrinology and Metabolism. 2007;92(3):797–800. doi: 10.1210/jc.2006-1239. [DOI] [PubMed] [Google Scholar]
- 5.Nebesio TD, Siddiqui AR, Pescovitz OH, Eugster EA. Time course to hypothyroidism after fixed-dose radioablation therapy of Graves’ disease in children. Journal of Pediatrics. 2002;141(1):99–103. doi: 10.1067/mpd.2002.125494. [DOI] [PubMed] [Google Scholar]
- 6.Rivkees SA, Cornelius EA. Influence of iodine-131 dose on the outcome of hyperthyroidism in children. Pediatrics. 2003;111(4):745–749. doi: 10.1542/peds.111.4.745. [DOI] [PubMed] [Google Scholar]
- 7.Cakir M, Samanci N, Balci N, Balci MK. Musculoskeletal manifestations in patients with thyroid disease. Clinical Endocrinology. 2003;59(2):162–167. doi: 10.1046/j.1365-2265.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 8.Galli-Tsinopoulou A, Stylianou C, Kokka P, Panagopoulou P, Nousia-Arvanitakis S. Rhabdomyolysis, renal failure, pericardial effusion, and acquired von Willebrand disease resulting from hypothyroidism in a 10-year-old girl. Thyroid. 2008;18(3):373–375. doi: 10.1089/thy.2006.0285. [DOI] [PubMed] [Google Scholar]
- 9.George G. Hypothyroidism presenting as puzzling myalgias and cramps in 3 patients. Journal of Clinical Rheumatology. 2007;13(5):273–275. doi: 10.1097/RHU.0b013e318156e2b9. [DOI] [PubMed] [Google Scholar]
- 10.Rabhi M, Chaari J, Toloune F. Rhabdomyolysis disclosing hypothyroidism. European Journal of Internal Medicine. 2006;17(3):p. 220. doi: 10.1016/j.ejim.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Finsterer J, Stöllberger C, Grossegger C, Kroiss A. Hypothyroid myopathy with unusually high serum creatine kinase values. Hormone Research. 1999;52(4):205–208. doi: 10.1159/000023462. [DOI] [PubMed] [Google Scholar]
- 12.Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocrine Journal. 2003;50(2):221–223. doi: 10.1507/endocrj.50.221. [DOI] [PubMed] [Google Scholar]
- 13.McKeran RO, Slavin G, Ward P, Paul E, Mair W. Hypothyroid myopathy. A clinical and pathological study. Journal of Pathology. 1980;132(1):35–54. doi: 10.1002/path.1711320105. [DOI] [PubMed] [Google Scholar]
- 14.Hekimsoy Z, Oktem IK. Serum creatine kinase levels in overt and subclinical hypothyroidism. Endocrine Research. 2005;31(3):171–175. doi: 10.1080/07435800500371706. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen D, Kim CS. Myositis associated with the decline of thyroid hormone levels in thyrotoxicosis: a syndrome? Thyroid. 2009;19(12):1413–1417. doi: 10.1089/thy.2009.0014. [DOI] [PubMed] [Google Scholar]
- 16.Kung AWC, Ma JTC, Yu YL, et al. Myopathy in acute hypothyroidism. Postgraduate Medical Journal. 1987;63(742):661–663. doi: 10.1136/pgmj.63.742.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espiritu RP, Stan MN. Rhabdomyolysis after withdrawal of thyroid hormone in a patient with papillary thyroid cancer. Endocrine Practice. 2008;14(8):1023–1026. doi: 10.4158/EP.14.8.1023. [DOI] [PubMed] [Google Scholar]
- 18.Andía Melero V, López-Guzmán A, Fraile Sáez Á, Arranz Martín A. Rhabdomiolysis secondary to antithyroid drugs. Medicina Clinica. 2007;129(18):p. 717. doi: 10.1157/13112515. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno H, Sugiyama Y, Nishi Y, Ueda N, Ohro Y, Togari H. Elevation of serum creatine kinase in response to medical treatment of Graves’ disease in children. Acta Paediatrica. 2006;95(2):243–245. doi: 10.1080/08035250500341444. [DOI] [PubMed] [Google Scholar]
- 20.Kurihara I. Rhabdomyolysis in a patient with postoperative hypothyroidism and hypoparathyroidism. Nippon Jinzo Gakkai Shi. 2008;50(1):59–63. [PubMed] [Google Scholar]
- 21.Riggs JE. Acute exertional rhabdomyolyses in hypothyroidism: the result of a reversible defect in glycogenolysis? Military Medicine. 1990;155(4):171–172. [PubMed] [Google Scholar]
- 22.Saha B, Maity C. Alteration of serum enzymes in primary hypothyroidism. Clinical Chemistry and Laboratory Medicine. 2002;40(6):609–611. doi: 10.1515/CCLM.2002.105. [DOI] [PubMed] [Google Scholar]
- 23.Soriano Guillén L, Martín Díaz M, Muñoz Calvo M, Pozo Román J, Argente Oliver J. Myositis secondary to antithyroid treatment. Anales de Pediatría. 2007;66:625–626. doi: 10.1157/13107401. [DOI] [PubMed] [Google Scholar]
- 24.Khaleeli AA, Griffith DG, Edwards RHT. The clinical presentation of hypothyroid myopathy and its relationship to abnormalities in structure and function of skeletal muscle. Clinical Endocrinology. 1983;19(3):365–376. doi: 10.1111/j.1365-2265.1983.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 25.McKeran RO, Slavin G, Andrews TM, Ward P, Mair W. Muscle fibre type changes in hypothyroid myopathy. Journal of Clinical Pathology. 1975;28(8):659–663. doi: 10.1136/jcp.28.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellegrini G, Moggio M. Hypothyroid myopathy: histochemical and ultrastructural features with physiopatological correlations (author’s transl) Rivista di Patologia Nervosa e Mentale. 1979;99(5):275–288. [PubMed] [Google Scholar]