Abstract
It has been reported that overproduction of reactive oxygen species occurs after brain injury and mediates neuronal cells degeneration. In the present study, we examined the role of Ras signaling on hydrogen peroxide-induced neuronal cells degeneration in dopaminergic neuroblastoma SH-SY5Y cells. Hydrogen peroxide significantly reduced cell viability in SH-SY5Y cultured cells. An inhibitor of the enzyme that catalyzes the farnesylation of Ras proteins, FTI-277, and a competitive inhibitor of GTP-binding proteins, GDP-beta-S significantly decreased hydrogen peroxide-induced reduction in cell viability in SH-SY5Y cultured cells. The results of this study might indicate that a Ras-dependent signaling pathway plays a role in hydrogen peroxide-induced toxicity in neuronal cells.
1. Introduction
Induction of reactive oxygen species (ROS) formation has been implicated in many neurological diseases such as ischemia, traumatic brain injury, Alzheimer's disease, and Parkinson's disease [1]. Hydrogen peroxide (H2O2) belongs to nonradical form of ROS and is easily converted to hydroxyl radical which cause damage to many cellular components or even cell death [2]. It is increasingly apparent that H2O2 plays a key role in cell death of neuronal [3] and glial cells [4]. Several studies have indicated that H2O2 activates a number of signaling cascades including extracellular signal-regulated kinase (ERK) [5], c-Jun-N-terminal kinase (JNK) [6], and nuclear factor kappa B (NF-κB) [3]. However, the upstream elements that lead to this committed stage of H2O2-induced cellular death signaling in neuronal cells need further investigation.
It has been reported that a major pathway involved in ERK stimulation in various types of cells requires the sequential activation of Ras, Raf, and mitogen-activated/ERK-activated kinase (MEK) [7]. Therefore, in the present study, exposure to exogenous H2O2 was used to determine the effects of H2O2 on activation of Ras-dependent death signaling cascades in cultured human neuroblastoma cell lines, SH-SY5Y cells.
2. Materials and Methods
SH-SY5Y cells were grown in completed media which were made with 45% Minimum Essential Media (MEM), 45% Ham's F-12, 10% inactivated fetal bovine serum, and 100 units/ml penicillin/streptomycin. The cells were maintained at 37°C under 5% CO2/95% humidified air incubator for indicated time. The toxic effect of H2O2 on cell viability was determined in cultured cells. The cultured cells were exposed to H2O2 for 24 hours. The control-cultured cells were incubated with cultured medium for 24 hours. Cell viability was measured by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay, which is based on the conversion of MTT to dark blue formazan crystals by mitochondrial dehydrogenase enzyme. MTT in Dulbecco's Phosphate Buffer Saline (D-PBS) was added into each well and incubated at 37°C for 4 hours. The solution was discarded, then the extraction buffer (0.04 N HCl in isopropanol) was added. The optical densities were measured at 570 nm spectral wavelength using microtiter plate reader. Data were expressed as mean ± SEM. Significance was assessed by one-way analysis of variance (ANOVA) and Tukey-Kramer Multiple Comparisons Test using the scientific statistic software SigmaStat version 2.03. Probability (P) values of less than.05 were considered significant.
3. Results
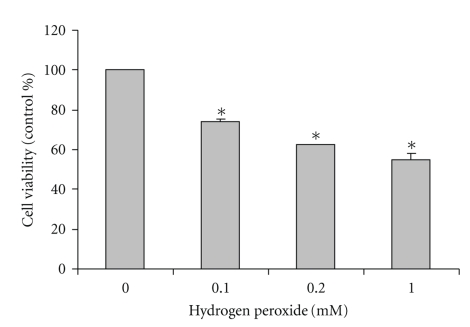
SH-SY5Y cultured cells were exposed to H2O2 at 0.1, 0.2, and 1 mM for 24 hours. The control-cultured cells were incubated with cultured medium for 24 hours. The highertheH2O2 concentrationswere, thegradual reduction in cell viability was observed. H2O2 at 0.1, 0.2, and 1.0 mM significantly decreased cell viability to 73 ± 1.5%, 62 ± 0.1%, and 55 ± 2.7% of the untreated (0 mM) control values, respectively (Figure 1). The results indicate that H2O2 produced a dose-dependent reduction in cell viability.
Figure 1.
Effect of H2O2-induced reduction in cell viability in SH-SY5Y cultured cells. SH-SY5Y cells were treated with H2O2 at 0.1, 0.2, and 1.0 mM for 24 hours. Cell viability was assessed using MTT assay and presented as percentage of untreated (0 mM) control cells. The results are expressed as mean ± SEM of four independent experiments. The ANOVA was performed for statistical analysis (*P <.05 compared with control).
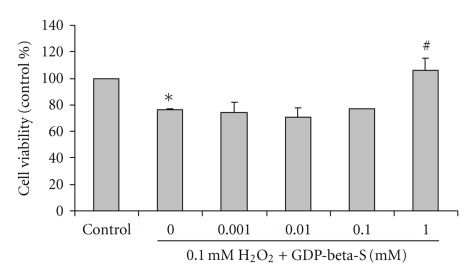
SH-SY5Y cells were exposed to 0.1 mM H2O2 with or without pretreatment with GDP-beta-S, a GDP analog for 3 hours. After incubation, the cell viability was determined using MTT assay. H2O2 at 0.1 mM for 24 h significantly decreased cell viability (76 ± 0.66% of the control) when compared with untreated control cells. Viability of SH-SY5Y cells, pretreated with 0.001, 0.01, 0.1 and 1.0 mM GDP-beta-S for 3 hours prior to incubation with 0.1 mM H H2O2 for another 24 hours, was 74 ± 7.5%, 70 ± 7.5%, 77 ± 0% and 106 ± 8.9% of the control values, respectively (Figure 2). The pretreatment of GDP-beta-S at 1.0 mM significantly increased cell viability in H2O2-treated cells when compared with H2O2-treated cells without GDP-beta-S. GDP-beta-S at 0.001, 0.01, 0.1, and 1.0 mM had no effect on cell viability when compared with control untreated cells (data not shown).
Figure 2.
Effect of an inhibitor of G-protein activation, GDP-beta-S, on H2O2-induced reduction in cell viability in SH-SY5Y cultured cells. SH-SY5Y cells were treated with 0.001, 0.01, 0.1, and 1.0 mM GDP-beta-S for 3 hours prior to incubation with 0.1 mM H2O2 for another 24 hours. Cell viability was assessed using MTT assay and presented as percentage of untreated (0 mM) control cells. The results are expressed as mean ± SEM of five independent experiments. The ANOVA was performed for statistical analysis (*P <.05 compared with control and #P <.05 compared with H2O2-treated cells).
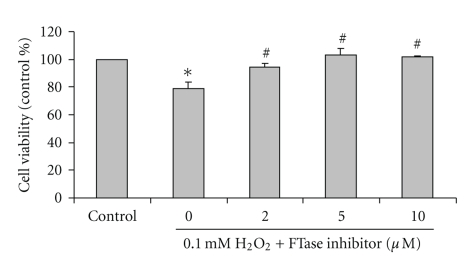
SH-SY5Y cells were exposed to 0.1 mM H2O2 with or without pretreatment with FTI-277, an inhibitor of farnesyltransferase, for 3 hours. After incubation, the cell viability was determined using MTT assay. H2O2 at 0.1 mM for 24 hours significantly decreased cell viability (78 ± 5.0% of the control) when compared with untreated control cells. Viability of SH-SY5Y cells, pretreated with 2.0, 5.0, and 10.0 μM FTI-277 for 3 hours prior to incubation with 0.1 mM H2O2 for another 24 hours, was 94 ± 2.6%, 102 ± 4.9%, and 101 ± 0.8% of the control values, respectively (Figure 3). The pretreatment of FTI-277 at 2.0, 5.0, and 10.0 μM significantly increased cell viability in H2O2-treated cells when compared with H2O2-treated cells without FTI-277. FTI-277 at 2.0, 5.0 and 10.0 μM had no effect on cell viability when compared with control untreated cells (data not shown).
Figure 3.
Effect of farnesyltransferase (FTase) inhibitor, FTI-277, on H2O2-induced reduction in cell viability in SH-SY5Y cultured cells. SH-SY5Y cells were treated with 2.0, 5.0, and 10.0 μM FTI-277 for 3 hours prior to incubation with 0.1 mM H2O2 for another 24 hours. Cell viability was assessed using MTT assay and presented as percentage of untreated (0 mM) control cells. The results are expressed as mean ± SEM of four independent experiments. The ANOVA was performed for statistical analysis (*P <.05 compared with control and #P <.05 compared with H2O2-treated cells).
4. Discussion
Several in vitro [8] and in vivo [9] studies have suggested that a Ras-dependent signaling pathway plays a role in the regulation of cell death cascades. Ras is the prototype small guanine nucleotide-binding proteins (G-proteins) or GTPase which cycle between inactive GDP-bound and active GTP-bound states. Active Ras is able to stimulate many effector proteins such as JNK, ERK, and NF-κB [10]. In the present study, the role of GDP-to-GTP exchange in Ras activation was investigated in H2O2-induced cell death using GDP-beta-S, a GDP analog that competitively inhibits G-protein activation by GTP. The pretreatment of GDP-beta-S at 1.0 mM significantly increased cell viability in H2O2-treated cells when compared with H2O2-treated cells without GDP-beta-S. These results clearly showed that GDP-beta-S reverses the toxic effects of H2O2 in reduction in cell viability in SH-SY5Y cells. It is presumably that intracellular GDP-beta-S inactivates G-proteins which would otherwise initiate cell death after exposure to H2O2. Exposure to stressful stimuli has demonstrated to induce G-protein activation in several cell systems. For example, hair cells of rat treated with GDP-beta-S are protected from gentamicin-induced ototoxicity. The results of potent protection of Ras inhibitors, B581 and FTI-277, against gentamicin-induced c-Jun activation and hair cell damage suggest that activation of Ras is functionally involved in this toxic cell damage [11].
In the present study, the potential role of Ras activation in H2O2-induced neuronal toxicity was explored with inhibitors of farnesyltransferase (FTase). These compounds block the activity of Ras by inhibiting the prenylation that is required for membrane insertion of Ras, which is in turn necessary for activation of downstream Ras signaling [12]. The results of the present study showed that FTI-277 reverses the toxic effects of H2O2 on reduction in cell viability in SH-SY5Y cultured cells. FTI-277 is a highly potent and selective inhibitor of FTase. Its inhibition is specific to Ras proteins whereas Rho isoforms and Rac are geranylgeranylated rather than farnesylated [13]. FTI-277 inhibits Ras processing in whole cells; however, it does not inhibit geranylgeranylated processing at concentrations up to 10 μM [14], the highest dose (10 μM) used in the present study, suggesting that the inhibitory effects of FTase inhibitor within the Ras superfamily are limited to Ras isoforms.
Ras proteins have been characterized into three major isoforms, H-Ras, N-Ras, and K-Ras [7]. Although they are almost identical, distinct cellular functions for the Ras isoforms have been documented. For example, the H-Ras protein increases resistance to the ionizing radiation; on the other hand, K-Ras decreases the radiation resistance in Rat2 fibroblast cells [15]. It has been demonstrated that H-Ras processing is inhibited at concentrations as low as 10 nM of FTI-277. N-Ras processing is inhibited at 5 μM of FTI-277 while complete inhibition of K-Ras requires 10 μM of FTI-277 [16]. Thus the data from the present study suggest that FTI-277 concentrations (2–10 μM) which can inhibit H2O2-induced toxicity in SH-SY5Y cells may protect SH-SY5Y cells through inhibition of Ras.
5. Conclusion
In conclusion, the results of the present study emphasize that Ras proteins may contribute as molecular elements in H2O2-induced cell death in neuroblastoma SH-SY5Y cells. Inhibition of Ras farnesyltransferase (FTase) may have potential in the management of oxidative stress-induced neuronal cell degeneration. Further exploration of the mechanism by which reduced Ras activity is able to decrease oxidative stress-induced neuronal cell degeneration seems to be established.
Acknowledgments
This paper was supported by a research Grant from the Mahidol University and the Thailand Research Fund to Banthit Chetsawang (RMU5180010) and Piyarat Govitrapong (Senior Scholar Fellowship). The authors thank Dr. Lyken Ghose (Editorial Office of Graduate Studies, MU) for revising the language of the paper.
References
- 1.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. The New England Journal of Medicine. 2003;348(14):1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 3.Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. Journal of Pineal Research. 2006;41(2):116–123. doi: 10.1111/j.1600-079X.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 4.Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Progress in Neurobiology. 2004;72(2):111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochemical Research. 2005;30(2):263–270. doi: 10.1007/s11064-005-2449-y. [DOI] [PubMed] [Google Scholar]
- 6.Hojo Y, Saito Y, Tanimoto T, et al. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circulation Research. 2002;91(8):712–718. doi: 10.1161/01.res.0000037981.97541.25. [DOI] [PubMed] [Google Scholar]
- 7.Clerk A, Sugden PH. Ras: the stress and the strain. Journal of Molecular and Cellular Cardiology. 2006;41(4):595–600. doi: 10.1016/j.yjmcc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 8.Tsai F-M, Shyu R-Y, Jiang S-Y. RIG1 suppresses Ras activation and induces cellular apoptosis at the Golgi apparatus. Cellular Signalling. 2007;19(5):989–999. doi: 10.1016/j.cellsig.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Zhou C, Nanda A, Zhang JH. Ras protein contributes to cerebral vasospasm in a canine double-hemorrhage model. Stroke. 2004;35(7):1750–1755. doi: 10.1161/01.STR.0000129898.68350.9f. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Sala D, Rebollo A. Novel aspects of Ras proteins biology: regulation and implications. Cell Death and Differentiation. 1999;6(8):722–728. doi: 10.1038/sj.cdd.4400557. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia A, Pak K, Brors D, Bodmer D, Frangos JA, Ryan AF. Involvement of ras activation in toxic hair cell damage of the mammalian cochlea. Neuroscience. 2003;122(4):1025–1035. doi: 10.1016/j.neuroscience.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Casey PJ, Seabra MC. Protein prenyltransferases. Journal of Biological Chemistry. 1996;271(10):5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 13.Leonard DM. Ras farnesyltransferase: a new therapeutic target. Journal of Medicinal Chemistry. 1997;40(19):2971–2990. doi: 10.1021/jm970226l. [DOI] [PubMed] [Google Scholar]
- 14.Garcia AM, Rowell C, Ackermann K, Kowalczyk JJ, Lewis MD. Peptidomimetic inhibitors of ras farnesylation and function in whole cells. Journal of Biological Chemistry. 1993;268(25):18415–18418. [PubMed] [Google Scholar]
- 15.Choi J-A, Park M-T, Kang C-M, et al. Opposite effects of Ha-Ras and Ki-Ras on radiation-induced apoptosis via differential activation of PI3K/Akt and Rac/p38 mitogen-activated protein kinase signaling pathways. Oncogene. 2004;23(1):9–20. doi: 10.1038/sj.onc.1206982. [DOI] [PubMed] [Google Scholar]
- 16.Lerner EC, Qian Y, Blaskovich MA, et al. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. Journal of Biological Chemistry. 1995;270(45):26802–26806. doi: 10.1074/jbc.270.45.26802. [DOI] [PubMed] [Google Scholar]