Abstract
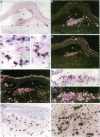
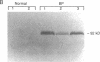
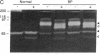
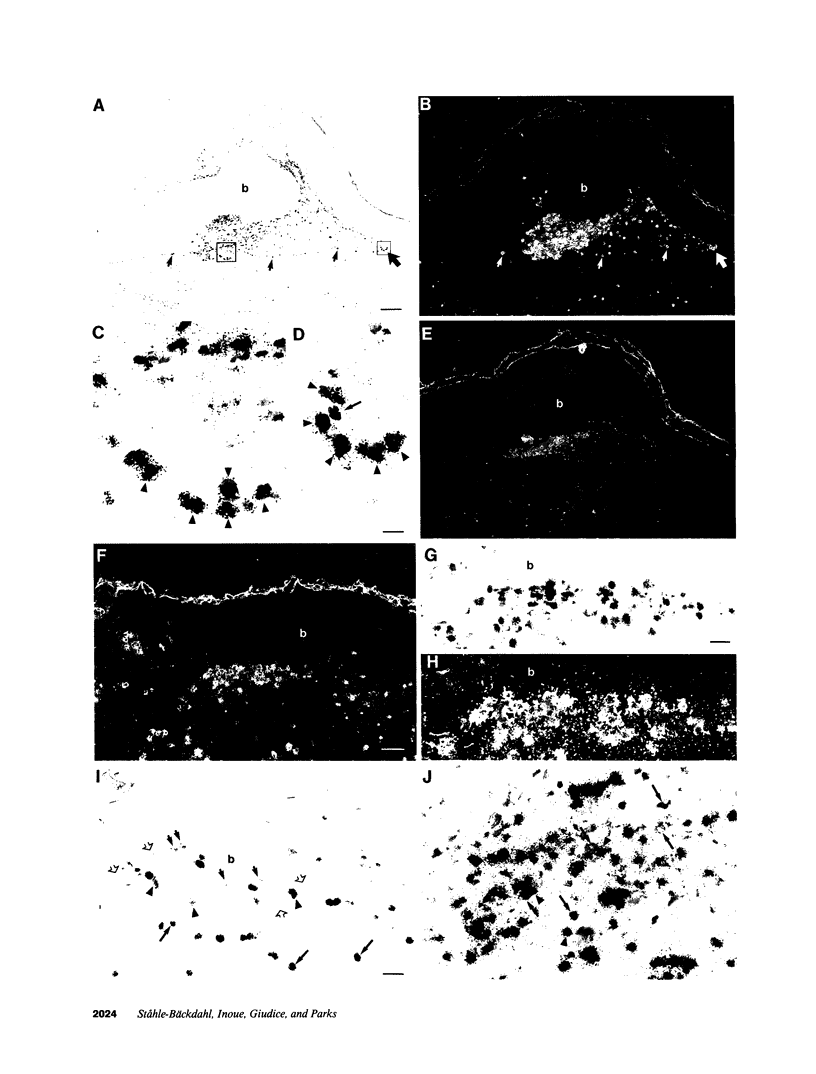
Eosinophils are prominent in bullous pemphigoid (BP), and proteases secreted from these and other inflammatory cells may induce disruption of the basement membrane. We used in situ hybridization and immunohistochemistry to localize the sites of 92-kD gelatinase expression in BP lesions. In all samples (20/20), a strong signal for gelatinase mRNA was detected only in eosinophils and was most pronounced where these cells accumulated at the floor of forming blisters. No other cells were positive for enzyme mRNA. Both eosinophils and neutrophils, however, contained immunoreactive 92-kD gelatinase indicating that active expression occurred only in eosinophils. Degranulated eosinophils were also seen near blisters, and as demonstrated by gelatin zymography, immunoblotting, and ELISA, 92-kD gelatinase protein was prominent in BP blister fluid. No other gelatinolytic activity was specifically detected in BP fluid, and only small amounts of 92-kD gelatinase were present in suction blister fluids. As demonstrated in vitro, 92-kD gelatinase cleaved the extracellular, collagenous domain of recombinant 180-kD BP autoantigen (BP180, BPAG2, HD4, type XVII collagen), a transmembrane molecule of the epidermal hemidesmosome. Our results suggest that production and release 92-kD gelatinase by eosinophils contributes significantly to tissue damage in BP.
Full text
PDF


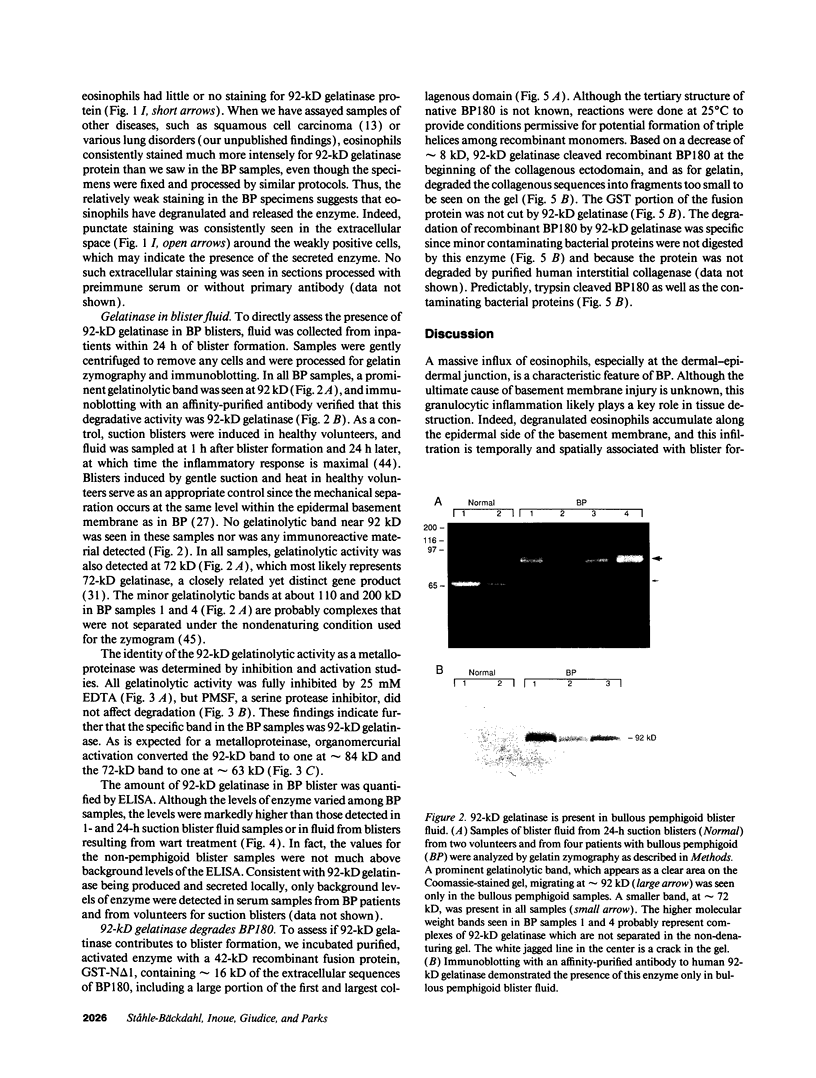
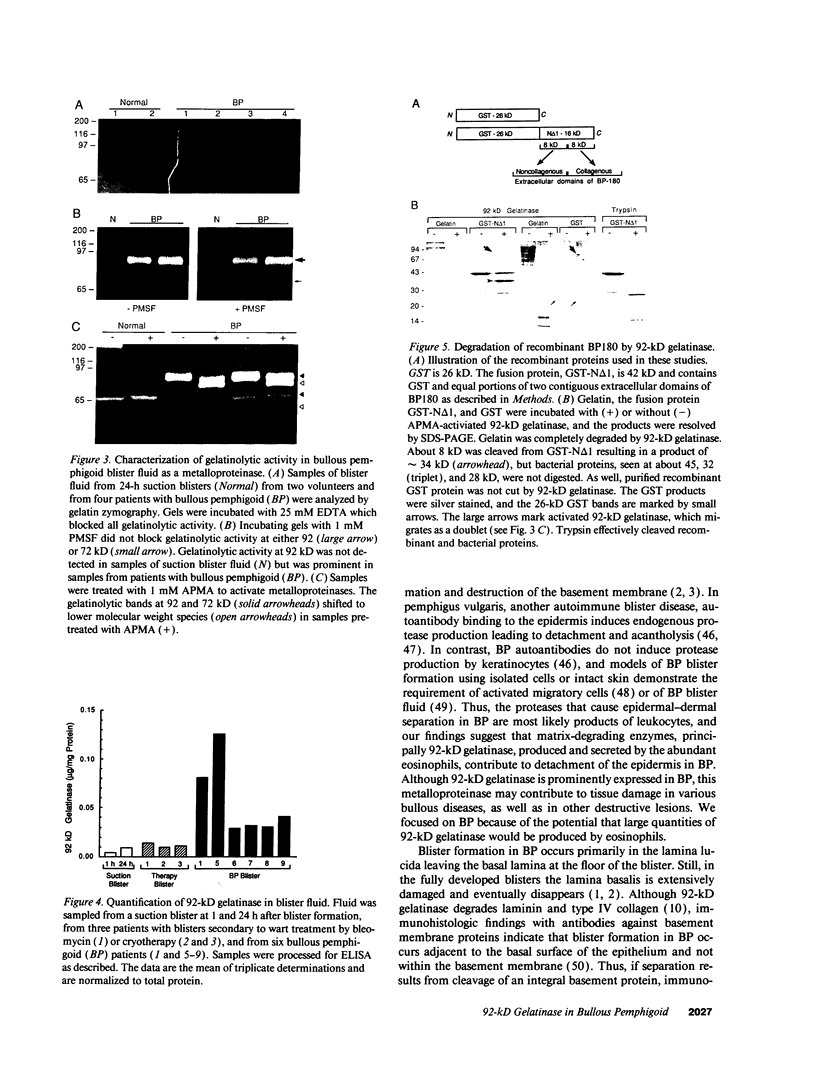



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busiek D. F., Ross F. P., McDonnell S., Murphy G., Matrisian L. M., Welgus H. G. The matrix metalloprotease matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem. 1992 May 5;267(13):9087–9092. [PubMed] [Google Scholar]
- Diaz L. A., Ratrie H., 3rd, Saunders W. S., Futamura S., Squiquera H. L., Anhalt G. J., Giudice G. J. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990 Oct;86(4):1088–1094. doi: 10.1172/JCI114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubertret L., Bertaux B., Fosse M., Touraine R. Cellular events leading to blister formation in bullous pemphigoid. Br J Dermatol. 1980 Dec;103(6):615–624. doi: 10.1111/j.1365-2133.1980.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Mihm M. C., Jr, Osage J. E., Kwan T. H., Austen K. F., Wintroub B. U. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J Invest Dermatol. 1982 Feb;78(2):91–101. doi: 10.1111/1523-1747.ep12505711. [DOI] [PubMed] [Google Scholar]
- Farb R. M., Dykes R., Lazarus G. S. Anti-epidermal-cell-surface pemphigus antibody detaches viable epidermal cells from culture plates by activation of proteinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):459–463. doi: 10.1073/pnas.75.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. H., Kotler D., Tierney A., Wilson C. S., Fauci A. S. Detection of HIV-1 RNA in the lamina propria of patients with AIDS and gastrointestinal disease. J Infect Dis. 1989 Mar;159(3):467–471. doi: 10.1093/infdis/159.3.467. [DOI] [PubMed] [Google Scholar]
- Gammon W. R., Merritt C. C., Lewis D. M., Sams W. M., Jr, Carlo J. R., Wheeler C. E., Jr An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982 Apr;78(4):285–290. doi: 10.1111/1523-1747.ep12507222. [DOI] [PubMed] [Google Scholar]
- Gammon W. R., Merritt C. C., Lewis D. M., Sams W. M., Jr, Wheeler C. E., Jr, Carlo J. R. Functional evidence for complement-activating immune complexes in the skin of patients with bullous pemphigoid. J Invest Dermatol. 1982 Jan;78(1):52–57. doi: 10.1111/1523-1747.ep12497912. [DOI] [PubMed] [Google Scholar]
- Giudice G. J., Emery D. J., Diaz L. A. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992 Sep;99(3):243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Giudice G. J., Emery D. J., Zelickson B. D., Anhalt G. J., Liu Z., Diaz L. A. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol. 1993 Nov 15;151(10):5742–5750. [PubMed] [Google Scholar]
- Giudice G. J., Squiquera H. L., Elias P. M., Diaz L. A. Identification of two collagen domains within the bullous pemphigoid autoantigen, BP180. J Clin Invest. 1991 Feb;87(2):734–738. doi: 10.1172/JCI115054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Grando S. A., Glukhenky B. T., Drannik G. N., Epshtein E. V., Kostromin A. P., Korostash T. A. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol. 1989 Jul;125(7):925–930. [PubMed] [Google Scholar]
- Grando S. A., Glukhenky B. T., Drannik G. N., Kostromin A. P., Chernyavsky A. I. Cytotoxic proteases in blister fluid of pemphigus and pemphigoid patients. Int J Tissue React. 1989;11(4):195–201. [PubMed] [Google Scholar]
- Hayakawa T., Yamashita K., Tanzawa K., Uchijima E., Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992 Feb 17;298(1):29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson S. B., Riddelle K. S., Jones J. C. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992 Sep;99(3):264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968 Feb;50(2):129–137. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- Kuhns D. B., DeCarlo E., Hawk D. M., Gallin J. I. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992 Jun;89(6):1734–1740. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Tamai K., Tan E. M., Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5'-end of the gene and the 3'-untranslated region of the mRNA. J Biol Chem. 1993 Apr 25;268(12):8825–8834. [PubMed] [Google Scholar]
- Liptay M. J., Parks W. C., Mecham R. P., Roby J., Kaiser L. R., Cooper J. D., Botney M. D. Neointimal macrophages colocalize with extracellular matrix gene expression in human atherosclerotic pulmonary arteries. J Clin Invest. 1993 Feb;91(2):588–594. doi: 10.1172/JCI116238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Diaz L. A., Haas A. L., Giudice G. J. cDNA cloning of a novel human ubiquitin carrier protein. An antigenic domain specifically recognized by endemic pemphigus foliaceus autoantibodies is encoded in a secondary reading frame of this human epidermal transcript. J Biol Chem. 1992 Aug 5;267(22):15829–15835. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffey R. J., Jr, Matrisian L. M. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4(6):527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K., Morioka S., Ogawa H. The pathogenic mechanisms of blister formation in bullous pemphigoid. J Invest Dermatol. 1982 Nov;79(5):303–306. doi: 10.1111/1523-1747.ep12500082. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y., Uematsu J., Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem. 1993 Apr;113(4):493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- Oikarinen A. I., Zone J. J., Ahmed A. R., Kiistala U., Uitto J. Demonstration of collagenase and elastase activities in the blister fluids from bullous skin diseases. Comparison between dermatitis herpetiformis and bullous pemphigoid. J Invest Dermatol. 1983 Sep;81(3):261–266. doi: 10.1111/1523-1747.ep12518285. [DOI] [PubMed] [Google Scholar]
- Patterson S., Gross J., Webster A. D. DNA probes bind non-specifically to eosinophils during in situ hybridization: carbol chromotrope blocks binding to eosinophils but does not inhibit hybridization to specific nucleotide sequences. J Virol Methods. 1989 Feb;23(2):105–109. doi: 10.1016/0166-0934(89)90124-9. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W. H., Osteen K. G., Matrisian L. M., Navre M., Giudice L. C., Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993 Jan;168(1 Pt 1):253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest. 1992 Nov;90(5):1952–1957. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Expression of interstitial collagenase, 92-kDa gelatinase, and tissue inhibitor of metalloproteinases-1 in granuloma annulare and necrobiosis lipoidica diabeticorum. J Invest Dermatol. 1993 Mar;100(3):335–342. doi: 10.1111/1523-1747.ep12470032. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Kovacs S. O., Pentland A. P., Olerud J. E., Welgus H. G., Parks W. C. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993 Dec;92(6):2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Welgus H. G., Parks W. C. Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem. 1993 Aug 15;268(23):17354–17361. [PubMed] [Google Scholar]
- Sarret Y., Woodley D. T., Goldberg G. S., Kronberger A., Wynn K. C. Constitutive synthesis of a 92-kDa keratinocyte-derived type IV collagenase is enhanced by type I collagen and decreased by type IV collagen matrices. J Invest Dermatol. 1992 Dec;99(6):836–841. doi: 10.1111/1523-1747.ep12614800. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Sawamura D., Li K., Chu M. L., Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991 Sep 25;266(27):17784–17790. [PubMed] [Google Scholar]
- Schaumburg-Lever G., Orfanos C. E., Lever W. P. Electron microscopic study of bullous pemphigoid. Arch Dermatol. 1972 Nov;106(5):662–667. [PubMed] [Google Scholar]
- Schiltz J. R., Michel B., Papay R. Appearance of "pemphigus acantholysis factor" in human skin cultured with pemphigus antibody. J Invest Dermatol. 1979 Dec;73(6):575–581. doi: 10.1111/1523-1747.ep12541618. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Sires U. I., Griffin G. L., Broekelmann T. J., Mecham R. P., Murphy G., Chung A. E., Welgus H. G., Senior R. M. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993 Jan 25;268(3):2069–2074. [PubMed] [Google Scholar]
- Sirum K. L., Brinckerhoff C. E. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989 Oct 31;28(22):8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Parks W. C. 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma. Am J Pathol. 1993 Apr;142(4):995–1000. [PMC free article] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sudbeck B. D., Eisen A. Z., Welgus H. G., Parks W. C. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992 Oct;99(4):497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- Sudbeck B. D., Jeffrey J. J., Welgus H. G., Mecham R. P., McCourt D., Parks W. C. Purification and characterization of bovine interstitial collagenase and tissue inhibitor of metalloproteinases. Arch Biochem Biophys. 1992 Mar;293(2):370–376. doi: 10.1016/0003-9861(92)90408-o. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Nishida Y., Suzuki F., Kishi J., Yamashita K., Hayakawa T. Induction of angiogenesis in chick yolk-sac membrane by polyamines and its inhibition by tissue inhibitors of metalloproteinases (TIMP and TIMP-2). Biochem Biophys Res Commun. 1990 Sep 28;171(3):1264–1271. doi: 10.1016/0006-291x(90)90822-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Parry D. A., Klaus-Kovtun V., Steinert P. M., Stanley J. R. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991 Jul 5;266(19):12555–12559. [PubMed] [Google Scholar]
- Welgus H. G., Bauer E. A., Stricklin G. P. Elevated levels of human collagenase inhibitor in blister fluids of diverse etiology. J Invest Dermatol. 1986 Nov;87(5):592–596. doi: 10.1111/1523-1747.ep12455837. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate G. E., Weaver A. C., Couchman J. R. Bullous pemphigoid antigen localization suggests an intracellular association with hemidesmosomes. J Invest Dermatol. 1985 Mar;84(3):218–224. doi: 10.1111/1523-1747.ep12265229. [DOI] [PubMed] [Google Scholar]
- Wiche G., Becker B., Luber K., Weitzer G., Castañon M. J., Hauptmann R., Stratowa C., Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J Cell Biol. 1991 Jul;114(1):83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]