Abstract
A novel model of nutritionally induced hypertension in the rat is described. Dietary obesity was produced by providing sweet milk in addition to regular chow, which elicited a 52% increase in caloric intake. Despite 54% greater body weight gain and 139% heavier retroperitoneal fat pads, 120 days of overfeeding failed to increase systolic pressure in the conscious state (125 ± 8 vs. 121 ± 4 mmHg in chow-fed controls) or mean arterial pressure under urethan anesthesia (71 ± 4 vs. 63 ± 3 mmHg). In contrast, mild hypertension developed in intermittantly fasted obese animals (a 21-mmHg increase in systolic blood pressures measured in the conscious state and a 16-mmHg increase in mean arterial pressure under anesthesia relative to chow-fed controls). The first 4-day supplemented fast was initiated 4 wk after the introduction of sweet milk, when the animals were 47 g overweight relative to chow-fed controls. Thereafter, 4 days of starvation were alternated with 2 wk of refeeding for a total of 4 cycles. A rapid fall in systolic blood pressure (12 ± 2 mmHg at 2 days) accompanied the onset of supplemented fasting and was maintained thereafter (2.7 ± 2.6 mmHg further decrease during the latter half of the fast). With refeeding, blood pressure rose precipitously (13 ± 3 mmHg in the 1st 2 days), despite poststarvation anorexia. Blood pressure tended to rise slightly over the remainder of the realimentation period (5.2 ± 2.8 mmHg). After the 4th supplemented fast, hypertension was sustained during 30 days of refeeding. Cumulative caloric intake in starved-refed rats fell within 2% of that in chow-fed controls. Despite normophagia, intermittently fasted rats gained 30% more weight and had 96% heavier retroperitoneal fat pads. Refeeding hypertension appeared to be due to increased sympathetic nervous activity, since 1) cardiac β-adrenergic receptors were downregulated, as indicated by a 40% decrease in the maximum binding of [3H]dihydroalpranolol; and 2) the decrease in heart rate as a result of β-blockade was enhanced. Refeeding hypertension in the dietary obese rat may be a potential animal model for some forms of human obesity-related hypertension.
Keywords: feeding, hypertension, sympathetic nervous system, heart rate, beta-adrenergic receptors, rat
Although Hypertension is Associated with increased adiposity in humans (17), evidence for a similar association in animals is thus far lacking. Genetically hypertensive rats, including spontaneously hypertensive (SHR), Dahl salt-sensitive, Milanese, and New Zealand rats, weigh the same or somewhat less than normotensive control strains (37). SHR are resistant to dietary obesity, because they gain less weight on a high-fat diet than normotensive controls (34). Furthermore, the development of most forms of experimental hypertension is accompanied by depletion of adipose stores. For example, chronic treatment with deoxycorticosterone acetate (DOCA) plus NaCl decreases body weight by one-fourth and induces severe hypertension, whereas treatment with either DOCA or NaCl alone has no effect on either blood pressure or body weight (26).
Conversely, obese animals generally do not exhibit hypertension. The blood pressure of the obese Zucker rat is unchanged relative to lean littermates, and the pressor response to hypothalamic stimulation is also unaltered (21). Spontaneously obese aged rats are also normotensive (7). Obesity-inducing lesions of the ventromedial hypothalamus (VMH) in weanling rats prevent the increase in blood pressure seen during maturation, and thus produce obese hypotensive adults (4). VMH lesions in older rats have no effect on blood pressure, despite an increased sodium intake secondary to hyperphagia. VMH lesions can in fact prevent hypertension induced by either adrenal regeneration (3) or renal ischemia (14). Similarly, lesions of the paraventricular nucleus (PVN) can produce obesity (19) and can also prevent genetic hypertension (1).
Reduced activity of the sympathetic nervous system in animal models of obesity may account for the absence of elevated blood pressure. Decreased norepinephrine turnover in sympathetic target tissues, including the heart, has been noted in VMH-lesioned and Zucker rats as well as ob/ob mice. This decrease in sympathetic activity may in fact be a contributing factor in obesity (18).
The sole example of coexisting obesity and hypertension in an animal model is the obese SHR. These rats are less hypertensive than lean SHR littermates (35). When an inbred strain was created from obese SHR, only lean animals were hypertensive; the SHR-corpulent rat is normotensive (23). Thus the cp/cp genotype simultaneously conveys obesity and protection from hypertension. Further evidence of the independence of obesity and hypertension in this model is that dexamethasone treatment or starvation normalizes body weight in obese SHR without altering blood pressure (35). In addition, induction of dietary obesity in genetically lean SHR by high-fat feeding largely prevents the rise of blood pressure in SHR (34). To complete this seeming paradox, sympathomimetic drugs used as anorectic agents for weight loss elevate blood pressure (8), whereas sympatholytic drugs used as antihypertensive agents may promote weight gain as a significant side effect (2).
There is thus considerable evidence supporting a negative relationship between blood pressure and adiposity in the rodent, in contrast to the positive relationship in humans. The discrepancy between human and animal data may be accounted for by differences in the stability of human and animal obesities. Fat humans characteristically exhibit erratic swings in food intake, resulting in large and rapid weight loss and regain (13), whereas obese animals maintain relatively constant levels of food intake and body weight. Therefore we sought to develop an animal model of human obese hypertension by duplicating the erratic weight history of the fat human in a rodent model.
METHODS
Twenty-eight male Sprague-Dawley rats 8 wk of age were used. Blood pressure and heart rate were determined at the beginning of the experiment and at least biweekly thereafter, by use of tail-cuff sphygmomanometry and an automated physiograph system (Narco Biosystems) on prewarmed restrained animals. The rats were handled repeatedly and familiarized with the restraint chamber. Vocalizations, struggling, or other signs of distress were absent during blood pressure measurement. After repeated determinations of blood pressure and heart rate of 10 wk of age, the rats were divided into two groups matched on the basis of body weight and blood pressure. Nineteen rats were provided with a sweet milk preparation consisting of sweetened condensed milk (Borden) diluted with deionized water and fortified with vitamins and minerals (AIN-76). The diet provided 11% of total calories from protein, 23% from fat, and 66% from carbohydrates, primarily sucrose and lactose. This sweet milk preparation was provided ad libitum in addition to regular chow (Ralston-Purina). Sodium intake in the groups receiving sweet milk was matched to the control level of ~ 1.4 mmol/day. A control group of nine rats continued to be maintained solely on regular chow throughout the experiment.
After 4 wk on the high-sucrose diet, the obese rats were divided into two groups matched on the basis of weight and blood pressure. Nine rats received no additional treatment and were maintained on the sweet milk for the remainder of the experiment. The other 10 rats were fasted for 4 days, during which a solution of vitamins, minerals, and NaCl was provided in a quantity matched to prefast base-line intake. Average daily mi-cronutrient intake was thus held constant during the fast period, whereas daily caloric intake was reduce to 1.2 kcal. Four-day fasts were then alternated with 2 wk of refeeding for a total of four cycles. Blood pressure and heart rate were determined at the onset of the fast, 2 days later, on the 4th day of the fast immediately before the reintroduction of high-sucrose diet, and after 2 days of refeeding. Blood pressure was also determined after 4 h of refeeding after the first fast.
At the end of the refeeding period following the fourth fast, four rats from each experimental group were anesthetized with urethan (1 g/kg) and fitted with femoral arterial and venous catheters for the direct measurement of arterial pressure and intravenous administration of drugs. The peak pressor response to a series of phenylephrine injections was determined. Five minutes after the last phenylephrine injection, each rat was injected with 1.0 mg/kg metoprolol. Ten minutes later, each rat was injected with 2.0 mg/kg of AT-methylscopolamine. The rats were killed 30 min after the final injection.
The 5–6 rats in each group not used in acute experiments were anesthetized with pentobarbital sodium (35 mg/kg). Nasoanal length was measured, and the Lee index was calculated as the cube root of weight divided by the nasoanal length in millimeters (×l0,000). The heart was quickly removed and weighed. Left ventricular wall thickness was determined by use of calipers, and the heart was flash frozen in a dry ice-acetone bath for receptor binding assays. The epididymal and retroperitoneal fat pads, the liver, and the kidneys were also removed and weighed.
For receptor binding assays, hearts were slowly thawed and homogenized in 10 vol of ice-cold 50 mM tris(hydroxymethyl)aminomethane (Tris)-HCl buffer, pH 7.7 at 25°C, containing 5.0 mM EDTA (Tris-EDTA) by use of a Brinkman polytron (setting 6, 30 s). Homogenates were centrifuged at 300 g for 10 min at 4°C. The supernatants were then centrifuged at 50,000 g for 10 min, and the pellets washed twice by centrifugation, once in Tris-EDTA, and again in Tris-HCl buffer without EDTA. The final resuspension was in 50 vol of Tris-HCl and contained 0.5 mg protein/ml.
As previously described (9), β-adrenergic receptor binding assays using [3H]dihydroalpranolol (3H-DHA, 105 Ci/mmol, New England Nuclear) were carried out at 25°C for 20 min in a total volume of 1.0 ml. Membrane suspensions were incubated in triplicate with six concentrations of 3H-DHA ranging from 0.18 to 1.7 nM. Nonspecific binding was defined by parallel incubations with 0.5 μM (−)propranolol. This concentration of (−)propranolol is ~1,000 times its Ki value at 3H-DHA sites and displaced the same proportion of binding as did 10 μM (-)isoprotorenol (data not shown). Nonspecific binding was ~50% of the total at a 3H-DHA concentration close to the dissociation constant (KD) and was a linear function of radioligand concentration. Incubation was terminated by rapid filtration under reduced pressure over Whatman GF/B filters, which were subsequently rinsed three times with 5.0 ml ice-cold 50 mM Tris-HCl buffer.
Data were analyzed by analysis of variance with Newman-Keuls tests. For longitudinal comparisons, analysis of variance for repeated measures was performed and each data point was compared with the prediet base line. For starved-refed rats, each data point was also compared with the prefasting base line. To examine the time course of the effects of fasting, measurements taken on the 2nd and 4th days of fasting were compared. Receptor binding data were analyzed by weighted linear regression of Hofstee plots (40).
RESULTS
Body and organ weights
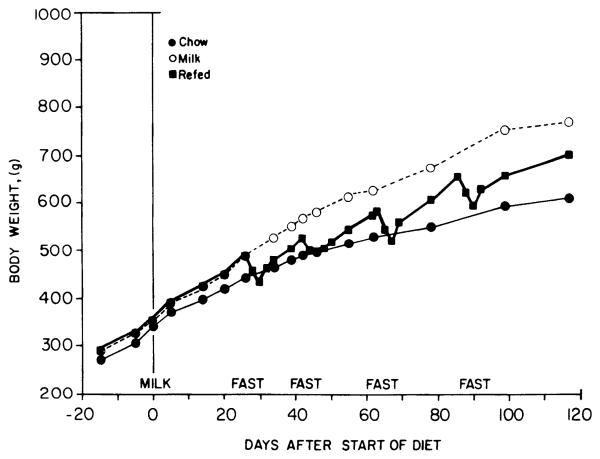
Uninterrupted access to sweet milk produced an increased rate of weight gain throughout the experiment (Fig. 1). Milk-fed rats were significantly heavier than chow-fed controls by the 3rd wk of the diet (P < 0.05, t test, df = 28). A 4-day supplemented fast resulted in a 11.6 ± 0.3% decrease in body weight the first time it was instituted, but relative weight loss declined over successive fasting periods until the fourth fast produced only a 9.7 ± 0.5% decrease in weight. Prefast weight was reattained after ~6 days of refeeding.
FIG. 1.
Effects of high-sucrose diet with and without starvation and refeeding on body weight. Shown are mean body weights of male rats fed chow only (●), given access to sweet milk in addition to chow (○), or given access to sweet milk and chow but intermittently fasted (■). SE were in all cases <5% of mean.
At the end of the experiment, both groups receiving sweet milk were obese, as indicated by increases in body weight, Lee index, and fat pad weights (Table 1). The continuously fed obese control rats were 26% overweight relative to chow-fed controls, whereas starved-refed rats were only 15% overweight (P < 0.05 vs. chow-fed controls, Newman-Keuls test). However, when assessed by the Lee index, the two groups fed sweet milk were equally obese. Fat pad weights in the two groups were likewise similar, although the continuously fed rats tended toward heavier fat pads. Liver, heart, and kidney weights were unchanged, although heart and kidney masses comprised a smaller proportion of total body weight in the obese groups (P < 0.05, Newman-Keuls test). Myocardial hypertrophy, as indicated by left ventricular wall thickness, did not occur.
TABLE 1.
Effects of high-sucrose diet with and without starvation and refeeding on organ weights at death
| Variable | Controls | High Sucrose | Starved Refed |
|---|---|---|---|
| Body wt, g | 609±31 | 770±22* | 701±24* |
| Nasoanal length, mm | 255±5 | 259±4 | 255±3 |
| Lee index, 104 × g−3/mm | 338±9 | 355±8* | 349±6* |
| Epididymal fat pad wt, g | 11±2 | 21±1* | 18±2* |
| g/kg body wt | 18±2 | 30±1* | 28±4* |
| Retroperitoneal fat pad wt, g | 18±4 | 43±2* | 36±3* |
| g/kg body wt | 24±5 | 60±7* | 56±6* |
| Liver wt, g | 17±1 | 21±2 | 18±1 |
| Kidney wt, g | 3.6±0.2 | 3.7±0.2 | 3.3±0.04 |
| Heart wt, g | 1.5±0.07 | 1.4±0.08 | 1.4±0.05 |
| Left ventricular wall thickness, mm | 4.2±0.2 | 4.5±0.1 | 4.5±0.2 |
Values are means ± SE of measurements on 4 animals in each group.
Differs significantly from control (Purina chow), P < 0.05, by analysis of variance with Newman-Keuls test.
Food Intake
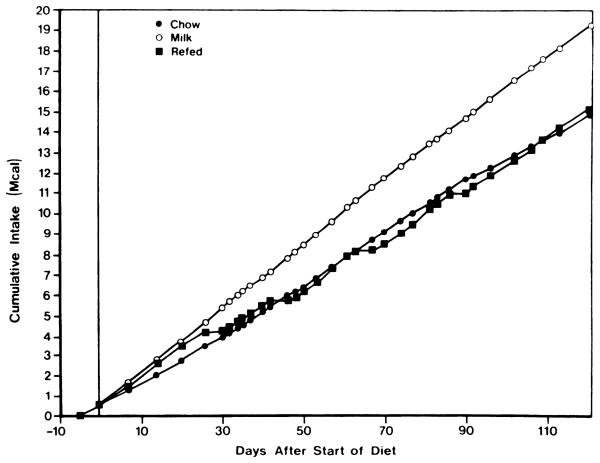
Access to sweet milk induced immediate and sustained hyperphagia (Fig. 2). Daily caloric intake in rats fed sweet milk increased from a base line of 104 ± 1 kcal/day (n = 3 cages) during the last 5 days of chow feeding to 158 ± 4 kcal/day (n = 4, 2 cages × 2 wk) during the first two weeks after the start of the diet (P < 0.05, analysis of variance for repeated measures). Caloric intake remained relatively constant in chow-fed and milk-fed controls throughout the remainder of the experiment. Starved-refed rats consumed fewer calories during the first 2 days of refeeding after the first three fasts (100 ± 7 kcal/day) than during the 2 days just before fasting (140 ± 8 kcal/day; P < 0.05, analysis of variances for repeated measures). This reduction of food intake after fasting has previously been described as poststarvation anorexia (11). During the first 4 h of refeeding, however, fasted rats consumed 55 ± 4 kcal, whereas during the 4 h after the second fast, chow-fed and milk-fed controls ingested only 49 and 45 kcal, respectively. By the 3rd and 4th days of refeeding, pre-fasting levels of intake were reattained (150 ± 7 kcal/day). Beyond the 2nd day after the reintroduction of food, caloric intake in fasted-refed rats (143 ± 3 kcal/day, n = 21) remained slightly below the mean for all weekly measurements in milk-fed controls (155 ± 2 kcal/day, n = 17; P < 0.05 unpaired t test). Poststarvation anorexia was not observed after the fourth fast; intake during the first 2 days of refeeding actually exceeded base line by 28%.
FIG. 2.
Cumulative food intake in starved-refed, dietary obese, and control rats. Shown is mean cumulative food intake of male rats fed chow only (●), given access to sweet milk in addition to chow (○), or given access to sweet milk and chow but intermittently fasted (■). SE were <7% of mean in all cases.
The percentage of total caloric intake obtained from chow among rats with access to sweet milk declined over the 1st wk of exposure to the diet but stabilized for the remainder of the experiment (Table 2). Starved-refed rats consumed a greater proportion of chow during the first 2 days of refeeding, but the proportion of intake derived from chow subsequently declined toward base line. Although the proportion of total intake derived from chow varied substantially during the refeeding period, it should be noted that protein intake remained relatively constant between 12 and 14%.
TABLE 2.
Proportion of total caloric intake derived from chow in rats provided with sweet milk
| % of Calories from Chow |
n | ||
|---|---|---|---|
| Refed | Milk fed | ||
| Week 1 | 27.6 | 23.7 | 1 |
| Week 2 | 16.4 | 13.1 | 1 |
| Weeks 3–18 | 18.4±1.5 | 18.0±1.4 | 16 |
| Weeks 14–18 | 25.1±0.8 | 24.9±1.4 | 5 |
| Prefast base line (2–3 days) | 11.9±1.7 | 4 | |
| Refeeding periods | |||
| First 4 h | 35.3±2.5 | 4 | |
| Days 1 and 2 | 27.5±2.6 | 4 | |
| Days 3 and 4 | 20.6±0.7 | 4 | |
| Days 5–7 | 19.0±1.8 | 4 | |
| Remainder (Day 8+) | 17.2±1.9 | 4 | |
Total no. of calories consumed in form of chow or sweet milk preparation were computed for each period and used to calculate the proportion of total calories derived from chow.
Cumulative caloric intake over the 4 mo of observation in starved-refed rats exceeded that of chow-fed controls by <2% (Fig. 2). Yet, these animals were nearly as obese as those continuously fed sweet milk, even though the latter were markedly more hyperphagic, consuming over 29% more food energy than the lean controls. The starved-refed dietary obese rat thus represents a model of obesity occurring in the absence of an overall increase in caloric intake. Overall food efficiency was 23% greater in starved-refed rats (0.0234 g wt gain/kcal consumed) relative to chow-fed controls (0.0191 g/kcal). Continuously fed obese control rats shows a 17% increase in food efficiency (0.0223 g/kcal).
Blood pressure
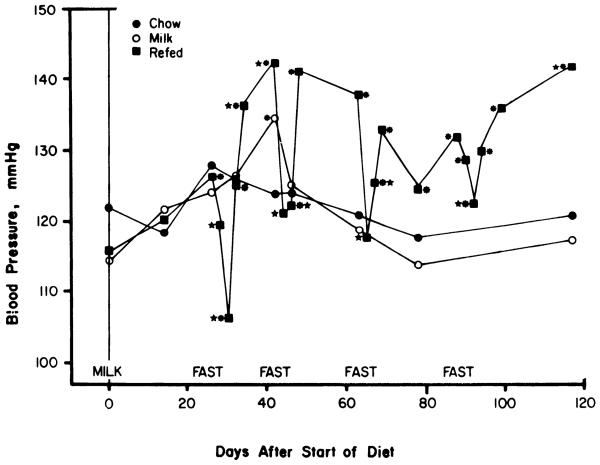
The consumption of sweet milk in addition to regular chow, with the concomitant development of hyperphagia and obesity, did not affect blood pressure over the course of the experiment [F(7,54) = 1.31, P > 0.05]. Blood pressure in the chow-fed control group also did not change over time [F(7,54) = 1.34, P > 0.05]. Fasting and refeeding did have significant effects on blood pressure [Fig. 3; F(19,171) = 8.03, P < 0.01]. A depressor response to the first fast was evident by the 2nd day, and an additional fall in pressure took place between the 2nd and 4th days of the fast (P < 0.05, Newman-Keuls test). During the second and third fasts, the fall in blood pressure in response to fasting was complete within 2 days with no further depressor effect from 2 additional days of fasting. After all four fasts blood pressure returned to prefasting base line within 2 days of the reintroduction of food, then continued to rise until moderately hypertensive levels were attained by the end of the refeeding period. Blood pressure was subsequently normalized during fasting, except during the fourth fast that produced an attenuated depressor response apparent only on the 4th day.
FIG. 3.
Effects of high-sucrose diet with and without starvation and refeeding on systolic blood pressure. Shown are mean systolic blood pressures with groups designated as in Fig. 1. * Points differ from prediet base line, P < 0.05 by analysis of variance and Newman-Keuls test. * Points for refed group differ from immediately preceding prefasting base line. Blood pressure was measured by tail cuff in prewarmed restrained rats. Each measurement represents mean of 6 or more determinations in each rat. Residual SE by analysis of variance for repeated measures was 1.1, 3.2, and 1.5 mmHg for the refed, milk-fed, and chow-fed groups, respectively. Overall residual SE by two-way analysis of variance for repeated measures was 0.91 mmHg.
Blood pressure was increased over prediet base line by the 2nd day of refeeding after the first fast and remained above base line for the remainder of the experiment (P < 0.05, Newman-Keuls test), except during the second and third fasts. Blood pressure was significantly below prediet base line only at the end of the first fast. Blood pressure was elevated above prediet base line in the milk-fed group at a single point (day 40), but this isolated effect should be interpreted with caution, since the overall F test was not significant.
A two-way analysis of variance by repeated measures indicated main effects of experimental group [F(2,154) = 18.5, P < 0.01], and time [F(6,154) = 3.26, P < 0.01], as well as a group by time interaction [F(2,154) = 5.44 P < 0.01]. The latter indicates that changes over time differed between groups. Overall mean blood pressure of the fasted-refed rats was higher than both the milk-fed and chow-fed groups (P < 0.05, Newman-Keuls test). The two control groups did not differ from each other.
Heart rate
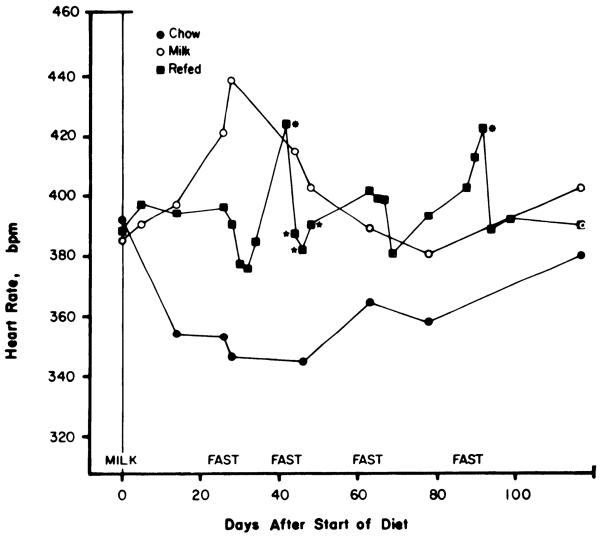
Fasting and refeeding had no effect on heart rate over the course of the experiment [F(19,171) = 0.32, P > 0.05]. Similarly, heart rate did not change over the course of the experiment in the milk-fed or chow-fed groups [F(6,53) = 0.01 and 0.12, respectively]. Heart rate tended to fall during the first three fasts, but this change was only significant during the second fast (Fig. 4; P < 0.05, Newman-Keuls test). Heart rate increased above prediet base line at the end of the first refeeding period and at the end of the fourth fast but otherwise remained within 5% of the initial value. Apparent changes in heart rate in the two control groups did not reach significance by the Newman-Keuls test.
FIG. 4.
Effects of high-sucrose diet with and without starvation and refeeding on heart rate. Shown are mean heart rates with groups designated as in Fig. 1. Each measurement represents mean of 3 or more determinations in each rat. Residual SE by analysis of variance for repeated measures was 7.9, 38.1, and 28.4 beats/min for refed, milk-fed, and chow-fed groups, respectively. Overall residual SE by two-way analysis of variance for repeated measures was 11.9 beats/min. See legend of Fig. 3 for definition of probability symbols.
A two-way analysis of variance by repeated measures did not reveal a significant group effect [F(2,154) = 2.23], although when compared with chow-fed controls both the refed and milk-fed groups had higher heart rates by post hoc analysis (P < 0.05, Newman-Keuls test). Access to sweet milk thus appeared to produce a modest tachycardia, independent of the fasting and refeeding regimen.
In terminal studies conducted under urethan anesthesia, dietary obesity in the absence of intermittent fasting treatment was without effect on blood pressure determined by direct measurement (Table 3). Blood pressure as determined by direct measurement was elevated in fasted-refed rats relative to either milk-fed or chow-fed controls, thus confirming data on systolic blood pressure obtained by tail cuff (Fig. 3). The elevation in systolic pressure in the refed group relative to chow-fed controls in the anesthetized state (19.6%) was similar to that obtained by tail cuff the previous day (19.1%).
TABLE 3.
Effects of high-sucrose diet with and without starvation and refeeding on cardiovascular function and autonomic tone
| Variable | Controls | High Sucrose | Starved Refed |
|---|---|---|---|
| Measurements in anesthetized state | |||
| Blood pressure, mmHg | |||
| Systolic | 92±3 | 100±4 | 110±2*† |
| Diastolic | 49±3 | 57±4 | 64±0.2* |
| Mean, D + [(S −D)/3] | 63±3 | 71±4 | 79±1* |
| Heart rate, beats/min | 236±15 | 281±28 | 322±32* |
| Decrease in heart rate after 1 mg/kg metoprolol | 15±11 | 36±11 | 56±17* |
| Increase in heart rate after 2 mg/kg N-methylscopolamine | 63±16 | 16±13* | 9±10* |
| Measurements in conscious state | |||
| Systolic blood pressure | 121±4 | 125±8 | 142±4*† |
| Heart rate | 347±14 | 422±31* | 433±11* |
Values are means ± SE of measurements on 4 animals in each group. D, diastolic; S, systolic.
Differs significantly from control (Purina chow), P < 0.05, by analysis of variance with Newman-Keuls test.
Differs significantly from high-sucrose group, P < 0.05.
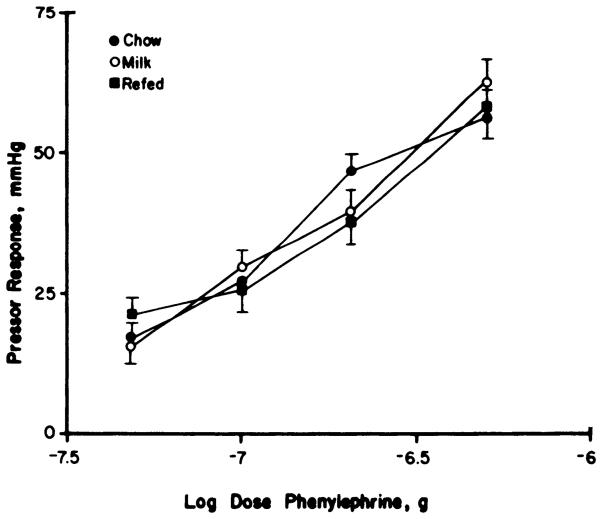
For the heart rate, the rank order of refed > milk fed > chow fed was similar under conscious and anesthetized conditions. An increase in heart rate after N-methyl scopolamine, readily observed in controls, was absent in milk-fed rats, suggestive of a withdrawal of vagal tone. Milk-fed rats did demonstrate a significant decrease in heart rate in response to injection of the β-antagonist metopolol (P < 0.01, paired t test), whereas controls fed Purina chow lacked this response. These results are compatible with a shift in autonomic balance in favor of the sympathetic nervous system. The modest tachycardia observed in the conscious state was maintained under anesthesia (Table 3). The decrease in heart rate after β-blockade was enhanced threefold m fasted-refed animals, suggesting an increase in sympathetic outflow to the heart. The failure of the vagolytic agent N-methylscopolamine to subsequently increase heart rate is compatible with a withdrawal of parasympathetic input. Refeeding hypertension was not associated with any changes in vascular reactivity to phenylephrine (Fig. 5).
FIG. 5.
Effects of high-sucrose diet with and without starvation and refeeding on pressor responses to graded doses of intravenous phenylephrine. Peak systolic pressor responses to phenylephrine injection via femoral venous catheter, ± SE, for chow-fed controls (●), rats fed sweet milk plus chow throughout (○), and intermittently fasted rats (■). Rats were anesthetized with urethan (1 g/kg), and blood pressure was measured via a pressure transducer. Latency and duration of the pressor responses did not differ between groups (data not shown).
Receptor binding studies utilizing cardiac tissue provide further support for enhanced sympathetic outflow with starvation and refeeding (Table 4). β-Receptor density, as measured with 3H-DHA binding, was decreased 40% in starved-refed rats (P < 0.05, Newman-Keuls test) but only 16% in obese controls (P > 0.05). Differences in radioligand affinity were not significant.
TABLE 4.
Effects of high-sucrose diet with and without starvation and refeeding on cardiac β-adrenergic receptor binding
| Variable | Controls | High Sucrose | Starved Refed |
|---|---|---|---|
| Bmax, fmol/mg protein | 215±21 | 181±38 | 128±11* |
| KD, nM | 1.69±0.23 | 0.92±0.10 | 1.01±0.16 |
Values are means ± SE of measurements on 4 animals in each group, each based on 6 radioligand concentrations assayed in triplicate. Maximum binding (Bmax) and dissociation constant (KD) values of [3H]-dihydroalpranolol were obtained from weighted linear regression of Hofstee plots by method of Zivin and Waud (40).
Differs significantly from control (Purina chow), P < 0.05, by analysis of variance with Newman-Keuls test.
DISCUSSION
The present study demonstrates that hypertension can be induced in obese rats by a regimen of repeated starvation and refeeding. Refeeding hypertension has previously been described in humans (6), dogs (36), swine (30), and mice (31). The sympathetic nervous system is implicated in the pathogenesis of this form of hypertension, since the bradycardic response to β-blockade is enhanced and cardiac β-adrenergic receptors are down-regulated, implying chronic overstimulation. A role for noradrenergic neurons is also supported by previous evidence that surgical sympathectomy prevents refeeding hypertension in the dog (36). Furthermore, although fasting in the rat has no effect on urinary epinephrine and produces only a slight fall in urinary norepinephrine, refeeding more than doubles urinary norepinephrine and epinephrine (18). In humans, the parallel decreases in blood pressure, heart rate, and urinary catecholamine metabolites resulting from a 21-day supplemented fast are completely reversed by only 3 days of refeeding (16).
A proposed contributing factor in refeeding hypertension is altered vascular function. Morphological changes in blood vessels and decreased arterial distensibility have been described in starved-refed animals (12, 30, 31). However, it is not clear to what extent the observed changes may be the result of hypertension rather than its cause. In the present study, vascular responsiveness to phenylephrine was unchanged when animals were examined after an extended refeeding period, suggesting no more than a limited role for increased vascular reactivity in this model of hypertension.
Refeeding hypertension cannot be attributed to dietary excess, because blood pressure shows no consistent relationship with caloric intake. During the first 2 days of refeeding, while blood pressure was rising rapidly, food consumption was decreased relative to continuously fed obese controls. Even during the first 4 h of refeeding, only 24% more calories were consumed by fasted rats than by unfasted animals fed the same diet. Even excluding periods of fasting and the first 2 days of refeeding, the mean of all other intake measurements in starved-refed rats was significantly less than the mean intake of rats with continuous access to sweet milk. Despite a sustained excessive caloric intake, the milk-fed obese controls remained normotensive. Refeeding hypertension described in this study also developed independently from alterations in the intake of sodium, potassium, calcium, or magnesium because similar amounts of these minerals were provided by chow, fortified sweet milk preparation, and the supplement provided during fasting. Thus constant levels of these nutrients were provided during cycles of supplemented fasting and refeeding, and intake of these minerals was similar among the experimental groups. The hypertension is not related to diet composition or degree of obesity, because heavier rats continuously fed an identical diet remained normotensive. Blood pressure elevation is thus specifically dependent on the restriction and restoration of food intake.
Increased heart rate, however, was equally present in both milk-fed groups. This tachycardia appears to be related to sympathetic nervous system activity, because it is reversed by β-blockade with metoprolol. The tachycardia is unlikely to be a direct result of obesity, since it reached its greatest intensity 4 wk after the introduction of the diet when the animals were only 10% overweight, and failed to further increase as the obese controls accumulated an average of 280 g in additional weight. Alterations in either the composition of the diet or total caloric intake could be responsible for the tachycardia. Available evidence favors a more critical role for hyperphagia, because overfeeding with either high-sucrose or high-fat diets produces equivalent increases in cardiac norepinephrine turnover (29).
A decrease in cardiac β-receptor number has been reported in several hypertensive models known to be associated with an increase in sympathetic nerve activity, including Goldblatt one-kidney, DOCA-sodium, and SHR hypertension (32). The association between increased sympathetic activity and cardiac β-receptor downregulation is sufficiently close to suggest that the finding of decreased β-receptor number after starvation and refeeding is indicative of persistently elevated cardiac sympathetic drive. Changes in β-adrenergic receptor binding cannot be attributed to obesity per se, since cardiac 3H-DHA binding is unchanged in Zucker fa/fa rats (21).
Increases in heart weight in obese rats were anticipated, since heart and body weights are correlated in humans (22). However, both absolute and relative heart weights were unchanged in the present study. In humans, left ventricular wall thickness is correlated with degree of overweight (22). However, no changes were observed in obese or starved-refed rats in the present study. Cardiac hypertrophy may be detected in future studies with longer periods of observation.
Sucrose overfeeding has been shown to increase sympathetic nervous system activity (38). However, recent long-term studies have shown that elevated sympathetic activity occurs only during the initial stages of dietary obesity, since brown adipose tissue β-receptors were downregulated after 2 wk of excess caloric intake but not after 3 mo (20). The present data tend to support the hypothesis that major increases in sympathetic activity are absent in long-term dietary obesity. Both the enhancement of the bradycardic response to β-blockade and the downregulation of β-adrenergic receptor number failed to attain statistical significance in the dietary obese controls. As suggested elsewhere (20), sympathetic responses to increased caloric intake may participate primarily in short-term physiological regulation.
The present study demonstrates that starved and refed rats can become markedly obese while consuming no more calories than chow-fed controls. The increase in actual efficiency of energy accumulation may be considerably greater, because previous studies have shown that repeated episodes of starvation and refeeding facilitate deposition of fat at the expense of lean tissue (33). Increased efficiency of food utilization during realimen-tation is independent of changes in locomotor activity and is the result of reduced O2 consumption at rest and in response to a meal (5). Thus transient caloric restriction has the paradoxical ability to promote adiposity. Multiple episodes of starvation and refeeding have greater than additive metabolic effects (33), which may account for the large increase in efficiency of weight gain observed in refed rats in the present study.
Consistent with findings in other forms of experimental obesity (3, 4, 7, 14, 21, 23, 34, 35), dietary-induced excess adiposity did not cause sustained elevations in blood pressure, despite prolonged hyperphagia and the deposition of massive adipose stores. Together with previous animal and human data, these findings militate against direct or static effect of adipose tissue mass on blood pressure, suggesting instead an indirect and dynamic relationship. The present study is the first report of an obesity-related hypertension in the rat.
High-sucrose diets have been shown to produce a modest pressor effect (5–10 mmHg) in the rat (25). An initial rise in blood pressure in response to high-sucrose feeding was observed, although this increase was significant only at 6 wk after initiation of the diet. This transient elevation in blood pressure may reflect a short-term induction of sympathetic nervous system activity by overfeeding (20). Diets low in protein (8% of total calories) have been shown to stimulate the sympathetic nervous system in young rats (28). Although the protein intake of milk-fed rats was moderately low (13%) relative to chow-fed controls (22%), this reduction in protein intake may have been insufficient to evoke sustained sympathetic activation. The ability of low-protein diets to stimulate sympathetic outflow declines between 22 and 55 days of age (28) and may have been further attenuated in the 70-day-old rats used in the present study. Because a diet high in sucrose and moderately low in protein failed to increase blood pressure in milk-fed rats, it is unlikely that changes in diet composition can account for the hypertension in the refed group.
The depressor effect of fasting has previously been demonstrated in SHR and WKY rats (39) and is confirmed here in obese rats. The blood pressure decrease with fasting in dietary obesity is greater than that previously described in lean animals; if it can be assumed that overall sympathetic activity is increased, and not just the cardiac component, the exaggerated depressor response to fasting may be due to the abrupt withdrawal of an enhanced sympathetic tone that is acting to maintain blood pressure in the overfed rat. It is unlikely that the exaggerated depressor effect was related to ketouria-dependent natriuresis, since overfed rats are resistant to ketosis and ketouria. In contrast to the present findings, a loss of up to 30% of total body weight in aged spontaneously obese male Sprague-Dawley rats was reported to be without effect on mean arterial pressure (7). However, the animals underwent gradual weight loss on 60% of control rations. The failure of mild caloric restriction to elicit a depressor response is consistent with the hypothesis that the blood pressure is affected more by the rate of change in body weight, or by net energy balance, than by the absolute gain or loss of weight (Ernsberger and Nelson, unpublished observations).
As reported previously in larger animals (30, 36), cardiovascular responses to starvation were attenuated with repeated episodes of fasting. The depressor response to the fourth fast was less than half that observed during the first fast, and the slight bradycardic response to the early fasts was transformed into a tachycardic response by the fourth fast.
Refeeding hypertension in the dietary obese rat shares several features with human obese hypertension. Like refeeding hypertension, human obese hypertension is associated with a sustained increase in sympathetic tone (15, 16). The hypertension is mild in degree (24) as it is in refeeding hypertension. The obese hypertensive human, like the obese hypertensive starved-refed rat, does not overeat relative to lean controls (17). Tachycardia is present in at least some obese populations, but high cardiac output is a more reliable characteristic (22). The persistent tachycardia of starved-refed animals suggests a high-output hypertension, but more detailed studies of cardiac function are required to substantiate this.
The present study describes a novel hypertensive rodent model in which consistent, reproducible, and self-sustaining blood pressure elevations are generated by a pattern of caloric intake, through a mechanism involving activation of the sympathetic nervous system. The apparent changes in the neural input to the heart in this model may be relevant to cardiovascular disease in obese humans. Further experimentation is needed to elucidate the mechanism linking changes in caloric intake and activation of the sympathetic nervous system, and to characterize refeeding schedules that minimize cardiovascular and metabolic complications. These are important questions for the many human patients who undergo large variations in energy influx and stores (13).
Acknowledgments
The authors thank Carol A. Graham for excellent technical assistance throughout the course of this work and Vangie Walker for secretarial help.
This work was supported by National Heart, Lung, and Blood Institute Grants HL-29033 and HL-39444, the Schweppe Foundation, and a National Science Foundation predoctoral fellowship to P. Ernsberger.
REFERENCES
- 1.Anzar S, Ernsberger P, Livingston S, Azar P, Iwai J. Paraventricular-suprachiasmatic lesions prevent Dahl hypertension. Clin. Sci. Lond. 1981;61:49s–51s. doi: 10.1042/cs061049s. [DOI] [PubMed] [Google Scholar]
- 2.Bai TR, Webb D, Hamilton M. Treatment of hypertension with beta-adrenoceptor blocking drugs. J. R. Coll. Physicians Lond. 1982;16:239–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardis LL, Brownie AC, Molteni A, Skeleton FR. Effect of ventromedial hypothalamic lesions on adrenal regeneration hypertension in young-adult rats. Lab. Invest. 1967;16:516–525. [PubMed] [Google Scholar]
- 4.Bernardis LL, Skeleton FR. Blood pressure in female and male rats following ventromedial hypothalamic lesions placed at four different ages. Proc. Soc. Exp. Biol. Med. 1965;120:756–760. doi: 10.3181/00379727-120-30648. [DOI] [PubMed] [Google Scholar]
- 5.Boyle PC, Storlien LH, Harper AE, Keesey RE. Oxygen consumption and locomotor activity during restricted feeding and realimentation. Am. J. Physiol. 1981;241:R392–R397. doi: 10.1152/ajpregu.1981.241.5.R392. (Regulatory Integrative Comp. Physiol. 10) [DOI] [PubMed] [Google Scholar]
- 6.Brozek J, Chapman CB, Keys A. Drastic food restriction: effect on cardiovascular dynamics in normotensive and hypertensive conditions. J. Am. Med. Assoc. 1948;137:1569–1574. doi: 10.1001/jama.1948.02890520001001. [DOI] [PubMed] [Google Scholar]
- 7.Crandall DL, Goldstein BM, Gabel RA, Cervoni P. Hemodynamic effects of weight reduction in the obese rat. Am. J. Physiol. 1984;247:R266–R271. doi: 10.1152/ajpregu.1984.247.2.R266. (Regulatory Integrative Comp. Physiol. 16) [DOI] [PubMed] [Google Scholar]
- 8.Douglas JG, Munro JF. Drug treatment and obesity. Pharmacol. Ther. 1982;18:351–373. doi: 10.1016/0163-7258(82)90037-7. [DOI] [PubMed] [Google Scholar]
- 9.Ernsberger P, U'Prichard DC. Para-azidoclonidine: a novel photoaffinity ligand for the alpha2-receptor. Life Sci. 1986;38:1557–1563. doi: 10.1016/0024-3205(86)90494-7. [DOI] [PubMed] [Google Scholar]
- 10.Goodman MN, Ruderman NB. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA and protein. Am. J. Physiol. 1980;239:E269–E276. doi: 10.1152/ajpendo.1980.239.4.E269. (Endocrinol. Metab. 2) [DOI] [PubMed] [Google Scholar]
- 11.Hamilton CL. Problems of refeeding after starvation in the rat. Ann. NY Acad. Sci. 1969;157:1004–1017. doi: 10.1111/j.1749-6632.1969.tb12933.x. [DOI] [PubMed] [Google Scholar]
- 12.Hembrough FB, Riedesel DH. Mechanical behavior change in a major artery after a series of starvation-refeeding episodes. Am. J. Physiol. 1970;219:742–746. doi: 10.1152/ajplegacy.1970.219.3.742. [DOI] [PubMed] [Google Scholar]
- 13.Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. Saunders; Philadelphia, PA: 1980. [Google Scholar]
- 14.Johnson AK, Buggy J, Fink GD, Brody MJ. Prevention of renal hypertension and of the central pressor effect of angiotensin by ventromedial hypothalamic ablation. Brain Res. 1981;205:255–264. doi: 10.1016/0006-8993(81)90337-1. [DOI] [PubMed] [Google Scholar]
- 15.Jung RT, Shetty PS, Barrand M, Callingham BA, James WPT. Role of catecholamines in hypotensive response to dieting. Br. Med. J. 1979;1:12–13. doi: 10.1136/bmj.1.6155.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung RT, Shetty PS, James WPT. The effect of refeeding after semistarvation on catecholamine and thyroid metabolism. Int. J. Obes. 1980;4:95–100. [PubMed] [Google Scholar]
- 17.Keen H, Thomas BJ, Jarrett RJ. Obesity and cardiovascular risk. Int. J. Obes. 1982;6(Suppl. 1):83–89. [PubMed] [Google Scholar]
- 18.Lansberg L, Young JB. Fasting, feeding and regulation of the sympathetic nervous system. N. Engl. J. Med. 1978;298:1295–1301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- 19.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 1981;27:1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 20.Levin BE, Finwegan MB, Triscari JT, Sullivan AC. Effect of diet and obesity on brown adipose metabolism. Am. J. Physiol. 1984;246:E418–E425. doi: 10.1152/ajpendo.1984.246.5.E418. (Endocrinol. Metab. 9) [DOI] [PubMed] [Google Scholar]
- 21.Levin BE, Stoddard-Apter S, Sullivan AC. Central activation and peripheral function of sympatho-adrenal and cardiovascular systems in the Zucker rat. Physiol. Behav. 1984;32:295–299. doi: 10.1016/0031-9384(84)90144-6. [DOI] [PubMed] [Google Scholar]
- 22.Messerli FH. Cardiovascular adaptations to obesity and arterial hypertension: detrimental or beneficial? Int. J. Cardiol. 1983;3:94–97. doi: 10.1016/0167-5273(83)90069-4. [DOI] [PubMed] [Google Scholar]
- 23.Michaelis OE, Ellwood KC, Judge JM, Schoene NW, Hansen CT. Effect of dietary sucrose on the SHR/N-corpulent rat: a new model for insulin-independent diabetes. Am. J. Clin. Nutr. 1984;39:612–618. doi: 10.1093/ajcn/39.4.612. [DOI] [PubMed] [Google Scholar]
- 24.Perera GA, Damon A. Height, weight and their ratio in the accelerated form of primary hypertension. Arch. Int. Med. 1957;100:263–265. doi: 10.1001/archinte.1957.00260080089017. [DOI] [PubMed] [Google Scholar]
- 25.Preuss HG, Fournier RD. Effects of sucrose ingestion on blood pressure. Life Sci. 1982;30:879–886. doi: 10.1016/0024-3205(82)90615-4. [DOI] [PubMed] [Google Scholar]
- 26.Reid JL, Zivin JA, Kopin IJ. Central and peripheral adrenergic mechanisms in the development of deoxycorticosterone-saline hypertension in rats. Circ. Res. 1975;37:569–579. doi: 10.1161/01.res.37.5.569. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell NJ, Saville ME, Stock MJ. Metabolic responses to fasting and refeeding in lean and genetically obese rats. Am. J. Physiol. 1983;244:R615–R620. doi: 10.1152/ajpregu.1983.244.5.R615. (Regulatory Integrative Comp. Physiol. 13) [DOI] [PubMed] [Google Scholar]
- 28.Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Metabolism. 1983;32:257–261. doi: 10.1016/0026-0495(83)90190-7. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. J. Clin. Invest. 1983;72:361–270. doi: 10.1172/JCI110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GS, Smith JL, Mameesh MS, Simon J, Johnson BC. Hypertension and cardiovascular abnormalities in starved-refed swine. J. Nutr. 1964;82:173–182. doi: 10.1093/jn/82.2.173. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Vaniz GT, Ashburn AD, Williams WL. Diet-induced hypertension and cardiovascular lesions in mice. Yale J. Biol Med. 1970;43:61–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Stiles GL, Lefkowitz RJ. Cardiac adrenergic receptors. Ann. Rev. Med. 1984;35:149–164. doi: 10.1146/annurev.me.35.020184.001053. [DOI] [PubMed] [Google Scholar]
- 33.Szepesi B, Vegors R, Michaelis OE, Demouy JM. Long-term effects of starvation-refeeding in the rat. Nutr. Metab. 1975;19:45–54. doi: 10.1159/000175646. [DOI] [PubMed] [Google Scholar]
- 34.Wexler BC. Inhibition of the pathogenesis of spontaneous hypertension in SHR's by feeding a high fat diet. Endocrinology. 1981;108:981–989. doi: 10.1210/endo-108-3-981. [DOI] [PubMed] [Google Scholar]
- 35.Wexler BC, McMurty JP. Dexamethasone suppression of cushingold degenerative changes in obese spontaneously hypertensive rats. Metabolism. 1984;33:281–288. doi: 10.1016/0026-0495(84)90051-9. [DOI] [PubMed] [Google Scholar]
- 36.Wilhemj CM, Carnazzo AJ, McCarthy HH. The effect of fasting and realimentation with diets high in carbohydrate or protein on the blood pressure and heart rate of sympathecto-mized dogs. Am. J. Physiol. 1957;191:103–110. doi: 10.1152/ajplegacy.1957.191.1.103. [DOI] [PubMed] [Google Scholar]
- 37.Yamori Y. The SHR model and its relation to human essential hypertension. In: Sleight P, Freis ED, editors. Hypertension. Butterworths; London: 1982. [Google Scholar]
- 38.Young JB, Landsberg L. Stimulation of the sympathetic nervous system during sucrose feeding. Nature Lond. 1977;269:615–617. doi: 10.1038/269615a0. [DOI] [PubMed] [Google Scholar]
- 39.Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1978;27:1711–1714. doi: 10.1016/0026-0495(78)90256-1. [DOI] [PubMed] [Google Scholar]
- 40.Zivin JA, Waud DR. How to analyze binding, enzyme and uptake data: the simplest case, a single phase. Life Sci. 1982;30:1407–1422. doi: 10.1016/0024-3205(82)90554-9. [DOI] [PubMed] [Google Scholar]