Abstract
Background
Inhaled corticosteroids (ICS) are considered first-line treatment for persistent asthma; yet, there is significant variability in treatment response. Dual specificity phosphatase-1 (DUSP1) appears to mediate the anti-inflammatory action of corticosteroids.
Objective
To determine whether variants in the DUSP1 gene are associated with clinical response to ICS treatment.
Methods
Study participants with asthma were drawn from the following multi-ethnic cohorts: the Genetics of Asthma in Latino Americans (GALA) study, the Study of African Americans, Asthma, Genes & Environments (SAGE), and the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE). We screened GALA participants for genetic variants that modified the relationship between ICS use and bronchodilator response. We then replicated our findings in SAGE and SAPPHIRE participants. In a group of SAPPHIRE participants treated with ICS for 6 weeks, we examined whether a DUSP1 polymorphism was associated with changes in forced expiratory volume at one second (FEV1) and self-reported asthma control.
Results
DUSP1 polymorphisms, rs881152 and rs34507926, localized to different haplotype blocks and appeared to significantly modify the relationship between ICS use and bronchodilator response among GALA participants. This interaction was also seen for rs881152 among SAPPHIRE, but not SAGE participants. Among the group of SAPPHIRE patients prospectively treated with ICS for 6 weeks, rs881152 genotype was significantly associated with changes in self-reported asthma control but not FEV1.
Conclusion
DUSP1 polymorphisms were associated with clinical response to ICS therapy, and therefore, may be useful in the future to identify asthma patients more likely to respond to this controller treatment.
Clinical implications
These findings further our understanding of ICS pharmacogenetics and will hopefully result in improved tailoring of this controller therapy among individuals with asthma and in better disease control.
Capsule summary
We identified genetic variants in DUSP1 which appeared to mediate the clinical response to inhaled corticosteroid (ICS) medication. These findings may eventually assist in identifying individuals with asthma most likely to respond this controller therapy.
Keywords: Asthma, inhaled corticosteroids, dual specificity phosphatase-1, DUSP1, corticosteroid responsiveness
INTRODUCTION
Inhaled corticosteroid (ICS) therapy is considered to be first line treatment for the control of persistent asthma.(1) However, there appears to be substantial variation in ICS treatment response, which may contribute to a significant proportion of disease-related morbidity.(2–4)
In-vitro models have identified dual specificity phosphatase-1 (DUSP1), also known as MAP kinase phosphatase-1 (MKP1), as a potential master regulator of corticosteroid response.(5–7) The induction of DUSP1 activity by corticosteroids results in the dephosphorylation and inhibition of mitogen activated protein kinases (MAPKs), thereby reducing the expression and production of pro-inflammatory cytokines.(8–13)
Prior studies have shown bronchodilator responsiveness to be a consistent predictor of therapeutic response to ICS medication.(14) Therefore, in this study we assess whether single nucleotide polymorphisms (SNPs) in the DUSP1 gene are associated with bronchodilator responsiveness in 3 separate, ethnically diverse cohorts and whether these relationships are modified by the concurrent use of ICS medication. We also assess whether implicated SNPs are prospectively associated with ICS treatment response among participants in the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity (SAPPHIRE).
METHODS
Study population and setting
The Institutional Review Boards of Henry Ford Health System and the University of California, San Francisco approved the study. Informed written consent was obtained from study participants or their guardians, and written assent was also obtained from all participants who were minors. Study participants comprised 3 separate cohorts, the Genetics of Asthma in Latino Americans (GALA) study, the Study of African Americans, Asthma, Genes & Environments (SAGE), and the SAPPHIRE.
The first two studies, GALA and SAGE, were cross-sectional and have been described elsewhere.(15;16) The GALA study began as 399 Puerto Rican and 301 Mexican asthma trios, recruited from four clinical centers located in San Francisco, New York City, Puerto Rico and Mexico City. The SAGE study included individuals recruited from the San Francisco Bay Area. Participants in both studies were age 8–40 years, had physician-diagnosed asthma or >12% bronchodilator responsiveness, and had symptoms of coughing, wheezing, or shortness of breath for at least 2 years. Biological parents and grandparents were required to identify themselves as either Puerto Rican or Mexican American for GALA participants, and as African American for SAGE participants. Patients were excluded from these studies if they had >10 pack-year smoking history, smoked in the previous 12 months, or had a history of other lung diseases. Concurrent ICS use was assessed by questionnaire in these cohorts.
Individuals in the SAPPHIRE cohort all received care through a large health system serving southeast Michigan. Patients were invited for screening if they met the following criteria: age 12–56 years, a prior clinical diagnosis of asthma, and no recorded diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Longitudinal health care data was available for participants who had coverage through an affiliated provider. These data included pharmacy records for patients with prescription riders, which permitted assessment of concurrent ICS use in the larger group of screened participants.
As SAPPHIRE was specifically designed to assess ICS responsiveness as one of its objectives, a subset of screened patients was invited to undergo 6 weeks of ICS treatment (i.e., 160µg beclomethasone dipropionate HFA [Teva Specialty Pharmaceuticals LLC, Horsham, PA] twice daily per metered dose inhaler [MDI]), as has been done elsewhere.(14) In order to qualify for this additional assessment, participants had to meet the following criteria: a baseline forced expiratory volume at one second (FEV1) between 40–90% predicted, bronchodilator reversibility (i.e., improvement in FEV1 of >12% from baseline), no smoking in the preceding year or <10 pack-year smoking history total, no oral or inhaled corticosteroid use in the 4 weeks preceding enrollment, not pregnant at the time of enrollment and not intending to get pregnant during the 6-week treatment period. Participants were instructed in proper MDI technique including the use of a provided spacer (AeroChamber Plus® Z STAT, Monahan Medical Corp., Plattsburgh, NY). All SAPPHIRE participants completed a survey at their baseline assessment, and individuals who underwent the 6-weeks of ICS treatment also completed a survey at the follow-up visit. Asthma control was assessed at each visit using the Asthma Control Test (ACT) (QualityMetric Inc., Lincoln, RI).(17)
Assessment of pulmonary function
Spirometry was performed in accordance with 1995 American Thoracic Society(18) and more recent 2005 ATS/ERS recommendations depending on the time of enrollment.(19;20) A Fleisch-type pneumotachometer (KoKo PFT Spirometer®, nSpire Health Inc., Louisville, CO) was used to measure pulmonary function parameters, and expected values were obtained using standard equations derived from the U.S. population.(21) If possible, patients were asked to withhold their bronchodilator medications 12 hours before lung function tests. Bronchodilator response was determined by administering 360µg albuterol sulfate from a standard MDI, and a second dose (180µg for children <16 years, and 360µg for persons ≥16 years) was administered if >12% reversibility was not achieved following the first dose. A >12% improvement in FEV1 was considered reversible(22;23) and was required for enrollment in the 6-week treatment trial. However, for the purposes of assessing ICS by genotype interactions on bronchodilator response the difference in FEV1 after the first dose of albuterol was used (i.e., between the first and second set of spirometry measurements). Pulmonary function was reassessed after 6 weeks in the subset of individuals in the SAPPHIRE trial who received beclomethasone dipropionate HFA.
DNA isolation, genotyping, and assessment of ancestry
All study patients had genomic DNA extracted from whole blood samples. Genotyping was performed with iPLEX® gold (Sequenom, Inc., San Diego, CA). Each individual was genotyped for 107 single nucleotide polymorphisms (SNPs), which were informative for determining individuals’ ancestry due to their differing allele frequencies in continental population groups. This set of ancestry informative markers (AIMs) has been described in detail elsewhere,(24) but was developed from West African individuals living in London, U.K. and South Carolina, U.S.; individuals of European ancestry from Coriell's North American Caucasian panel (Coriell Institute for Medical Research, Camden, New Jersey); and Native American individuals (i.e., Mayan and Nahua) from villages in Tlapa in the state of Guerrero, Mexico.
For the GALA and SAGE cohorts we estimated individual ancestry using the program STRUCTURE, which employs a Bayesian approach.(25) For the SAPPHIRE cohort we used the software package PSMIX, which uses maximum likelihood estimation to estimate ancestry.(26;27) We have previously shown that both methods produce highly correlated ancestry estimates.(28)
Eight tagging SNPs were selected to provide coverage of the DUSP1 gene (7 intronic and 1 synonymous), and 2 additional SNPs were selected that had minor allele frequencies ≥5%. This genotyping was performed by allelic discrimination using a Taqman SNP genotyping assay (Applied Biosystems, Foster City, California).
Statistical analysis
Given the reported association between bronchodilator responsiveness and ICS responsiveness,(14) we first screened the DUSP1 SNPs for association with bronchodilator responsiveness. We hypothesized that polymorphisms related to ICS responsiveness would demonstrate differential associations with the bronchodilator responsiveness between individuals currently treated and those not treated with an inhaled steroid. Alternatively stated, given the biologic association between ICS use and beta-agonist response,(29;30) genetic variants (i.e., SNP genotypes) which mediate steroid response should therefore demonstrate differing patterns of association with bronchodilator response in the presence and absence of ICS use. Accordingly, we assessed for effect modification by ICS use (i.e., genotype by steroid use interactions on bronchodilator response). The dependent variable, bronchodilator responsiveness, was measured as the percent change between the pre- and post-bronchodilator FEV1. Since our analysis involved an assessment of ICS by genotype interactions, we restricted the study population to just individuals with asthma in all cohorts (i.e., case-only analysis).
We first assessed for significant associations between both genotype and the genotype by ICS use interaction term on bronchodilator responsiveness in the GALA cohort using multivariable linear regression models. An additive (or co-dominant) genetic model was used for the initial screening of each genotype. In other words, heterozygote individuals were assumed to have a quantitative phenotype half-way between those of the homozygote individuals (i.e., the SNP genotypes were coded as 0, 1, and 2) as has been done elsewhere.(31;32) These regression models also included potential confounders, such as age, sex, proportion of African ancestry, proportion of Native American ancestry, duration of asthma, and study recruitment site. Among the GALA cohort we also stratified by Puerto Rican and Mexican ethnicity, as these groups have previously shown pharmacogenetic differences in drug responsiveness.(33;34)
Polymorphisms that demonstrated significant direct and interaction associations with bronchodilator responsiveness were reassessed in the SAGE and SAPPHIRE cohorts (this SAPPHIRE group included only individuals enrolled in the health plan and in whom we have previously demonstrated near complete capture of ICS use).(35) We again assessed for significant associations between both genotype and the genotype by ICS interaction term on bronchodilator responsiveness, adjusting for age, sex, proportion of African ancestry, proportion of Native American ancestry, and duration of asthma. Models for the SAGE cohort included an additional covariate for study recruitment site, whereas all SAPPHIRE patients were recruited from a single health system. Based on the plot of these relations by rs881152 genotype, we also assessed a dominant/recessive genetic model for this genotype (i.e., GG vs. AG and AA) in the GALA, SAGE, and SAPPHIRE cohorts.
We assessed the relationship between rs881152 genotype and ICS responsiveness in the subgroup of SAPPHIRE participants treated with 6 weeks of beclomethasone therapy. ICS responsiveness was defined as both the change in FEV1 between the baseline visit and after 6 weeks of ICS therapy and as the change in self-reported asthma control between baseline and follow-up (i.e., the change in ACT score after 6 weeks of treatment).
Pearson’s chi squared test was used to assess for deviations from Hardy-Weinberg equilibrium (HWE). Assessments of linkage disequilibrium between SNPs and haplotype block analyses were performed with Haploview 4.1, which is available at http://www.broad.mit.edu/mpg/haploview.(36) The outcome variables used in the regression analyses (i.e., bronchodilator response, percent change in FEV1, and change in ACT score) were assess for their distribution. All of these distributions satisfied the assumption of normality and were therefore used in the regression analyses without additional transformations. Analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC)(37) and R v2.9.0.(38)
RESULTS
Study populations
The baseline characteristics of individuals with asthma in the 4 cohorts, GALA, SAGE, SAPPHIRE, and SAPPHIRE treatment subgroup are shown in Table 1. Individuals in GALA and SAGE were younger when compared with the two SAPPHIRE groups with average ages of 15.2, 19.4, and 30.9, and 32.2 years, respectively. All participants in the GALA study were of Latino ethnicity, with 291 (45.0%) considering themselves Mexican and 355 (55.0%) considering themselves Puerto Rican. In contrast, all of the individuals from the SAGE and SAPPHIRE cohorts self-identified as non-Hispanic, African American. Likewise the average proportion of genetic African ancestry in the SAGE and the two SAPPHIRE groups was 79.2%, 77.8%, and 78.9% respectively. The distribution of genetic ancestry among the entire GALA cohort was 20.5% African, 48.8% European, and 30.7% Native American. However, this distribution differed between Mexican and Puerto Rican individuals in GALA as previously reported.(39) Among Mexican individuals, the average proportion of African, European, and Native American ancestry was 13.1%, 37.7%, and 49.2%, respectively. Among Puerto Rican individuals, the average proportion of African, European, and Native American ancestry was 26.6%, 57.9%, and 15.5%, respectively.
Table 1.
Baseline characteristics of study individuals with asthma grouped according to the source cohort
| Characteristic | Study Cohorts | |||
|---|---|---|---|---|
| GALA (n = 646) |
SAGE (n = 264) |
SAPPHIRE* (n = 430) |
SAPPHIRE group treated with 6 weeks of ICS therapy*† (n = 165) |
|
| Age (years) - mean ±SD |
15.2 ±7.7 | 19.4 ±9.3 | 30.9 ± 15.2 | 32.2 ± 13.5 |
| Female – no. (%) | 295 (45.7) | 157 (59.5) | 268 (62.3) | 95 (57.6) |
| Latino ethnicity – no. (%) |
646 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mexican | 291 (45.0) | -- | -- | -- |
| Puerto Rican | 355 (55.0) | -- | -- | -- |
| Self-reported race – no. (%) |
-- | -- | -- | -- |
| Black | NA | 264 (100.0) | 430 (100.0) | 165 (100.0) |
| White | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Proportion of ancestral admixture (%) – mean ± SD |
-- | -- | -- | -- |
| African | 20.5 ± 10.8 | 79.2 ± 10.6 | 77.8 ± 10.0 | 78.9 ± 10.7 |
| European | 48.8 ± 16.9 | 17.0 ± 9.5 | 18.7 ± 10.0 | 17.1 ± 10.3 |
| Native American | 30.7 ± 20.7 | 3.8 ± 4.0 | 3.5 ± 3.5 | 2.7 ± 3.4 |
| Duration of asthma (years) – mean ± SD |
8.8 ±5.9 | 12.2 ± 8.4 | 17.5 ± 12.4 | 20.7 ± 12.6 |
| ICS use at baseline – no. (%) |
369 (57.1%) | 144 (54.5%) | 45 (10.5%) | 90 (5.8%) |
| FEV1 percent predicted at baseline – mean ± SD |
85.9 ± 17.4 | 92.5 ± 16.4 | 89.5 ± 17.4 | 76.1 ± 17.4 |
| FEV1 percent reversibility at baseline – mean ± SD |
8.2 ± 12.8 | 9.2 ± 9.7 | 10.6 ± 11.9 | 21.4 ± 12.3 |
| Asthma Control Test score at baseline – mean ± SD |
-- | -- | 19.8 ± 4.3 | 17.8 ± 5.1 |
GALA denotes the Genetics of Asthma in Latino Americans study; SAGE, the Study of African Americans, Asthma, Genes & Environments; SAPPHIRE, the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity; SD, standard deviation; FEV1, forced expiratory volume in one second; ICS, inhaled corticosteroid; and NA, not available.
The SAPPHIRE cohort (n = 430) analytic set was restricted to those who members of the health plan and who reported African American race-ethnicity. This number included 48 (29%) of the 165 individuals who subsequently were enrolled in the 6-week ICS treatment trial.
The treatment group included African American patients with asthma who qualified for and consented to 6 weeks of beclomethasone dipropionate HFA treatment.
Screening for DUSP1 genotypes that modify the relationship between ICS use and bronchodilator responsiveness
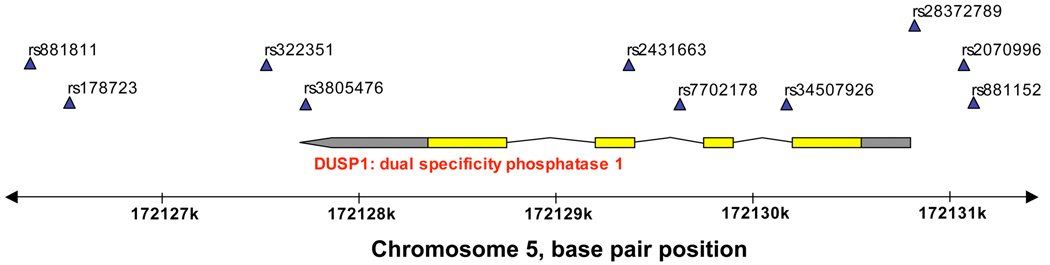
Given the ancestral diversity of the GALA participants, we first used this population to screen for associations between DUSP1 genotypes and bronchodilator responsiveness. In particular, we assessed for significant interactions between these genotypes and concurrent ICS use (i.e., to assess which DUSP1 polymorphisms appeared to affect the relationship between ICS use and bronchodilator response) while adjusting for age, sex, proportion of African ancestry, proportion of Native American ancestry, duration of asthma, and study recruitment site. The location of these polymorphisms on chromosome 5 and their relationship to DUSP1 are shown in Figure 1. Assessments of HWE are shown in Table E1 of the online supplement.
Figure 1. Chromosomal and DUSP1 position of single nucleotide polymorphisms assessed.
Polymorphism positions are indicated with the blue triangles. Exons are shown in yellow, untranslated (UTL) regions in gray, and introns as the connecting lines. The 3’ UTL is denoted by the pointed end. DUSP1 denotes dual specificity phosphatase-1.
Among the entire GALA cohort only one SNP, rs881152, was significantly associated with bronchodilator responsiveness, and none showed a significant genotype-ICS interaction (Table 2). However, after stratifying by ethnicity, we observed a number of significant associations among Puerto Rican patients with asthma, but none among the Mexican patients. In particular, SNPs rs881152, rs34507926, rs7702178, and rs3805476 showed both significant genotype and ICS interaction associations with bronchodilator responsiveness.
Table 2.
DUSP1 single nucleotide polymorphism that modify the relationship between ICS use and bronchodilator responsiveness among GALA cohort participants with asthma.*
| SNP number |
Polymorphism† | All patients (n = 646) |
Mexican American patients (n = 291) |
Puerto Rican patients (n = 355) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minor allele frequency |
Genotype associated P-value |
Genotype x ICS use interaction P-value |
Minor allele frequency |
Genotype associated P-value |
Genotype x ICS use interaction P-value |
Minor allele frequency |
Genotype associated P-value |
Genotype x ICS use interaction P-value |
||
| rs881152 | G/A | 0.14 | 0.021 | 0.100 | 0.14 | 0.744 | 0.886 | 0.14 | 0.004 | 0.026 |
| rs2070996 | C/T | 0.22 | 0.391 | 0.633 | 0.27 | 0.667 | 0.789 | 0.25 | 0.445 | 0.373 |
| rs28372789 | A/-‡ | 0.38 | 0.753 | 0.654 | 0.36 | 0.347 | 0.408 | 0.40 | 0.202 | 0.176 |
| rs34507926 | C/G | 0.11 | 0.090 | 0.594 | 0.15 | 0.307 | 0.102 | 0.08 | <0.001 | 0.005 |
| rs7702178 | T/C | 0.21 | 0.050 | 0.256 | 0.24 | 0.760 | 0.886 | 0.18 | 0.005 | 0.041 |
| rs2431663 | T/G | 0.07 | 0.142 | 0.579 | 0.04 | 0.980 | 0.438 | 0.10 | 0.109 | 0.274 |
| rs3805476 | G/A | 0.21 | 0.057 | 0.257 | 0.25 | 0.646 | 0.960 | 0.18 | 0.009 | 0.043 |
| rs322351 | C/T | 0.39 | 0.517 | 0.522 | 0.37 | 0.361 | 0.416 | 0.42 | 0.102 | 0.123 |
| Rs178723 | A/G | 0.44 | 0.886 | 0.755 | 0.45 | 0.865 | 0.880 | 0.43 | 0.775 | 0.973 |
| rs881811 | C/T | 0.14 | 0.635 | 0.830 | 0.16 | 0.257 | 0.103 | 0.13 | 0.043 | 0.115 |
DUSP1 denotes dual specificity phosphatase-1; GALA, Genetics of Asthma in Latino Americans; SNP, single nucleotide polymorphism; and ICS, inhaled corticosteroid
The linear regression equations used were of the following form: ΔFEV1 (% change post- vs. pre-bronchodilator) = α + β1(genotype) + β2(current ICS use) + β3(genotype x ICS use) + β4(age) + β5(sex) + β6(proportion of African ancestry) + β7(proportion of Native American ancestry) + β8(duration of asthma) + β9(study recruitment site) + error. An additive genetic model was used for each genotype. The P-values for the genotype coefficient (β1) and the genotype x ICS use interaction term (β3) are presented in the table.
Presented in the format of major allele/minor allele
Deletion polymorphism
Linkage disequilibrium for the DUSP1 SNPs and their estimated haploltype blocks in the GALA cohort are shown in Figure E1A–C of the online supplement. Among the Puerto Rican participants, SNPs rs34507926, rs7702178, and rs3805476, which demonstrated the aforementioned significant ICS interactions, were attributed to the same haplotype block (Figure E1C of the online supplement). Therefore, for replication in SAGE and SAPPHIRE we selected the most significant SNP, rs34507926, in this group for further analysis. We also selected SNP rs881152 for further analysis because it represented different haplotype blocks in Mexicans and Puerto Ricans.
Replication of DUSP1 genotypes that modify the relationship between ICS use and bronchodilator responsiveness
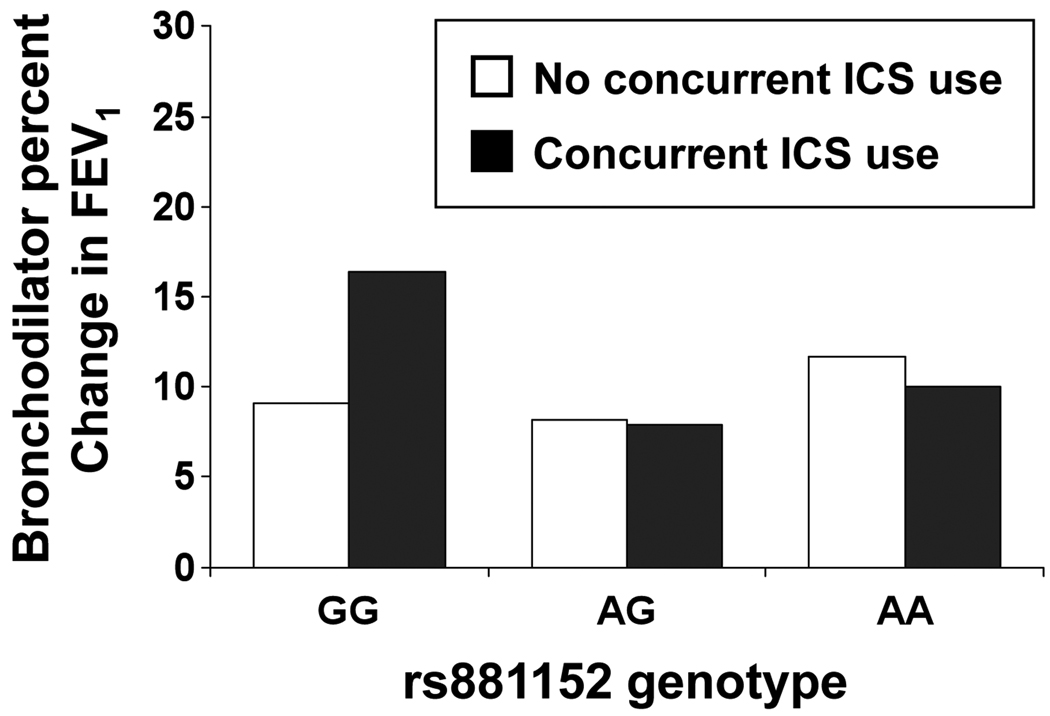
Replication of rs881152 and rs34507926 in the SAGE and SAPPHIRE cohorts is shown in Table 3. In both cohorts we observed a consistent relationship between ICS use and bronchodilator responsiveness. However, only in the SAPPHIRE cohort did genotype rs881152 appear to modify the relationship between ICS use and bronchodilator responsiveness (the magnitude and direction of interaction estimate was similar to that seen in GALA – data not shown). The negative interaction term in SAPPHIRE implied that among those on inhaled steroids the individuals with the GG genotype had greater bronchodilator response than those with the AA genotype (P=0.044 and P=0.043 in models 2 and 3, respectively). This is shown graphically in Figure 2. Figure 2 also suggests that the relationship may dominant/recessive with the GG genotype individuals showing the greatest bronchodilator response in the setting of ICS use. Reassessing the ICS by rs881152 genotype interaction with this relationship (i.e., GG vs. AG and AA) strengthen the interaction in all of the GALA participants (P=0.060), the subset of Puerto Rican participants in GALA (P=0.014), and in SAPPHIRE participants (P=0.039) (data not shown).
Table 3.
Replication of interactions between DUSP1 genotypes and inhaled corticosteroid use on baseline bronchodilator responsiveness among patients with asthma (SAGE [n = 264] and SAPPHIRE [n = 430] cohorts).*
| Covariates | SAGE cohort | SAPPHIRE cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ |
Model 1† | Model 2‡ | Model 3§ | |||||||
| Broncho dilator % change in FEV1 |
P-value | Broncho dilator % change in FEV1 |
P-value | Broncho dilator % change in FEV1 |
P-value | Broncho dilator % change in FEV1 |
P-value | Broncho dilator % change in FEV1 |
P-value | Broncho dilator % change in FEV1 |
P-value | |
| rs881152 genotype |
−0.08 | 0.936 | −0.56 | 0.722 | −1.28 | 0.432 | −0.46 | 0.635 | 0.19 | 0.851 | 0.31 | 0.770 |
| ICS use | 2.813 | 0.030 | 2.45 | 0.122 | 2.99 | 0.069 | 3.96 | 0.037 | 7.00 | 0.004 | 7.58 | 0.003 |
| rs881152 genotype by ICS interaction |
-- | -- | 0.847 | 0.685 | 1.29 | 0.554 | -- | -- | −6.55 | 0.044 | −6.72 | 0.043 |
| rs34507926 genotype |
1.49 | 0.535 | 3.34 | 0.366 | 7.97 | 0.042 | 3.50 | 0.118 | 3.45 | 0.138 | 4.29 | 0.077 |
| ICS use | 2.37 | 0.036 | 2.53 | 0.029 | 2.83 | 0.020 | 4.18 | 0.028 | 4.14 | 0.033 | 4.53 | 0.025 |
| rs34507926 genotype by ICS interaction |
-- | -- | −3.21 | 0.509 | −6.38 | 0.191 | -- | -- | 0.84 | 0.926 | 1.27 | 0.888 |
DUSP1 denotes dual specificity phosphatase-1; SAGE, Study of African Americans, Asthma, Genes & Environments; SAPPHIRE, Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity; FEV1, forced expiratory volume in one second; and ICS, inhaled corticosteroid
An additive genetic model was used for each genotype. The genotypes for rs881152, GG, AG, and AA, were coded as 0, 1, and 2, respectively. The genotypes for rs34507926, CC, CG, and GG, were coded as 0, 1, and 2, respectively. However, there were no individuals with the rs34507926 genotype GG in the SAPPHIRE analytic group, such that CC and CG were coded as 0 and 1, respectively for this analysis.
Model 1 follows the form ΔFEV1 (% change post- vs. pre-bronchodilator) = α + β1(genotype) + β2(current ICS use) + error
Model 2 follows the form ΔFEV1 (% change post- vs. pre-bronchodilator) = α + β1(genotype) + β2(current ICS use) + β3(genotype x ICS use) + error
Model 3 follows the form ΔFEV1 (% change post- vs. pre-bronchodilator) = α + β1(genotype) + β2(current ICS use) + β3(genotype x ICS use) + β4(age) + β5(sex) + β6(proportion of African ancestry) + β7(proportion of Native American ancestry) + β8(duration of asthma) + error. An additional term for study recruitment site was added for the SAGE cohort, but this term was not included for the SAPPHIRE cohort since all individuals in the latter were enrolled from a single, large health system. The parameter estimates for the first three terms are shown in the table.
Figure 2. Relationship between DUSP1 genotype rs881152 and bronchodilator responsiveness among individuals in the SAPPHIRE cohort, stratified by inhaled corticosteroid (ICS) use.
Bronchodilator responsiveness was measured as the percentage change in pre- and post-bronchodilator FEV1 at the initial screening visit for individuals in the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE). Concurrent ICS use at the time of this screening was determined from patient report and from pharmacy claims. The number of individuals with the rs881152 genotypes GG, AG, and AA were 239, 125, and 21, respectively among those without concurrent ICS use, and 26, 17, and 2, respectively among those currently using ICS medication.
In contrast, rs34507926 genotype was significantly associated with bronchodilator responsiveness in the SAGE cohort (P=0.042), but not in the SAPPHIRE cohort (P=0.143) after adjusting for potential confounders (Table 3). The interaction term estimate for rs34507926 genotype and ICS use did not reach statistical significance in any of the models.
Assessing the relationship between DUSP1 genotype rs881152 and steroid response
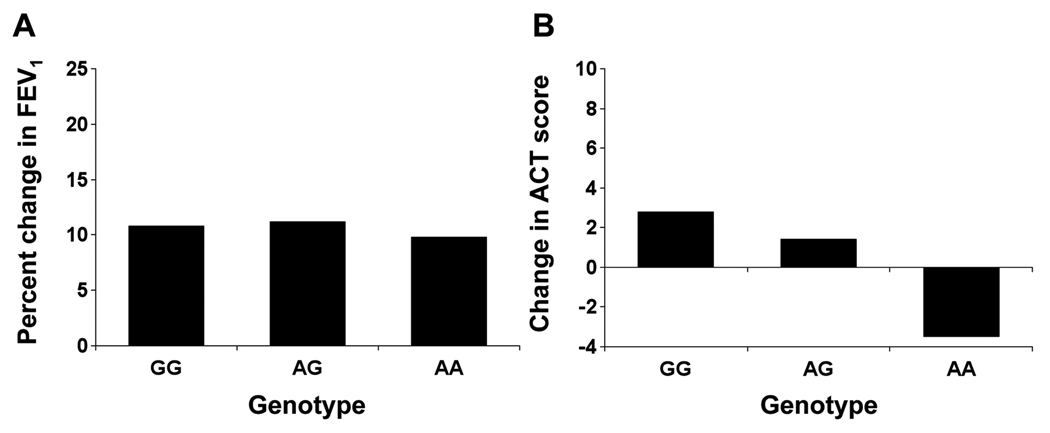
Since the aforementioned significant interactions suggested that these DUSP1 polymorphisms may affect response to ICS medication, we specifically assessed this relationship in a separate subgroup of SAPPHIRE participants treated for 6 weeks with beclomethasone dipropionate HFA. Here the outcome was the percent improvement in pre-bronchodilator FEV1 over the 6-week treatment period and the change in self-reported asthma control. The rs881152 genotype was not associated with change in FEV1 over 6 weeks of ICS treatment (Table 4 and Figure 3A). However, DUSP1 genotype at rs881152 was significantly associated a change in self-reported asthma control over the 6-week ICS treatment period. Here asthma control was measured using the Asthma Control Test, and the change in control, as the absolute change in this measure. As shown in Figure 3B, the mean change in ACT by rs881152 genotype was 2.81 (± 6.22 SD) for GG, 1.38 (± 7.36 SD) for AG, and −3.5 (±9.13 SD) for AA. The trend across rs881152 genotypes was statistical significance before and after adjusting for potential confounders (P-value = 0.011 and 0.013, respectively) (Table 4).
Table 4.
Relationship between DUSP1 rs881152 genotype and both change in pulmonary function and self-reported asthma control following 6 weeks of inhaled corticosteroid use among patients with asthma (SAPPHIRE treatment group; n = 165).*
| Variable | % change in FEV1 from baseline† | Change in Asthma Control Test score from baseline‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted mean ± SD |
P- value |
Adjusted mean ± SD§ |
P- value |
Unadjusted mean ± SD |
P- value |
Adjusted mean ± SD§ |
P- value |
|
| rs881152 genotype ‖ | −0.05 ± 2.06 | 0.981 | −0.42 ± 1.78 | 0.812 | −2.28 ± 0.89 | 0.011 | −2.21 ±0.88 | 0.013 |
DUSP1 denotes dual specificity phosphatase-1; SAPPHIRE, Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity; FEV1, forced expiratory volume in one second; and SD, standard deviation.
The SAPPHIRE subgroup are those patients with asthma who were eligible and agreeable to 6-weeks of treatment with inhaled beclomethasone dipropionate HFA. An additive genetic model was used for the rs881152 genotype whereby GG=0, AG=1, and AA=2.
The percent change in FEV1 was measured as a difference between the pre-bronchodilator FEV1 measurements at the initial visit and the 6-week follow-up visit using the following equation: ((FEV1 (6-wk follow-up) - FEV1 (initial))/ FEV1 (initial)) x 100%.
The Asthma Control Test has a score range of 5–25 with higher scores signifying better control. Therefore, a negative coefficient suggests a worsening of control between baseline and the 6-week follow-up exam
Adjusted for patient age, sex, duration of asthma, and baseline percent of predicted FEV1.
Figure 3. Relationship between DUSP1 genotype rs881152 and inhaled corticosteroid (ICS) responsiveness among the subgroup of individuals treated as part of SAPPHIRE.
Inhaled corticosteroid responsiveness was measured both as the percentage change in pre-bronchodilator FEV1 (A) and the change in asthma control (B) between the initial screening visit and following 6 weeks of ICS treatment. The change in asthma control was measured as the absolute difference in Asthma Control Test (ACT) score at these two time points. The number of individuals with rs881152 genotypes GG, AG, and AA were 88, 63, and 10, respectively, for the analysis looking at percentage change in pre-bronchodilator FEV1. The number of individuals with rs881152 genotypes GG, AG, and AA were 86, 63, and 10, respectively, for the analysis looking at the change in ACT score. As shown in Table 4, the P-values for the association between rs881152 genotype (i.e., coded GG=0, AG=1, and AA=2) and both FEV1 change (A) and ACT score change (B) were 0.981 and 0.011, respectively. DUSP1 denotes dual specificity phosphatase-1.
We also performed the following post hoc analyses. First, the relationship between asthma control and a dominant/recessive genetic model for rs881152 genotype (i.e., GG vs. AG and AA) produced results of borderline statistical significance (P=0.060 and P=0.085 for the unadjusted and adjusted models, respectively – data not shown). Second, there was no relationship between rs881152 genotype and change in bronchodilator responsiveness over the 6-week ICS treatment period (data not shown).
DISCUSSION
Here we report a relationship between polymorphisms in DUSP1 and response to inhaled corticosteroids. To identify risk alleles we took a staged approach, first screening for polymorphisms that appeared to affect the cross-sectional relationship between ICS use and bronchodilator response, then validating in additional cohorts, followed by confirmation in a prospective study of ICS responsiveness.
The rationale for this approach is that steroid resistant asthmatics may experience greater proteolytic and inflammatory activity leading to airway remodeling and decreased bronchodilator response.(40) Furthermore an increased inflammatory milieu, as found in steroid resistant asthma, may reduce airway smooth muscle relaxation by inhibiting cAMP production or by directly inhibiting β2-receptor mediated gene expression.(41) Corticosteroids can enhance β2-agonist induced cAMP production(29) and have been shown to prevent inflammatory cytokine mediated uncoupling of β2-receptor activation of its downstream targets.(30) Together, these observations provide a potential mechanism whereby polymorphisms which appear to modify the relationship between ICS use and bronchodilator response may also influence steroid responsiveness as measured more directly (e.g., as an improvement in pulmonary function or self-reported asthma control). As such, our novel approach may prove useful to screen for other polymorphisms associated with steroid response.
Corticosteroids induce the expression and prevent the proteolytic degradation of DUSP1, a key inhibitor of the MAPK cascade.(42;43) For example, Kang et al found that dexamethasone is able to induce DUSP1 and subsequently decrease JNK and p38 MAPK activity in human airway smooth muscle.(11) Conversely, in mouse models, corticosteroid inhibition of JNK and p38 MAPK is impaired in DUSP1−/− macrophages(8) and these cells produce excessive amounts of inflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6).(44) Bhavsar and colleagues found increased activation of p38 MAPK in airway macrophages from severe asthmatics when compared to non-severe asthmatics, and the severe asthmatic group also exhibited impaired corticosteroid induction of DUSP1.(45) Therefore, although not previously examined, our observation of a relationship between DUSP1 polymorphisms and ICS-related improvement in self-reported asthma control is not altogether surprising.
Nevertheless, we do not currently know whether the polymorphisms identified here affect DUSP1 function or if they are just in linkage disequilibrium with genetic variants that do. In particular, rs881152, which was most significantly associated with ICS response, is located in the promoter region of DUSP1 (i.e., 357 base pairs proximal to the transcription start site). This was the most 5’ SNP that we investigated in this study. However, a recent study by Johansson-Hague et al. suggests that the promoter site involved in glucocorticoid-induced gene expression may be further upstream,(46) and Tchen and colleagues have identified glucocorticoid-responsive regions approximately 1.3 and 4.6 kilobases upstream of the DUSP1 transcription start site.(47) Therefore, it is possible that polymorphisms upstream of those identified here may have even stronger associations with clinical ICS response than we demonstrated.
This study must be interpreted in light of its other limitations, as well. First, the observed associations were not consistent across all of the race-ethnic groups studied. For example, the gene-drug interactions observed in Puerto Rican patients with asthma were not observed in Mexican individuals. However, we have previously observed different pharmacologic and pharmacogenetic interactions between these two ethnic groups.(16;33;48) Although we adjusted for individual genetic ancestry in our models, it is still possible that the between group differences that we observed were due to additional gene-gene or gene-environment interactions that were specific to these groups or their areas of residence. In addition, different patterns of linkage disequilibrium between the polymorphisms examined here and the causal variants could have resulted in between group differences. Moreover, concurrent ICS use was determined by patient report in the GALA and SAGE cohorts, but was assessed by actual pharmacy fills in SAPPHIRE. This may account in part for the differences in estimated ICS use at baseline between the GALA, SAGE, and SAPPHIRE cohorts, and it may also explain some of the between group differences in pharmacogenetic interactions.
We assessed genotype by ICS interactions in the GALA cohort without accounting for multiple comparisons. We felt that this was a reasonable approach given the underlying linkage disequilibrium between polymorphisms in DUSP1 (Figure E1 of the online repository). While the appropriate significance threshold for interactive effects is less well defined, a principal component approach can used to account for correlation between SNPs.(49) Applying this approach suggests that the threshold for genotype significance in GALA should be downwardly adjusted by a factor of 5 (i.e., P-value<0.01) (data not shown). Therefore, the genotype effects for the SNPs promoted for replication in SAGE and SAPPHIRE still met this threshold. Moreover, the significant interaction for rs881152 was replicated in SAPPHIRE, providing further evidence for this relationship.
It is also uncertain which genetic model is most appropriate for SNP rs881152 (i.e., co-dominate vs. dominant/recessive), as both appeared plausible in our analyses. This will require further functional analysis of this genetic variant. Lastly, among participants in the treatment trial we noted a prospective difference in self-reported asthma control by rs881152 genotype, which was not accompanied by a commensurate change in pulmonary function. This unlinking of symptoms and physiologic measures may be due in part to the short-treatment period; however, an even longer prospective study of ICS use noted symptomatic improvements without significant changes in FEV1.(50)
In conclusion, we demonstrate here that a DUSP1 polymorphism that appears to modify the relationship between steroid use and bronchodilator response is also associated with symptomatic response to ICS therapy (i.e., a change in ACT score). Our findings suggest that this polymorphism (or one in linkage disequilibrium) may be useful in identifying asthma patients more likely to respond to ICS controller treatment. However, the between group differences that we observed suggest that additional genetic and environmental effects may exist, which deserve further study.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the families and the patients for their participation. The authors would also like to thank the numerous health care providers and community clinics for their support and participation in the GALA, SAGE and SAPPHIRE studies. The authors would like to especially thank Jeffrey M. Drazen, M.D., Scott Weiss, M.D., Ed Silverman, M.D., Ph.D., Homer A. Boushey, M.D., and Jean G. Ford, M.D., for all of their effort toward the creation of the GALA study.
This work was supported by grants from the American Asthma Foundation Strategic Program for Asthma Research, the National Institutes of Health (AI079139, AI061774, HL079055, DK064695), and the Fund for Henry Ford Hospital to LKW and National Institutes of Health (HL078885, AI077439, HL088133, U01 GM61390, ES015794) and the Flight Attendant Medical Research Institute (FAMRI) to EGB.
Abbreviations
- ACT
Asthma Control Test
- DUSP1
Dual specificity phosphatase-1
- FEV1
Forced expiratory volume at one second
- GALA
Genetics of Asthma in Latino Americans
- ICS
Inhaled corticosteroids
- SAGE
Study of African Americans, Asthma, Genes & Environments
- SAPPHIRE
Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity
- SNP
Single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 3.Sobande PO, Kercsmar CM. Inhaled corticosteroids in asthma management. Respir Care. 2008;53(5):625–633. [PubMed] [Google Scholar]
- 4.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109(3):410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 5.Abraham SM, Clark AR. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochem Soc Trans. 2006;34(Pt 6):1018–1023. doi: 10.1042/BST0341018. [DOI] [PubMed] [Google Scholar]
- 6.Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J Endocrinol. 2003;178(1):5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- 7.Clark AR, Lasa M. Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr Opin Pharmacol. 2003;3(4):404–411. doi: 10.1016/s1471-4892(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 8.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203(8):1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhavsar P, Ahmad T, Adcock IM. The role of histone deacetylases in asthma and allergic diseases. J Allergy Clin Immunol. 2008;121(3):580–584. doi: 10.1016/j.jaci.2007.12.1156. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 11.Kang BN, Jude JA, Panettieri RA, Jr, Walseth TF, Kannan MS. Glucocorticoid regulation of CD38 expression in human airway smooth muscle cells: role of dual specificity phosphatase 1. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L186–L193. doi: 10.1152/ajplung.00352.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelaia G, Cuda G, Vatrella A, Gallelli L, Caraglia M, Marra M, et al. Mitogen-activated protein kinases and asthma. J Cell Physiol. 2005;202(3):642–653. doi: 10.1002/jcp.20169. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280(9):8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- 14.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119(1):73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai HJ, Kho JY, Shaikh N, Choudhry S, Naqvi M, Navarro D, et al. Admixture-matched case-control study: a practical approach for genetic association studies in admixed populations. Hum Genet. 2006;118(5):626–639. doi: 10.1007/s00439-005-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naqvi M, Tcheurekdjian H, DeBoard JA, Williams LK, Navarro D, Castro RA, et al. Inhaled corticosteroids and augmented bronchodilator responsiveness in Latino and African American asthmatic patients. Ann Allergy Asthma Immunol. 2008;100(6):551–557. doi: 10.1016/S1081-1206(10)60055-5. [DOI] [PubMed] [Google Scholar]
- 17.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 23.National Heart LaBIN. Global Initiative for Asthma: Global Stategy for Asthma Management and Prevention. 2002 02-3659. [Google Scholar]
- 24.Yaeger R, vila-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, et al. Comparing genetic ancestry and self-described race in african americans born in the United States and in Africa. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu B, Liu N, Zhao H. PSMIX: an R package for population structure inference via maximum likelihood method. BMC Bioinformatics. 2006;7:317. doi: 10.1186/1471-2105-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005;28(4):289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]
- 28.Yang JJ, Burchard EG, Choudhry S, Johnson CC, Ownby DR, Favro D, et al. Differences in allergic sensitization by self-reported race and genetic ancestry. J Allergy Clin Immunol. 2008;122(4):820–827. doi: 10.1016/j.jaci.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aksoy MO, Mardini IA, Yang Y, Bin W, Zhou S, Kelsen SG. Glucocorticoid effects on the beta-adrenergic receptor-adenylyl cyclase system of human airway epithelium. J Allergy Clin Immunol. 2002;109(3):491–497. doi: 10.1067/mai.2002.122154. [DOI] [PubMed] [Google Scholar]
- 30.Mak JC, Hisada T, Salmon M, Barnes PJ, Chung KF. Glucocorticoids reverse IL-1beta-induced impairment of beta-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br J Pharmacol. 2002;135(4):987–996. doi: 10.1038/sj.bjp.0704545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhry S, Ung N, Avila PC, Ziv E, Nazario S, Casal J, et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am J Respir Crit Care Med. 2005;171(6):563–570. doi: 10.1164/rccm.200409-1286OC. [DOI] [PubMed] [Google Scholar]
- 34.Naqvi M, Thyne S, Choudhry S, Tsai HJ, Navarro D, Castro RA, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma. 2007;44(8):639–648. doi: 10.1080/02770900701554441. [DOI] [PubMed] [Google Scholar]
- 35.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 37.SAS Institute Inc. SAS/STAT Users Guide. Cary, NC: SAS Institute Inc; 2004. Version 9.1 ed. [Google Scholar]
- 38.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 39.Choudhry S, Coyle NE, Tang H, Salari K, Lind D, Clark SL, et al. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006;118(5):652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 40.Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol. 2007;120(5):1065–1072. doi: 10.1016/j.jaci.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shore SA, Moore PE. Regulation of beta-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol. 2003;137(2–3):179–195. doi: 10.1016/s1569-9048(03)00146-0. [DOI] [PubMed] [Google Scholar]
- 42.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22(22):7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20(24):7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119(Pt 22):4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 45.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63(9):784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 46.Johansson-Haque K, Palanichamy E, Okret S. Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. J Mol Endocrinol. 2008;41(4):239–249. doi: 10.1677/JME-08-0015. [DOI] [PubMed] [Google Scholar]
- 47.Tchen CR, Martins JR, Paktiawal N, Perelli R, Saklatvala J, Clark AR. Glucocorticoid regulation of mouse and human dual specificity phosphatase 1 (DUSP1) genes: unusual cis-acting elements and unexpected evolutionary divergence. J Biol Chem. 2010;285(4):2642–2652. doi: 10.1074/jbc.M109.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tcheurekdjian H, Thyne SM, Williams LK, Via M, Rodriguez-Santana JR, Rodriguez-Cintron W, et al. Augmentation of bronchodilator responsiveness by leukotriene modifiers in Puerto Rican and Mexican children. Ann Allergy Asthma Immunol. 2009;102(6):510–517. doi: 10.1016/S1081-1206(10)60126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32(4):361–369. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- 50.Long-578 term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]