Abstract
Acid-sensing ion channels (ASICs) respond to acidosis that normally occurs after inflammation. We examined the expression of ASIC1, ASIC2, and ASIC3 mRNAs in lumbar DRG neurons before and 24h after carrageenan-induced muscle inflammation. Muscle inflammation causes bilateral increases of ASIC2 and ASIC3, but not ASIC1 (neither ASIC1a nor ASIC1b) mRNA, suggesting differential regulation of ASIC1 versus ASIC2 and ASIC3 mRNA. Similar mRNA increases were observed following inflammation in knockout mice: ASIC2 mRNA increases in ASIC3−/− mice; ASIC2 and ASIC3 mRNAs increase in ASIC1−/− mice. Prior behavioral studies in ASIC3−/− mice showed deficits in secondary hyperalgesia (increased response to noxious stimuli outside the site of injury), but not primary hyperalgesia (increased response to noxious stimuli at the site of injury). In this study, we show that ASIC1−/− mice surprisingly do not develop primary muscle hyperalgesia, but develop secondary paw hyperalgesia. In contrast and as expected, ASIC3−/− mice develop primary muscle hyperalgesia, but do not develop secondary paw hyperalgesia. The pharmacological utility of the non-selective ASIC inhibitor A-317567, given locally, was tested. A-317567 reverses both primary and the secondary hyperalgesia induced by carrageenan muscle inflammation. Thus, peripherally located ASIC1 and ASIC3 play different roles in the development of hyperalgesia after muscle inflammation.
Keywords: ASIC, muscle inflammation, hyperalgesia, DRG neurons, carrageenan, A-317567
Introduction
Acid-sensing ion channels (ASICs) are proton-gated voltage-independent ion channels located on neurons in the peripheral and central nervous systems. ASICs belong to the epithelial sodium channel/degenerin (ENaC/DEG) family of amiloride-sensitive transmembrane ion channel proteins (see (37, 41, 78)). Four genes within mammalian genomes encode seven subunits to date – ASIC1a, ASIC1b, ASIC1b2, ASIC2a, ASIC2b, ASIC3 and ASIC4.(1, 15, 26, 27, 40, 54, 69, 73) Homomeric and heteromeric ASIC subunits combine to form trimeric ASICs,(13, 34) which depending on the subunit composition in the DRG display differences in pH sensitivity, current kinetics and ion selectivity.(11, 20, 29, 53) ASICs respond to acidosis, play a significant role in nociceptive processing of hyperalgesia both peripherally and centrally.(1, 3, 6, 8–10, 15, 16, 21, 27, 28, 37, 40–42, 54, 65, 66, 68, 70, 72–74, 76)
Despite a number of well-designed and controlled studies, the role of ASICs in nociception has led to conflicting results. Some prior behavioral studies generated from ASIC knockout or dominant negative mutant mice show no differences or increases in hyperalgesia of the paw after intraplantar inflammation.(16, 45, 56, 68) Previous studies show increases in ASIC1, ASIC2, and ASIC3 mRNAs after cutaneous paw inflammation (30, 71). Our laboratory, however, has consistently shown that ASIC3 plays a critical role in the development of inflammatory and non-inflammatory hyperalgesia induced by deep tissue insult,(30, 65, 66) which Yen et al. also reported recently.(81) We have suggested that these differences could be related to differences between cutaneous and deep tissue injury.
However, differences between pain at the site of injury, termed primary hyperalgesia, and pain outside the site of injury, termed secondary hyperalgesia, could also explain these differences. Animal models of cutaneous inflammation typically measure primary hyperalgesia at the paw; while those of deep tissue insult have typically measured secondary hyperalgesia at the paw. One purpose of this study was to determine if there were differences in expression of ASICs with deep tissue inflammation, and behavioral deficits in ASIC1 and ASIC3 knockout mice with regard to primary and secondary hyperalgesia after muscle insult.
Animal models of pain have not sorted out whether ASICs play a peripheral or central role in the continued manifestation of the hyperalgesia after the inflammation is established. Previous literature suggests that peripherally located ASIC3 is important at the site of muscle inflammation since re-expression of ASIC3 into muscle of ASIC3−/− mice prior to induction of inflammation restores the hyperalgesia.(66) Co-administration of the ASIC3 antagonist, APETx2 with CFA into the paw prevents the development of cutaneous hyperalgesia 4h later in rats, whereas blockade of peripheral ASIC1 with Psalmotoxin 1(PcTx1) at the time of intraplantar CFA injection has no effect on cutaneous hyperalgesia.(21) Intrathecal or intracerebroventricular blockade of ASIC1 induces analgesia and reverses hyperalgesia after nerve injury.(21, 44) Systemic application of the non-selective antagonists A-317567 and amiloride reduce primary hyperalgesia induced by CFA and skin incision.(24) In this study, we therefore used a non-selective inhibitor of ASICs, A-317567, injected directly into the muscle 24h after muscle inflammation to test if activation of peripheral ASICs were important in maintaining hyperalgesia. We further tested the effectiveness of this drug in ASIC1−/− and ASIC3−/− mice to examine whether ASIC1 or ASIC3 were critical for the behavioral effects.
We therefore hypothesized that there would be differential expression of ASICs after inflammation, and that ASIC3, but not ASIC1, would mediate the secondary hyperalgesia associated with muscle inflammation. Since there were behavioral deficits in both ASIC1−/− and ASIC3−/− mice, we tested the ability of A-317567 to reverse both primary and secondary hyperalgesia in WT and in ASIC3−/− and ASIC1−/− mice with muscle inflammation.
Methods
Animal Care and Use
All animal experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines. Congenic ASIC1−/− and ASIC3−/− on a C57Bl/6 background and congenic C57Bl/6 (WT) mice were bred at the University of Iowa Animal Care Facility.(56, 76) New breeding pairs of mice are started every six months. The congenic ASIC1−/− is a knockout of ASIC1a, and not ASIC1b.(76) Male and female mice, 6–10 weeks of age, WT (n=64), ASIC3−/− (n=32), ASIC1−/− (n=24) were used in these studies.
Induction of Inflammation
Mice were briefly anesthetized with 4% isoflurane and one gastrocnemius muscle (left) was injected with 20 µl of 3% carrageenan dissolved in sterile isotonic saline, which has a slightly acidic pH (6.0), typical of commercially available saline preparations. The pH of the final 3% carrageenan solution was 6.0. Behavior measurements were made before and 24 hours after carrageenan injection, and after administration of drug, A-317567.
A-317567
Mice were given one dose of 0.025 µmol A-317567 (C-{6-[2-(1-isopropyl-2-methyl-1,2,3,4-tetrahydro-isoquinolin-7-yl)-cyclopropyl]-naphthalen-2-yl}-methanediamine) (10µl) injected into the left gastrocnemius muscle 24 hours after induction of inflammation. As a control for systemic effects one group of WT mice (n=8) received 0.025 µmol A-317567 (10µl) in the contralateral muscle. Isotonic saline (10 µl) was used as the vehicle control in separate mice (n=45). Animals were tested 15 minutes after administration of the drug or vehicle.
Behavioral Testing
Mice were acclimated for 2 days before testing for muscle sensitivity and cutaneous mechanical sensitivity, as described previously.(30) Muscle sensitivity was tested by squeezing the gastrocnemius muscle of the mice with a calibrated pair of tweezers until the mouse withdrew from the stimulus. The force at which the mouse withdrew was measured in mN. A decrease in threshold was interpreted as primary muscle hyperalgesia. Sensitivity in both the ipsilateral and contralateral muscles was measured. Muscle sensitivity was tested as follows: before carrageenan injection of the muscle, 24h after the injection, and 15 min after A-317567 injection. Baseline responses for muscle sensitivity for WT, ASIC1−/−, and ASIC3−/− mice did not differ significantly, and baseline responses were similar for left and right sides. Cutaneous mechanical sensitivity was tested bilaterally by assessing the number of responses to repeated application of a 0.4 mN von Frey filament to the plantar surface of the paw. The number of withdrawals out of 5 was assessed in 10 trials and an average of all 10 trials was determined for each time period. A significant increase in the number of responses was interpreted as secondary mechanical hyperalgesia. Cutaneous mechanical sensitivity was tested as follows: before carrageenan injection of the muscle, 24h after the injection, 15 min after A-317567 injection. Testing was blinded for genotype and drug status of the animals. WT, ASIC1−/−, and ASIC3−/− mice did not differ in baseline cutaneous mechanical sensitivity responses.
Quantitative RT-PCR
RNA was purified from ipsilateral and contralateral lumbar (L4, L5, L6) DRGs using the Trizol reagent (Invitrogen, Carlsbad, CA). DRGs were collected 24h after carrageenan injection or from control mice. All of the mice were subjected to behavioral testing for muscle and cutaneous mechanical sensitivity 1–2 hours prior to collecting the DRGs. RNA concentration and purity was assessed by spectrophotometric measurement at 260 and 280nm. First strand cDNA was synthesized from 0.2-1ug of each RNA sample using Superscript III or VILO reverse transcriptase (Invitrogen, Carlsbad, CA). Taqman PCR was carried out using an ABI prism 7900 sequence detector (Applied Biosystems, Inc., Foster City, CA) on diluted cDNA samples (University of Iowa, DNA Facility, Iowa City, IA). Reactions were carried out for 40 cycles in triplicate. ASIC1 (ASIC1a and ASIC1b) (Mm01305997_m1), ASIC2 (Mm00475691_m1), ASIC3 (Mm00805460_m1) and the mouse control assay for glyceraldehyde-3-P-dehydrogenase (GAPDH) were obtained from Applied Biosystems, Inc. (Foster City, CA). All of the assays would generate a single PCR product which spans the boundary of two exons, thus reducing the possibility of genomic DNA contamination in the results. Control samples without reverse transcriptase did not produce any background PCR products. Quantitative RT-PCR data were normalized with GAPDH mRNA levels and relative amounts of mRNA were determined by using the comparative cycle thresholds (CT).
Statistical Analysis
Data are represented as the mean ± S.E.M. Behavioral data were analyzed with a repeated measures ANOVA followed by post-hoc testing with a Tukey’s test. Differences were considered significant at p< 0.05. Quantitative RT-PCR was analyzed with a two-way ANOVA for group (WT, WT-inflamed, ASIC1−/−, ASIC3−/−) and side (ipsilateral vs. contralateral).
Results
Increased expression of ASIC2 and ASIC3 but not ASIC1 mRNA in DRG after muscle inflammation
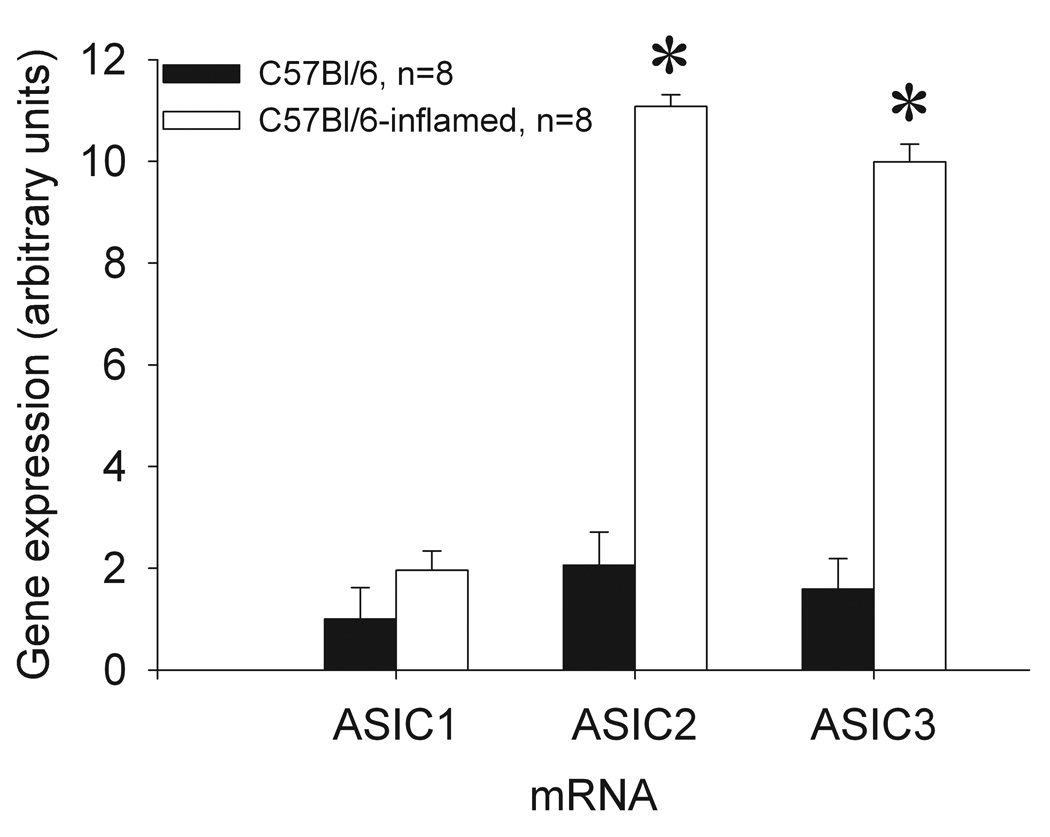
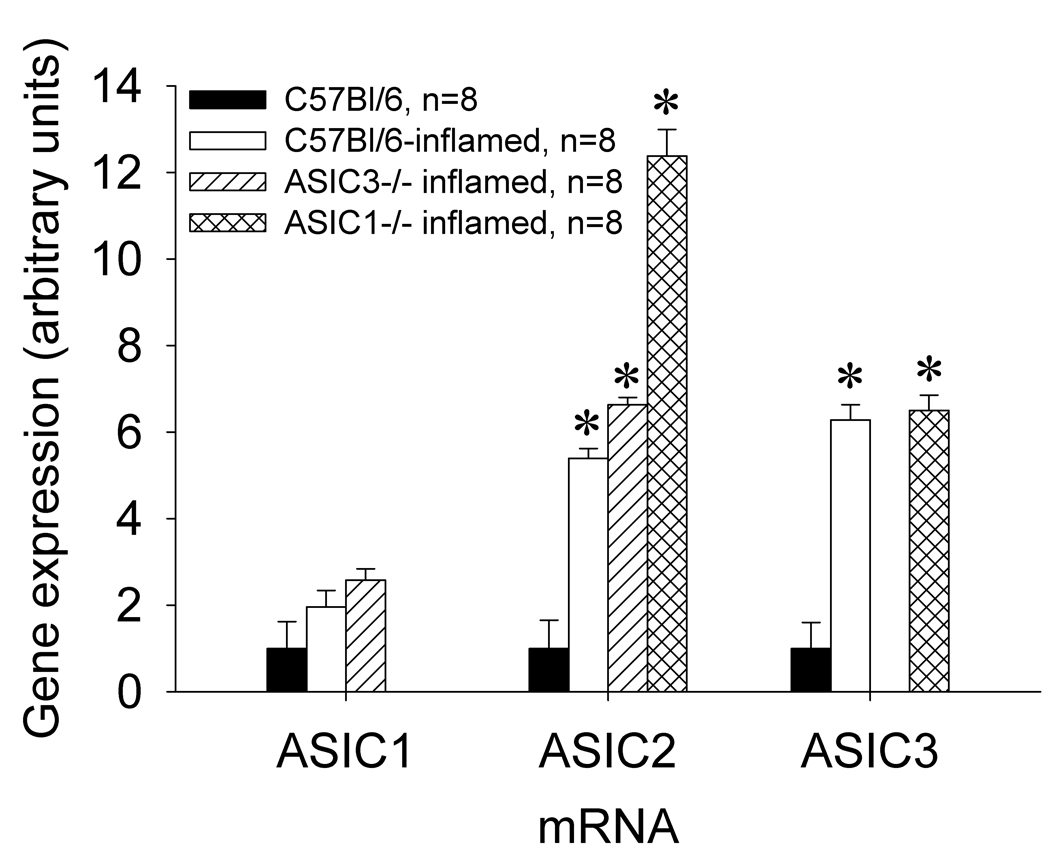
There is a significant induction (approximately 10-fold increases) of ASIC2 and ASIC3, but not ASIC1 (ASIC1a and ASIC1b) mRNA in the lumbar DRG innervating muscle 24h following carrageenan-induced muscle inflammation when compared to DRG from mice without muscle inflammation (Figure 1). Statistical analysis showed a significant effect for inflammation for ASIC2 (F1,40=13.7, p=0.001) and ASIC3 (F1,32=13.7, p=0.001) but not ASIC1. However there was no effect for side (ipsilateral or contralateral) indicating a bilateral increase in mRNA in the DRG after unilateral muscle inflammation. Since there was not a significant difference between the results for the ipsilateral and contralateral mRNAs, the data for the two sides were pooled graphically. Twenty-four hours after carrageenan-induced muscle inflammation, ASIC3−/− mice showed increases in ASIC2 mRNA (6-fold) and ASIC1−/− mice showed increases in ASIC2 and ASIC3 mRNAs, at 12- and 6-fold, respectively; these increases were not significantly different from WT mice (Figure 2). Thus, ASIC2 and ASIC3 increased with inflammation and ASIC1 did not, suggesting a differential regulation of ASICs.
Figure 1.
Comparative qRT-PCR analysis for ASIC1, ASIC2, and ASIC3 mRNAs of L4, L5, and L6 DRGs from C57Bl/6 versus C57Bl/6-inflamed mice. There are bilateral increases in ASIC2 and ASIC3, and not ASIC1 mRNA levels, 24 hours after muscle inflammation. No differences were measured between the ipsilateral and contralateral mRNAs. (* = significantly greater than uninflamed mice, P<0.05).
Figure 2.
Comparative qRT-PCR analysis for ASIC1, ASIC2, and ASIC3 mRNAs in L4, L5, and L6 DRGs from C57Bl/6, C57Bl/6-inflamed, ASIC3−/− inflamed, and ASIC1−/− inflamed mice. ASIC2 mRNA was increased in C57Bl/6-inflamed, ASIC3−/− inflamed and ASIC1−/− inflamed mice. ASIC3 mRNA increased in C57Bl/6-inflamed and in ASIC1−/− inflamed mice. ASIC1 mRNA levels were not significantly different between the groups. (* = significantly increased from C57Bl/6 uninflamed mice, P<0.05).
Hyperalgesia of the muscle does not develop in ASIC1−/− mice
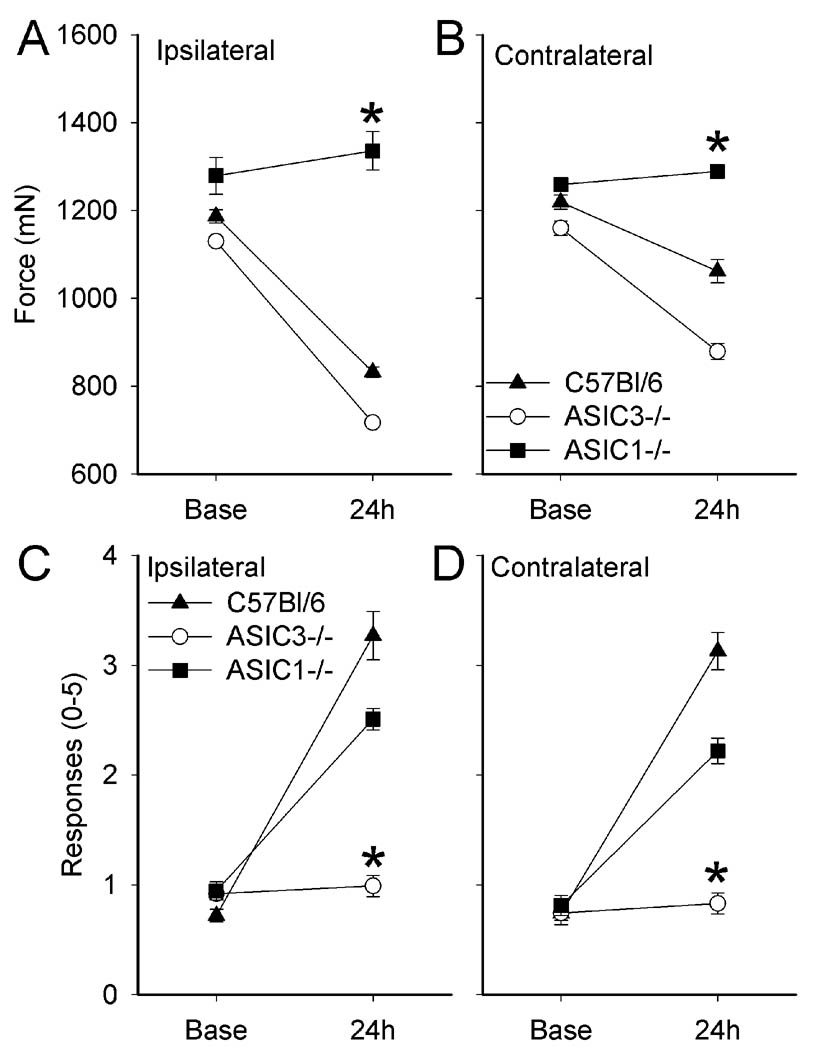
C57Bl/6 (WT) mice showed a significant bilateral decrease in the withdrawal threshold of the muscle 24h after carrageenan-induced muscle inflammation; the decrease was greater for the ipsilateral inflamed muscle compared to the contralateral muscle (p<0.05) (Figure 3A and B). A similar decrease in withdrawal threshold of the muscle was observed in ASIC3−/− mice 24h after muscle inflammation (Figure 3A and B). Muscle withdrawal thresholds in ASIC1−/− mice, however, were unchanged 24h after carrageenan-induced muscle inflammation, on both the ipsilateral or contralateral sides, and the withdrawal thresholds were significantly greater than WT mice (P<0.05).
Figure 3.
A and B) Muscle hyperalgesia (tweezers) develops similarly in both C57Bl/6 (WT) and ASIC3−/− mice after muscle inflammation on both ipsilateral and contralateral sides. ASIC1−/− mice do not develop muscle hyperalgesia after muscle inflammation. C and D) Cutaneous mechanical hyperalgesia (von Frey) develops similarly in both C57Bl/6 (WT) and ASIC1−/− mice on both sides. Cutaneous hyperalgesia does not develop in ASIC3−/− mice after muscle inflammation. (* = P<0.05).
Hyperalgesia of the paw does not develop in ASIC3−/− mice
C57Bl/6 (WT) mice showed a significant bilateral increase in the number of withdrawals to a 0.4 mN force applied to the paw 24h after carrageenan-induced muscle inflammation (Figure 3C and D). There was a similar increase in the number of withdrawals for both the ipsilateral and contralateral paws of ASIC1−/− mice 24h after carrageenan-induced muscle inflammation (Figure 3C and D). However, ASIC3−/− mice showed a significant reduction (p<0.05, compared to C57Bl/6 (WT) mice) in the number of withdrawals on both the ipsilateral and contralateral sides 24h after muscle inflammation (Figure 3C and D) in agreement with a prior study.(66)
A-317567 reverses muscle and paw hyperalgesia
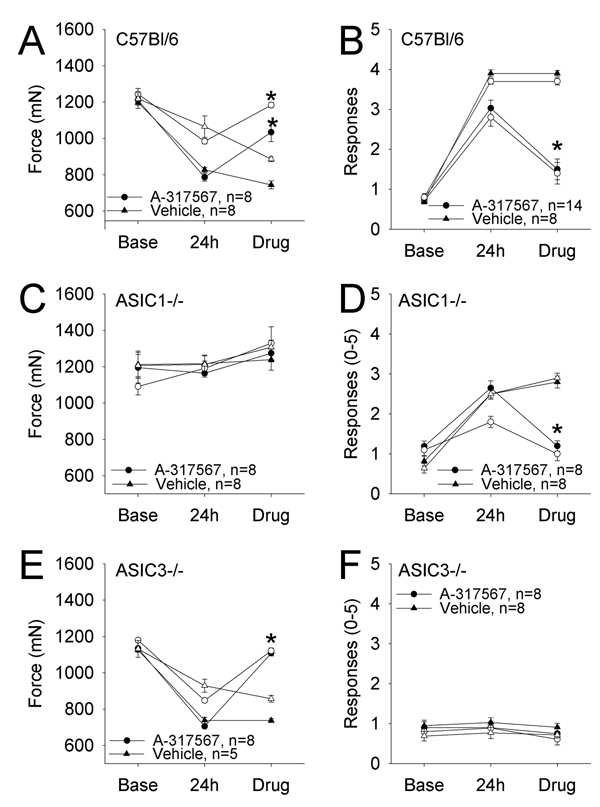
To determine if ASICs are important for maintaining hyperalgesia once developed, we next tested if peripheral blockade of ASICs would reverse the hyperalgesia in C57Bl/6 mice. Injection of 0.025 µmole A-317567, a non-selective ASIC antagonist, into the inflamed gastrocnemius muscle (24h after inflammation) reversed the decrease in muscle withdrawal threshold in C57Bl/6 (WT)(Figure 4A and B). Surprisingly, the unilateral injection of A-317567 reversed the hyperalgesia on both the ipsilateral and the contralateral sides. To confirm that this dose did not produce a systemic effect, we injected 0.025 µmol A-317567 into the contralateral gastrocnemius muscle of animals with carrageenan induced inflammation of the ipsilateral muscle. The withdrawal thresholds of both sides remained unchanged 15 min after injection of the drug into the contralateral gastrocnemius muscle (798 ± 31 mN ipsilateral; 990 ± 50 mN contralateral), which is comparable to the withdrawal thresholds prior to drug administration (880 ± 24 mN ipsilateral; 1128 ± 40 mN contralateral) in WT mice with muscle inflammation of the ipsilateral gastrocnemius muscle.
Figure 4. Effect of A-317567 on muscle inflammation in C57Bl/6 (WT), ASIC1−/− and ASIC3−/− mice.
A, C and E Muscle sensitivity (tweezers) in mice before and 24 h after carrageenan-induced muscle inflammation, and after A-317567 treatment. B, D and F Cutaneous mechanical sensitivity before and after muscle inflammation, and after A-317567 treatment. Closed symbols = ipsilateral, open symbol = contralateral sides. C57Bl/6 (WT) mice (A and B) develop muscle hyperalgesia (tweezers) (A) and cutaneous mechanical hyperalgesia (B) 24 h after carrageenan-induced muscle inflammation and is reversed by A-317567. Hyperalgesia and reversal with A-317567 is seen in both the ipsilateral and contralateral sides. ASIC1−/− mice (C and D), do not develop muscle hyperalgesia after muscle inflammation and A-317567 has no effect on muscle withdrawal thresholds (C). Cutaneous mechanical hyperalgesia develops in ASIC1−/− mice after muscle inflammation and A-317567 reverses the hyperalgesia on both sides (D). ASIC3−/− mice (E and F) develop muscle hyperalgesia after muscle inflammation and A-317567 reverses the effect on both ipsilateral and contralateral sides (E). A-317567 has no effect on mechanical responsiveness in ASIC3−/− mice that do not develop mechanical hyperalgesia after muscle inflammation (F). (* = P<0.05 when compared to the vehicle).
To further confirm the role of ASIC1 in muscle sensitivity and ASIC3 in cutaneous sensitivity we tested the effect of A-317567 in ASIC3−/− and ASIC1−/− mice, respectively. In ASIC1−/− mice where there is an increased number of withdrawals to mechanical stimulation of the paw, unilateral intramuscular injection of 0.025 µmol A-317567, 24h after carrageenan induced muscle inflammation, decreases the number of withdrawals bilaterally (Figure 4D), However, in ASIC1−/− mice that do not show a decrease in muscle withdrawal threshold after inflammation, A-317567 had no effect (Figure 4C). In contrast, in ASIC3−/− mice intramuscular injection of 0.025 µmole A-317567 reduces the inflammation-induced decrease in withdrawal threshold of the muscle (Figure 4E). However, in ASIC3−/− mice, where there is no change in the number of withdrawal thresholds of the paw to mechanical stimulation, at 24h after carrageenan induced muscle inflammation, A-317567 has no effect (Figure 4F).
Discussion
Bilateral changes in ASIC expression after injury
The current study shows an upregulation of ASIC2 and ASIC3 mRNAs in lumbar DRGs following muscle inflammation, not only ipsilateral to the inflamed muscle, but also contralaterally. Surprisingly, the bilateral increases in mRNAs were of similar magnitude, despite a unilateral muscle inflammation, and a greater hyperalgesia ipsilaterally. This is a uniquely different pattern to that observed after cutaneous inflammation induced by CFA injection where increases were observed unilaterally for ASIC1a, ASIC1b, ASIC2b, and ASIC3.(71) The differences between the magnitude of hyperalgesia and mRNA levels could be the result of protein modification and interactions ipsilaterally in the inflamed tissue. Inflammatory mediators and protein phosphorylation can modulate and enhance ASIC currents.(2, 31, 38, 71) Thus, although mRNA levels are similar bilaterally, there could be enhanced ASIC activity ipsilaterally from the inflamed muscle that would be manifested as a greater degree of hyperalgesia.
Similar to the results with mRNA expression, local blockade of ASICs with A-317567 reversed the hyperalgesia not only on the injected side, but also on the contralateral side. The reversal of hyperalgesia in knockout mice was specific for ASIC1 and muscle hyperalgesia, and for ASIC3 and paw hyperalgesia, whether ipsilateral or contralateral to the inflamed muscle. The bilateral effect was unexpected but suggests activation of ASICs in inflamed muscle is critical for maintaining hyperalgesia through enhancing central excitability. In support of a role for ASICs in central sensitization, dorsal horn neurons from ASIC3−/− mice do not develop enhanced central excitability that normally occurs after repeated intramuscular acid injection.(65)
The bilateral increases in expression of mRNA for ASIC in the lumbar DRG following muscle inflammation could result from generation of dorsal root reflexes (DRR) bilaterally. Dorsal root reflexes are antidromic action potentials generated at the central terminals of primary afferent fibers, which then result in peripheral release of inflammatory mediators to enhance inflammation and pain.(67) Previously we showed that carrageenan-induced inflammation generates DRRs at the level of the spinal cord that enhance the inflammatory process ipsilaterally.(57, 63, 64) Unilateral inflammation also produces DRRs not only on the ipsilateral side, but also the contralateral side.(12, 36, 58) There are also measurable bilateral effects indicative of inflammation such as edema, vasodilation and plasma extravasation.(36, 58) Thus, we conclude that unilateral muscle inflammation results in the generation of DRRs ipsilaterally and contralaterally that then increases expression of ASIC mRNAs in both the ipsilateral and contralateral DRGs. Bilaterally increased expression of ASIC mRNAs in the DRGs could result in increased sensitivity to peripherally applied stimuli manifested as hyperalgesia. Blocking input from the inflamed muscle, with the local injection of A-317567 into the ipsilateral side, would prevent this input from reaching the spinal cord to generate the central excitability and dorsal root reflexes.
With muscle inflammation, WT, ASIC1−/−, and ASIC3−/− mice showed a significant upregulation of ASIC2 mRNA. We propose that ASIC2 might modulate channel activity. In cell culture experiments, ASIC2/ASIC3 heteromeric channels demonstrate increased responses to decreased pH over that of ASIC3 homomeric channels(7) and ASIC2a enhances ASIC1a’s response to the regulatory neuropeptide FMRF-amide.(5) In our studies, the ASIC1 mRNA levels in the lumbar DRGs before and after inflammation remain unchanged. Our mRNA data suggest that ASIC1, ASIC2, and ASIC3 are differentially regulated with inflammation.
ASIC1 and ASIC3 play distinct roles in muscle and cutaneous hyperalgesia
Genetically modified knockout mice or transgenic animals for ASICs have not always demonstrated a positive result for their role in nociception after peripheral inflammation or acid injections.(22, 45, 49, 55, 56, 59, 68) Some prior literature focusing on ASIC3−/− mice show no difference or even enhanced hyperalgesia of the paw after paw inflammation, i.e. primary hyperalgesia.(16, 56, 68) However, the current study, and our prior studies consistently show deficits in secondary hyperalgesia after muscle or joint insult in ASIC3−/− mice,(65, 66) again suggesting differences between deep tissue hyperalgesia and cutaneous hyperalgesia.
We also show for the first time that muscle hyperalgesia at both the site of inflammation and the contralateral hindlimb does not develop in ASIC1−/− mice. This result is distinctly different from prior work showing that there is still increased mechanical hyperalgesia of the paw after carrageenan paw inflammation, and after repeated intramuscular acid injection.(65, 68, 81) We suggest the differences between the prior reports and the current study are directly related to differences between processing of cutaneous and muscle nociceptive information. Muscle afferents express more ASIC3, calcitonin gene-related peptide (CGRP), and substance P and less isolectin B4 and somatostatin when compared to cutaneous afferents.(46, 47, 52) Injection of neuropeptides into skin or muscle results in different responses. Substance P produces spontaneous pain when injected into skin and a decrease in the pressure pain threshold, without spontaneous pain, when injected into muscle.(35) CGRP does not produce pain when injected alone into either skin or muscle but produces pain when injected with substance P into muscle.(51) Centrally there are also differences in processing of nociceptive information from muscle when compared to skin. For example, stimulation of C-fibers innervating muscle produces prolonged discharges in flexor motor neurons that outlasts the stimulus, is longer lasting than cutaneous stimulation of C-fibers, and increases response to noxious stimuli bilaterally.(75, 79) Formalin injected into the skin of the lower back increases c-fos expression throughout laminae I–V, but when injected into the muscles of the lower back there is no labeling in laminae II.(48) Thus, differences in processing of nociceptive stimulation from skin and muscle likely underlie the differences between prior studies utilizing cutaneous models of inflammation and the current study using a model of muscle inflammation.
Differences in the tissue expression of ASIC subunits in peripheral neurons or peripheral tissues could influence nociceptive behavior. The current data shows that blockade of peripheral receptors with A-317567 at the site of inflammation reverses the muscle and cutaneous hyperalgesia once developed suggesting peripheral receptor activation is critical to the development of hyperalgesia. In addition to expression in sensory neurons and brain, ASIC3 is located in non-neuronal tissues such as testis, muscle, lung, bone and synovium.(6, 30, 32, 33) The role of ASIC3 in these non-neuronal cells is unclear but ASIC3 may also modulate nociception indirectly. ASIC1 is abundantly expressed in spinal cord neurons and brain,(1, 3, 6, 8–10, 15, 19, 27, 40, 54, 73, 74, 76–78, 80) and its action is thought to primarily be through central mechanisms. It is unclear if centrally expressed ASIC1a plays a distinct role in the processing of hyperalgesia after muscle inflammation. This study highlights the action of ASIC1 in the peripheral sensory neurons, since blockade of peripheral ASICs completely reverses the hyperalgesia once developed.
Inhibition of ASICs reduces nociceptive behaviors
The pharmacology of ASICs has been extensively studied with non-selective drugs such as amiloride and A-317567.(4, 14, 17, 18, 21, 24, 43, 50, 60, 70, 71) A-317567 produces a concentration dependent inhibition of acid-evoked ASIC currents in DRG neurons(24, 39). Intramuscular injection of amiloride also prevents the onset of hyperalgesia induced by repeated intramuscular acid injections suggesting ASIC3 and ASIC1 are important for mediating peripheral nociception at the site of injury.(65, 66) Re-expression of ASIC3 in primary afferent fibers innervating muscle of ASIC3−/− mice restores the mechanical hyperalgesia of the paw,(66) supporting a peripheral role for ASICs in deep tissue hyperalgesia.
In this study, blockade of ASICs in the inflamed muscle reverses the inflammation-induced hyperalgesia bilaterally. In contrast, we previously showed that intramuscular lidocaine in rats reversed carrageenan-induced muscle hyperalgesia ipsilaterally, but not contralaterally.(61) This difference is surprising since lidocaine should reduce all afferent input to the spinal cord. However, differences could be related to the mode of action of the drug (specific blockade of ASICs vs. sodium channels), differences in hyperalgesia between mice and rats, or the magnitude of the change and power in the previous study (3 vehicle and 7 lidocaine) compared to the current study (8 vehicle and 8 A-317567).
Prior reports suggest that ASIC1 plays a role in the generation of hyperalgesia through the central nervous system.(44) Intrathecal application of amiloride or the ASIC1 antagonist PcTx1, however, prevents development of nocifensive behaviors that develop after formalin, acetic acid, glutamate, nerve injury, and repeated intramuscular acid injections.(25, 44, 62) Intrathecal delivery of siRNA to ASIC1a or ASIC3 prevents development of hyperalgesia induced by paw inflammation.(21, 23, 24) However our data show a reversal of muscle hyperalgesia with local injection of A-317567 in WT and ASIC3−/− mice, suggesting that ASIC1 at the site of muscle inflammation is important in the generation of muscle inflammation. While prior data support a central role for ASIC1, our data for the first time show a peripheral role of ASIC1 in the generation of hyperalgesia.
In summary, growing bodies of evidence indicate that ASICs play a role in the sensory signaling associated with inflammatory pain. In this study, we find that ASIC1 and ASIC3 play distinct roles in the development of muscle inflammatory hyperalgesia. Analgesics targeted to specific subunits of ASICs may be useful in alleviating inflammatory muscle pain. Pharmaceuticals designed to reduce ASIC1a could result in decreases in pain at the site of muscle inflammation. Inhibitors of ASIC3 could reduce the development of secondary hyperalgesia, and referred pain following inflammation.
Perspective
This study shows changes in ASIC mRNA expression and behavioral hyperalgesia of C57Bl/6 (wild type), ASIC1−/−, and ASIC3−/− mice before and after the induction of muscle inflammation. A-317567 was effective in reversing hyperalgesia in these animals, suggesting the potential of ASICs as therapeutic targets for muscle inflammatory pain.
Acknowledgements
Supported by National Institutes of Health AR053509 (KAS), NS057821 (ARL), Grant from Department of Anesthesiology, University of Utah (ARL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 2.Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol. 2002;543:521–529. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- 5.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 6.Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 7.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ J Biol Chem. 2000;275:28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- 8.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem. 1997;272:28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- 11.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U SA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bileviciute I, Stenfors C, Theodorsson E, Lundeberg T. Unilateral injection of calcitonin gene-related peptide (CGRP) induces bilateral oedema formation and release of CGRP-like immunoreactivity in the rat hindpaw. Br J Pharmacol. 1998;125:1304–1312. doi: 10.1038/sj.bjp.0702192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carnally SM, Dev HS, Stewart AP, Barrera NP, Van Bemmelen MX, Schild L, Henderson RM, Edwardson JM. Direct visualization of the trimeric structure of the ASIC1a channel, using AFM imaging. Biochem Biophys Res Commun. 2008;372:752–755. doi: 10.1016/j.bbrc.2008.05.100. [DOI] [PubMed] [Google Scholar]
- 14.Catarsi S, Babinski K, Seguela P. Selective modulation of heteromeric ASIC protongated channels by neuropeptide FF. Neuropharmacology. 2001;41:592–600. doi: 10.1016/s0028-3908(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 15.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, protongated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Kalbacher H, Grunder S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol. 2006;127:267–276. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. J Biol Chem. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- 21.Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira J, Santos AR, Calixto JB. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci. 1999;65:1059–1066. doi: 10.1016/s0024-3205(99)00336-7. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 28.Häbler C. Untersuchungen zur Molekularpathologie der Gelenkexsudate und ihre klinischen Ergebnisse. Arch Klin Chir. 1929;156:20–42. [Google Scholar]
- 29.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 30.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Immke DC, McCleskey EW. ASIC3: a lactic acid sensor for cardiac pain. ScientificWorldJournal. 2001;1:510–512. doi: 10.1100/tsw.2001.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi K, Marumo F. Molecular cloning of a DEG/ENaC sodium channel cDNA from human testis. Biochem Biophys Res Commun. 1998;245:589–593. doi: 10.1006/bbrc.1998.8483. [DOI] [PubMed] [Google Scholar]
- 33.Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 34.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 35.Jensen K, Tuxen C, Pedersen-Bjergaard U, Jansen I. Pain, tenderness, wheal and flare induced by substance-P, bradykinin and 5-hydroxytryptamine in humans. Cephalalgia. 1991;11:175–182. doi: 10.1046/j.1468-2982.1991.1104175.x. [DOI] [PubMed] [Google Scholar]
- 36.Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci. 2007;26:935–942. doi: 10.1111/j.1460-9568.2007.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 38.Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, Welsh MJ. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci U S A. 2003;100:2029–2034. doi: 10.1073/pnas.252782799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 41.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 42.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907–48913. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- 44.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- 45.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005:1–35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 48.Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine. 2006;31:2048–2052. doi: 10.1097/01.brs.0000231756.56230.13. [DOI] [PubMed] [Google Scholar]
- 49.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007;133:150–160. doi: 10.1016/j.pain.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen-Bjergaard U, Nielsen LB, Jensen K, Edvinsson L, Jansen I, Olesen J. Calcitonin gene-related peptide, neurokinin A and substance P: effects on nociception and neurogenic inflammation in human skin and temporal muscle. Peptides. 1991;12:333–337. doi: 10.1016/0196-9781(91)90022-h. [DOI] [PubMed] [Google Scholar]
- 52.Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- 53.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 55.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 56.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 57.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol. 1995;484(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rees H, Sluka KA, Lu Y, Westlund KN, Willis WD. Dorsal root reflexes in articular afferents occur bilaterally in a chronic model of arthritis in rats. J Neurophysiol. 1996;76:4190–4193. doi: 10.1152/jn.1996.76.6.4190. [DOI] [PubMed] [Google Scholar]
- 59.Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol. 2004;558:659–669. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J Physiol. 2006;570:339–354. doi: 10.1113/jphysiol.2005.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Sluka KA, Radhakrishnan R, Price MP, Welsh MJ. ASIC3 Mediates Mechanical Hyperalgesia Induced by Muscle Injury. In: Brune K, Handwerker HO, editors. Hyperalgesia : molecular mechanisms and clinical implications. Seattle: IASP Press; 2004. pp. 105–112. [Google Scholar]
- 63.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 64.Sluka KA, Rees H, Westlund KN, Willis WD. Fiber types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol. 1995;74:981–989. doi: 10.1152/jn.1995.74.3.981. [DOI] [PubMed] [Google Scholar]
- 65.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 66.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sluka KA, Westlund-High KN. Neurologic regulation of inflammation. In: Firestein GS, Budd RC, Harris ED Jr, McInnes IB, Ruddy S, Sergent JS, editors. Kelley's textbook of rheumatology. eighth edition. Philadelphia, PA: Saunders/Elsevier; 2009. pp. 411–419. [Google Scholar]
- 68.Staniland AA, McMahon SB. Mice lacking acid-sensing ion channels (ASIC) 1 or 2 but not ASIC3, show increased pain behaviour in the formalin test. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Ugawa S, Ueda T, Takahashi E, Hirabayashi Y, Yoneda T, Komai S, Shimada S. Cloning and functional expression of ASIC-beta2, a splice variant of ASIC-beta. Neuroreport. 2001;12:2865–2869. doi: 10.1097/00001756-200109170-00022. [DOI] [PubMed] [Google Scholar]
- 70.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs) Curr Drug Targets Inflamm Allergy. 2004;3:71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 73.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 74.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 75.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 77.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 79.Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986;6:1433–1442. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 81.Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]