Abstract
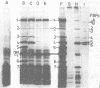
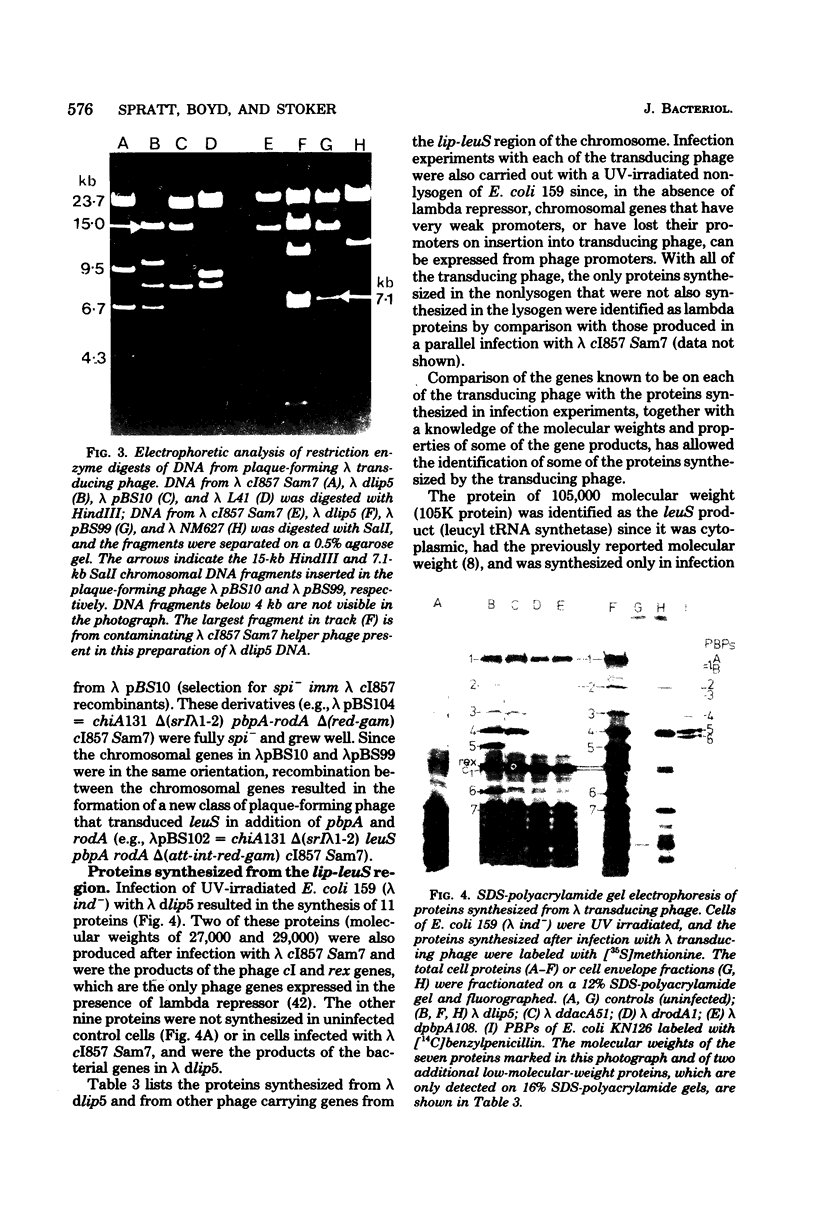
A series of defective lambda transducing phage carrying genes from the lip-leuS region of the Escherichia coli chromosome (min 14 on the current linkage map) has been isolated. The phage defined the gene order as lac---lip-dacA-rodA-pbpA-leuS---gal. These included the structural genes for penicillin-binding protein 2 (pbpA) and penicillin-binding protein 5 (dacA) as well as a previously unidentified cell shape gene that we have called rodA. rodA mutants were spherical and very similar to pbpA mutants but were distinguishable from them in that they had no defects in the activity of penicillin-binding protein 2. The separation into two groups of spherical mutants with mutations that mapped close to lip was confirmed by complementation analysis. The genes dacA, rodA, and pbpA lie within a 12-kilobase region, and represent a cluster of genes involved in cell shape determination and peptidoglycan synthesis. A restriction map of the lip-leuS region was established, and restriction fragments were cloned from defective transducing phage into appropriate lambda vectors to generate plaque-forming phage that carried genes from this region. Analysis of the proteins synthesized from lambda transducing phage in ultraviolet light-irradiated cells of E. coli resulted in the identification of the leuS, pbpA, dacA, and lip gene products, but the product of the rodA gene was not identified. The nine proteins that were synthesized from the lip-leuS region accounted for 57% of its coding capacity. Phage derivatives were constructed that allowed about 50-fold amplification of the levels of penicillin-binding proteins 2 and 5 in the cytoplasmic membrane.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Fletcher G., Irwin C. A., Henson J. M., Fillingim C., Malone M. M., Walker J. R. Identification of the Escherichia coli cell division gene sep and organization of the cell division-cell envelope genes in the sep-mur-ftsA-envA cluster as determined with specialized transducing lambda bacteriophages. J Bacteriol. 1978 Jan;133(1):91–100. doi: 10.1128/jb.133.1.91-100.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G., Richmond K. M. Translocation of the tetracycline resistance determinant from R100-1 to the Escherichia coli K-12 chromosome. J Bacteriol. 1975 Dec;124(3):1153–1158. doi: 10.1128/jb.124.3.1153-1158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. The concept of the penicillin target from 1965 until today. The thirteenth marjory stephenson memorial lecture. J Gen Microbiol. 1977 Jul;101(1):13–33. doi: 10.1099/00221287-101-1-13. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Knowles J. R., Katze J. R., Lapointe J., Söll D. Purification of leucyl transfer ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1401–1406. [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Goldman R., Tipper D. J., Feingold B., Strominger J. L. Morphology of an Escherichia coli mutant with a temperature-dependent round cell shape. J Bacteriol. 1978 Dec;136(3):1143–1158. doi: 10.1128/jb.136.3.1143-1158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Jones C. W., Khorana J., Strominger J. L. Mapping of the mecillinam-resistant, round morphological mutants of Escherichia coli. J Bacteriol. 1978 Jan;133(1):196–202. doi: 10.1128/jb.133.1.196-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Donachie W. D. Identification of the ftsA gene product. J Bacteriol. 1979 Mar;137(3):1088–1094. doi: 10.1128/jb.137.3.1088-1094.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Maruyama I. N., Takagaki Y., Tamaki S., Nishimura Y., Hirota Y. Isolation of a mutant of Escherichia coli lacking penicillin-sensitive D-alanine carboxypeptidase IA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2631–2635. doi: 10.1073/pnas.75.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Tamaki S., Curtis S. J., Strominger J. L. Mutational evidence for identity of penicillin-binding protein 5 in Escherichia coli with the major D-alanine carboxypeptidase IA activity. J Bacteriol. 1979 Jan;137(1):644–647. doi: 10.1128/jb.137.1.644-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A., Brammar W. J. The use of specialised transducing phages in the amplification of enzyme production. Mol Gen Genet. 1976 Nov 24;149(1):87–99. doi: 10.1007/BF00275963. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of the left arm of the chromosome of bacteriophage lambda. Genetics. 1968 Jul;59(3):311–325. doi: 10.1093/genetics/59.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Biogenesis of the wall in bacterial morphogenesis. Adv Microb Physiol. 1979;19:1–62. doi: 10.1016/s0065-2911(08)60197-6. [DOI] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Comparison of the binding properties of two 6 beta-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob Agents Chemother. 1977 Jan;11(1):161–166. doi: 10.1128/aac.11.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Crasemann J. M., Stahl M. M. Rec-mediated recombinational hot spot activity in bacteriophage lambda. III. Chi mutations are site-mutations stimulating rec-mediated recombination. J Mol Biol. 1975 May 15;94(2):203–212. doi: 10.1016/0022-2836(75)90078-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J. A genetic map of several mutations affecting the mucopeptide layer of Escherichia coli. Genet Res. 1972 Aug;20(1):65–74. doi: 10.1017/s0016672300013598. [DOI] [PubMed] [Google Scholar]