Abstract
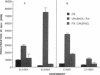
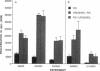
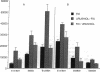
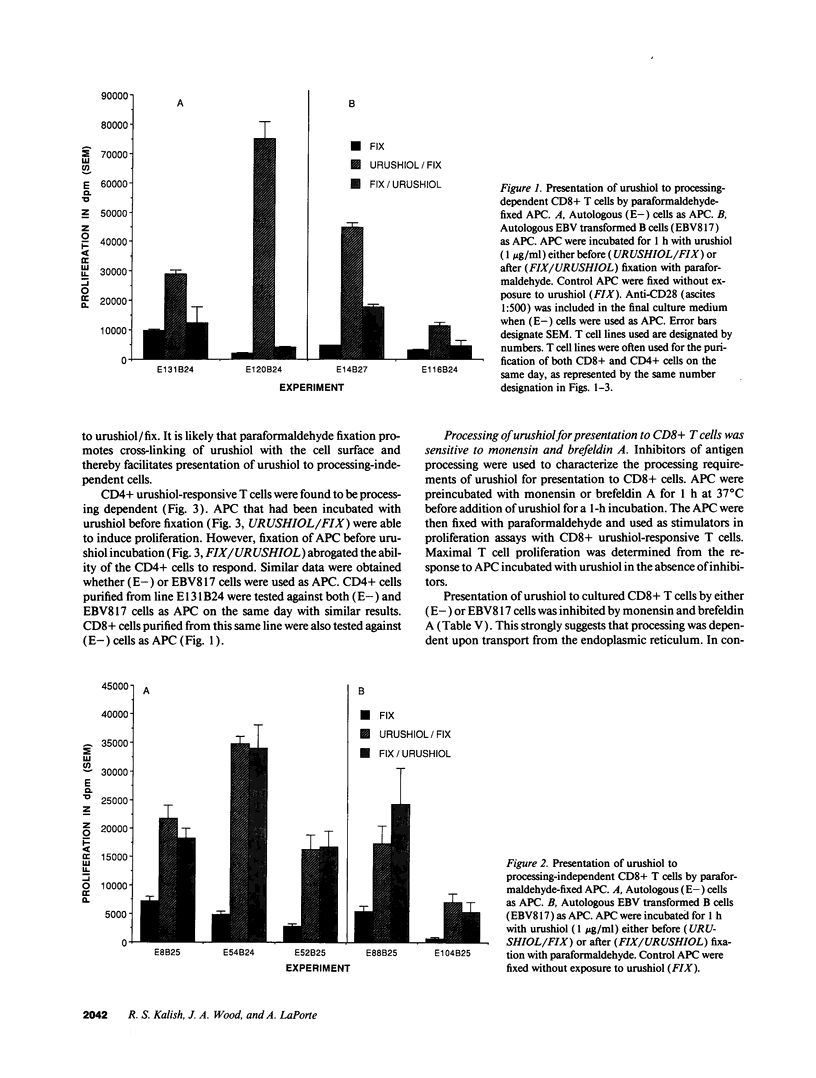
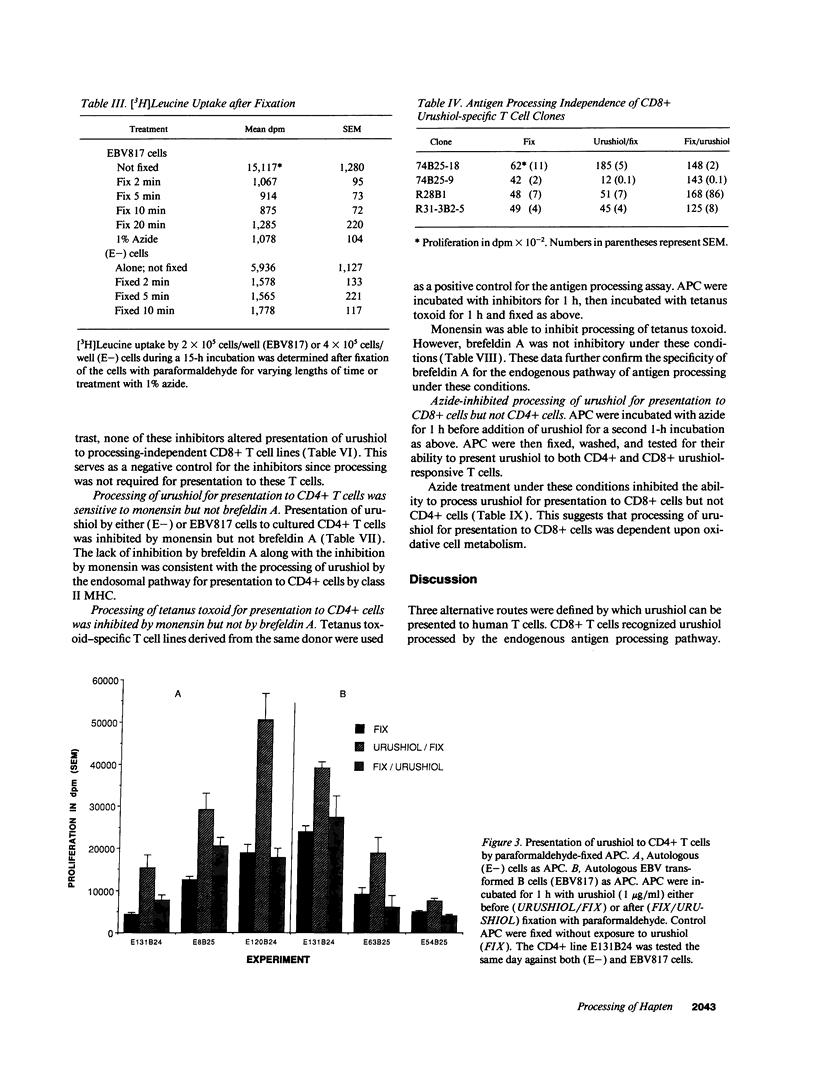
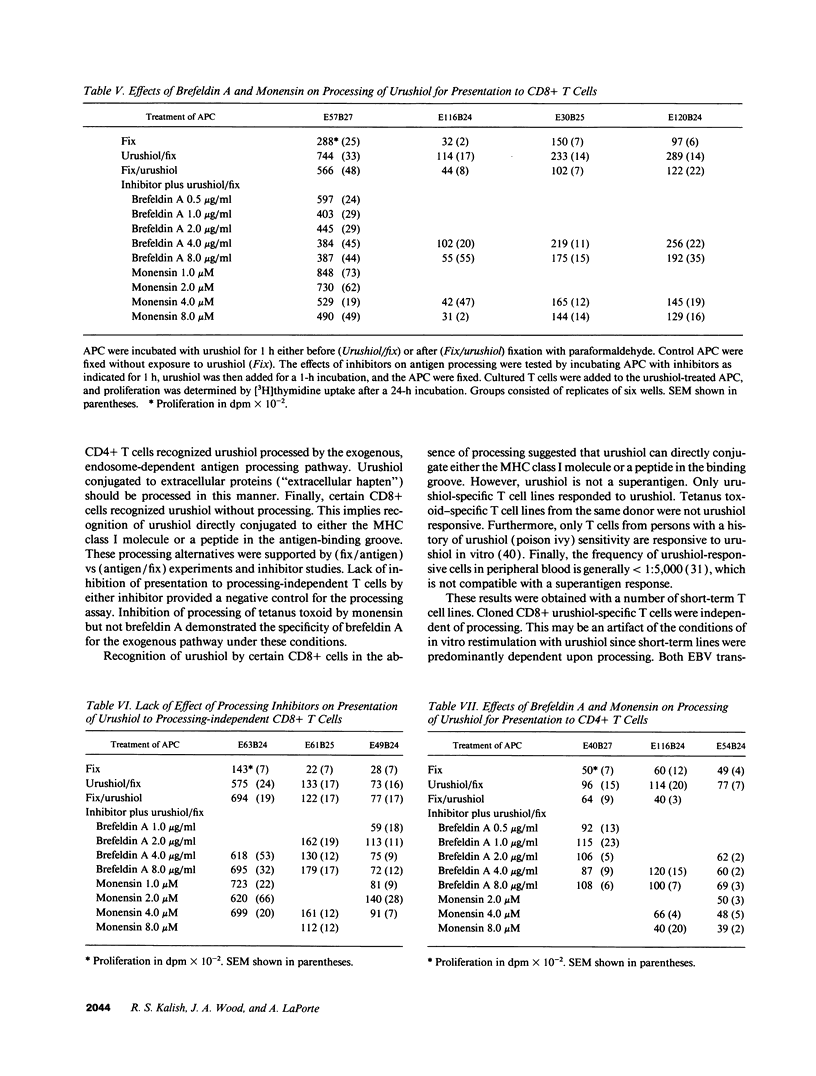
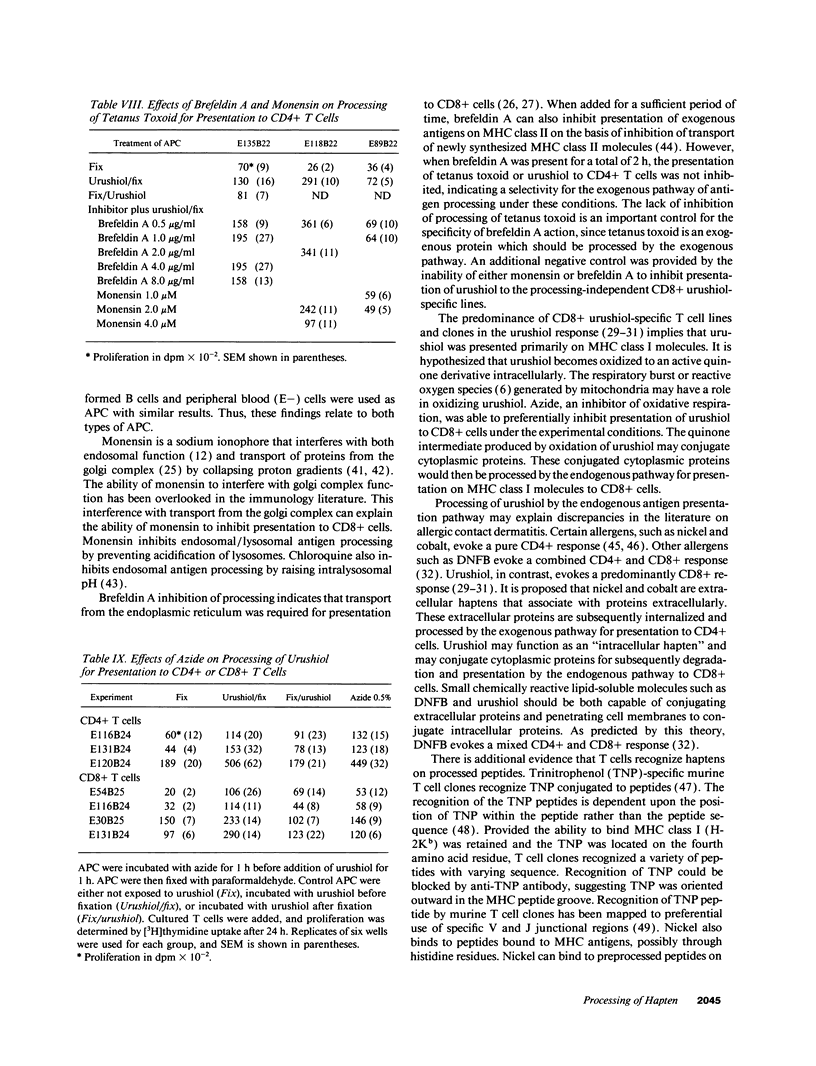
The antigen processing requirements for urushiol, the immunogen of poison ivy (Toxicodendron radicans), were tested by presentation of urushiol to cultured human urushiol-responsive T cells. Urushiol was added to antigen-presenting cells (APC) either before or after fixation with paraformaldehyde. Three distinct routes of antigen processing were detected. CD8+ and CD4+ T cells, which were dependent upon processing, proliferated if urushiol was added to APC before fixation, but did not proliferate when urushiol was added to APC after fixation. Processing of urushiol for presentation to CD8+ T cells was inhibited by azide, monensin, and brefeldin A. This suggests that urushiol was processed by the endogenous pathway. In contrast, presentation of urushiol to CD4+ T cells was inhibited by monensin but not by brefeldin A. This was compatible with antigen processing by the endosomal (exogenous) pathway. Finally, certain CD8+ T cells recognized urushiol in the absence of processing. These cells proliferated in response to APC incubated with urushiol after fixation. Classification of contact allergens by antigen processing pathway may predict the relative roles of CD4+ and CD8+ cells in the immunopathogensis of allergic contact dermatitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Ullrich S. J., Appella E., Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature. 1990 Jul 5;346(6279):63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- Attaya M., Jameson S., Martinez C. K., Hermel E., Aldrich C., Forman J., Lindahl K. F., Bevan M. J., Monaco J. J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992 Feb 13;355(6361):647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- Baer H. Chemistry and immunochemistry of poisonous Anacardiaceae. Clin Dermatol. 1986 Apr-Jun;4(2):152–159. doi: 10.1016/0738-081x(86)90074-x. [DOI] [PubMed] [Google Scholar]
- Bauer A., Rutenfranz I., Kirchner H. Processing requirements for T cell activation by Mycoplasma arthritidis-derived mitogen. Eur J Immunol. 1988 Dec;18(12):2109–2112. doi: 10.1002/eji.1830181239. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V., Colston M. J. The processing and presentation of mycobacterial antigens by human monocytes. Eur J Immunol. 1988 May;18(5):691–696. doi: 10.1002/eji.1830180506. [DOI] [PubMed] [Google Scholar]
- Byck J. S., Dawson C. R. Assay of protein-quinone coupling involving compounds structurally related to the active principle of poison ivy. Anal Biochem. 1968 Oct 24;25(1):123–135. doi: 10.1016/0003-2697(68)90086-9. [DOI] [PubMed] [Google Scholar]
- Byers V. S., Epstein W. L., Castagnoli N., Baer H. In vitro studies of poison oak immunity. I. In vitro reaction of human lymphocytes to urushiol. J Clin Invest. 1979 Nov;64(5):1437–1448. doi: 10.1172/JCI109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesen M., Moustafa M. A., Adline J., Vandervorst D., Poupaert J. H. Evidence for an arene oxide-NIH shift pathway in the metabolic conversion of phenytoin to 5-(4-hydroxyphenyl)-5-phenylhydantoin in the rat and in man. Drug Metab Dispos. 1982 Nov-Dec;10(6):667–671. [PubMed] [Google Scholar]
- Colonna M., Bresnahan M., Bahram S., Strominger J. L., Spies T. Allelic variants of the human putative peptide transporter involved in antigen processing. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3932–3936. doi: 10.1073/pnas.89.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Crumpacker D. B., Alexander J., Cresswell P., Engelhard V. H. Role of endogenous peptides in murine allogenic cytotoxic T cell responses assessed using transfectants of the antigen-processing mutant 174xCEM.T2. J Immunol. 1992 May 15;148(10):3004–3011. [PubMed] [Google Scholar]
- DAWSON C. R. The chemistry of poison ivy. Trans N Y Acad Sci. 1956 Mar;18(5):427–443. doi: 10.1111/j.2164-0947.1956.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocinski B. L., Tigelaar R. E. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990 Jun 1;144(11):4121–4128. [PubMed] [Google Scholar]
- Hertl M., Bohlen H., Jugert F., Boecker C., Knaup R., Merk H. F. Predominance of epidermal CD8+ T lymphocytes in bullous cutaneous reactions caused by beta-lactam antibiotics. J Invest Dermatol. 1993 Dec;101(6):794–799. doi: 10.1111/1523-1747.ep12371697. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- KLIGMAN A. M. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958 Feb;77(2):149–180. doi: 10.1001/archderm.1958.01560020001001. [DOI] [PubMed] [Google Scholar]
- Kalish R. S., Johnson K. L. Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J Immunol. 1990 Dec 1;145(11):3706–3713. [PubMed] [Google Scholar]
- Kalish R. S., Morimoto C. Quantitation and cloning of human urushiol specific peripheral blood T-cells: isolation of urushiol triggered suppressor T-cells. J Invest Dermatol. 1989 Jan;92(1):46–52. doi: 10.1111/1523-1747.ep13070998. [DOI] [PubMed] [Google Scholar]
- Kalish R. S., Morimoto C., Schlossman S. F. Generation of CD8 (T8) cytotoxic cells has a preferential requirement for CD4+2H4- inducer cells. Cell Immunol. 1988 Feb;111(2):379–389. doi: 10.1016/0008-8749(88)90101-3. [DOI] [PubMed] [Google Scholar]
- Kalish R. S., Morimoto C. Urushiol (poison ivy)-triggered suppressor T cell clone generated from peripheral blood. J Clin Invest. 1988 Sep;82(3):825–832. doi: 10.1172/JCI113685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs G. L., Rotstein O. D., Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990 Dec 5;265(34):21099–21107. [PubMed] [Google Scholar]
- Löfström A., Wigzell H. Antigen specific human T cell lines specific for cobalt chloride. Acta Derm Venereol. 1986;66(3):200–206. [PubMed] [Google Scholar]
- Martin S., Ortmann B., Pflugfelder U., Birsner U., Weltzien H. U. Role of hapten-anchoring peptides in defining hapten-epitopes for MHC-restricted cytotoxic T cells. Cross-reactive TNP-determinants on different peptides. J Immunol. 1992 Oct 15;149(8):2569–2575. [PubMed] [Google Scholar]
- Martinez C. K., Monaco J. J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991 Oct 17;353(6345):664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Benacerraf B., Rock K. L. The class II MHC-restricted presentation of endogenously synthesized ovalbumin displays clonal variation, requires endosomal/lysosomal processing, and is up-regulated by heat shock. J Immunol. 1992 Feb 15;148(4):1016–1024. [PubMed] [Google Scholar]
- Michalek M. T., Benacerraf B., Rock K. L. The class II MHC-restricted presentation of endogenously synthesized ovalbumin displays clonal variation, requires endosomal/lysosomal processing, and is up-regulated by heat shock. J Immunol. 1992 Feb 15;148(4):1016–1024. [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann B., Martin S., von Bonin A., Schiltz E., Hoschützky H., Weltzien H. U. Synthetic peptides anchor T cell-specific TNP epitopes to MHC antigens. J Immunol. 1992 Mar 1;148(5):1445–1450. [PubMed] [Google Scholar]
- Romagnoli P., Labhardt A. M., Sinigaglia F. Selective interaction of Ni with an MHC-bound peptide. EMBO J. 1991 Jun;10(6):1303–1306. doi: 10.1002/j.1460-2075.1991.tb07648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. J., Khan L., Chung L. Y. Are free radicals and not quinones the haptenic species derived from urushiols and other contact allergenic mono- and dihydric alkylbenzenes? The significance of NADH, glutathione, and redox cycling in the skin. Arch Dermatol Res. 1990;282(1):56–64. doi: 10.1007/BF00505646. [DOI] [PubMed] [Google Scholar]
- Shear N. H., Spielberg S. P., Grant D. M., Tang B. K., Kalow W. Differences in metabolism of sulfonamides predisposing to idiosyncratic toxicity. Ann Intern Med. 1986 Aug;105(2):179–184. doi: 10.7326/0003-4819-105-2-179. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Scheidegger D., Garotta G., Scheper R., Pletscher M., Lanzavecchia A. Isolation and characterization of Ni-specific T cell clones from patients with Ni-contact dermatitis. J Immunol. 1985 Dec;135(6):3929–3932. [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Cerundolo V., Colonna M., Cresswell P., Townsend A., DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992 Feb 13;355(6361):644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- Sweetser M. T., Morrison L. A., Braciale V. L., Braciale T. J. Recognition of pre-processed endogenous antigen by class I but not class II MHC-restricted T cells. Nature. 1989 Nov 9;342(6246):180–182. doi: 10.1038/342180a0. [DOI] [PubMed] [Google Scholar]
- Tapper H., Sundler R. Role of lysosomal and cytosolic pH in the regulation of macrophage lysosomal enzyme secretion. Biochem J. 1990 Dec 1;272(2):407–414. doi: 10.1042/bj2720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Wei M. L., Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992 Apr 2;356(6368):443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U., Hebbelmann S., Pflugfelder U., Ruh H., Ortmann B., Martin S., Iglesias A. Antigen contact sites in class I major histocompatibility complex-restricted, trinitrophenyl-specific T cell receptors. Eur J Immunol. 1992 Mar;22(3):863–866. doi: 10.1002/eji.1830220335. [DOI] [PubMed] [Google Scholar]
- Yang Y., Waters J. B., Früh K., Peterson P. A. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]