Abstract
Ghrelin and leptin, putative controllers of human appetite, have no effect on human meal-to-meal appetite but respond to variations in energy availability. Nonhomeostatic characteristics of appetite and spontaneous activity stem from inhibition by leptin and ghrelin of brain reward circuit that is responsive to energy deficit, but refractory in obesity, and from the operation of a meal-timing circadian clock.
Keywords: Leptin, insulin, ghrelin, motivation, reward, obesity
INTRODUCTION
Recent epidemic increases in the incidence of obesity in developed countries is closely related to increased availability and intake of palatable, energy-dense food (23) and declining levels of physical activity (3). As both food intake and energy expenditure determine the plateau around which body weight and fat are regulated, factors controlling feeding and spontaneous activity hold the key to prevent and possibly reduce obesity. The thesis of this review is that our understanding of the regulation of energy balance is impeded by preoccupation with a homeostatic view of this mechanism and comparative inattention to nonhomeostatic motivational and physiological processes controlling feeding, spontaneous physical activity, and metabolism.
Non-homeostatic control of meal-to-meal eating
The inference of homeostatic mechanism of energy regulation was made by E.F. Adolph over 60 yr ago when he noted that body weight in rats stabilizes and is defended at a given plateau at the end of the growth period. The contribution of feeding to this process was in that ad-libitum fed rat eats relatively constant amounts of standard lab chow sufficient to maintain a stable weight plateau (1). After lesions of the ventromedial hypothalamus (VMH) animals gain excess fat and weight, but when weight again stabilizes, an identical amount of food per unit weight is consumed as in intact rats to maintain the new weight plateau (16). Partial starvation and weight loss in both neurologically intact, and in the obese VMH lesioned rats is followed by overeating and hypoactivity, when unlimited access to food is restored, until a complete recovery of weight to pre-starvation levels is achieved. Gordon Kennedy demonstrated that spontaneous physical activity is a component of the mechanism contributing to weight stability in mature rodents. He noted low spontaneous activity levels during rapid growth. With attainment of sexual maturity and a stable weight plateau, spontaneous physical activity level reaches its peak and contributes to energy balance through daily oscillations in energy expenditure around a stable weight plateau (16). These early studies suggested that both feeding and spontaneous physical activity contribute to weight stability in a homeostatic fashion.
A contemporary view of energy regulation adopts the premise that feeding and spontaneous physical activity act as negative feedback controls in this process. An important distinction between the early and contemporary views of energy regulation is in the concept of what is being regulated. The early studies implicated feeding and spontaneous physical activity in maintenance and defense of any stable body weight and fat plateau. The contemporary definition focuses on regulation of body fat levels by way of the hormones insulin and leptin that signal deviation from normative fatness to the controlling hypothalamic centers. A sustained inhibitory influence over feeding, and a stimulatory influence over energy expending processes (presumed to include physical activity), are postulated to operate in the basal, meal-to-meal state, under the influence of insulin and leptin, and to be relaxed when body weight and fat as well as plasma concentrations of insulin and leptin decline to allow for restoration of predeprivation fat level (25). A more detailed formulation of this homeostatic view is that the gut peptide ghrelin stimulates feeding in response to negative energy balance while meal size and energy balance are jointly regulated by the satiating gut hormones cholecystokinin, glucagon-like peptide-1 (GLP-1), and peptide YY (PYY) and by the adiposity satiety hormones insulin and leptin (21). The pivotal evidence in support of the contemporary homeostatic view of energy regulation hinges on insulin and leptin exerting tonic inhibition over feeding and stimulation of physical activity and metabolism by acting in a corrective homeostatic fashion by binding to their receptors on hypothalamic neurons (25).
We noted a number of lines of evidence that did not support the homeostatic view of the role of feeding and spontaneous physical activity in energy regulation. Feeding in humans and animals is strongly influenced by palatability, sensory diversity, food availability, portion size, and social facilitation (19). Humans and animals also do not respond homeostatically to increased caloric density of food as illustrated by rapid fat gain and elevation of body weight plateau on cafeteria and high-fat diets (15, 27). Deficient caloric metering is also seen in the failure of intravenous nutrient infusions (34), enforced inactivity (28), or energy cost of exercise (17) to stimulate corrective appetitive and ingestive responses. Human and animal weights level off at vastly different plateaus depending on nonhomeostatic aspects of food presentation and availability or variable opportunities and necessity for physical activity (27). Perhaps the strongest evidence contradicting the contemporary model of energy regulation is the absence of a dose-dependent increase in hormonal negative feedback over feeding and physical activity with increases in body fatness (26). Instead, the effectiveness of leptin and insulin to suppress feeding and activate spontaneous physical activity progressively declines with increases in obesity (21, 25).
NEW FINDINGS
The inconsistencies between the contemporary homeostatic concept of energy regulation and evidence implicating nonhomeostatic controls prompted us to examine the role of leptin and insulin in the control of human meal-to-meal eating and appetite. We manipulated energy balance by varying energy content of the meal, imposing energy cost of exercise, and infusing nutrients intravenously (4). Concurrent measurements of putative hormonal mediators of the homeostatic energy regulatory mechanism were carried out, which was not the case in most of the previous studies.
In our study, reduction in short-term changes in energy availability was generated by either providing a small 100 Kcal morning meal at 6 a.m. or by imposing a 550 Kcal morning energy deficit by moderate-intensity exercise between 10 and 12 a.m. in nine healthy postmenopausal women who served as their own controls. Short-term energy availability was increased by either providing a large 500 Kcal morning meal or by replacing 364 calories missing from the small meal and expended in exercise by infusing intravenously total parenteral nutrients (TPN) between 6 and 11 a.m. TPN contained the same macronutrient composition as the morning meal.
Changes in appetite were measured with a 10-cm visual analog scale (VAS) under these four treatment trials: a small meal (Rest), exercise (EX), and intravenous replacement of calories missing in the small meal (Rest-TPN) or expended during exercise (EX-TPN). A sedentary trial in which a large morning meal was provided (Sed) served as a control condition. Subjects rated their hunger and satiation on the VAS by marking a point on a scale that ranged from “not at all” to “extreme.” Concurrent measurements were carried out of the putative hunger hormone ghrelin, and of the putative adiposity negative feedback hormones leptin and insulin. Gastric insulinotropic peptide (GIP) was measured as a prototype gut hormone responsive to meal intake. In addition, food consumed during the ad libitum mid-day meal was measured for evidence of possible delayed increases in eating as a compensation for the morning energy shortfall or the energy cost of exercise. Measurements were terminated at 5 p.m.
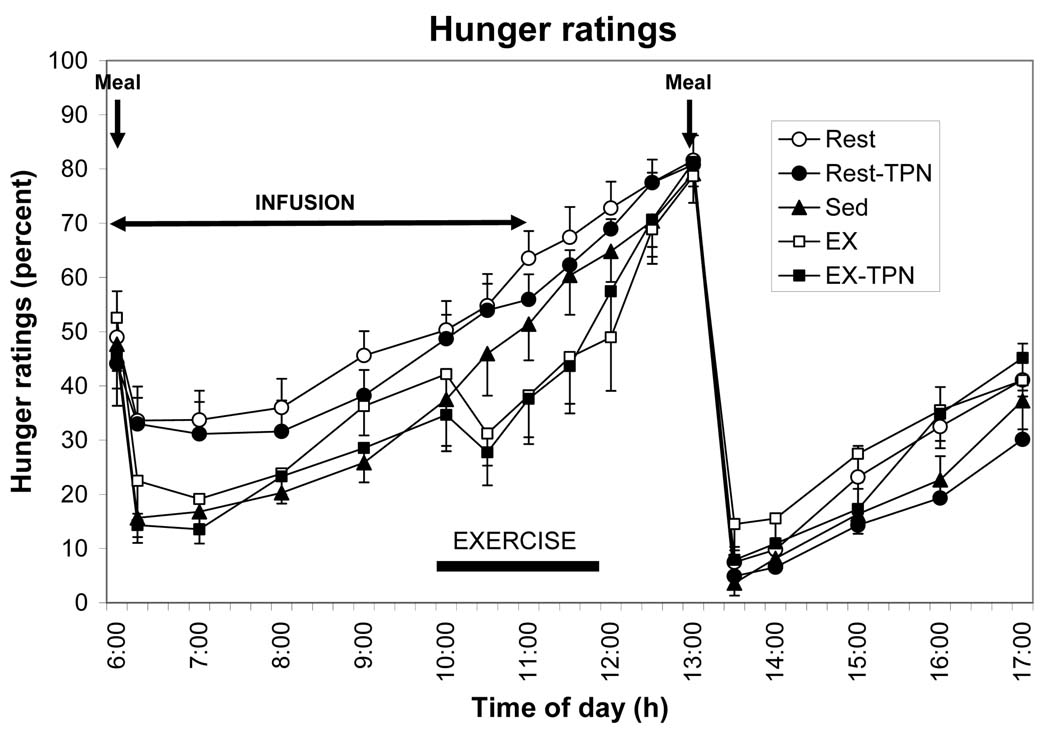
The results (Fig. 1) rather unambiguously revealed the sensitivity of human perception of hunger to changes in energy content of different size meals. By contrast, hunger was insensitive to short-term fluctuations in energy availability caused by exercise or intravenous nutrient infusions. In addition, exercise suppressed hunger and increased perception of fullness (Fig. 2, left) corroborating similar reports by others (17) and demonstrating again the insensitivity of human appetite to exercise-induced short-term energy deficit.
Figure 1.
Hunger ratings on a 10-cm visual analog scale in response to a 100 Kcal (open circles) or a 500 Kcal (solid circles) breakfast at 6 am, 550 Kcal exercise energy expenditure between 10 and 12 h, presence of intravenous replacement of 364 Kcal between 6 and 11 h, and ad-libitum food intake at 13 h in 10 postmenopausal women. Four treatment trials: a small meal (Rest), exercise (EX), and intravenous replacement of calories missing in the small meal (Rest-TPN) or expended during exercise (EX-TPN). A sedentary trial in which a large morning meal was provided (Sed) served as a control condition. TPN, total parenteral nutrients. [Adapted from Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab 2009;94:2290–2298. Copyright © 2009 The Endocrine Society. Used with permission.]
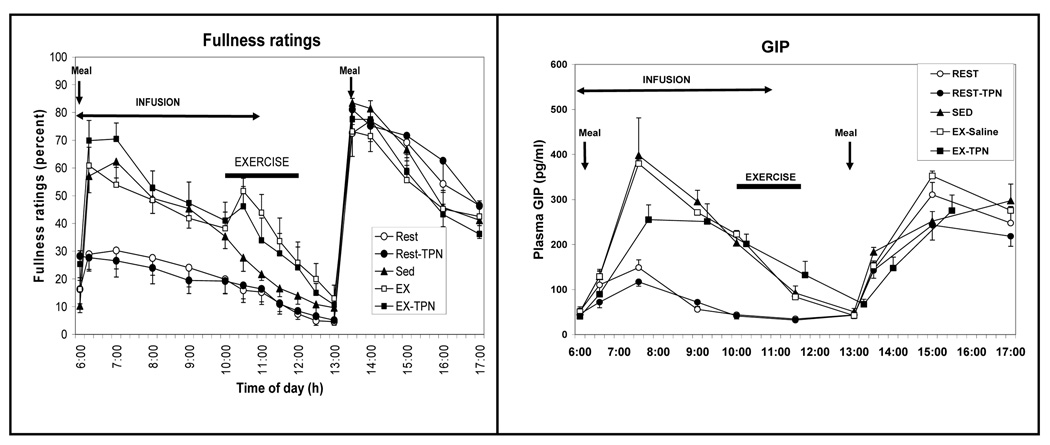
Figure 2.
Ratings of fullness (left) and changes in the concentration of gastric insulinotropic peptide (GIP; right) in response to differences in meal size, exercise energy expenditure, and total parenteral nutrients (TPN). Symbols as in Figure 1. Four treatment trials: a small meal (Rest), exercise (EX), and intravenous replacement of calories missing in the small meal (Rest-TPN) or expended during exercise (EX-TPN). A sedentary trial in which a large morning meal was provided (Sed) served as a control condition. [Adapted from Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab 2009;94:2290–2298. Copyright © 2009 The Endocrine Society. Used with permission.]
There was a strong correspondence between the GIP secretory response (Fig. 2, right) and the magnitude and time course of reported fullness in response to manipulation of meal size (Fig. 2, left) suggesting the important role of nutrient transit through the gastrointestinal tract on this measure of short-term satiation.
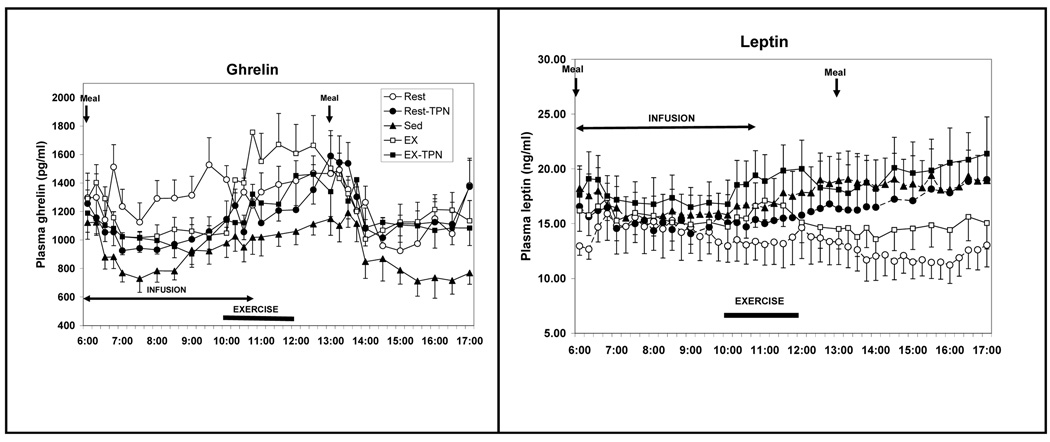
In contrast to the insensitivity of appetite response, the putative appetite controlling hormones responded appropriately to negative morning energy balance caused by either a small meal or exercise energy expenditure: ghrelin with increases (Fig. 3, left), and leptin with decreases (Fig. 3, right), and both to correction of energy imbalance via TPN infusions.
Figure 3.
Changes in plasma total ghrelin (left) and leptin (right) concentrations in response to differences in meal size, exercise energy expenditure and total parenteral nutrients (TPN). Symbols as in Figure 1. Four treatment trials: a small meal (Rest), exercise (EX), and intravenous replacement of calories missing in the small meal (Rest-TPN) or expended during exercise (EX-TPN). A sedentary trial in which a large morning meal was provided (Sed) served as a control condition. [Adapted from Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab 2009;94:2290–2298. Copyright © 2009 The Endocrine Society. Used with permission.]
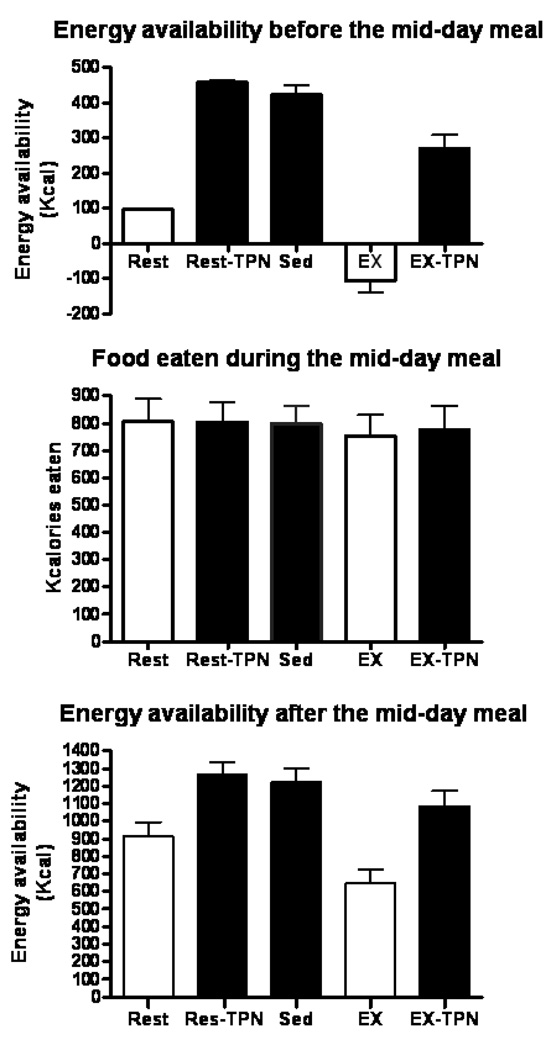
The failure of appetite to respond to short-term fluctuations in energy balance was paralleled by the failure of these fluctuations to produce increased food consumption during the mid-day meal (Fig. 4). As was the case in studies manipulating energy density of food (15), appetite and meal-to-meal eating appeared to be controlled by stomach capacity rather than to preceding energy deficit. In the absence of sustained energy restriction or weight loss, human appetite and meal-to-meal eating were unresponsive to short-term variations in energy availability caused by either inadequate meal size, energy expenditure of exercise, or intravenous increases in nutrient energy.
Figure 4.
Energy balance before (top), food consumed during (center), and energy balance after (bottom) the ad-libitum meal at 13 h. Four treatment trials: a small meal (Rest), exercise (EX), and intravenous replacement of calories missing in the small meal (Rest-TPN) or expended during exercise (EX-TPN). A sedentary trial in which a large morning meal was provided (Sed) served as a control condition. (Reprinted from Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab 2009;94:2290–2298. Copyright © 2009 The Endocrine Society. Used with permission.)
REINTERPRETATION OF THE ENERGY REGULATORY MECHANISM
Collectively, our research coupled with other lines of evidence on the opportunistic responses of human appetite to environmental circumstances and its unresponsiveness to short-term variations in energy availability support the concept of its non-homeostatic control when not constrained by food restriction or a substantial weight loss. In fundamental design, a human’s meal-to-meal consumption pattern does not differ from that of a blowfly. When a blowfly steps into a sugar solution, its taste receptors on the foot pads trigger ingestion of sweet fluid in proportion to its concentration and palatability. A full crop inhibits further feeding through a message sent by a recurrent nerve to the brain, and avulsion of this negative feedback from the crop leads to overeating (7).
Nonhomeostatic contribution of physical activity to energy regulation
According to the current view of energy regulation, increases in body fat level should increase adiposity hormone negative feedback on feeding and facilitate physical activity and other forms of energy expenditure to produce a loss of calories and body fat. Conversely, decreases in body fat level should reduce adiposity hormone negative feedback over feeding and suppress catabolic processes that include physical activity and metabolic energy expenditure to restore energy balance (25). Injections of leptin into leptin-deficient mice that are obese and profoundly hypoactive appear to support this concept by stimulating levels of physical activity (22).
Physical activity can produce weight losses and reduce the level at which body fat is regulated in both rodents (27) and humans (13), regardless of the type of prevailing maintenance diets, when it is done either spontaneously or enforced. In addition, during recovery from enforced weight loss, exposure to forced exercise results in a lower recovery weight plateau than does ad libitum eating without exercise (20). Physical activity may contribute to lowering weight plateaus in humans and rodents, in part, by suppressing appetite (Fig. 1) and preventing increases in feeding in compensation for exercise energy expenditure (Fig. 4).
The question of whether these effects of physical activity are homeostatic needs to be examined in the context of manipulations that affect energy balance. Much evidence overwhelmingly fails to support a homeostatic role of physical activity in energy regulation. First, there is a strong negative relationship between spontaneous physical activity and body fat in both animals and humans. Obesity in rodents is invariably associated with profound hypoactivity whether induced by high-fat diet feeding (27), lesions of medial basal hypothalamus (16), or the absence or inactivation of leptin or its receptors (22). Morbidly obese humans also are reported to be almost completely inactive (32). Conversely, maintaining rats on restricted access to food leads to 300% to 500% increases in spontaneous running as their weight loss increases. If the experiment is not terminated, rats virtually run themselves to death from energy depletion (24). This animal model is paralleled by human anorexia nervosa, a condition of suppressed food intake, sustained weight loss, and high motivation for, and involvement in, physical activity (6). Thus, the prevailing evidence indicates that spontaneous physical activity levels are related to body fat in inverse and nonhomeostatic fashion.
Leptin and insulin act on brain substrates of reward to mediate nonhomeostatic eating and locomotion
A way of reconciling the conflicting evidence regarding the restraining effects of insulin and leptin on appetite and body weight and fat gain (25) and the evidence that they do not influence meal-to-meal eating (4) is to consider actions of the two hormones on the brain substrates of reward. Evidence links actions of insulin and leptin to spontaneous food seeking and locomotor behaviors through their suppression of the relevant brain substrates of reward. Insulin secretion accompanies ingestion of food, and the magnitude of this response is related to size and composition of the meal. Increases in insulin concentration associated with meal eating suppress both motivation to eat (25) and the motivation for physical activity. The well known behavioral sequence in animals (and perhaps many humans) is to display somnolence and engage in sleep following a meal. Direct suppression by insulin injections of spontaneous physical activity in rats is seen at plasma concentrations that are too low to cause hypoglycemia and hunger (5). Similarly, hyperinsulinemia, that either accompanies lesions of VMH (9) or overeating after enforced weight loss (20), is accompanied by reductions in the levels of spontaneous physical activity or motivation to run on a treadmill. When hyperinsulinemia following the VMH lesions is prevented with subdiaphragmatic vagotomy, hyperphagia, obesity, and hypoactivity do not develop (9). Increased insulin secretion during the absorptive period stimulates increased transcription of the leptin gene. Leptin, in turn contributes to suppression of locomotor behavior as is shown for hyperactivity in anorexic subjects (8) and semi-starved rats (10). Thus the absorptive increases in insulin and leptin contribute to postmeal suppression of behavioral activation.
Absorption of a meal and decline of insulin to its basal levels are also associated with a decline in plasma leptin concentration (Fig. 3, right). As concentrations of both hormones decline, behavioral activation and spontaneous physical activity increase (14). Thus declines in insulin and leptin secretion after digestion and absorption of a meal increase the motivation for locomotor behavior. This implicates the two hormones at their low postabsorptive concentrations in facilitation of nonhomeostatic meal-to-meal feeding and stimulation of nonhomeostatic spontaneous locomotor activity.
In view of exercise-associated appetite suppression and increases in lipolysis, it would be logical to expect that these effects would be mediated by increased leptin release during exercise. This inference has to date not been substantiated, although several studies report exercise-associated increases in plasma leptin concentration (12, 18). Moderate exercise-associated leptin increases also are noticeable in our study (Fig. 4, right) and coincide with temporary suppression of hunger (Fig. 1). If more definitive support is found for the role of leptin in exercise-induced appetite suppression, this leptin action could serve as evidence for its role in functional linkage of meal eating and behavioral activation, and it could reveal a mechanism through which post-meal restraint over feeding contributes to reduction in the weight plateau that is observed in exercising animals and humans.
Substantial evidence suggests that involvement of insulin and leptin in nonhomeostatic control of meal eating and physical activity are mediated through their actions on the brain substrates of reward. Two hypothalamic circuits serving motivation and reward have been described (2). One responds to fluctuation in short-term energy availability and body fat levels (and, for convenience, will be called homeostatic) and the second one is activated in the obese condition and preferentially responds to external sensory aspects of food (hedonic circuit). The homeostatic circuit comprises a large area of the medial shell of the nucleus accumbens, the ventral pallidum, the medial part of the lateral hypothalamus, and the ventral tegmentum. It is activated by μ opioid, cannabinoid CB 1, and dopamine receptor stimulation. Such stimulation increases the drive to eat, obtain addictive rewards, and locomote. Both insulin and leptin receptors are present in, and are shown to influence, the homeostatic brain circuit of reward (11). Low insulin and leptin enhance, while high insulin and leptin dampen the rewarding value of stimulation of this circuit by suppressing release of dopamine and related neurotransmitters (14).
How nonhomeostatic meal-to-meal eating and locomotion help stabilize different weight plateaus
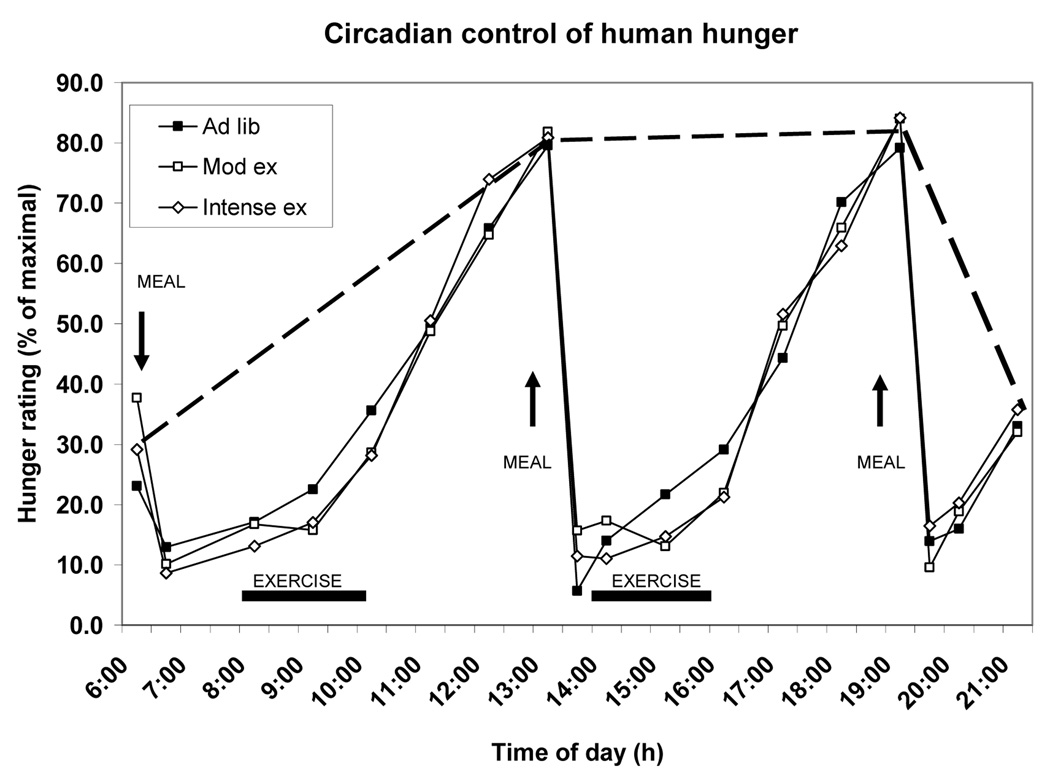
The evidence that meal-to-meal eating (4) and spontaneous physical activity operate in nonhomeostatic fashion leaves open the question regarding how stable weight plateaus that range from extreme leanness to morbid obesity are established and maintained. The resolution may reside in the roles of insulin and leptin within the temporal organization of feeding and behavioral activation during a 24 h day. This organization involves a circadian pattern of feeding and behavioral activation during approximately one half of the day, and behavioral quiescence and suppression of feeding during the other half. The circadian pattern to human hunger ratings is evident from Figure 5. In this study, 10 healthy postmenopausal women engaged in a sedentary trial and in two exercise trials requiring two bouts of treadmill walking differing in intensity but producing a similar workload (35) Two aspects of the circadian appetite organization are seen.
Figure 5.
Circadian changes in human hunger (broken line) in a study where three ad-libitum meals (Ad-lib) were offered at 6 to 7 h intervals, and two 2-h periods of exercise (ex) of different intensity but equivalent work load were provided. Mod, moderate. Based on data from (35).
First, hunger ratings are lower at the transition between waking and nocturnal phases of the day and are absent during the night. Second, hunger ratings are equally high at habitual feeding times in the sedentary and exercise trials and unaffected by the energy cost of exercise. The same was true in the previously described study despite variations in energy availability caused by exercise, different size meals, or intravenous nutrient infusions (Fig. 1). That meal-associated ratings of hunger show an ultradian (less than 24 h) periodicity that approximates the duration of time that it takes to digest and absorb a meal, and that at such times the hunger ratings are refractory to changes in energy availability, suggests a presence of a nonhomeostatic coordinating central nervous influence that activates hunger at habitual meal times in an all-or-none fashion.
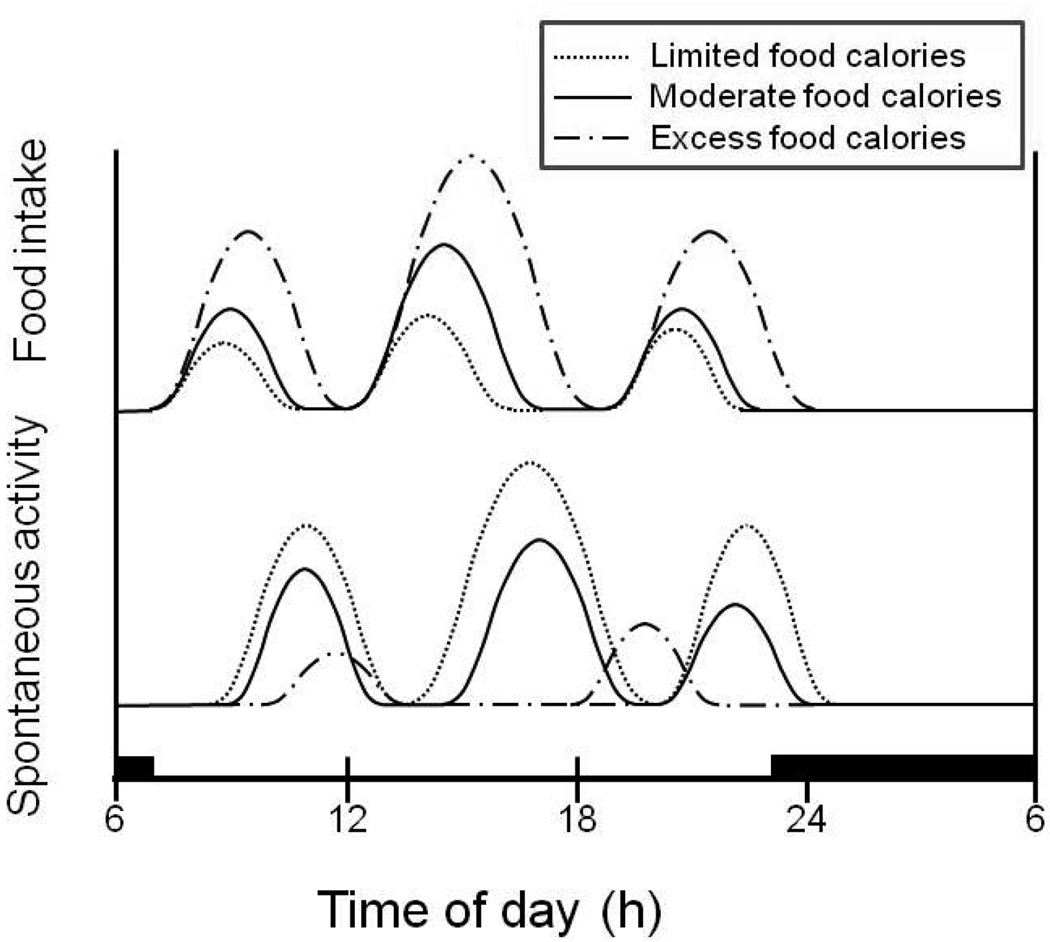
No feeding mechanism can effectively support survival without a capacity to respond to energy depletion. A functional connection between the nonhomeostatic control of meal-to-meal appetite and eating, and of spontaneous locomotor activity, resides in the sensitivity of the neural substrates of reward to variations in energy availability and levels of body fat. The reward valuation in the homeostatic drive circuit that motivates food seeking, hypothalamic self-stimulation, and locomotion is potentiated by food deprivation and body fat losses (2). This is accomplished, in part, by decrease in the inhibitory effects of circulating insulin and leptin on the neural substrates of reward when they reach low levels during negative energy balance and body fat loss. Therefore increased hunger and behavioral activation can be viewed as a mechanism that operates only when the size and caloric content of meals falls short of producing increased plasma insulin and leptin between habitual feeding times. If the ingested meal is small as was the case with 100-Kcal morning meal in our study, ratings of hunger are increased between the habitual meal times (Fig. 1), and behavior activation also is expected to be higher. The greater the shortfall in meal energy or weight loss and concomitant decline in insulin and leptin concentrations, the greater the activation of the homeostatic substrates of reward and increased motivation to seek food and locomote (Fig. 6). Operation of this chain of events is illustrated by the progressive and ultimately fatal increases in locomotor activity in rats maintained on restricted access to food that produces ongoing body fat and body weight losses (24).
Figure 6.
A concept of ultradian and nycthemeral oscillations in energy balance caused by a reciprocal relationship between the amount of food eaten and post-meal behavioral activation during day and suppression of both behaviors during night. Small meals (hatched line) lead to increased physical activity, while excess energy intake (broken line) suppresses locomotion.
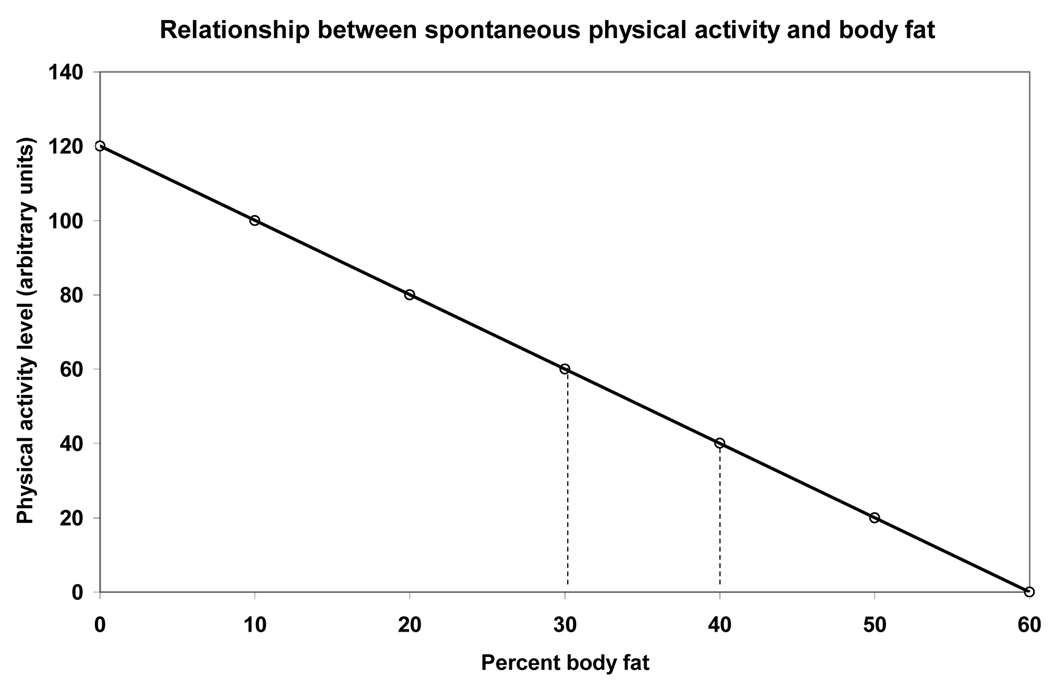
As meal eating raises the basal concentrations of both insulin and leptin, the already mentioned sequence of satiation and behavioral suppression is enacted. In step with increases in body fat, concentrations of the two hormones rise and inhibit locomotor behavior. At a morbidly obese state, they cause almost complete inactivity (32). Inhibitory effects of increased basal plasma insulin and leptin concentrations over locomotor activation account for the established reciprocal relationship between spontaneous physical activity and body fat levels (Fig. 7).
Figure 7.
The inverse relationship between spontaneous physical activity and body fat. Hatched vertical lines hypothetically define activity levels that should support body fat levels corresponding to body mass indices of between 20 and 30 kg/m2.
Metabolic contributions of insulin and leptin to maintenance of variable weight plateaus
Epidemic increases in human obesity and the ease of producing dietary obesity in animals may be attributed in part to the hedonic brain circuit of reward (2). This circuit comprises a smaller area of nucleus accumbens and ventral pallidum, orbitofrontal, anterior cingulate, and insular cortex, hippocampus and amygdala in the limbic forebrain, and mesolimbic dopamine projections to the nucleus accumbens and cortical sites. As with the homeostatic circuit, a hedonic pathway is activated by dopamine, μ opioids, and endocannabinoids. The hedonic circuit typically does not respond to declines in energy balance but is engaged at stable body weight plateaus in association with body fat increases and obesity, and is responsible for heightened finickiness to taste of food. Obese animals overeat palatable food, but lose weight and undereat unpalatable or bland foods (27). Both brain substrates of reward thus contribute to acquisition and storage of nutrient energy, the homeostatic one by increasing appetite and locomotion in response to energy shortage, and hedonic one by facilitating seeking palatable food uncoupled from energy shortage.
Leptin and insulin also contribute to stability of body weight and body fat levels through their metabolic actions. Insulin resistance develops at high body fat levels and limits further nutrient storage and fat synthesis by blunting antilipolytic and lipogenic insulin actions. In this way high basal plasma insulin concentrations contribute to maintenance of obese body fat levels. During food restriction and body weight losses, higher sensitivity of adipose and other peripheral tissues to insulin favors glycogen and lipid synthesis and drives the restoration of higher body fat levels.
The magnitude of a nocturnal rise in leptin concentration is proportional to the nutritional state at the onset of darkness. Nocturnal leptin concentration, and presumably its lipolytic action (33), are greater in response to energy surplus, and lower after energy depletion (31). Dawn and dusk nadirs of human hunger (Fig. 5) may reflect circadian transition from higher nocturnal leptin concentrations when lipolysis is increased and behavioral activation blocked to the lower diurnal leptin concentrations when leptin may affect the motivation to locomote in conjunction with meal eating. When humans deliberately truncate the period of nocturnal rest, declines in plasma leptin and increases in plasma ghrelin are associated with increased appetite and presumably greater fat synthesis (29). However, as levels of obesity rise and tissue resistance to leptin action increases (26), nocturnal lipolytic effects of leptin would be expected to decline and tend to maintain higher body fat levels.
CONCLUSION
Absence of a controlling role for leptin and insulin in meal-to meal eating prompts a reinterpretation of their role in energy regulation. Our research and other evidence indicate that both meal-to-meal feeding, appetite, and spontaneous physical activity operate in a nonhomeostatic fashion, while plasma leptin and insulin respond to short-term fluctuations in energy availability and bear no relationship to human appetite. The nonhomeostatic character of meal-to meal feeding and spontaneous physical activity is mediated by inhibitory actions of insulin and leptin on the brain substrates of reward. After meals and in response to body fat gain, increases in the concentration of these two hormones reduce the incentive value of food and behavioral activation. Negative energy balance and body fat losses reduce basal concentrations of both hormones and increase the motivation to seek food and locomote. A circadian clock produces maximal facilitation of appetite at habitual meal times regardless of the prevailing energy balance and thus contributes to a nonhomeostatic pattern of feeding. The contribution of both hormones to weight stability is mediated by their modulation of the responsiveness of brain substrates of reward to changes in momentary energy availability and body fat level and by producing metabolic effects that favor retention of higher body energy stores. The responsiveness of brain substrates of reward to reduced energy availability and body fat losses favors increases in food seeking motivation and behavioral activation and drives weight and fat gain after body fat losses. Homeostatic brain substrate of reward becomes refractory at high body fat levels when resistance to both hormones develops. In addition, a hedonic brain circuit that is uncoupled from responsiveness to energy deficit favors opportunistic ingestion of palatable food.
Human feeding behavior is thus designed for nonhomeostatic meal-to-meal eating and is influenced by social forces that interfere with optimal operation of the energy regulatory mechanism. Environmental circumstances and the design of controls of eating behavior enhance the motivation for food seeking when energy deprived and after body fat loss but also encourage humans to overeat highly palatable and energy rich foods when not energy deprived. This suppresses physical activity and engages the hedonic brain circuit both of which contribute to excess fat synthesis and accumulation. Our currently inadequate understanding of the energy regulatory mechanism limits the opportunity to adjust conditions that would allow optimal operation of feeding behavior and physical activity for maintenance of healthy weight. The need for additional knowledge is particularly acute in four areas:
Definition of quantities and palatability characteristics of human meals that would preserve the ultradian motivation to be physically active;
Characterization of types, volumes, and intensity of physical activity that would produce optimal diurnal between-meal appetite suppression and nocturnal lipolysis;
Coordinated research on the role of the hormones that track the changes in energy balance (e.g., leptin, insulin, ghrelin, and gut peptides) by interacting with the neurochemical and neuroanatomical control of feeding and physical activity in the context of ultradian and nycthemeral organization; and finally,
Assessment of the feasibility of restructuring the human environment to reduce exposure to highly palatable and energy-rich food and increase the opportunity for physical activity in the workplace (30) and elsewhere.
Acknowledgements
Because of the journal limit on the number of references, the author regrets her inability to cite the work of many authors deserving recognition.
Funding: NIH grants R15 DK066286 to KTB, DK20572 to Michigan Diabetes Research and Training Center, and M01-RR00042 to Michigan Clinical Research Unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adolph EF. Urges to eat and drink in rats. Amer J Physiol. 1947;151:110–125. doi: 10.1152/ajplegacy.1947.151.1.110. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. “Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 4.Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab. 2009;94:2290–2298. doi: 10.1210/jc.2008-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell BA, Fibiger HC. Effects of insulin on spontaneous activity during food deprivation. J Comp Physiol Psychol. 1970;71:341–346. doi: 10.1037/h0029132. [DOI] [PubMed] [Google Scholar]
- 6.Casper RC. The “drive for activity” and “restlessness” in anorexia nervosa: potential pathways. J Affect Disord. 2006;92:99–107. doi: 10.1016/j.jad.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Dethier VG, Solomon RL, Turner LH. Sensory input and central excitation and inhibition in the blowfly. J Comp Physiol Psychol. 1965;60:303–313. doi: 10.1037/h0022557. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich S, Burghardt R, Schneider N, Broecker-Preuss M, Weiss D, Merle JV, Craciun EM, Pfeiffer E, Mann K, Lehmkuhl U, Hebebrand J. The role of leptin and cortisol in hyperactivity in patients with acute and weight-recovered anorexia nervosa. Prog Neuro-Psychopharm Biol Psychiat. 2009;33:658–662. doi: 10.1016/j.pnpbp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Eng R, Gold RM, Sawchenko PE. Hypothalamic hypoactivity prevented but not reversed by subdiaphragmatic vagotomy. Physiol Behav. 1978;20:637–641. doi: 10.1016/0031-9384(78)90257-3. [DOI] [PubMed] [Google Scholar]
- 10.Exner C, Hebebrand J, Remschmidt H, Wewetzer C, Ziegler A, Herpertz S, Schweiger U, Blum WF, Preibisch G, Heldmaier G, Klingenspor M. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry. 2000;5:476–481. doi: 10.1038/sj.mp.4000771. [DOI] [PubMed] [Google Scholar]
- 11.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: Update 2008. Am J Physiol. 2009;296:R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JS, Van Pelt RE, Zinder O, Landt M, Kohrt WM. Acute exercise effect on postabsorptive serum leptin. J Appl Physiol. 2001;91:680–686. doi: 10.1152/jappl.2001.91.2.680. [DOI] [PubMed] [Google Scholar]
- 13.Franz MJ, VanWormer JJ, Crain L, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J Am Diet Assoc. 2007;107:755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 15.Kendall A, Levitsky DA, Strupp BJ, Lissner L. Weight loss on a low-fat diet: consequence of the imprecision of the control of food intake in humans. Am J Clin Nutr. 1991;53:1124–1129. doi: 10.1093/ajcn/53.5.1124. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy GC, Mitra J. Hypothalamic control of energy balance and the reproductive cycle in the rat. J Physiol. 1963;166:396–407. doi: 10.1113/jphysiol.1963.sp007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin Nut. 1994;48:715–724. [PubMed] [Google Scholar]
- 18.Kraemer RR, Durand RJ, Acevedo EO, Johnson EO, Synovitz LB, Kraemer GR, Gimpel T, Castracane VD. Effects of high-intensity exercise on leptin and testosterone concentrations in well-trained males. Endocrine. 2003;21:261–265. doi: 10.1385/ENDO:21:3:261. [DOI] [PubMed] [Google Scholar]
- 19.Levitsky DA. The non-regulation of food intake in humans: Hope of reversing the epidemic of obesity. Physiol. Behav. 2005;86:623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 20.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackson MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol. 2009;297:R793–R802. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 22.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 23.Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychol Bull. 2001;127:325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- 24.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psych. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 28.Shepard TY, Weil KM, Sharp TA, Grunwald GK, Bell ML, Hill JO, Eckel RH. Occasional physical inactivity combined with a high-fat diet may be important in the development and maintenance of obesity in human subjects. Am J Clin Nutr. 2001;73:703–708. doi: 10.1093/ajcn/73.4.703. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter Eve. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 30.Thompson WG, Foster RC, Eide DS, Levine JA. Feasibility of a walking workstation to increase daily walking. Br J Sports med. 2008;42:225–228. doi: 10.1136/bjsm.2007.039479. [DOI] [PubMed] [Google Scholar]
- 31.van Aggel-Leijssen DP, van Baak MA, Tenenbaum R, Campfield LA, Saris WH. Regulation of average 24 h human plasma leptin level; the influence of exercise and physiological changes in energy balance. Int J Obes Relat Metab Disord. 1999;23:151–158. doi: 10.1038/sj.ijo.0800784. [DOI] [PubMed] [Google Scholar]
- 32.Vanhecke TE, Frankin BA, Miller WM, deJong AT, Coleman CJ, McCullough PA. Cardiorespiratory fitness and sedentary lifestyle in the morbidly obese. Clin Cardiol. 2009;32:121–124. doi: 10.1002/clc.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang MY, Leem Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 34.Welch I, Saunders K, Read NW. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroeneterology. 1985;89:1293–1297. doi: 10.1016/0016-5085(85)90645-6. [DOI] [PubMed] [Google Scholar]
- 35.Wuorinen E. Ph.D. Dissertation. University of Michigan; 2007. Detection of exercise energy expenditure. [Google Scholar]