Abstract
This experimental investigation tested two different strains of rat, Sprague–Dawley (SD) and Fischer 344 (F344), in their ability to learn lever pressing for food (autoshaping) or intracranial self-administration (ICSA) of dextroamphetamine (AMPH) into the nucleus accumbens (NAcc). Additionally, a unique method of intracranial drug delivery was utilized, via reverse dialysis, by the use of a microdiaylsis probe. The experiments revealed definite behavioral differences between SD and F344 animals. The autoshaping data indicated that SD rats, on average, acquired lever pressing for food in fewer training days than F344 rats. Also, the ICSA experiment revealed that SD rats self-administered AMPH at a 30 μg/μl concentration. Lever pressing was significantly greater in those SD rats receiving AMPH than in the F344 drug group. Furthermore, the F344 rats never acquired lever pressing for intra-NAcc delivery of AMPH under our testing regime. These data reveal differences in performance of positively reinforced operant tasks between the inbred F344 rats as compared to the outbred SD strain.
Keywords: Self-administration, Dextroamphetamine, Reverse microdialysis, Lever pressing, Intracranial
1. Introduction
Neural substrates of reward and of drug self-administration potentially differ between strains of rats. Accordingly, strain differences have been reported in experiments involving reinforcement. A number of studies have shown differences in drug preference between F344 and other inbred rat strains. For example, nicotine produces conditioned place preference, a measure of drug reward, in Lewis but not F344 rats (Horan et al., 1997), and nicotine is self-administered by Lewis but not F344 rats (Brower et al., 2002). Similar differences have been reported with other compounds. For instance, Lewis rats self-administer cocaine after less training and at lower doses than do F344 rats (Kosten et al., 1997), and ethanol is a strong reinforcer in the Lewis strain, but a weak reinforcer in the F344 strain (Suzuki et al., 1988).
Conversely, the same strain differences have not been obtained with all compounds. For instance, F344 rats were found to consume significantly greater amounts of morphine in a two-bottle choice procedure and maintained significantly greater morphine intravenous self-administration than another inbred strain, WAG/GSto rats (Sudakov et al., 1993). Similarly, AMPH produces a more pronounced conditioned place preference in F344 than in Lewis rats, and AMPH-induced locomotion is higher in F344 than Lewis rats (Stohr et al., 1998).
Thus, previous studies suggest that F344 rats appear to differ in responding to drugs of abuse as compared to other inbred strains of rat. Therefore, we sought to determine if there were performance differences in responding for positive reinforcers as compared to an outbred strain of rat. To examine this question, we compared the self-administration of AMPH by F344 rats with that of SD rats, an outbred strain known to self-administer AMPH (Fletcher, 1998). To minimize the role of pharmacokinetic factors in the possible strain differences, we used a procedure involving ICSA of AMPH directly into the NAcc via reverse dialysis. To examine the selectivity of the effects, the animals’ ability to learn lever pressing for food was analyzed in addition to their acquisition of self-administration of AMPH.
2. Methods
All procedures were approved in advance by the Institutional Animal Care and Use Committee.
2.1. Subjects
Male (275–299 g) Sprague–Dawley (Harlan) and Fischer 344 (Charles River) rats were allowed 1 week to acclimate to the vivarium. Thereafter, two animals from each strain were randomly selected to serve as weight controls, and the remaining animals were food restricted until they were at 85% of the controls’ weight. Upon reaching the weight criterion, 37 animals (19 SD and 18 F344) were autoshaped in an operant chamber with food as a reinforcer (Oscos et al., 1988). Additionally, a different set of 16 animals underwent stereotaxic surgery for subsequent ICSA testing (8 SD rats, n =4 for vehicle and n =4 for AMPH; 8 F344 rats, n =3 for vehicle and n =5 for AMPH).
2.2. Behavioral apparatus
The training and test procedures used computer-controlled operant chambers in sound-attenuating boxes (Coulbourn Instruments, Allentown, PA USA). One wall of each chamber contained a central feeder bin and a left and right lever assembly with one being active and the other inactive. The active lever would retract and extend while the inactive lever remained extended. A cue light was positioned directly above the active lever and was illuminated only during lever extension for both experiments. Responding on the inactive lever had no programmed consequence but was measured as an index of locomotor activity. An overhead houselight was illuminated throughout the behavioral procedures. Food pellets (45 mg, BioServ, San Diego, CA USA) were used during autoshaping. The same chambers, excluding the central feeder bin, were used for AMPH self-administration testing.
2.3. Autoshaping
Autoshaping consisted of daily training sessions of 20 trials, using a fixed-ratio 1 schedule of reinforcement, with 45-second intertrial intervals. A trial is the term used to denote the active lever extension period (10 s), which gave the animal an opportunity to press. The intertrial interval is defined as the time-out period between the active lever’s retraction and its subsequent extension (i.e., the amount of time the lever was retracted). During a trial, if the animal did not press the lever within the 10-second period, a food pellet would still be delivered. However, pressing the extended lever during the trial shortened the waiting time for the food reinforcer. To be considered trained, animals had to lever press to a criterion of 90% (lever press 18 out of 20 trials) for 2 consecutive days.
2.4. In vitro determination of radiolabeled methamphetamine diffusion across a microdialysis membrane
Before beginning the experiments described here, we first determined how much radiolabeled AMPH would permeate through the microdialysis probe we would be using. We prepared 30 μg/μL methamphetamine (Sigma-Aldrich Co., St. Louis, MO) in 1.0 mL of lactated Ringer’s solution. To this was added 5.4 μL of the stock [3H] radiolabeled methamphetamine (1.5 mM) (National Institute for Drugs of Abuse, Bethesda MD). This diluted [3H] methamphetamine-plus-cold-methamphetamine was the solution that went in the syringe (7.9 μM radiolabeled methamphetamine). Before filling the syringe, we set aside three 1.0-μL aliquots from the tritiated drug stock solution and put them into separate scintillation vials. These samples were used to relate radiation quantification to concentration. We then added 200 μL of lactated Ringer’s solution (vehicle) to a 1.5-mL eppendorf tube; this would be the bath solution for the microdialysis probe filled with radiolabeled drug to equilibrate in. Next, the microdialysis probe was placed into the eppendorf tube with the dialysis membrane submerged in the vehicle solution. The drug syringe was placed in the syringe pump and connected to the probe with tubing. The pump was started for 1 minute at a speed of 2 μL per minute and then stopped to allow the infused drug to equilibrate with the vehicle in the tube for another minute. This starting and stopping of the pump was repeated, to mimic 5 and 10 lever press conditions for drug infusions, with three different probes. Subsequently, we aspirated the radiolabeled drug perfusate solution from the tube and added 10 mL of scintillation fluid to measure counts per minute (cpm) with a liquid scintillation counter. The above process was repeated three times with different probes to determine the mean disintegrations per minute (dpm). We calculated the probes to be 10% permeable to the drug.
2.5. Drugs
Dextroamphetamine sulfate was used as the drug reinforcer (30 μg/μL) (Sigma-Aldrich Co., St. Louis, MO). Previous ICSA experiments performed in our laboratory, using SD rats, revealed that this concentration of AMPH is reinforcing (Rodriguez et al., 2001). Additionally, a dose response curve using F344 rats was performed with the 30 μg/μL concentration producing the most lever presses as compared with two lower concentrations (3 and 10 μg/μL). Albeit, in that preliminary F344 dose response study the number of animals was low (n = 2/group) and our criteria of ICSA (10 or more presses for 3 consecutive days) was not attained with any of the concentrations. This drug dose falls within the range used in other studies using different strains of rat (Hoebel et al., 1983; Phillips et al., 1994; Chevrette et al., 2002) and takes into account the 10% efficacy of the microdialysis probe. Vehicle was lactated Ringer’s solution composed of electrolytes mEq/l: Na+ 130, K+ 4, Ca2+ 3, Cl− 110, and Lactate 28 (B. Braun Medical Inc., Irvine, CA).
2.6. Surgery
Naïve animals were anesthetized with an intraperitoneal injection of 50 mg/kg sodium pentobarbital (Nembutal, Abbott Laboratories, Chicago, IL, USA) and underwent stereotaxic surgery. Unilateral administration of AMPH has been shown to affect operant behaviors (Schildein et al., 1998); thus surgery consisted of implanting a guide cannula unilaterally, dimensions of 0.38-mm outer diameter and 14-mm length (CMA/Micro-dialysis AB, Stockholm, Sweden), directed to the right NAcc using coordinates: +1.5 mm anterior to bregma, +1.5 mm lateral to bregma, and −5.5 mm ventral to bregma (Paxinos and Watson, 1998). Three surgical grade screws attached to the skull and dental cement held the guide cannula in place. Topical antibiotic ointment was applied to the surgical wound to prevent infection, and ibuprofen (80 mg/day) was put into the drinking water for 2–3 days in order to alleviate discomfort. Also, 0.10 mL (30 K units) of penicillin was injected intramuscularly to combat any possible bacterial infection due to the surgical procedure. Animals were allowed 1 week to recover from surgery before AMPH self-administration testing.
2.7. Reverse microdialysis
A microdialysis probe was used to deliver the drug by reverse dialysis to reduce high pressure infusions as compared with an open-ended cannula. The physical properties of the dialysis membrane enable solutions to diffuse along their concentration gradient. The AMPH solution thus diffuses out into the NAcc rather than being crudely administered as a bolus injection, which would occur with an open-ended cannula. The use of reverse microdialysis has the advantage of maintaining a drug concentration due to its slow flow rate with the volume being dialyzed over a long period of time and without spread beyond the site of microdialyzed administration (Quan and Blatteis, 1989). This technique also prevents tissue damage due to aqueous solution droplets (Quan and Blatteis, 1989). The reverse microdialysis technique offers reliable drug diffusion over the desired area without pressure injection variability (Bazzett et. al., 1991). In addition, it is a powerful technique for the study of local actions of drugs in different tissues such as specific brain nuclei (Hocht et al., 2003). Furthermore, the probe could be advantageous for both the administration of drug and concomitant collection of neurotransmitter levels. Finally, we have not directly compared open-ended cannulae and reverse dialysis for behavioral advantages. But using the microdiaylsis probe has its benefits as mentioned above.
2.8. Intracranial self-administration testing
In our drug self-administration paradigm, acquisition of intra-NAcc self-administration is defined as 10 or more responses on at least 3 consecutive days. On all test days the dummy cannula was removed and replaced with a microdialysis probe, which had a 0.24-mm outer diameter and 2.00-mm membrane length (CMA/Microdialysis AB). Next, the animal was immediately placed into the chamber and the dialysis probe was connected to the preloaded infusion (no dead volume).
Daily testing sessions consisted of 30 trials (30 lever extensions) with a fixed-ratio 1 schedule of reinforcement every 24 h for 8 days. Each trial lasted 1 minute. If the animal did not press the lever during the 1-minute presentation, then a 1-minute intertrial interval followed. If a lever press did occur during the 1-minute trial, then the lever would retract, the cue light would turn off and activation of the infusion pump would result. The drug or vehicle infusion lines were preloaded with their respective solution to avoid dead space. The AMPH group was tested only with drug whereas the control group was tested only with vehicle solution. The flow rate was 2 μL per minute with a 1-minute flow of either vehicle or 30 μg/μL AMPH solution followed by a 1-minute intertrial interval. Animals were euthanized 24 h after their last self-administration session with subsequent brain removal for histological verification. This behavioral paradigm is reproducible and has previously been performed in our laboratory (Rodriguez et al., 2001).
2.9. Data acquisition and analysis
Pump drug delivery (Harvard Apparatus, Holliston, MA USA) and the lever presses were controlled and data were recorded using the Winlinc Behavioral Experiment Control Software (Coulbourn Instruments, Allentown, PA USA) run by a desktop computer. The number of lever presses was recorded and exported to an Excel file for further analysis. Total active lever presses versus days were measured and plotted to compare groups. A two-way analysis of variance with repeated measures and Student–Newman Keuls post-hoc analysis were used to detect significant differences by means of Sigma Stat software.
2.10. Histological verification
Animals were euthanized via a 1-mL injection of sodium pentobarbital 75 mg/kg solution (Nembutal, Abbott Laboratories, Chicago, IL, USA). Prior to heart beat cessation, transcardial perfusions were performed by the administration of 0.9% physiological saline followed by formaldehyde fixation. The brains were extracted and placed in fixative until sectioning. A vibrotome was used to cut the brain into 200- to 300-micron sections.
3. Results
The autoshaping experimental procedure revealed a behavioral difference between SD and F344 rats. Fig. 1 shows that in the first 5 days of training SD rats lever-pressed more for food than did F344 rats. By day 6, most SD rats had reached criterion and no longer needed training (Table 1). Training continued beyond what Fig. 1 represents with all the SD rats learning by day 7. As shown in Table 1, most of the F344 rats learned by day 8; however, 3 of the 18 F344 rats never acquired the autoshaping procedure. Comparison between SD and F344 rats (Fig. 1) in the first 5 days revealed a significant effect between strains (F(1,35) =15.05; p <0.001) and day (F(4,140) =40.08; p <0.001) by two-way ANOVA. Analysis also revealed a significant interaction between strains and days ( p =0.006). Student Newman–Keuls posthoc analysis showed significantly more lever pressing in the SD than in the F344 rats on days 2, 3, 4, and 5 ( p <0.001). Those SD rats which reached performance criterion prior to day 5 were included in the statistical analysis as a mean group/day substitution performance score for days 4 and 5 (total of 2 observations for day 4 and 9 observations for day 5). Also, three F344 rats which never attained performance criterion were included in the statistical analysis.
Fig. 1.
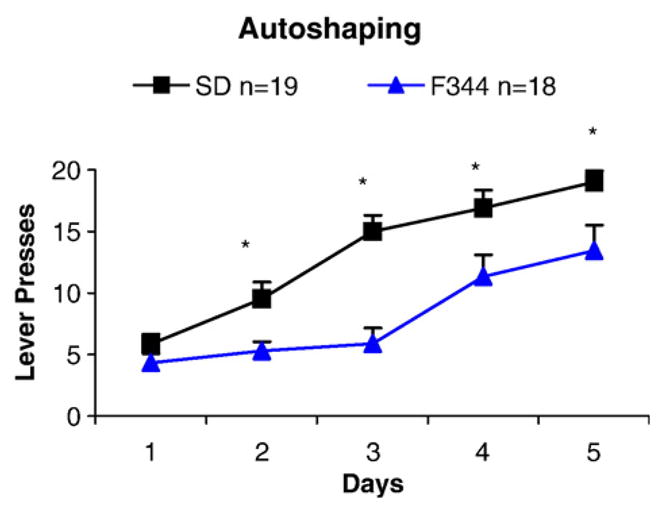
Comparison between Sprague–Dawley (SD) and Fischer 344 (F344) rats in responding for food reinforcement revealed a significant effect of strain and days of training. Student Newman–Keuls posthoc analysis showed significantly more lever pressing in the SD than the F344 strain on days 2, 3, 4, and 5 (*p< 0.001). SD rats which attained lever pressing criterion prior to day 5 were included in the statistical analysis as a mean group/day substitution performance score for days 4 and 5 (total of 2 observations for day 4 and 9 observations for day 5).
Table 1.
Time table of daily lever pressing for food reinforcement in SD and F344 rats
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 2 | 10 | 16 | 19 | 19 | ||||
| 2 | 1 | 0 | 12 | 19 | 20 | |||||
| 3 | 3 | 11 | 13 | 20 | 19 | |||||
| 4 | 10 | 19 | 20 | |||||||
| 5 | 7 | 13 | 11 | 20 | 20 | |||||
| 6 | 8 | 15 | 20 | 20 | ||||||
| 7 | 1 | 3 | 4 | 2 | 3 | 18 | 20 | |||
| 8 | 7 | 5 | 20 | 20 | ||||||
| 9 | 7 | 15 | 19 | 20 | ||||||
| 10 | 7 | 13 | 20 | 2 | 20 | 20 | ||||
| 11 | 4 | 9 | 16 | 20 | 20 | |||||
| 12 | 4 | 10 | 19 | 20 | ||||||
| 13 | 8 | 15 | 19 | 20 | ||||||
| 14 | 6 | 12 | 19 | 20 | ||||||
| 15 | 9 | 9 | 18 | 20 | ||||||
| 16 | 14 | 18 | 20 | |||||||
| 17 | 0 | 4 | 5 | 13 | 20 | 20 | ||||
| 18 | 4 | 4 | 14 | 20 | 20 | |||||
| 19 | 6 | 4 | 6 | 9 | 20 | 20 | ||||
| 20 | 5 | 3 | 13 | 18 | 19 | |||||
| 21 | 4 | 5 | 6 | 6 | 20 | 19 | ||||
| 22 | 2 | 6 | 2 | 0 | 4 | 0 | 2 | 0 | 2 | 0 |
| 23 | 1 | 2 | 3 | 12 | 18 | 19 | ||||
| 24 | 1 | 7 | 6 | 11 | 20 | 1 | 20 | 20 | ||
| 25 | 8 | 5 | 9 | 20 | 20 | |||||
| 26 | 7 | 8 | 6 | 14 | 20 | 20 | ||||
| 27 | 4 | 13 | 16 | 20 | 20 | |||||
| 28 | 9 | 10 | 16 | 19 | 20 | |||||
| 29 | 6 | 3 | 2 | 15 | 20 | 20 | ||||
| 30 | 8 | 8 | 4 | 8 | 15 | 20 | 20 | |||
| 31 | 5 | 3 | 2 | 2 | 1 | 6 | 18 | 20 | ||
| 32 | 7 | 6 | 0 | 2 | 3 | 20 | 20 | |||
| 33 | 7 | 2 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 |
| 34 | 3 | 1 | 1 | 14 | 18 | 20 | ||||
| 35 | 1 | 2 | 1 | 2 | 3 | 2 | 2 | 5 | 0 | 2 |
| 36 | 0 | 5 | 8 | 19 | 1 | 20 | 20 | |||
| 37 | 0 | 6 | 11 | 19 | 20 |
Raw data demonstrating Sprague–Dawley rats (rows 1–19) and Fischer 344 (rows 20–37) rats lever pressing for food.
Table shows the learning rate for each animal in acquiring the task and responding at a 90% criterion (respond for 18 of 20 possible reinforcers) for 2 consecutive days. All SD rats learned the task, whereas 15 of the 18 F344 rats reached criterion and 3 failed to respond for food.
The self-administration paradigm also revealed different behavioral profiles for the two strains. Following our testing regime, SD rats acquired ICSA of AMPH (Fig. 2), but the F344 rats did not. Comparison of vehicle control groups between strains revealed no difference (F(1,5) =0.001; p=0.968), and the two groups of controls were subsequently pooled for further analysis (SD n=4, F344 n=3 for total vehicle n=7). Comparison between SD rats and vehicle controls revealed a significant effect of treatment (F(1,9) =5.94; p =0.038). Student Newman–Keuls post-hoc analysis showed significantly more lever pressing by the SD rats receiving AMPH than by controls on days 6, 7, and 8 (p<0.05). By contrast, comparison of F344 rats and vehicle controls revealed no significant effect of treatment (F(1,10) =0.27; p=0.617). Comparison of SD and F344 AMPH self-administering groups revealed a significant main effect of strain (F(1,7) =14.029; p=0.007) and a significant interaction between strains and days (p<0.001). Student Newman–Keuls posthoc analysis showed significantly more lever pressing for AMPH by SD than by F344 rats on days 4, 6, 7, and 8 (p<0.05).
Fig. 2.
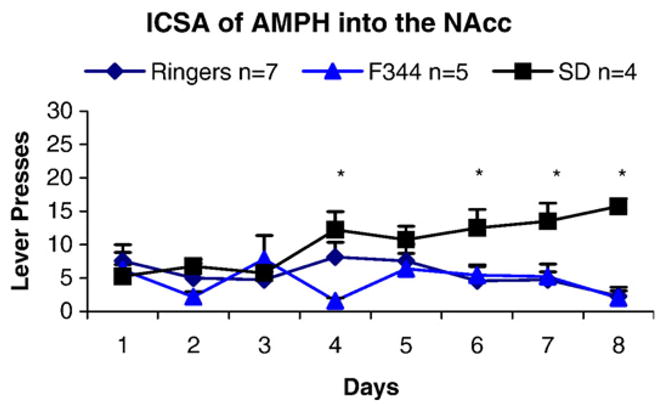
Comparison of SD and F344 AMPH self-administering groups revealed a significant main effect of strain (F(1,7) =14.029; p =0.007) and a significant interaction between strains and days (p=0.001). Student Newman–Keuls posthoc analysis showed significantly more lever pressing for AMPH by SD than by F344 rats on days 4, 6, 7, and 8 (*p< 0.05). ICSA=intracranial self-administration, AMPH=amphetamine, NAcc=nucleus accumbens, SD=Sprague–Dawley, F344=Fischer 344.
During the intertrial interval, lever presses from the AMPH groups (F344 and SD) were observed on both the active and inactive levers. A two-tailed t-test analysis revealed no significant differences between the two strains in the number of presses on the active lever (p =0.40), but the F344 rats did press more on average over the course of the 8-day study (Fig. 3). Activity on the inactive lever was more pronounced in SD than in F344 rats during the intertrial interval when the only lever extended was the inactive one (p =0.08) (Fig. 3).
Fig. 3.
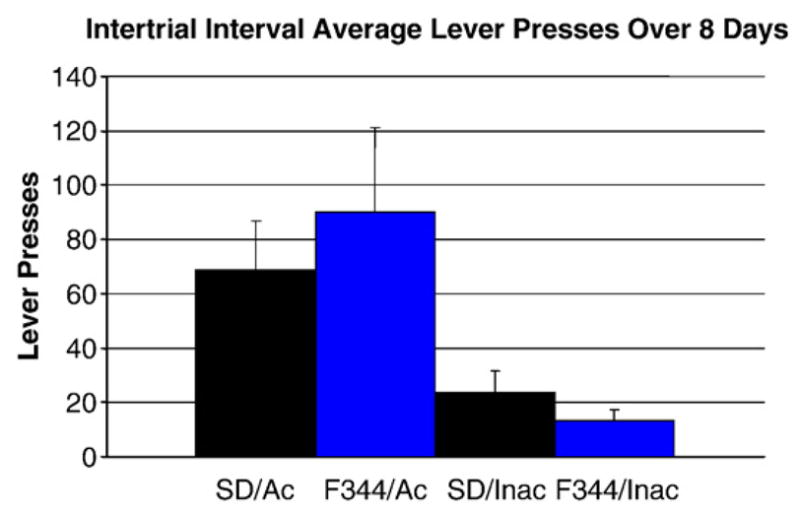
Comparison between F344 and SD rats’ average number of active (Ac) and inactive (Inac) lever presses during the intertrial interval revealed no significant effect of strain, by t-test (p=0.40 and p=0.08, respectively).
Histology results showed that all animals included in the self-administration study had correct probe placement, the probe tips were within both the core and shell subterritories, and hence no animals were excluded. Fig. 4 is a composite illustration of the microdialysis probe tip locations for all 16 animals showing the lateral variations with no anterior/posterior differences.
Fig. 4.
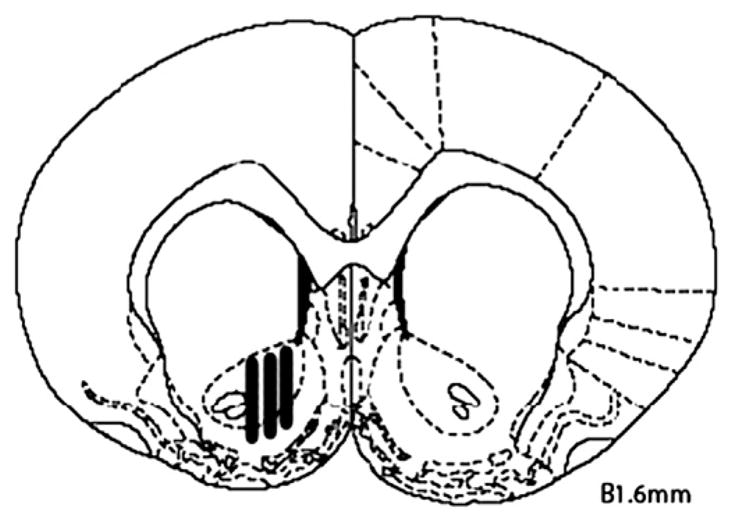
A frontal view of a coronal section, redrawn from the 1998 rat brain atlas of Paxinos and Watson, showing probe locations (B1.6 denotes +1.6 mm anterior to bregma). The black bars represent the locations of the 2-mm dialysis tips, within the NAcc, for all animals showing the lateral variations with no anterior/ posterior differences. Histological examination showed that all probes lay within these positions. No animals were excluded for incorrect probe placement.
4. Discussion
In this study, F344 rats did not respond as well as SD rats in two positive reinforcement tasks. The SD rats, on average, reached lever pressing criterion for food responding with fewer training sessions than the F344 rats required, and the SD rats acquired intra-NAcc self-administration whereas the F344 rats did not. The novelty of our findings pertains to the behavioral differences observed with regards to the ICSA study. The results suggest that the local application of AMPH into the NAcc reveals possible neural biological differences in the reward circuitry between the inbred and outbred strains. Additionally, this method of drug delivery for intra-NAcc self-administration has not previously been reported. However, Hernandez et al. (1987) did report experimenter administered AMPH into the NAcc via a microdialysis probe with subsequent detection of dopamine levels. Our technique of ICSA could potentially allow for concomitant detection of dopamine levels within the NAcc. That behavioral and neurochemical study may assist in the explanation of the results reported here.
The mesolimbic dopamine system may underlie the differences between the strains. In previous studies Nestler’s group reported that tyrosine hydroxylase and neurofilament proteins are modified by chronic morphine and chronic cocaine treatments in the ventral tegmental area (VTA) in SD rats and that the inbred Lewis and F344 rat strains, under drug naïve conditions, show different levels of these proteins in the VTA (Beitner-Johnson et al., 1991, 1993). That group also showed that levels of adenylyl cyclase and cAMP-dependent protein kinase activity in the NAcc and locus coeruleus are higher in Lewis than in F344 rats (Guitart et al., 1993). Furthermore, Camp et al. (1994) reported that compared with F344 rats, Lewis rats showed greater behavioral activation and an enhanced extracellular concentration of dopamine following an acute injection of methamphetamine or cocaine, as indicated by a shift to the left in the dose-effect curve. They also found that Lewis rats were more susceptible than F344 rats to methamphetamine sensitization. They contended that differences in pharmacokinetics between the two strains might account for the behavioral and neuro-chemical differences since the Lewis rats had higher plasma and brain levels of methamphetamine and cocaine than did F344 rats. Because neurochemical measurements were not performed in our study, it is not known whether there was a difference in extracellular neurotransmitter concentrations between the SD and F344 rats during ICSA.
In this study, the F344 rats did press the active lever during the intertrial interval, which suggests a nonspecific psychos-timulant effect from the few infusions that were self-administered. Even so, it was the lack of pressing during the time the active lever was extended that demonstrated profound behavioral differences between the two strains. This behavioral difference may be attributed to the possible sensitivity the F344 rats have to AMPH. However, numerous reports suggest the contrary with regard to psychostimulant effects on F344 rats (George et al., 1991; Kosten et al., 1997; Camp et al., 1994). Conversely, Kosten et al. (2007) has recently reported that F344 rats self-administer more cocaine intravenously than Lewis rats. So, the extent of drug-taking behavior by F344 rats remains equivocal in the literature.
Although our drug self-administration paradigm eliminated any pharmacokinetic differences between the two strains (both strains received local drug infusions which eliminates systemic metabolism), it did not affect the possible pharmacodynamic differences that may explain the results reported here (the subjective affect to the drug, i.e. hedonic or aversive). Also, we can not rule out that the F344 rats expressed gliosis as an explanation for the lack of responding for AMPH. So, the use of F344 rats for studies of reinforcement tasks may need to be reconsidered. Nonetheless, additional work needs to be performed to investigate the neurochemical and genetic expression events associated with these behaviors that could underlie the differences reported here.
Acknowledgments
Our sincerest appreciation goes to Dr. Robert Renthal for all his assistance with the in vitro experiment. Supported by DA 04195, RR 013646-06A1, the Ewing Halsell Foundation, and the Kleberg Foundation to JLM and a Minority Supplement to JSR.
Footnotes
There is no conflict of interest for all involved in this manuscript preparation.
References
- Bazzett TJ, Becker JB, Albin RL. A novel device for chronic intracranial drug delivery via microdialysis. J Neurosci Methods. 1991;40(1):1–8. doi: 10.1016/0165-0270(91)90111-c. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine- and cocaine-regulated phosphoproteins. Brain Res. 1991;561(1):147–50. doi: 10.1016/0006-8993(91)90759-o. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis–Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61(5):1766–73. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930(1–2):12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res. 1994;668:180–93. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Chevrette J, Stellar JR, Hesse GW, Markou A. Both the shell of the nucleus accumbens and the central nucleus of the amygdala support amphetamine self-administration in rats. Pharmacol Biochem Behav. 2002;71:501–7. doi: 10.1016/s0091-3057(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. A comparison of the effects of risperidone, raclopride, and ritanserin on intravenous self-administration of d-amphetamine. Pharmacol Biochem Behav. 1998;60(1):55–60. doi: 10.1016/s0091-3057(97)00559-5. [DOI] [PubMed] [Google Scholar]
- George FR, Porrino LJ, Ritz MC, Goldberg SR. Inbred rat strain comparisons indicate different sites of action for cocaine and amphetamine locomotor stimulant effects. Psychopharmacology. 1991;104:457–62. doi: 10.1007/BF02245649. [DOI] [PubMed] [Google Scholar]
- Guitart X, Kogan JH, Berhow M, Terwilliger RZ, Aghajanian GK, Nestler EJ. Lewis and Fischer rat strains display differences in biochemical, electro-physiological and behavioral parameters: studies in the nucleus accumbens and locus coeruleus of drug naïve and morphine-treated animals. Brain Res. 1993;611(1):7–17. doi: 10.1016/0006-8993(93)91770-s. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Lee F, Hoebel BG. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: increase in extracellular dopamine and serotonin. Brain Res Bull. 1987;19:623–8. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Hocht C, Opezzo JA, Gironacci M, Pena C, Taira CA. Study of the hypothalamic angiotensin system in aortic coarctated rats using the reverse microdialysis technique. Pharmacol Res. 2003;48(3):301–7. doi: 10.1016/s1043-6618(03)00126-9. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology. 1983;81:158–63. doi: 10.1007/BF00429012. (Berl) [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26(1):93–4. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nester EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci. 2007;121(2):380–8. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Oscos A, Martinez JL, Jr, McGaugh JL. Effects of post-training d-amphetamine on acquisition of an appetitive autoshaped lever press response in rats. Psychopharmacology. 1988;95(1):132–4. doi: 10.1007/BF00212781. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology. 1994;114:477–85. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Quan N, Blatteis CM. Microdialysis: a system for localized drug delivery into the brain. Brain Res Bull. 1989;22(4):621–5. doi: 10.1016/0361-9230(89)90080-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Phelix CF, Martinez JL., Jr D-Amphetamine self-administered into the nucleus accumbens via reverse microdialysis in a two lever operant chamber. Soc Neurosci Abstracts. 2001;27:878.15. [Google Scholar]
- Schildein S, Agmo A, Huston JP, Schwarting RK. Intra-accumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790(1–2):185–94. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Stohr T, Schulte Wermeling D, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacol Biochem Behav. 1998;59(4):813–8. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- Sudakov SK, Goldberg SR, Borisova EV, Surkova LA, Turina IV, Rusakov DJu, et al. Differences in morphine reinforcement property in two inbred rat strains: associations with cortical receptors, behavioral activity, analgesia and the cataleptic effects of morphine. Psychopharmacology. 1993;112(2–3):183–8. doi: 10.1007/BF02244908. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245(1):164–70. [PubMed] [Google Scholar]


