Abstract
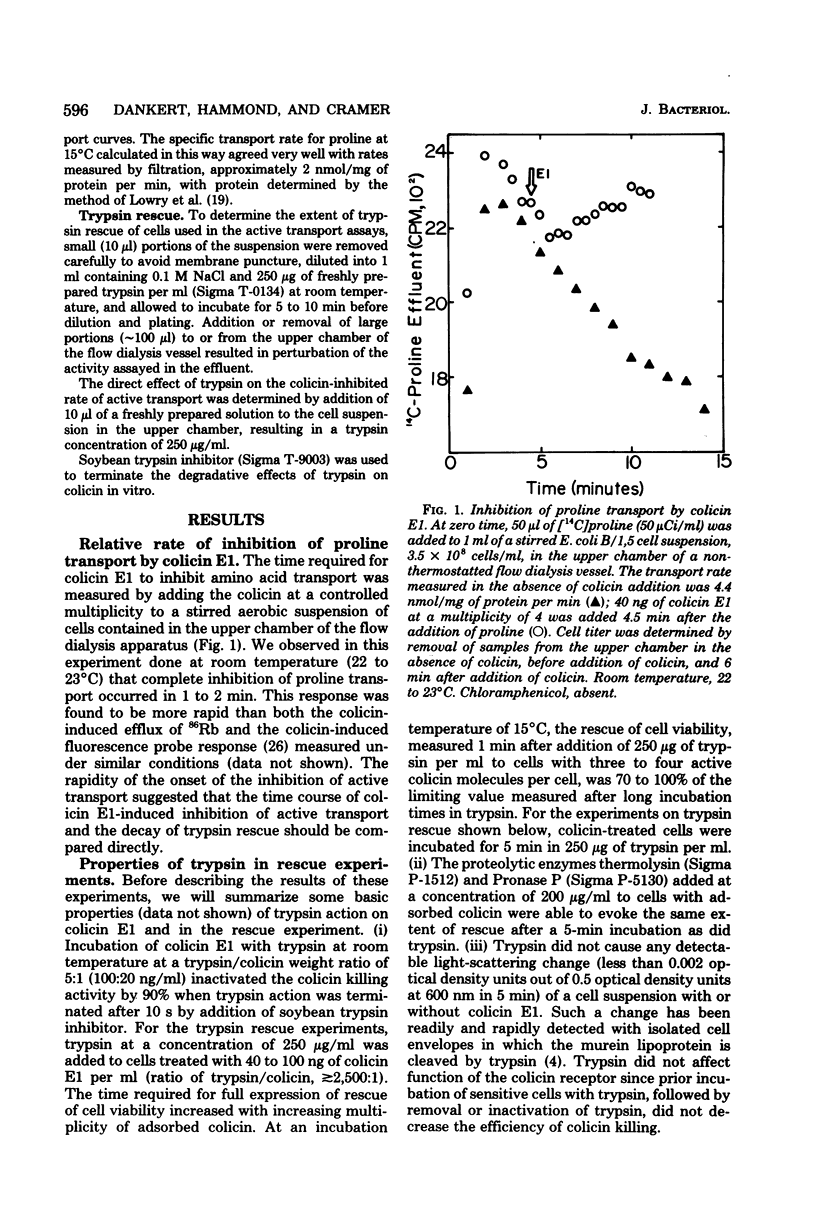
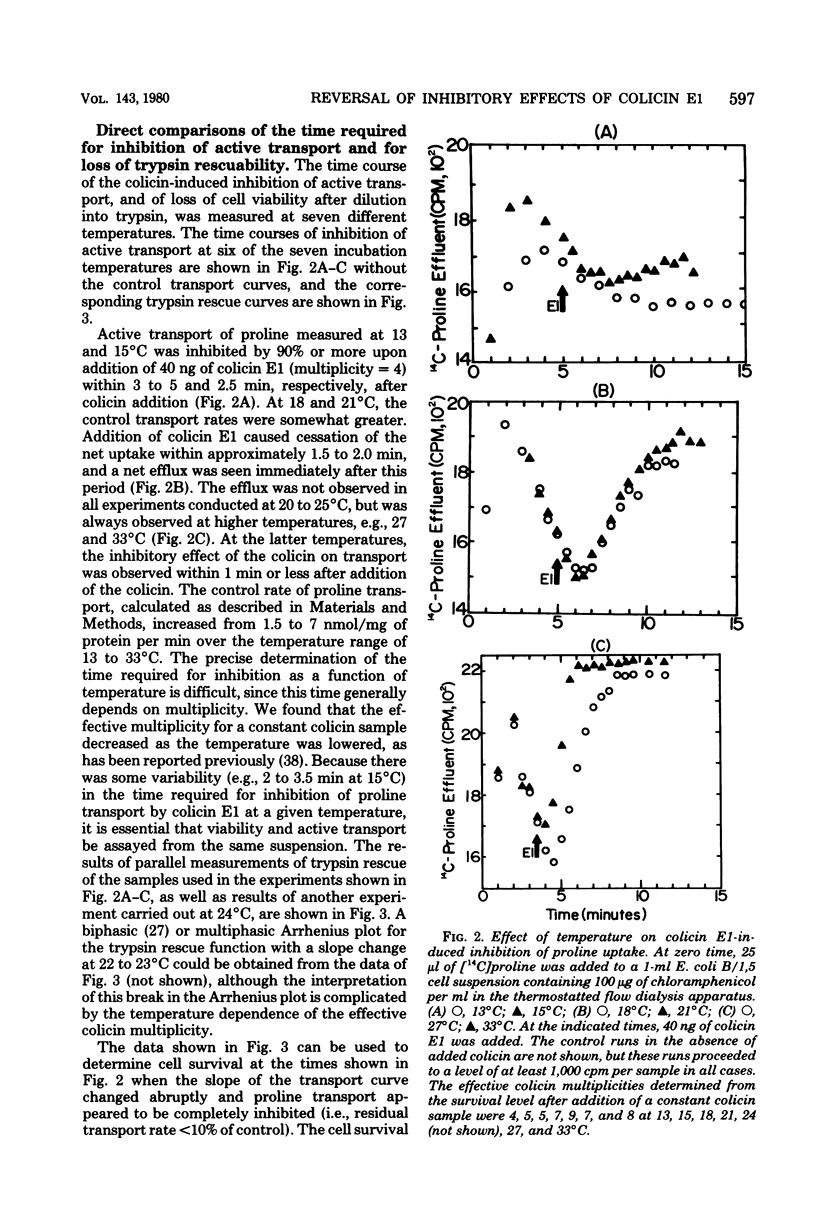
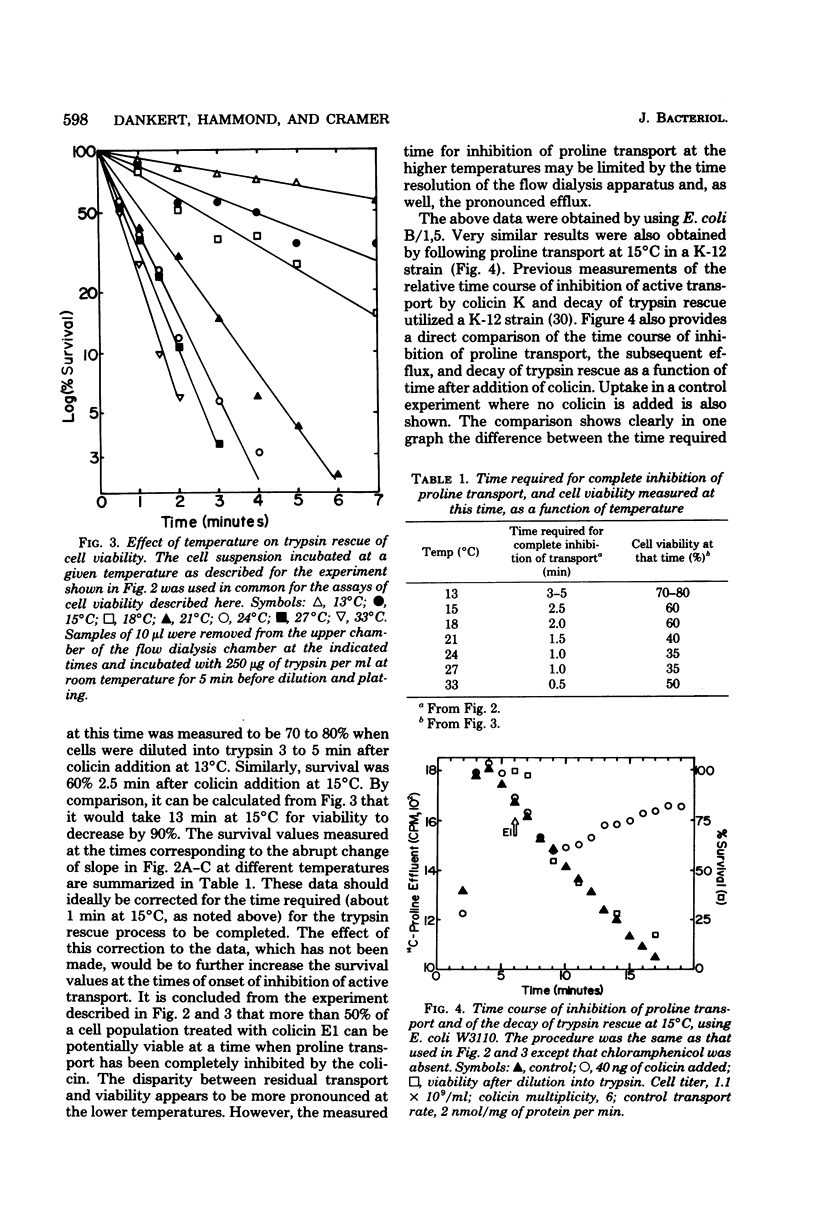
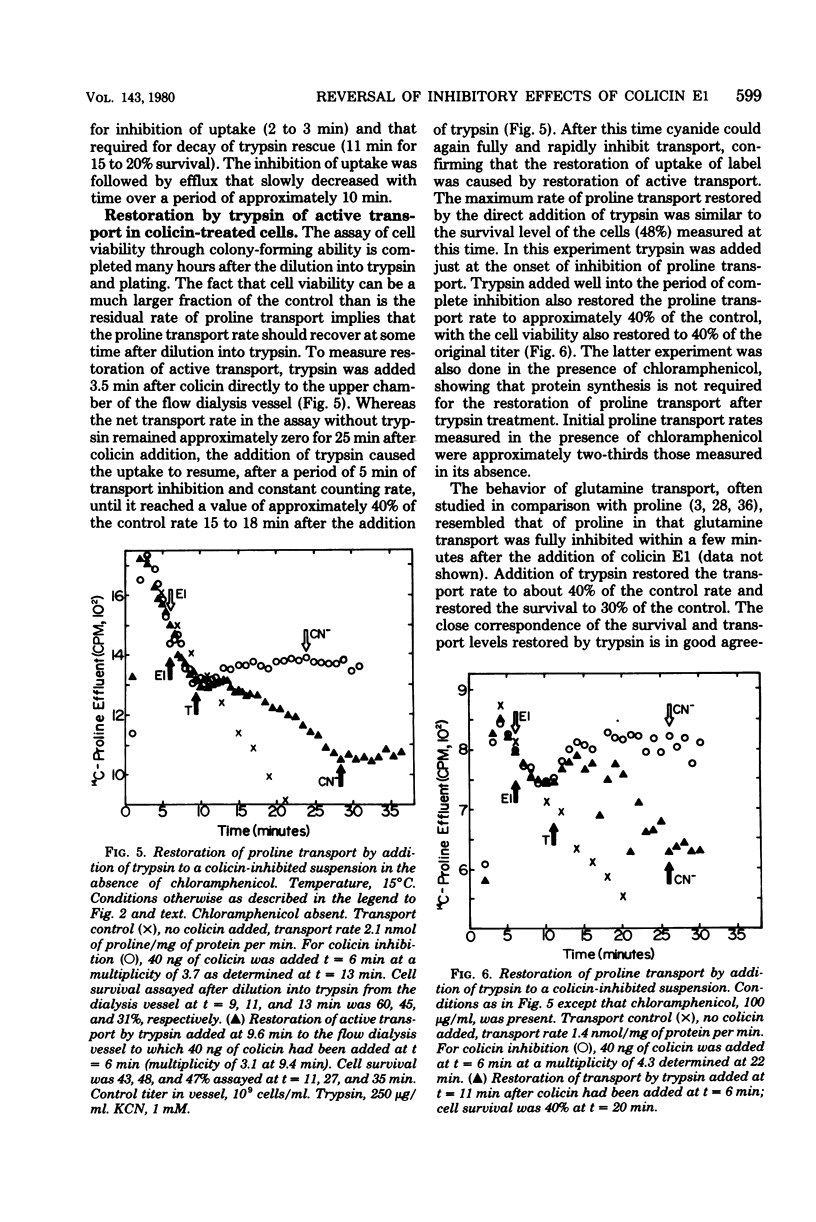
The time course for inhibition of proline transport and irreversible loss of cell viability after treatment with colicin E1 was measured as a function of temperature between 13 and 33 degrees C, using a thermostatted flow dialysis system. Complete inhibition of proline transport at 33 and 13 degrees C occurred in 0.5 min and 3 to 5 min, respectively, after addition of colicin E1 at an effective multiplicity of about 4. At these times, the fractional cell survival, assayed by dilution directly from the flow dialysis vessel into trypsin, ranged from 35 to 80%, with viability always greater than 50% at the lower incubation temperatures. Further studies were carried out at 15 degrees C. Complete inhibition of proline transport, which required 2 to 3 min, occurred much more rapidly at 15 degrees C than did the decay of trypsin rescue, which required 10 to 15 min to reach a survival level of 10 to 20%. The direct addition of trypsin to the flow dialysis vessel, after an addition of colicin E1 that caused complete inhibition of proline or glutamine transport, resulted in restoration of net transport. The restored level was typically about 40% of the control rate, and was very similar to the fractional cell viability measured after incubation in trypsin in the same vessel. It is concluded that trypsin can restore active transport to a significant fraction of a cell population in which transport has been initially inhibited by colicin E1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Beppu T., Kawabata K., Arima K. Specific inhibition of cell division by colicin E 2 without degradation of deoxyribonucleic acid in a new colicin sensitivity mutant of Escherichia coli. J Bacteriol. 1972 May;110(2):485–493. doi: 10.1128/jb.110.2.485-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cavard D., Marotel-Schirmann J., Barbu E. Réversion de la fixation des colicines. C R Acad Sci Hebd Seances Acad Sci D. 1971 Sep 27;273(13):1167–1170. [PubMed] [Google Scholar]
- Cavard D., Rampini C., Barbu E., Polonovski J. Activité phospholipasique et autres modifications du métabolisme des phospholipides consécutives a l'action des colicines sur E. coli. Bull Soc Chim Biol (Paris) 1968 Dec;50(9):1455–1471. [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Cramer W. A., Keenan T. W. Phospholipase A activity is not associated with early effects of colicin E1. Biochem Biophys Res Commun. 1974 Jan;56(1):60–67. doi: 10.1016/s0006-291x(74)80315-3. [DOI] [PubMed] [Google Scholar]
- Dandeu J. P., Billault A., Barbu E. Action des colicines sur la vitesse de sortie du potassium intracellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 17;269(20):2044–2047. [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Konisky J., Tokuda H. Mode of action of colicins Ia, E1 and K. Zentralbl Bakteriol Orig A. 1979 Jun;244(1):105–120. [PubMed] [Google Scholar]
- Kopecky A. L., Copeland D. P., Lusk J. E. Viability of Escherichia coli treated with colicin K. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4631–4634. doi: 10.1073/pnas.72.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Lau C., Richards F. M. Behavior of colicins E1, E2, and E3 attached to sephadex beads. Biochemistry. 1976 Feb 10;15(3):666–671. doi: 10.1021/bi00648a034. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Park M. H. Phospholipase activity plays no role in the action of colicin K. Biochim Biophys Acta. 1975 Jun 11;394(1):129–134. doi: 10.1016/0005-2736(75)90211-4. [DOI] [PubMed] [Google Scholar]
- Marotel-Schirmann J., Cavard D., Sandler L., Barbu E. Temps nécessaire à une colicine fixée sur une bactérie pour déclencher des processus irréversibles. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jan 5;270(1):230–233. [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M., NAKAMURA M. Reversibility of inhibition of nucleic acids and protein synthesis by colicin K. Biochem Biophys Res Commun. 1962 May 4;7:306–309. doi: 10.1016/0006-291x(62)90196-1. [DOI] [PubMed] [Google Scholar]
- Nose K., Mizuno D. Degradation of ribosomes in Escherichia coli cells treated with colicin E2. J Biochem. 1968 Jul;64(1):1–6. doi: 10.1093/oxfordjournals.jbchem.a128853. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Effects of temperature and of fatty acid substitutions on colicin K action. Antimicrob Agents Chemother. 1973 Jul;4(1):16–24. doi: 10.1128/aac.4.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Luria S. E. Stages in colicin K action, as revealed by the action of trypsin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2030–2034. doi: 10.1073/pnas.69.8.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- REYNOLDS B. L., REEVES P. R. Some observations on the mode of action of colicin F. Biochem Biophys Res Commun. 1963 Apr 23;11:140–145. doi: 10.1016/0006-291x(63)90081-0. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Reeves P. R. Kinetics of adsorption of colicin CA42-E2 and reversal of its bactericidal activity. J Bacteriol. 1969 Oct;100(1):301–309. doi: 10.1128/jb.100.1.301-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose P. Sedimentation analysis of DNA degradation products resulting from the action of colicin E2 on Escherichia coli. Biochim Biophys Acta. 1970 Aug 8;213(2):320–334. doi: 10.1016/0005-2787(70)90040-7. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Energetics of galactose, proline, and glutamine transport in a cytochrome-deficient mutant of Salmonella typhimurium. J Supramol Struct. 1977;6(3):389–398. doi: 10.1002/jss.400060312. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. In vivo proteolytic cleavage of colicins requires specific receptor binding. Nature. 1979 Mar 22;278(5702):362–364. doi: 10.1038/278362a0. [DOI] [PubMed] [Google Scholar]
- Wendt L. Mechanism of colicin action: early events. J Bacteriol. 1970 Dec;104(3):1236–1241. doi: 10.1128/jb.104.3.1236-1241.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



